Abstract
Brazilian porcupine poxvirus (BPoPV) is a new poxvirus recently described in porcupines (Coendou prehensilis) from Brazil. Herein, we described a free-ranging adult male Coendou (Sphiggurus) spinosus rescued after being found lethargic on the ground in a rural area. The animal presented crusty, edematous, and suppurative skin lesions on the face, tail, and perineum, and yellowish ocular secretion. The diagnosis was performed by histopathology, transmission electron microscopy (TEM), PCR, and sequencing. Microscopically, proliferative and necrotizing dermatitis, subacute, multifocal with ballooning degeneration, and eosinophilic intracytoplasmic viral inclusion bodies were observed. TEM confirmed large brick-shaped virions inside the keratinocyte cytoplasm, measuring about 200–280 × 120–180 nm. Partial fragment of intracellular mature virion membrane protein gene and putative metalloproteinase gene was successfully amplified and sequenced, and the strain herein denoted IAL/21 V-102 was classified as BPoPV, showing 99.4% of nucleotide identity to the reference strain UFU/USP001. Enrofloxacin 10% (10 mg/kg) was administered every 24 h through intramuscular injection for 10 days, dipyrone/metamizole (25 mg/kg) every 24 h orally (PO) for 3 days, 0.5 ml (mL) of thymomodulin every 24 h PO for 30 days, and each 48 h for another 15 days. The lesions were cleaned and debrided every 15 days. Seventy-five days after the beginning of the treatment, the cutaneous lesions regressed, the animal gained weight, and was clinically stable. After treatment, the skin biopsy showed only mild epidermal acanthosis, intra-cellular edema, and mild lymphoplasmacytic perivascular dermatitis. No viral particles were observed by TEM and no poxviral DNA was amplified by PCR. This study documents the first case of confirmed and treated BPoPV infection in a hairy dwarf porcupine. The implemented therapeutic plan eliminated the infection and improved the general state of the animal.
Supplementary information
The online version contains supplementary material available at 10.1007/s42770-022-00804-3.
Keywords: Chordopoxvirinae, Poxvirus, Erethizontidae, BPoPV
Introduction
Poxviridae is a family of large, brick-shaped or ovoid, linear double-stranded DNA viruses, divided into two subfamilies based on host range, consisting of the Entomopoxvirinae (poxviruses of insects) and the Chordopoxvirinae (poxviruses of vertebrates) [1]. Zoonotic poxviruses can cause infections in humans, livestock, and wild animals, representing potential threats to public health, management, and conservation species’ programs and leading to local economic impairment [2]. Recently, several novel poxviruses have been discovered after infecting humans, domestic and wild animals [3].
In this context, a newly proposed species not yet recognized by the International Committee on Taxonomy of Viruses (ICTV), the BPoPV, was a recently described poxvirus in a free-living porcupine (Coendou prehensilis) from Uberlândia, Minas Gerais, Brazil, associated with epidermal hyperplasia, ballooning degeneration, and intracytoplasmic inclusion bodies in keratinocytes [4]. In 2021, another infected Coendou prehensilis was identified in Cuiabá, Mato Grosso, with the same cutaneous lesions pattern [5]. New World porcupines (Erethizontidae family) are nocturnal and arboreal mammals characterized by hairs modified into sharped quills and prehensile tails [6]. Erethizontids are distributed from Canada to Uruguay and Argentina. Brazil has the highest diversity of erethizontids in a wide range of habitats, including the Atlantic forest, Cerrado (neotropical savanna), and Pantanal [7]. The increasingly close contact between porcupines, humans, and domestic animals due to the changes in the environments raises concerns about the zoonotic potential of BPoPV. This study aimed to report, for the first time, the clinical features, diagnostic techniques, and treatment in a free-ranging hairy dwarf porcupine, Coendu (Sphiggurus) spinosus (F. Cuvier 1823) infected with Brazilian porcupinepox virus from Araçoiaba da Serra, São Paulo, Brazil.
Material and methods
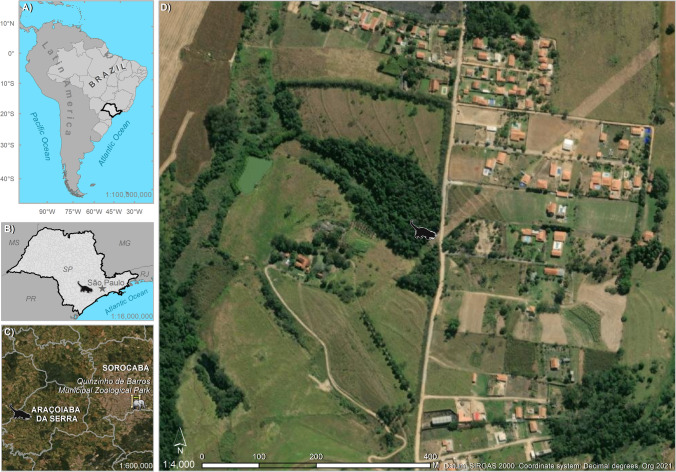
On March 17, 2021, a free-ranging, male, adult, hairy dwarf porcupine (Coendu (Sphiggurus) spinosus, F. Cuvier 1823) was found by the environmental surveillance service in a rural area with urban characteristics in the municipality of Araçoiaba da Serra, São Paulo, Brazil (− 23.5278, − 47.7132). The animal was found lethargic on the ground and was transferred to the Quinzinho de Barros Municipal Zoological Park in Sorocaba, São Paulo, Brazil, for veterinary evaluation. ArcGIS 10.2.2 software was used for the geographic illustration (Image, ESRI) (Fig. 1).
Fig. 1.
A Location of the state of Sao Paulo in Brazil and Latin America; B location of the porcupine and the capital of the state of Sao Paulo; C location of the zoological park and the porcupine on the municipal scale; D the landscape where the porcupine was found
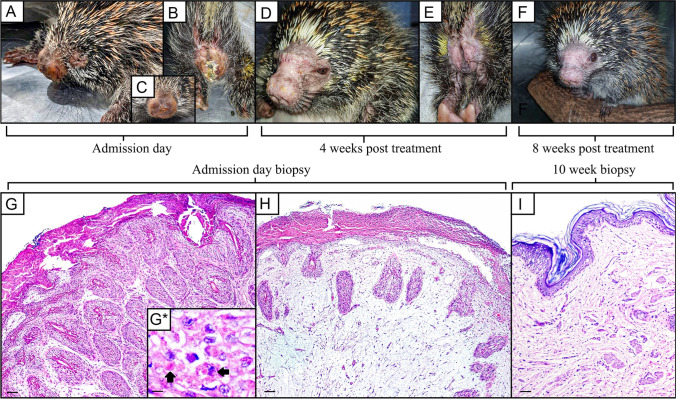
On clinical examination, the porcupine presented a regular body score and multiple erythematous and edematous skin lesions with ulcerative foci, exudation, and crust formation, especially on the muzzle, tail, and perineal regions (Fig. 2). Purulent ocular discharge was also observed.
Fig. 2.
Gross and microscopic findings. Multiple erythematous and edematous skin lesions with ulcerative foci, exudation, and crust formation, especially on the muzzle, and perineal regions. Admission day (A–C), 4 weeks post treatment (D–E), 8 weeks post treatment (F). On admission day, microscopically pattern characterized by irregular hyperplasia of the epidermal and follicular epithelium, with marked proliferation, up to five times of normal thickness, of the stratum spinosum cells (acanthosis) and long rete ridges (G H&E, × 4; scale bar = 100 µm). Variably keratinocytes contain eosinophilic intracytoplasmic viral inclusion bodies (arrows) (G*. H&E, × 40; scale bar = 10 µm). Deep dermis showing a moderate number of viable and degenerate neutrophils, macrophages, lymphocytes, and plasma cells separated by abundant, loose, and edematous matrix, sometimes with hemorrhagic areas (H H&E, × 4; scale bar = 100 µm). Mild epidermal acanthosis after the treatment (I H&E, × 4; scale bar = 100 µm)
Skin fragments of the perineum and tail were excised by incisional biopsy before and 75 days after the beginning of the treatment. Samples fixed immediately in 10% neutral buffered formalin were processed routinely, embedded in paraffin wax, sectioned at 4 μm thick, and stained with hematoxylin and eosin (H&E) for routine microscopic analysis for histopathological analysis. For transmission electron microscopy (TEM), formalin-fixed and paraffin-embedded skin samples were deparaffined in xylol, dehydrated, and then fixed in glutaraldehyde 2.5% (Sigma, St Louis, MO, USA) in 0.1 M sodium cacodylate buffer, pH 7.2, overnight. The tissue was post-fixed in a solution containing 1% osmium tetroxide and incubated for 2 h, then was stained in block with 0.5% uranyl acetate overnight, dehydrated in graded acetone, and embedded in EPON 812 resin (Sigma, St. Louis, MO, USA). Ultrathin sections were obtained from 100 nm cuts in Sorvall MT 2-B (Porter Blum) ultramicrotome (Sorvall, Newtown, CT, USA) stained with lead citrate and examined in a transmission electron microscope JEM-1011 (JEOL, Tokyo, Japan) operating at 80 kV.
Total DNA from fresh frozen skin fragments were extracted with BioGene Viral DNA/RNA extraction kit (Bioclin, Belo Horizonte, Brazil) according to the manufacturer’s instruction and subjected to a pan-pox universal PCR assay, as previously described [8], using the forward primer 5′-ACA CCA AAA ACT CAT ATA ACT TCT (insulin metalloproteinase-like protein gene), the reverse primer CCT ATT TTA CTC CTT AGT AAA TGA T (intracellular mature virion [IMV] membrane protein gene), and GoTaq® Green Master Mix (Promega, Madison, WI). PCR products were separated by electrophoresis in 2% agarose gels, stained with GelRed (Biotium), and examined under ultraviolet light. The amplified PCR fragment was purified (QIAquick PCR Purification Kit, Qiagen, Hilden, Germany) and directly sequenced through the ABI BigDye Terminator cycle sequencing kit, version 3.1, in an automated sequencer (ABI Prism 3500 Genetic Analyzer; Applied Biosystems, CA, USA). Sequencing chromatograms were edited manually using Sequencher™ 4.7 software (Gene Codes Corporation, Michigan, EUA). Assembled contigs were subjected to a homology search in the GenBank/EMBL/DDBJ database using Blast 2.10.1 software (http://www.ncbi.nlm.nih.gov/blast/).
The species verification (BPoPV) was conducted using the MEGA X software (Kumar et al. 2018). The IAL/21 V-102 BPoPV strain described in this study was aligned with the prototype UFU/USP001 BPoPV strain (MN692191), as well as 15 reference poxvirus sequences from distinct animal species available in GenBank, using the BioEdit software, version 7.0.5.2 (Ibis Therapeutics, USA). Neighbor-joining (NJ) trees were constructed based on a Kimura 2-parameter model determined by MEGA X [9] with 1000 bootstrap replicates. Nucleotide sequences determined in this study have been deposited in GenBank under the accession number ON322740.
Results
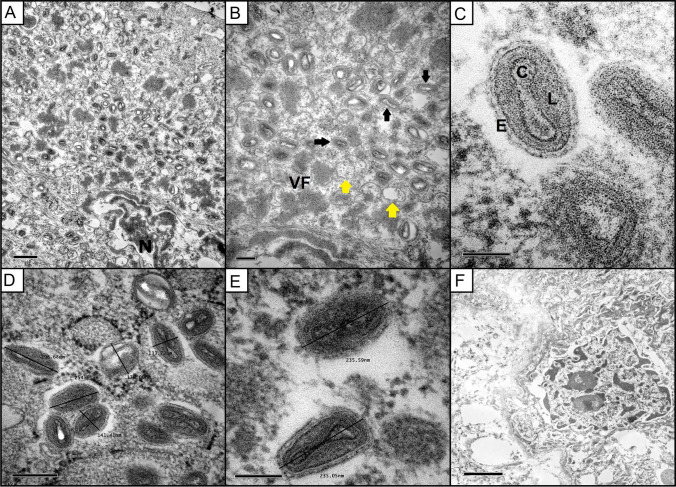
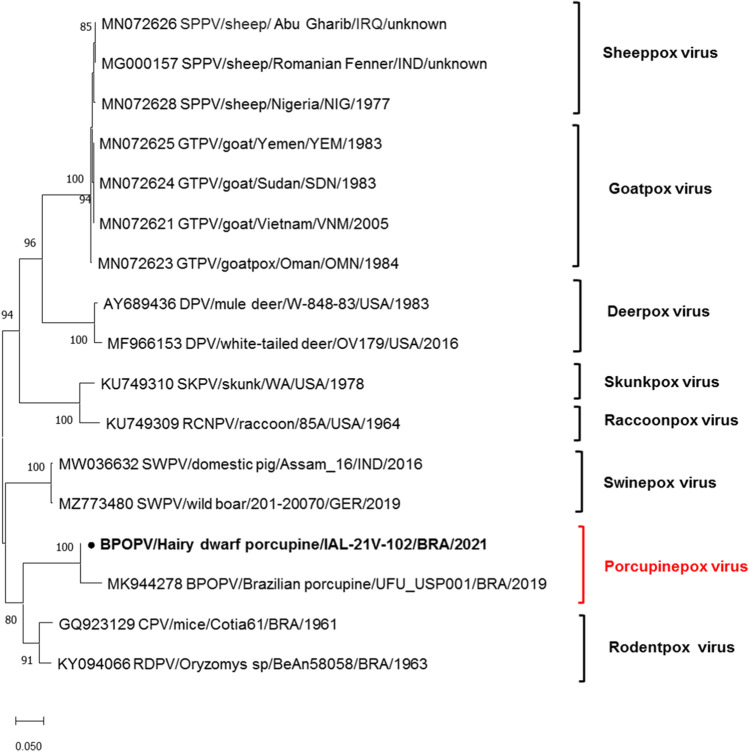
Microscopically, all sections of skin fragments obtained on the first day of hospitalization, before the treatment, revealed the same histopathological pattern characterized by irregular hyperplasia of the epidermal and follicular epithelium with marked proliferation, up to five times of normal thickness, of the stratum spinosum cells (acanthosis) and long rete ridges (Fig. 2). The majority of keratinocytes into stratum spinosum were remarkably hypertrophied, swollen, and vacuolated (ballooning degeneration), and variably contain eosinophilic intracytoplasmic viral inclusion bodies (type A or acidophilic-type inclusions), with 10 to 15 µm in diameter. Neutrophilic exocytosis and multiple single-cell necrotic/apoptotic keratinocytes were also presented. A thick serocellular crust composed of cytolytic and karyorrhetic debris mixed with bacterial colonies overlaid multifocally the eroded epidermis. Infiltrating into the superficial and deep dermis are a moderate number of viable and degenerate neutrophils, macrophages, lymphocytes, and plasma cells separated by abundant, loose, and edematous matrix, sometimes with hemorrhagic areas. Small caliber vessels within the superficial and deep dermis are lined by markedly hypertrophied endothelium, especially in these areas with extensive epidermal erosion and filled with red blood cells. TEM demonstrated enveloped, brick-shaped and ovoid virions, mature virus particles of approximately 200–280 120–180 nm, dumbbell-shaped core, flanked by two lateral bodies and surrounded by at least one complex coat or envelope in the cytoplasm of the keratinocytes, characteristic of poxvirus particles (Fig. 3). Aggregates of partially and fully formed viral particles accumulated in the cytoplasm. Progeny virus developed in the cytoplasm in association with aggregates of dense fibrous or granular material (“viroplasm”). Early immature particles appeared spherical, consisting of a unit membrane envelope from which project distinct spicules, surrounding a dense central nucleoid. During maturation, the particles became elongated and acquire lateral bodies and an external envelope. No viral particles were identified in dermal mesenchymal cells. A 231-pb PCR-fragment was obtained by sequencing (Supplementary material). The species verification and the phylogenetic analysis were performed with the PCR fragment for the insulin metalloproteinase-like gene. The genetic tree includes the strain sequenced in this study (highlighted in bold) along with prototype sequences obtained from GenBank. The IAL/21 V-102 strain was classified as Brazilian porcupinepox virus (BPoPV), forming a well-supported monophyletic group with a bootstrap value of 100% (Fig. 4). The IAL/21 V-102 BPoPV strain showed 99.4% similarity at nucleotide level compared to the UFU/USP001 prototype strain (GenBank accession number MN692191). In addition, IAL/21 V-102 BPoPV strain displayed 86.7 and 85.3% nucleotide similarity when compared to the newly described Brazilian rodent poxvirus species Cotia virus and BeAn58058 strains, respectively. Lower genetic homology was observed between IAL/21 V-102 BPoPV strain and poxviruses detected in sheep, goats, deers, skunks, raccoons, and pigs (72.1–82.6% nt) as stressed in the phylogenetic tree (Fig. 4).
Fig. 3.
Overview of ultra-thin sections of a porcupine keratinocyte infected with the Brazilian porcupinepox virus (BPoPV). A An area of replicating BPoPv contains virus particles at different stages of development, N = nuclei (scale bar = 0.5 µm). B Virus particles were formed in the cytoplasm from a “viral factory” (VF), aggregates of a dense fibrous or granular material. Immature spherical particles with external surface spicules (yellow arrows) are observed. And mature viral particles that appear elongated and acquire an outer envelope (black arrows). (Scale bar = 0.2 µm). C Thin sectioned intracellular mature poxvirus particle displaying dumbell-shapped core (C), a dense granular lateral body (L), and external coat (E) (scale bar = 100 nm). D and E Morphology and measurements of virions (scale bar = 200 and 100 nm, respectively). F Noninfected queratinocyte 75 days after the beginning of the treatment (scale bar = 2 µm)
Fig. 4.
Nucleotide-sequence-based similarity analysis of the IAL/21 V-102 BPoPV sample (indicated in bold) to other selected poxvirus strains. A neighbor-joining tree of partial putative metallo-protease gene sequences was generated using MEGA X software. Reference poxvirus strains were obtained from the GenBank database. The accession number, species, isolates, country, and year of each strain are indicated. The scale indicates the number of divergent nucleotide residues. Bootstrap values are shown as percentages at the branch nodes
The animal was treated with 10 mg/kg of enrofloxacin (Chemitril® 10%, Chemitecé®, Brazil) intramuscularly every 24 h for 10 days, and with dipyrone (metamizole, synonymously dipyrone, 25 mg/kg) orally every 24 h for 3 days. Fifteen milliliters of saline solution NaCl 0.9% was subcutaneously injected for hydration on the first day. Peptidic bovine thymus extract (Thymomodulin, Leucogen®, Aché Laboratórios Farmacêuticos, Brazil) was orally administered at a dose of 0.5 mL every 24 h from the second day until the 30th day of the hospitalization, and every 48 h for another 15 days. Every 15 days, the porcupine was chemically contained with 10% ketamine (20 mg/kg) and 2% xylazine (1.5 mg/kg) and kept in an isoflurane inhalation mask for debridement, irrigation, and wound cleaning with saline solution (NaCl 0.9%) and povidone-iodine 10%.
After treatment, the animal was clinically stable and gained 120 g of weight. The skin lesions completely regressed without any scar or sequelae. No further treatment was needed. Histopathological examination of the excised skin fragment on the 75th day after the beginning of the treatment revealed mild epidermal acanthosis and lymphoplasmacytic perivascular dermatitis (Fig. 2). PCR was negative for low-CG content poxvirus, and no viral particles were observed by transmission electron microscopy (Fig. 3).
Discussion
There are few studies on the detection and circulation of poxviruses in Brazilian wildlife. However, in recent years, a growing number of poxvirus isolates have been obtained from samples from humans, wild and domestic animals [10]. Furthermore, in Brazil, new cases of poxviruses infection in porcupines have been increasingly described in recent years [4, 5]. Here we report the second BPoPV strain described in Brazil. Phylogenetic analysis of IAL/21 V-102 BPoPV strain also exhibited moderately high nucleotide identity with Cotia virus, a poxvirus isolated from sentinel suckling mice in 1961 from São Paulo, Brazil, and with BeAn 58,058 poxvirus strain, isolated from a wild rodent Oryzomys sp. in the Utinga forest, Belém, state of Pará, Brazil in 1963 [11, 12]. However, the exact correlation between these viruses and the BPoPV was not established since the maximum-likelihood distances for this region of conserved nucleotides demonstrated that viruses probably belong to different genres [4].
The porcupine was rescued from a native vegetation agglomeration, near water bodies, a dirt road, and fewer than 150 m from households in an area of recent expansion with rural/tourism characteristics. Humans have been occupying natural areas and changing the environments, forcing wild animals to move and adapt to new habitats [2]. These changes should be investigated in the role of the emergence or reemergence of potentially infectious agents and zoonosis. In addition, the lesions caused by poxviruses in free-living animals can be indicators of low environmental quality and stress predisposing conditions [13].
The zoonotic potential and impact for porcupine conservation of BPoPV are still unknown. Therefore, treatment strategies seeking to heal skin lesions and reduce virus transmission are essential. In general, poxvirus infections are self-limiting, and treatment is primarily supportive care [14]. Cell-mediated immunity is particularly critical for the clearance of poxvirus-infected cells, requiring innate effector cells (natural killer cells) and adapted effector cells (cytotoxic T lymphocytes) [15, 16]. Also, poxviruses inhibit pro-inflammatory cytokines such as tumor necrosis factors (TNF) and interferons (IFN), leading to a downregulation expression of class I MHC molecules [16]. Thymomodulin (TMD) is a calf thymus acid lysate derivative, composed of several peptides that can enhance the functions of mature T-lymphocytes with cascading effects on B-cell, macrophage functions, and the cytotoxic activity of natural killer cells [17]. In vitro, TMD also increases the release of granulocyte–macrophage colony-stimulating factor and TNF, IFN-y, and interleukin-1β [18]. The use of immunomodulators molecules as a supportive treatment has already been described in cases of avipoxvirus infection in Psittaciformes [19]. Antibacterial therapy is necessary for poxviruses infections when secondary infection is present [13]. In this case, a combination of immunostimulatory and antimicrobial therapy with mechanical debridement and wound cleaning was demonstrated to be effective in the regression of lesions and viral clearance.
Conclusion
To the best of our knowledge, this is the first report describing the treatment options for Brazilian porcupine poxvirus infection in a free-ranging hairy dwarf porcupine, including clinical, molecular, and microscopic features. It reinforces the importance of wildlife surveillance to detect potential zoonotic diseases, especially in areas with anthropogenic environmental changes.
Supplementary information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank all professionals and fellows at the Biological Sample Management Center and Pathology Center of Adolfo Lutz Institute.
Author contribution
JMG and PEND collected the results and analyzed the data. TBE, EFM, and NCCA performed the histopathology analysis. KBF, ACSRC, and AL carried out the molecular analysis and sequencing. MLS, MGC, ALMC, RHFT, and TEMB performed the clinical follow-up, treatment, and collection of samples. GMN, LBL, and NNT performed the transmission electron microscopy technique and analysis. PSSM mapped and analyzed the location of the occurrence. JMG drafted the first version of the manuscript and PEND assembled picture panels. All the authors revised and improved the manuscript and finally approved the submitted version.
Funding
This work received financial support from the Special Health Fund for Mass Immunization and Disease Control (GAPS/FESIMA grant # 28/2020 and # 040/2019) of the Health Department of the State of São Paulo and the National Council for Scientific and Technological Development (CNPq) process number 404510/2021–3. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Declarations
Ethics approval
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to. This study involved the use of nonexperimental animals for diagnostic procedures and followed established recognized high standards (“best practice”) of individual veterinary clinical patient care. Ethical approval from a committee was not necessary, since the samples were taken by the attending veterinarian during the health monitoring of the animal, as part of the surveillance of infectious diseases in the wildlife animals program.
Consent for publication
The authors have consented to the submission of this case report to the journal.
Conflict of interest
The authors declare no competing interests.
Footnotes
Responsible Editor: Flavio Guimaraes Fonseca
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Oliveira G, Rodrigues R, Lima M, Drumond B, Abrahão J. Poxvirus host range genes and virus–host spectrum: a critical review. Viruses. 2017;9:331. doi: 10.3390/v9110331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Essbauer S, Pfeffer M, Meyer H. Zoonotic poxviruses. Vet Microbiol. 2010;140:229–236. doi: 10.1016/j.vetmic.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gigante CM, Gao J, Tang S, McCollum AM, Wilkins K, Reynolds MG, Davidson W, McLaughlin J, Olson VA, Li Y. Genome of alaskapox virus, a novel orthopoxvirus isolated from Alaska. Viruses. 2019;11:708. doi: 10.3390/v11080708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hora AS, Taniwaki SA, Martins NB, Pinto NNR, Schlemper AE, Santos ALQ, Szabó MPJ, Brandão PE. Genomic analysis of novel poxvirus Brazilian porcupinepox virus, Brazil 2019. Emerg Infect Dis. 2021;27:1177–1180. doi: 10.3201/eid2704.203818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maruyama FH, Neres RWP, Souza MA, Morgado TO, Ribeiro M, Correa SHR, Rosa JMA, Borges JC, Dutra V, Nakazato L (2021) Molecular detection of Brazilian porcupuinepox virus in Coendu Prehensilis in Mato Grosso, Brazil. In: Anais da Sociedade Brasileira de Microbiologia, R0928.
- 6.Menezes FH, Garbino GST, Semedo TBF, Lima M, Feijó A, Cordeiro-Estrela P, Costa IR. Major range extensions for three species of porcupines (Rodentia: Erethizontidae: Coendou) from the Brazilian Amazon. Biota Neotrop. 2020;20:2. doi: 10.1590/1676-0611-bn-2020-1030. [DOI] [Google Scholar]
- 7.Voss RS, Hubbard C, Jansa AS. Phylogenetic relationships of new world porcupines (Rodentia, Erethizontidae): implications for taxonomy, morphological evolution, and biogeography. Am Museum Novit. 2013;3769:1–36. doi: 10.1206/3769.2. [DOI] [Google Scholar]
- 8.Li Y, Meyer H, Zhao H, Damon IK. GC content-based pan-pox universal PCR assays for poxvirus detection. J Clin Microbiol. 2010;48:268–276. doi: 10.1128/JCM.01697-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35(6):1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Trindade G, Guimarães da FonsecaMarques SFJT, Nogueira ML, Mendes LCN, Borges AS, Peiró JR, Pituco EM, Bonjardim CA, Ferreira PCP, Kroon EG. Araçatuba virus: a vaccinialike virus associated with infection in humans and cattle. Emerg Infect Dis. 2003;9:155–160. doi: 10.3201/eid0902.020244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Afonso PP, Silva PM, Schnellrath LC, Jesus DM, Hu J, Yang Y, Renne R, Attias M, Condit RC, Moussatche N, Damaso CR. Biological characterization and next-generation genome sequencing of the unclassified cotia virus SPAn232 (Poxviridae) J Virol. 2012;86:5039–5054. doi: 10.1128/JVI.07162-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wanzeller ALM, Souza ALP, Azevedo RSS, Júnior ECS, Filho LCF, Oliveira RS, Lemos PS, Júnior JV, Vasconcelos PFC. Complete genome sequence of the BeAn 58058 virus isolated from Oryzomys sp rodents in the Amazon Region of Brazil. Genome Announc. 2017;2(5):9. doi: 10.1128/genomeA.01575-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cocumelli C, Fichi G, Marsili L, Senese M, Cardeti G, Cersini A, Ricci E, Garibaldi F, Scholl F, Di Guardo G, Terracciano G. Cetacean poxvirus in two striped dolphins (Stenella coeruleoalba) stranded on the Tyrrhenian coast of Italy: histopathological, ultrastructural, biomolecular, and ecotoxicological findings. Front Vet Sci. 2018;11:5. doi: 10.3389/fvets.2018.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tack DM, Reynolds MG. Zoonotic poxviruses associated with companion animals. Animals. 2011;1:377–395. doi: 10.3390/ani1040377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buller RM, Palumbo GJ. Poxvirus pathogenesis. Microbiol Rev. 1991;55:80–122. doi: 10.1128/mr.55.1.80-122.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seet BT, Johnston JB, Brunetti CR, Barrett JW, Everett H, Cameron C, Sypula J, Nazarian SH, Lucas A, McFadden G. Poxviruses and immune evasion. Annu Rev Immunol. 2003;21:377–423. doi: 10.1146/annurev.immunol.21.120601.141049. [DOI] [PubMed] [Google Scholar]
- 17.Kouttab NM, Prada M, Cazzola P. Thymomodulin: biological properties and clinical applications. Med Oncol Tumor Pharmacother. 1989;6:5–9. doi: 10.1007/BF02985217. [DOI] [PubMed] [Google Scholar]
- 18.Balbi B, Valle MT, Oddera S, Balleari E, Manca F, Rossi GA, Allegra L (1992) Thymomodulin increases release of granulocyte-macrophage colony stimulating factor and of tumour necrosis factor in vitro. Eur Respir J 1992 5:1097–103. http://www.ncbi.nlm.nih.gov/pubmed/1426220 [PubMed]
- 19.Godoy SN, Cubas ZS. Doenças virais e parasitárias em Psittaciformes - revisão. Clínica Veterinária. 2011;90:32–44. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.