Abstract
This paper aimed to screen the enzymatic activities and evaluate the carotenoid production level of twenty-two halophilic archaea isolated from Sfax solar saltern sediments. The molecular identification performed by sequencing the 16S rRNA genes showed that all strains have a high similarity degree (99.7–100%) with Halobacterium salinarum NRC-1. The strains were screened for the presence of eight hydrolase activities using agar plate-based assays. The most detected enzyme was gelatinase (77.27% of total strains), followed by protease (63.63%) and amylase activities (50%). The carotenoid production yields of the strains ranged between 2.027 and 14.880 mg/l. The UV–Visible spectroscopy of pigments revealed that it was a bacterioruberin type. When evaluated and compared to the standard β-carotene, the antioxidant capacities of these pigments showed a scavenging activity of more than 75% at a concentration of 5 μg/ml for three strains (AS16, AS17, and AS18). Then a sequence of one-step optimization processes was performed, using the one-factor-at-a-time approach, to define the optimum conditions for growth and carotenoid production of the highest carotenoid producing strain (AS17). Different environmental factors and nutritional conditions were tested. Variations in these factors were found to deeply influence growth and carotenoid production. A maximum carotenoid production (16.490 mg/l), higher than that of the control (14.880 mg/l), was observed at 37 °C, pH 7, 250 g/l of salinity, with 80% air phase in the flask at 110 rpm, in presence of light and in culture media containing (g/l) 10, yeast extract; 7.5, casamino acid; 20, MgSO4; 4, KCl; and 3, trisodium citrate.
Keywords: Archaea, Enzymes, Carotenoids, Bacterioruberin, Antioxidant
Introduction
Natural and artificial hypersaline environments are widely distributed around the world. They include solar salterns, coastal lagoons, natural salt lakes, hypersaline soils, springs, salt marshes, and salt deposits [1]. Halophilic archaea, the most abundant inhabitant of such environments face several oxidative stress conditions due to not only the high level of salinity but also to other extreme environmental parameters such as temperature and radiation. Furthermore, the significant effect of contaminants such as heavy metals could not be ignored [2, 3].
Microorganisms have developed different strategies to cope with extreme environmental conditions. The production of carotenoids is one of the most important strategies helping them to adapt, survive, and grow [4–7]. These archaea can be considered a commercial alternative source of carotenoids compared to macroalgae, plants, and chemical-production methods. They provide high growth rate, high productivity independently of the season and geographical conditions, and easy carotenoid extraction and purification with low cost [8, 9]. In addition, their high-salt tolerance enables their cultivation under non-sterile conditions reducing their production cost [10]. However, the bioactive properties of such haloarchaea remain scarcely studied compared to other groups (plants and algae) [11]. The carotenoids have received considerable attention due to their biotechnological potential, especially in the medical field as antioxidants, antitumor, antimicrobial, antiviral, and anti-inflammatory agents [12–17]. Nutritional and physical factors may influence the carotenoid synthesis by halophilic archaea [18]. They are involved in the regulation of carotenoid biosynthesis, as, for example, salinity, pH, oxygen concentration, and light exposure [19–21]. Besides, the oxidative stress has an impact on the carotenoid production, in particular bacterioruberin. The latter showed a positive correlation with increasing oxidant concentration (H2O2) [6].
Halophilic archaea are able to produce various extracellular enzymes involved in nutrient cycles of carbon and nitrogen [22], with stable and optimal activity under high temperature, salt concentration, and extreme pH [7, 11, 23–25]. Enzymes from extremely halophilic archaea are rich in acidic amino acids. They exhibit high-density surface charges [23]. Such halophilic enzymes are expected to be a very powerful tool and biocatalyst in industrial biotransformation processes (food industry, feed additive, washing, biomedical sciences, chemical industries, and environmental bioremediation) [24]. Several studies have been conducted to assess their industrial applicability, in particular, for cellulase, lipase, protease, amylase, xylanase, DNAase, nuclease, and others [18, 23, 24, 26, 27].
Sfax solar saltern, located in the central east area of Tunisia, is an extremely saline environment explored for NaCl production. Since the 1950s, it is fully exposed to industrial particulate fallouts enriched with heavy metals (Cd, Pb, Ni, Cu, and Zn) emanating mainly from the SIAPE factory (treatment of phosphate) and the lead melting industry [28]. Several geomicrobiological studies proved that the highly metal contaminated superficial sediments are mainly related three ponds called TS35, TS36, and S6 [29–31]. Through the isolation of twenty-two archaeal strains from the superficial sediments of such ponds, Baati et al. [32] showed their tolerance to high Pb, Cd, and Ni concentrations varying from 2.5 to 4.5 mM.
In this complementary study, the isolated strains were evaluated for other potential biotechnological applications, in terms of hydrolytic enzyme activities and carotenoid production. The effects of different environmental parameters such as temperature, pH, salinity, light, agitation, and nutrition on growth and carotenoid production of the most carotenoid-producing strain were also tested.
Materials and methods
Archaeal strains, growth conditions
The 22 archaeal strains used in this study were isolated from superficial sediment samples collected aseptically, during May 2017, from TS35, TS36, and S6 ponds of Sfax solar saltern (Tunisia). As previously described [29–32], these ponds were highly enriched with Cd, Pb, Ni, Cu, and Zn. These archaeal strains were selected based on both their morphology and pigmentation and were later purified through repeated sub-culturing. They grow at 37 °C in DSC-97 medium containing (g/l) yeast extract, 10; casamino acids, 7.5; NaCl, 250; MgSO4·7H2O, 20; KCl, 2; and trisodium citrate, 3 [33]. The pH was adjusted to 7. The strains were stored at − 80 °C in 20% glycerol (w/v).
Strain physiological characteristics
Cell shape and motility of the isolated strains were examined by light microscopy of exponentially growing liquid culture. Gram staining was tested according to Dussault [34]. Oxidase and catalase activities were detected as described by Oren et al. [35]. Nitrate and nitrite reduction and indole formation were performed according to the method of Gerhardt et al. [36]. The archaeal strain growth at different temperatures (4, 20, 30, 35, 40, 45, and 50 °C), pH (4, 5, 6, 7, 8, 9, and 10), and salt concentrations (0, 10, 15, 20, 25, 30, and 35%) was determined on a DSC-97 agar medium. The growth was carried out by spreading 20 μl of a culture suspension of each strain onto the surface of the respective media for 3 weeks.
DNA extraction, PCR amplification, DNA sequencing, and phylogenetic analysis
Cells were harvested by centrifugation (8000 g for 30 min), and the genomic DNA was extracted using Genomic DNA Purification Kit (NucleoSpin Tissue Kit, Macherey Nagel) according to the manufacturer’s protocol. The PCR amplification of the 16S rRNA genes was performed using TaKaRa Ex TaqTM (2.5 units, Promega) in 50-μl reaction buffer, containing 2 mM of each dNTP (dATP, dTTP, dGTP, dCTP), 20 μl of each primer (0.5 µM), and 5 μl of 10 × Ex Taq bufferTM [37]. The used primers were archaeal-specific 21 F [38] combined with the universal reverse primer 1390R [39]. The PCR amplification was carried out according to the following program: initial denaturation at 94 °C for 5 min and 24 cycles consisting of denaturation at 94 °C for 1 min, primer annealing at 59 °C for 1 min, and extension at 72 °C for 1.5 min. A final elongation step was performed for 15 min. The PCR product was sequenced using an automated Sanger sequencer at the DNA core facility at the National Center for Agricultural Utilization Research Center in Peoria, IL, USA. The resulting 16S rRNA genes sequences were compared to those available at the EzBiocloud [40]. The retrieved data were aligned, and nucleotide substitution model testing was performed using MEGA-X software [41]. The neighbor-joining tree was determined using the Tamura-Nei model (0.40, gamma distributed with invariant sites) based on model testing under MEGA X [41]. Measures of bootstrap support for internal branches were obtained from 1500 pseudoreplicates.
The sequence data obtained in this study has been submitted to EMBL/GenBank databases under the accession numbers: MT332425, MT735588, MT735589, MT735627, MT735628, MT738102, MT738362, MT738363, MT738364, MT738365, MT738366, MT738367, MT738368, MT738369, MT738370, MT738371, MT738372, MT738373, MT741675, MT741676, MT741677, and MT741679.
Screening for extracellular hydrolytic enzymes
All the strains were tested for their ability to produce enzymes by their streaking onto appropriate solid media containing the corresponding substrates. The pH of all media was adjusted to 7, and 25% total salt was added for detecting extracellular enzymatic activities. The archaeal strain proteolytic activity was detected by observing the formation of clear zones around colonies on skim milk agar media as described by Gonzalez et al. [42]. The amylase activity was revealed on starch agar based medium by observing clear zone around the colony after plates flooding with iodine-potassium iodide solution (Lugol’s iodine solution) according to Montalvo et al. [43]. The strains’ ability to degrade lipids was checked as described by Bhatnagar et al. [44]. The DNase activity was determined through DNase test agar by observing clear halos around the colonies after being flooded with HCl solution (1 N) as described by Jeffries et al. [45]. The pectinolytic activity was screened on pectin agar-based media by the presence of clear zones around the colonies after plates flooding with Lugol’s iodine solution according to Soares et al. [46]. The xylanase activity was revealed onto 2% agar saline medium supplemented with 1% xylan by the presence of clear zones around the colonies after plates flooding with 0.1% Congo red solution as described by Ghio et al. [47]. The cellulase activity was screened on carboxymethylcellulose (CMC) plates by observing clear halo around the colonies after plates flooding with 0.1% Congo red solution according to Kasana et al. [48]. The gelatin hydrolysis was checked on gelatin-based agar by the development of clear zones around the colonies after plates flooding with 15% (w/v) HgCl2, in 20% (v/v) concentrated HCl according to the method of Frazier [49].
Evaluation of carotenoid production
Extraction and quantification
The archaeal strains were cultivated in a DSC-97 medium. One percent of the mid-log culture was inoculated in 100 ml. All the flasks were incubated at 37 °C and 110 rpm into a shaking incubator (Daihan Lab Tech Co., Ltd.) for a 10-day period. The OD600 of different culture growth was determined by UV–Vis spectrophotometer (Genesys 10S UV–Vis). Referring to Giani and Martínez-Espinosa [6] and Sahli et al. [50], pigments were extracted, after harvesting the cells, by centrifugation at 8000 × g for 20 min at 4 °C with 10 ml of pure acetone (5% BHT). The mixture was centrifuged at 8000 g for 20 min until the entire pigment was extracted in the solvent. The solvent fraction containing the pigments was separated from the cell debris by centrifugation at 8000 × g for 10 min. The supernatant was then scanned between 350 and 650 nm using a UV–Vis spectrophotometer. In order to avoid the isomerization phenomena by light, the samples were wrapped with aluminum foil at all the steps. The total carotenoid content was determined according to Lobato et al. [21] by measuring the absorbance at 494 nm and calculated using an extinction coefficient, ε (1%), of 2540 using formula (1):
| 1 |
Antioxidant activities
The antioxidant powers of the extracted pigments from the archaeal strains were estimated using the 2,2′-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay according to the method of Hou and Cui [15]. A standard antioxidant (β-carotene) was used as a positive control. The carotenoid solutions and β-carotene were diluted yielding the same concentration of 5 µg/ml. DPPH was dissolved in methanol as 0.5 mM solution. The absorbances measured at 517 nm against methanol as a blank were recorded immediately after mixing 140 μl of the DPPH solution and 60 μl of carotenoid solution (Ai) and after incubation at 25 °C for 30 min in the dark (Aj). Ac was the absorbance of the mixture of 140 μl of DPPH solution and 60 μl of methanol. The radical scavenging activity (RSA) was calculated, as described by Hou and Cui [15], using Formula (2):
| 2 |
Effect of different cultural and nutritional conditions on carotenoid production
The optimal cultural conditions for growth and carotenoid production for the highest carotenoid-producing strain AS17 was achieved by inoculating 1 ml of the mid log culture in 100 ml of DSC-97 medium in a 250-ml Erlenmeyer flask and incubated at 37 °C and 110 rpm into a shaking incubator (Daihan Lab Tech Co., Ltd.) for a period of 10 days. Various parameters including NaCl concentration, pH, temperature, agitation rate, light, and medium components were optimized using a DSC-97 medium. Only one parameter was changed each time, while the other parameter values were kept constant. The NaCl concentrations were varied between 0 and 350 g/l. According to previous works [6, 51, 52], the pH values of media were adjusted between 4 and 10 by using 1 N HCl or 1 N NaOH. Temperature was varied between 25 and 40 °C. The effect of light on growth and carotenoid production was determined by comparing the growth in light and in dark (aluminum foil). The effects of aeration and oxygen were determined by the inoculation of 1 ml of the mid-log culture in 100 ml culture medium in 250-ml (60% air phase) and 500-ml Erlenmeyer flask (80% air phase) and incubation in a shaking incubator at 110 and 220 rpm for 10 days at 37 °C.
The medium components, yeast extract (0, 5, 10, 15, and 20 g/l), casamino acids (0, 5, 7.5, and 10 g/l), MgSO4·7H2O (0, 5, 10, 15, 20, and 25 g/l), KCl (0, 1, 2, 3, 4, and 5 g/l), and trisodium citrate (0, 3, 6, and 9 g/l) concentrations were also studied. All the flasks were incubated at 37 °C and 110 rpm for 10 days. The experiment was repeated three times to ensure reproducibility. The growth of all the cultures was determined by measuring turbidity at 24-h intervals at 600 nm by UV–Vis spectrophotometer. For each culture, the growth rate (μmax) was calculated using two measures during the exponential phase, according to Formula (3) [53]:
| 3 |
where OD1 and OD2 are the OD600 at times t1 and t2 (day), respectively. The generation time (tg) was calculated using Formula (4):
| 4 |
The carotenoid content was determined by measuring the absorbance at 494 nm after extraction as described above.
Statistical analysis
All assays were performed in triplicate. The mean values and standard deviation (± SD) of the three replicates were calculated using Microsoft Excel 2016. The effects of all the environmental and nutritional conditions on growth and carotenoid production were evaluated by one-way ANOVA using Microsoft Excel 2016. The p-value of 0.05 was used as a cut-off point at 95% confidence level. A p-value is significant when equal to or less than 0.05 [54].
Results and discussion
Physiological characteristics of the strains
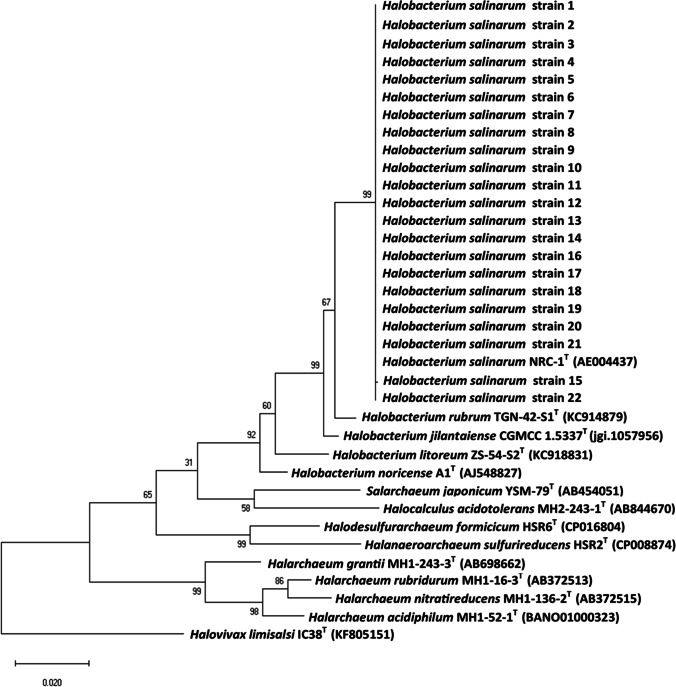
The morphological characteristics of the isolated strains showed they were Gram-negative, motile, and rod shaped. On solid media, they produced circular colonies of 1–2 mm in diameter with a pigmentation ranging from pale pink to red. The catalase and oxidase tests were positive. Nitrate was reduced to nitrite by 14 strains, and indole was not produced by any strain. Urease activity was found to be negative. All strains were extreme halophiles requiring at least 20% (w/v) salinity and tolerated NaCl up to the saturation level (> 30% w/v), but they grew optimally at 25% (w/v). They were able to grow at pH 5–9 (optimum pH 7, except for the strain AS1 that has an optimum pH of 6). All the strains were able to grow at 25–45 °C (optimum 37 °C). Their phylogenetic analysis revealed high degree of similarity (99.7–100%) with Halobacterium salinarum NRC-1 (Fig. 1). The diversity revealed by cultivation approach in contaminated sediments samples was more limited than that detected in other studies [18, 23, 24, 26]. The low diversity detected in the studied sediment samples could be attributed, besides the media composition effect, to their specific characteristics that were more stressful regarding salinity, organic matter, and heavy metal enrichment.
Fig. 1.
Phylogenetic tree based on 16S rRNA sequences of the 22 archaeal strains and other related archaeal sequences previously published in the databases determined by the neighbor-joining method using the Tamura-Nei model (0.40, gamma distributed with invariant sites) based on model testing under MEGA X. Halovivax limisalsi IC38T (KF805151) is used as an outgroup
The isolated strains were affiliated with the same genus, but they had different pigmentations and biochemical characteristics. Furthermore, they showed significant different heavy metal tolerances, as recently described by Baati et al. [32]. This could be explained by the fact that the halophilic archaea inhabiting Sfax solar saltern sediments could accumulate frequent spontaneous mutations in order to adapt to the environmental stress, due to the combined effect of the high salinity and metal concentrations. As a result, the strains changed their phenotypes and genotype in response to environmental stressors. Recently, the whole genome of five archaeal strains (AS1, AS2, AS8, AS11, and AS19) selected from the 22 strains and affiliated with Halobacterium salinarum were sequenced in order to provide information on genes that confer tolerance to high salinity and high heavy metal concentrations. Based on the 5 strain genome annotation results, many transposases were found (6 for AS8, 7 for AS1 and AS2, 12 for AS11 and AS19) suggesting a large number of insertion sequences in the genome [55].
The strains were affiliated to Halobacterium salinarum NRC-1 which was adapted to multiple stressors. In addition to high NaCl concentrations, H. salinarum NRC-1 was able to survive at high radiation, desiccation, toxic ions, and periodic changes of temperature and fluctuation of nutrient supplies [2]. Pfeiffer et al. [56] have noted that H. salinarum NRC-1 exhibited genome instability attributed to the large number of insertion sequence elements (ISH elements) affecting pigmentation and formation of gas vacuoles. The accumulation of frequent insertion sequence mutations (ISH) may mediate rapid adaptations to environmental stress [57]. Later, Charlebois [58] showed that the genome of H. salinarum NRC-1 contained 91 insertion sequences that were highly mobile elements. They were responsible for the high frequency of spontaneous mutations. Fileé et al. [59] and Pfeiffer et al. [60] supported previous reports of the ISH elements importance in haloarchaeal evolution. Recently, the genome analysis of 16 clones isolated after 500 generations in acid stress and compared to those of the Halobacterium sp NRC-1-ancestor revealed 378 mutations, 90% of which were haloarchaeal insertion sequences (ISH) and ISH-mediated large deletions [61].
Extracellular hydrolytic activities
The hydrolase activity screening results using agar plate-based assays are displayed in Table 1. The strains showed at least one enzyme activity. The gelatinase activity was detected by 77.27% of strains, while protease and amylase activities were detected by 63.63% and 50% of strains, respectively. Few strains were able to produce DNAase (27.27%), cellulase (22.72%), and lipase (18.18%). None of the strains was able to degrade xylan and pectin. Combined hydrolytic activities were detected in most of the strains (81%). Three strains presented 5 hydrolytic activities, 2 strains presented 4 hydrolytic activities, 4 strains presented 3 hydrolytic activities, and 9 strains presented 2 hydrolytic activities. No strains with all of the hydrolytic activities tested have been found in this study (Table 1).
Table 1.
Hydrolytic activities of the different archaeal strains
| Strains | Hydrolytic activity | Total of activities | ||||||
|---|---|---|---|---|---|---|---|---|
| Protease | Amylase | DNAase | Lipase | Cellulase | Gelatinase | Xylanase | ||
| AS1 | + | − | − | − | − | − | − | 1 |
| AS2 | + | − | − | − | − | + | − | 2 |
| AS3 | + | + | − | − | + | + | − | 4 |
| AS4 | + | + | + | + | − | + | − | 5 |
| AS5 | − | − | + | − | − | + | − | 2 |
| AS6 | − | − | − | − | − | + | − | 1 |
| AS7 | + | + | − | − | − | − | − | 2 |
| AS8 | + | − | − | − | − | − | − | 1 |
| AS9 | − | + | − | − | − | + | − | 2 |
| AS10 | − | − | − | − | − | + | − | 1 |
| AS11 | + | − | − | − | − | + | − | 2 |
| AS12 | − | + | − | − | + | + | − | 3 |
| AS13 | + | − | − | − | + | + | − | 3 |
| AS14 | + | + | − | − | − | + | − | 3 |
| AS15 | − | + | − | + | − | − | − | 2 |
| AS16 | + | − | − | − | − | + | − | 2 |
| AS17 | + | + | + | − | + | + | − | 5 |
| AS18 | + | + | + | + | − | + | − | 5 |
| AS19 | + | − | − | − | + | + | − | 3 |
| AS20 | + | − | + | + | − | + | − | 4 |
| AS21 | − | + | + | − | − | − | − | 2 |
| AS22 | − | + | − | − | − | + | − | 2 |
The most frequent hydrolytic activity detected was gelatinase, followed by protease and amylase activities. Cellulase and lipase activities were also detected. Makhdoumi et al. [26] noted high rate enzyme production including amylase, DNase, and lipase, respectively, from halophilic archaea isolated from a salted lake in Iran. Similarly, Kharroub et al. [27] reported a predominance of amylolytic activity while screening extracellular enzymes produced by halophilic archaea from an Algerian Sebkha. Nercessian et al. [23] demonstrated that protease and amylase were the dominant activities by halophilic archaea isolated from Argentinean salterns. Menasria et al. [24] revealed that esterase and inulinase were the most frequently detected activities produced by halophilic archaea isolated from Algerian arid and semi-arid wetlands followed by gelatinase. Recently, Sahli et al. [18] found that gelatinase, lipase, and amylase were the most detected hydrolytic activities produced by halophilic archaeal strains isolated from Algerian hypersaline environments.
According to Akmoussi et al. [62], the predominance of one type of enzyme over others could be attributed to the different biotope characteristics in these locations constituting a different reservoir of organic matter which can stimulate the metabolic activity of halophilic archaeal strains. These hydrolases play an important role in the geochemical cycling of nutrients in salterns, through hydrolyzing the high molecular weight biopolymers [22]. These hydrolases are able to degrade organic polymeric substances extracellularly, making small organic molecules available as carbon and energy sources [63]. Therefore, it can be deduced that the multi-stress-resistant archaeal strains isolated from Sfax solar saltern sediments could be potentially used to screen enzymes for industrial applications.
Carotenoid production
Quantification of carotenoid
In order to screen the greatest carotenoid producer among the archaeal strain collection, the pigments were extracted from the culture of each obtained strain in a DSC-97 medium. Table 2 regrouped the growth and carotenoid production level by strain. It shows that the degree of pigmentation (OD490/OD600) differs considerably among the strains (0.221 to 1.643). The carotenoid production yields range between 2.027 and 14.880 mg/l. The strain AS17 had the highest pigmentation degree value (1.643) indicating that it exhibited the highest carotenoid-producing ability (14.880 mg/l), followed by the AS1 strain (10.236 mg/l).
Table 2.
Growth and carotenoid production by the archaeal strains
| Strain | Growth (OD600) | Carotenoid (OD495) | Pigmentation degree (OD495/OD600) | Carotenoid concentration (mg/l) |
|---|---|---|---|---|
| AS1 | 2.105 | 2.600 | 1.235 | 10.236 |
| AS2 | 2.156 | 1.756 | 0.814 | 6.913 |
| AS3 | 2.445 | 1.522 | 0.622 | 5.992 |
| AS4 | 2.322 | 1.545 | 0.665 | 6.082 |
| AS5 | 2.275 | 1.203 | 0.528 | 4.736 |
| AS6 | 1.965 | 1.136 | 0.692 | 4.472 |
| AS7 | 2.028 | 1.078 | 0.531 | 4.244 |
| AS8 | 2.015 | 0.687 | 0.340 | 2.704 |
| AS9 | 1.655 | 0.823 | 0.497 | 3.240 |
| AS10 | 2.477 | 1.768 | 0.713 | 6.960 |
| AS11 | 2.201 | 1.905 | 0.865 | 7.500 |
| AS12 | 2.176 | 0.625 | 0.287 | 2.460 |
| AS13 | 2.139 | 0.551 | 0.257 | 2.169 |
| AS14 | 2.113 | 1.063 | 0.503 | 4.185 |
| AS15 | 1.941 | 1.202 | 0.619 | 4.732 |
| AS16 | 2.203 | 2.490 | 1.130 | 9.803 |
| AS17 | 2.300 | 3.780 | 1.643 | 14.880 |
| AS18 | 2.124 | 2.150 | 1.012 | 8.464 |
| AS19 | 2.279 | 1.575 | 0.691 | 6.200 |
| AS20 | 1.878 | 1.937 | 1.031 | 7.625 |
| AS21 | 1.455 | 1.088 | 0.747 | 4.283 |
| AS22 | 2.320 | 0.515 | 0.221 | 2.027 |
The extracted carotenoids occurring in each strain culture were analyzed by scanning spectrophotometric absorption in 350–650-nm range. The results showed that all the extracted pigments have identical absorption spectra characterized by three‐fingered peaks with absorption maxima at about 467, 494, and 527 nm and two cis absorption maxima at around 317 and 388 nm, which corresponded to C50-carotenoid (bacterioruberin) and its derivatives monoanhydro- and bisanhydro-bacterioruberin as previously reported by several authors [4–6, 10, 20, 21, 64]. Bacterioruberin has been identified as the main carotenoid in the following haloarchaea: Halobacterium salinarum strains NRC-1 and R1, Halorubrum sodomense, and Haloarcula vallismortis [65].
Compared to other halophilic archaea isolated from worldwide solar salterns for carotenoid production, some of the strains used in this study seem to be the highest carotenoid producer. They have a pigmentation degree ranging between 0.221 and 1.214. Fard et al. [66], however, showed that the pigmentation degree of Halobacterium salinarum R1 was only 0.573. The carotenoid production from many archaeal strains isolated from distinct saline habitats located in different regions of Algeria varied from 0.1 to 3.68 mg/l [18]. Abbes et al. [12] reported that carotenoid production yields of the halophilic archaea isolated from Sfax solar saltern brines (Tunisia) ranged only between 5.66 and 7.63 mg/l. Consequently, some archaeal strains used in this study were able to produce a higher amount of carotenoid than those isolated from brines samples. Chen et al. [8] demonstrated that to adapt to the stressful conditions in extreme environments, the halophilic archaea should produce a high amount of carotenoids.
Antioxidant activities
The antioxidant capacities of the extracted carotenoids from the archaeal strains were evaluated by DPPH assay. The scavenging capacity of the reactive species depended on the carotenoid concentration. Therefore, the carotenoid solutions and β-carotene were used in the same concentration (5 µg/ml). When compared to those of known antioxidant agent β-carotene, our results showed that all the pigments exhibited a capacity to act as antioxidants. The scavenging capacity of three extracted pigments produced by the strains AS16, AS17, and AS18 were important and significantly higher than the standard β-carotene (Fig. 2). Even bacterioruberin has established an antioxidant capacity and showed a higher radical scavenging ability than β-carotene due to the presence of more conjugated double bonds and hydroxyl groups [67]. Bacterioruberin has 13 conjugated double bonds and four hydroxyl groups versus the 9 conjugated double bonds and no oxygen atoms in β-carotene [14, 67]. The antioxidant property, attributed to the higher number of pairs of conjugated double bonds, makes these carotenoids remarkably interesting for human health, food, and pharmaceutical industries [2, 6, 10, 20]. Consequently, the halophilic archaeal strains isolated from sfax solar saltern sediments were shown to be able to overcome stressful conditions through the production of high amounts of carotenoids with a high antioxidant activity. Similar results were also reported elsewhere [6].
Fig. 2.

DPPH radical scavenging activity for the carotenoid extracts from the archaeal strains and the control (β-carotene) using equal concentration (5 µg/ml)
Optimal growth conditions and carotenoid production analysis
The yield of carotenoids in haloarchaea mainly depended on the used strain and culture conditions [21]. According to Vázquez-Madrigal [68], the archaeal metabolism is different, and the use of metabolic pathways does not entirely depend on the genetic but also on ecology. Only few works have described the effect of parameters like salt concentrations, pH, temperature, UV radiation, and nutrients’ availability on the carotenogenesis in haloarchaea [9, 18, 20, 21, 69–73]. The growth and the carotenoid production of the highest carotenoid producing strain (named AS17) were evaluated under various environmental and nutritional conditions to establish the most suitable conditions to maximize carotenoid production by one-step, using the one-factor-at-a-time approach.
Effect of environmental conditions
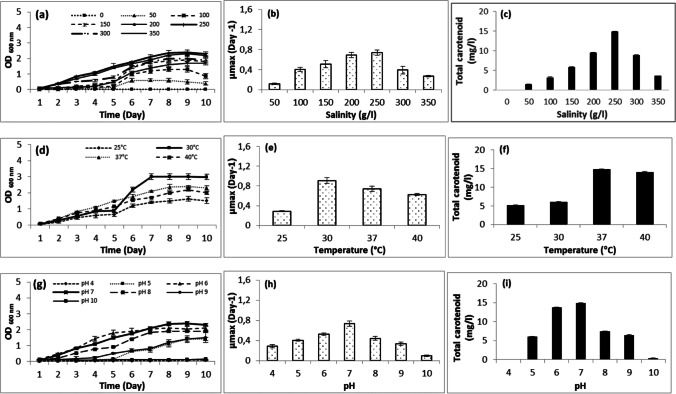
The NaCl concentration is the most important factor in determining halobacterial growth and total carotenoid production [70]. In our case, salinity was varied between 0 and 350 g/l, and the results displayed in Fig. 3a indicated that AS17 was not able to grow without NaCl. Growth was low at 50, 100, 150, 300, and 350 g/l with a lag phase of 5 days. The maximum growth was obtained at a salinity of 250 g/l, whereas slightly lower values were observed at 200 g/l NaCl. The growth rate (µmax) and doubling time were calculated in all salt concentrations. At 200 and 250 g/l, the growth rate (µmax) was higher compared to the other salinity values, achieving 0.691–0.738 day−1 (Fig. 3b). The generation time was 1.002 days at 200 g/l salt and 0.939 days at 250 g/l salt. The generation time values at the other tested salinities (50, 100, 150, 300, and 350 g/l) were higher (1.357–5.885 days). AS17 exhibited an increase in pigmentation as salinity increased. It produced 14.881 mg/l carotenoids at a salt concentration of 250 g/l NaCl; however, the carotenoid concentration decreased to 9.527 mg/l at 200 g/l NaCl (Fig. 3c). A similar increase in pigment production was observed by Halobacterium salinarum ATCC 33,170 [4]. This is consistent with the findings of previous studies conducted by Subramanian and Gurunathan [17]. They showed an increase in pigment production at higher NaCl concentration which can be explained by the carotenoids acting as protective agents against free radicals; hence, the biosynthesis of the carotenoids represented a stress response mechanism. Haloarchaea responded to osmotic stress by producing carotenoids acting as cellular membrane reinforcement by increasing the membrane rigidity, decreasing water permeability, and allowing permeability to oxygen [5, 15].
Fig. 3.
Effect of salinity, temperature, and pH on growth and carotenoid production of Halobacterium salinarum (AS17)
Temperature is one of the most important environmental factors affecting the growth of microorganisms. It modified the quantity of pigment by altering the concentration of enzymes involved in carotenoid production [74]. Regarding AS17, it was exposed to different temperature values (25, 30, 37, and 40 °C). The result showed it grew better at 30, 37, and 40 °C. The cultures reached OD600nm of 2.98, 2.3, and 2, respectively. The growth rate and doubling time values were affected by changes in temperature. The growth was optimal at 30 °C with a µmax of 0.910 day−1 (Fig. 3e). The highest carotenoid production was recorded at 37 °C (Fig. 3f).
pH is another important factor, affecting cell growth and total carotenoid production in a lot of microorganisms. According to Raghavan and Furtado [75], most of the extremely halophilic archaea display optimum growth at pH values close to 7. In this study, AS17 was unable to survive and grow at pH 4 and pH 10 (Fig. 2c). The maximum optical density (2.3) and the highest growth rate were reached at pH 7. Maximum pigments were produced at pH 7 (Fig. 3i).
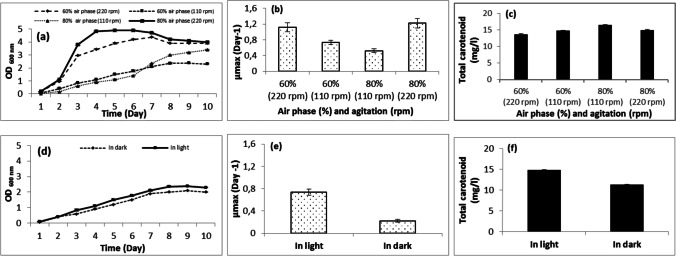
Oxygen is essential for optimal growth and carotenoid production in haloarchaea [19]. According to Schneegurt [76], when salinity and temperature increase or pH decreases, oxygen solubility in the culture is reduced, which has an impact on the availability of oxygen for the haloarchaea cells. In shake flasks, the oxygen supply depended on the liquid volume and agitation speed [21]. For our AS17, the effect of aeration and oxygen on growth and carotenoid production were tested by incubating 100 ml of DSC-97 medium in 250-ml (60% air phase) and 500-ml Erlenmeyer flask (80% air phase) at 110 and 220 rpm. The results showed that a maximum growth occurred with 80% air phase in the flask at 220 rpm (Fig. 4a) with a µmax of 1.221 day−1 (Fig. 4b), while the maximum production of pigments was observed with 80% air phase in the flask at 110 rpm (16.484 mg/l) (Fig. 4c).
Fig. 4.
Effect of air volume and agitation rate and light on growth and carotenoid production of Halobacterium salinarum (AS17)
Light is another important factor for the regulation of carotenoid synthesis by improving carotenogenesis. According to Lobato et al. [21], the effect of light on pigmentation of halophilic archaea greatly depended on species and strains. The effect of light on AS17 was tested by culturing the strain in light and in dark. The results showed that growth and carotenoid production were better in light than in dark (Fig. 4d–f). Another study found a similar result emphasizing that Halobacterium salinarum JCM10927 grown in light showed higher carotenoid content than when cultured in the dark [19]. Contrastingly, the strains Halobacterium salinarum ATCC 33,170, ATCC 43,214 showed no difference in pigmentation when cultivated in the absence or presence of light [77]. According to Sahli et al. [18], light had no significant effect on growth, but it influenced the carotenoid content.
Effect of nutritional conditions
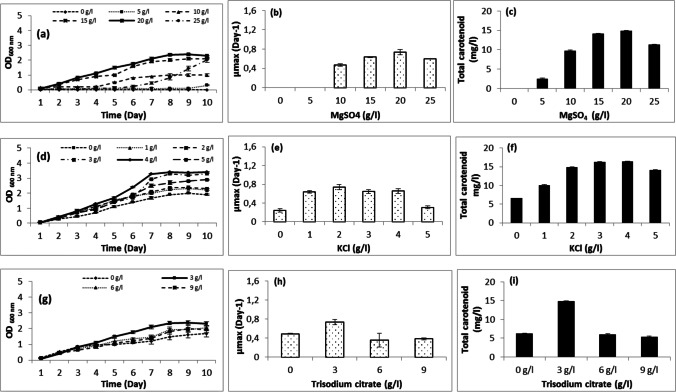
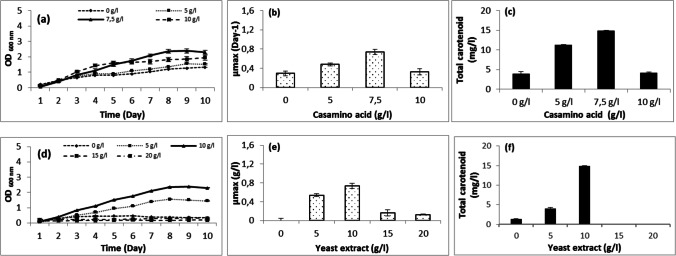
The effect of magnesium sulfate, potassium chloride, trisodium citrate, yeast extract, and casamino acid on Halobacterium salinarum growth was also studied in this paper. The achieved results show that the strain cannot grow without MgSO4. At 5 and 10 g/l, growth was very low. The optimal concentration was 20 g/l followed by 15 g/l. At 25 g/l, AS17 needed 7 days for growth (Fig. 5a). It produced carotenoids in media containing 5–25 g/l MgSO4 with an optimum at 20 g/l (Fig. 5c). The halophilic archaea required magnesium and sulfates ions for growth and carotenoid production. The high requirement for magnesium may be explained by its use for cell division, whereas the demand for sulfate ions may be due to their presence in the cell wall of halophilic archaea. High concentrations of magnesium were considered a serious stressor that limits microbial growth, mostly acting as a chaotropic agent affecting the structure and activity of macromolecules [78]. Furthermore, the haloarchaea magnesium requirement was dependent on genus and species [73]. The strain requirement of KCl was also tested in media containing 0–5 g/l. The results show that the growth and carotenoid production might occur without KCl but increased with KCl concentration increase (1–4 g/l) with an optimum at 4 g/l (Fig. 5d–f). Calegari-Santos et al. [4] demonstrated that KCl was essential for the osmotic equilibrium. The trisodium citrate, used as carbon source, was tested at concentrations ranging between 0 and 9 g/l. The results showed that the strain can grow and produce carotenoid without trisodium citrate. With the addition of trisodium citrate, the maximum of growth and carotenoid production were at a concentration of 3 g/l (Fig. 5g–i). Casamino acid and yeast extract, used as nitrogen source, were also tested in media containing 0–10 g/l and 0–20 g/l, respectively. The results show that the strain can grow and produce carotenoids (3.96 g/l) without casamino acid (Fig. 5a–c). The optimal production, however, was obtained with 7.5 g/l (14.881 g/l). Without yeast extract, AS17 grew slowly and produced only 1.33 g/l. The optimal concentration for growth and carotenoid production was 10 g/l. The growth rate (µmax) of such strain was affected by the addition of different concentrations of MgSO4, KCl, trisodium citrate, yeast extract, and casamino acid.
Fig. 5.
Effect of culture media (MgSO4, KCl, trisodium citrate, casamino acid, and yeast extract) on growth and carotenoid production of Halobacterium salinarum (AS17)
The ANOVA analysis showed that the influential factors on the archaeal strain growth were first the air volume and the agitation rate, followed by the pH with a p-value of 0.00001 and 0.00003, respectively, and then came temperature and salinity with a p-value of 0.0001 and 0.0004, respectively. Finally, the MgSO4 and yeast extract concentrations slightly affected growth. The other tested parameters were found to be insignificant with p-values ranging between 0.1229 and 0.8399. The influential factors affecting the carotenoid production were first the air volume and the agitation rate, the KCl concentration, and the salinity with a p-value ranging between 0.00002 and 0.0005 (Table 3). Second, temperature and light were also significant but at a less degree. Their p-values were equal to 0.0016 and 0.02422, respectively.
Table 3.
Analysis of variance of the different parameters; the significant values are shown in bold
| Responses | ||||||
|---|---|---|---|---|---|---|
| OD600 | Carotenoid concentration | |||||
| Variables | Sum of squares | F value | p-value | Sum of squares | F value | p-value |
| Environmental factors | ||||||
| Salinity | 137,775.90 | 23.62 | 0.00040 | 130,625.80 | 22.35 | 0.0005 |
| pH | 111.95 | 40.84 | 0.00003 | 0.00005 | 2.72 | 0.9987 |
| Temperature | 1897.77 | 81.83 | 0.00010 | 1052.39 | 29.21 | 0.0016 |
| Agitation + air volume | 9,845,300,229.12 | 147.68 | 0.00001 | 9,842,037,704.40 | 147.63 | 0.00002 |
| Light | 0.4225 | 1.55 | 0.3392 | 134.78 | 39.78 | 0.02422 |
| Nutritional factors | ||||||
| MgSO4 | 452.73 | 14.32 | 0.0054 | 49.94 | 1.15 | 0.3152 |
| KCl | 0.0835 | 0.0429 | 0.8399 | 335.89 | 35.40 | 0.0001 |
| Yeast extract | 206.32 | 6.51 | 0.0341 | 88.62 | 1.74 | 0.2237 |
| Casamino acid | 29.65 | 3.22 | 0.1229 | 17.19 | 0.7246 | 0.4273 |
| Trisodium citrate | 12.48 | 1.66 | 0.2454 | 26.62 | 1.51 | 0.2654 |
From the above analysis, it can be noticed that the air volume, agitation rate, salinity, and temperature were the main parameters affecting both of growth and carotenoid production.
Conclusions
16S rRNA gene sequencing of twenty-two archaeal strains isolated from Sfax solar saltern sediments showed that they were affiliated to the same genus H. salinarum despite their different biochemical characteristics, as well as their distinct resistance to heavy metals. The strains have undergone spontaneous mutations due to stressful conditions from which they are isolated. Their biotechnological potentials were evaluated in terms of hydrolytic enzyme activity and carotenoid production. They showed the ability to produce varying hydrolytic enzymes, mainly gelatinase, protease, and amylase, all of which may be useful in biotechnological applications. Further investigations should be performed to establish a biochemical characterization of these hydrolytic enzymes.
Carotenoids, a group of metabolites produced by different organisms, including haloarchaea, have gained massive attention due to their medicinal and industrial benefits for human beings. First, carotenoids from the different strains were extracted and quantified. The antioxidant activities were also evaluated. The results showed that the archaeal strains produce carotenoids with significant antioxidant activities. Then a sequence of optimization processes was carried out using the one-factor-at-a-time approach, to define the optimum growth conditions and carotenoid production of the highest carotenoid-producing strain (called AS17).
A maximum carotenoid production (16.490 mg/l), higher than that of the control (14.880 mg/l), was observed at 37 °C, pH 7, 250 g/l of salinity, with 80% air phase in the flask at 110 rpm, in the presence of light and in culture media containing (g/l) 10, yeast extract; 7.5, casamino acid; 20, MgSO4; 4, KCl; and 3, trisodium citrate. Further investigations should be performed to optimize carotenoid production by statistical approaches and the use of inexpensive industrial by-products as nutrient sources, on the one hand, and to determine their biological activities.
Acknowledgements
The authors would like to thank Mr Christopher Dunlap, professor in the National Center for Agricultural Utilization Research (Crop Bioprotection Research Unit 1815 N, University St Peoria, IL 61604, USA), for his help in strain identification. The authors would like to also thank Mr. Abdelmajid Dammak, English teacher at the University of Sfax, for further careful editing and proofreading of the revised manuscript.
Author contribution
HB isolated the strains, did the experimental part, and wrote the manuscript. MS contributed to the text preparation. EA was involved in result evaluation. CA and MT designed the study and revised the manuscript. All authors read and approved the final version of the manuscript.
Declarations
Conflict of interest
The authors declare no competing interests.
Human and animal participants
This study does not involve any human participants or animal experiments.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Oren A. Industrial and environmental applications of halophilic microorganisms. Environ Tech. 2010;31:825834. doi: 10.1080/09593330903370026. [DOI] [PubMed] [Google Scholar]
- 2.Jones DL, Baxter BK. DNA repair and photoprotection: mechanisms of overcoming environmental ultraviolet radiation exposure in halophilic archaea. Front Microbiol. 2017;8:1882–1898. doi: 10.3389/fmicb.2017.01882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matarredona L, Camacho M, Zafrilla B, Barrales GB, Esclapez J, Bonete MJ. The survival of Haloferax mediterranei under stressful conditions. Microorganisms. 2021;9:336–952. doi: 10.3390/microorganisms9020336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calegari-Santos R, Diogo RA, Fontana JD, Bonfim TMB. Carotenoid production by halophilic archaea under different culture conditions. Curr Microbiol. 2016;72:641–651. doi: 10.1007/s00284-015-0974-8. [DOI] [PubMed] [Google Scholar]
- 5.Giani M, Garbayo I, Vílchez C, Espinosa RMM. Haloarchaeal carotenoids: healthy novel compounds from extreme environments. Mar Drugs. 2019;17:524–537. doi: 10.3390/md17090524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giani M, Martínez-Espinosa RM. Carotenoids as a protection mechanism against oxidative stress in Haloferax mediterranei. Antioxidants. 2020;9:1060–1075. doi: 10.3390/antiox9111060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinez GM, Pire C, Martínez-Espinosa RM. Hypersaline environments as natural sources of microbes with potential applications in biotechnology: the case of solar evaporation systems to produce salt in Alicante County (Spain) Curr Res Microbial Sci. 2022;3:100136. doi: 10.1016/j.crmicr.2022.100136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen CW, Hsu SH, Lin MT, Hsu YH. Mass production of C50 carotenoids by Haloferax mediterranei using extruded rice bran and starch under optimal conductivity of brined medium. J Bioprocess Biosyst Eng. 2015;38:2361–2367. doi: 10.1007/s00449-015-1471-y. [DOI] [PubMed] [Google Scholar]
- 9.Fariq A, Azra Y, Jamil M. Production, characterization and antimicrobial activities of bio-pigments by Aquisalibacillus elongatus MB592, Salinicoccusnsesuvii MB597, and Halomonas aquamarina MB598 isolated from Khewra Salt Range, Pakistan. Extremophiles. 2019;23:435–449. doi: 10.1007/s00792-019-01095-7. [DOI] [PubMed] [Google Scholar]
- 10.Naziri D, Hamidi M, Hassanzadeh S, Tarhriz V, Maleki Zanjani B, Nazemyieh H, Hejazi MA, Hejazi MS. Analysis of carotenoid production by Halorubrum sp. TBZ126; an extremely halophilic archeon from Urmia Lake. Adv Pharm Bull. 2014;4:61–67. doi: 10.5681/apb.2014.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Villegas PG, Vigara J, Vila M, Varela J, Barreira L, Léon R. Antioxidant, antimicrobial, and bioactive potential of two new haloarchaeal strains isolated from odiel salterns (Southwest Spain) Biology. 2020;9:298–318. doi: 10.3390/biology9090298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abbes M, Baati H, Guermazi S, Messina C, Santulli A, Gharsallah N, Ammar E. Biological properties of carotenoids extracted from Halobacterium halobium isolated from a Tunisian solar saltern. BMC Complem Altern M. 2013;13:255–263. doi: 10.1186/1472-6882-13-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tuli HS, Chaudhary P, Beniwal V, Sharma AK. Microbial pigments as natural color sources: current trends and future perspectives. J Food Sci Technol. 2015;52:4669–4678. doi: 10.1007/s13197-014-1601-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Squillaci G, Parrella R, Carbone V, Minasi P, La Cara F, Morana A. Carotenoids from the extreme halophilic archaeon Haloterrigena turkmenica: identification and antioxidant activity. Extremophiles. 2017;21:933–945. doi: 10.1007/s00792-017-0954-y. [DOI] [PubMed] [Google Scholar]
- 15.Hou J, Cui HL. In vitro antioxidant, antihemolytic, and anticancer activity of the carotenoids from halophilic archaea. Curr Microbiol. 2018;75:266–271. doi: 10.1007/s00284-017-1374-z. [DOI] [PubMed] [Google Scholar]
- 16.Hegazy G, Abu-Serie MM, Abo-Elela GM, Ghozlan H, Sabry SA, Soliman NA, Abdel-Fattah YR. In vitro dual (anticancer and antiviral) activity of the carotenoids produced by haloalkaliphilic archaeon Natrialba sp. M6. Sci Rep. 2020;10:5986–6000. doi: 10.1038/s41598-020-62663-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Subramanian P, Gurunathan J. Differential production of pigments by halophilic bacteria under the effect of salt and evaluation of their antioxidant activity. Appl Biochem Biotechnol. 2020;190:391–409. doi: 10.1007/s12010-019-03107-w. [DOI] [PubMed] [Google Scholar]
- 18.Sahli K, Gomri MA, Esclapez J, VillegasPG, Ghennai O, Bonete MJ, LeónR, Kharroub K (2020) Bioprospecting and characterization of pigmented halophilic archaeal strains from Algerian hypersaline environments with analysis of carotenoids produced by Halorubrum sp. BS2. J Basic Microbiol 1–15 [DOI] [PubMed]
- 19.El-Sayed WSM, Takaichi S, Saida H, Kamekura M, Abu-Shady M, Seki H, Kuwabara T. Effects of light and low oxygen tension on pigment biosynthesis in Halobacterium salinarum, revealed by a novel method to quantify both retinal and carotenoids. Plant Cell Physiol. 2002;43:379–383. doi: 10.1093/pcp/pcf044. [DOI] [PubMed] [Google Scholar]
- 20.Rodrigo-Baños M, Garbayo I, Vílchez C, Bonete MJ, Martínez-Espinosa RM. Carotenoids from Haloarchaea and their potential in biotechnology. Mar Drugs. 2015;13:5508–5532. doi: 10.3390/md13095508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lobato ZM, Merchante AR, Fuentes JL, Sayago A, Recamales AF, Espinosa RMM, Vega JM, Vílchez C, Garbayo I. Optimization of growth and carotenoid production by Haloferax mediterranei using response surface methodology. Mar Drugs. 2018;16:372. doi: 10.3390/md16100372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung D, Kim H, Park HS, Kim HS, Yang D, Kim JG. Extracellular enzyme activities in the solar saltern sediments. Korean J Microbiol. 2020;56:133–139. [Google Scholar]
- 23.Nercessian D, Meglio LD, Castro RD, Paggi R. Exploring the multiple biotechnological potential of halophilic microorganisms isolated from two Argentinean salterns. Extremophiles. 2015;19:1133–1143. doi: 10.1007/s00792-015-0785-7. [DOI] [PubMed] [Google Scholar]
- 24.Menasria T, Aguilera M, Hocine H, Benammar L, Ayachie A, Bachirf A, Dekak A, Sánchez MM. Diversity and bioprospecting of extremely halophilic archaea isolated from Algerian arid and semi-arid wetland ecosystems for halophilic-active hydrolytic enzymes. Microbiol Res. 2018;207:289–298. doi: 10.1016/j.micres.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 25.Pfeifer K, Ergal I, Koller M, Basen M, Schuster B, Rittmann SKMR. Archaea biotechnology. Biotechnol Advances. 2021;47:107668. doi: 10.1016/j.biotechadv.2020.107668. [DOI] [PubMed] [Google Scholar]
- 26.Makhdoumi KA, Amoozegar MA, Khaledi ME. Diversity of hydrolytic enzymes in haloarchaeal strains isolated from Salt Lake. Int J Environ Sci Technol. 2011;8:705–714. [Google Scholar]
- 27.Kharroub K, Gomri MA, Aguilera M, Monteoliva-Sánchez M. Diversity of hydrolytic enzymes in haloarchaea isolated from Algerian sabkhas. Afr J Microbiol Res. 2014;8:3992–4001. [Google Scholar]
- 28.Azri C, Maalej A, Medhioub K, Rosset R. Evolution of atmospheric pollutants in the city of Sfax (Tunisia) (October 1996-June 1997) Atmosfera. 2007;20:223–246. [Google Scholar]
- 29.Bahloul M, Chabbi I, Sdiri A, Amdouni R, Medhioub K, Azri C (2015) Spatiotemporal variation of particulate fallout instances in Sfax City, Southern Tunisia: influence of sources and meteorology. Adv Meteorol 4[396:11
- 30.Bahloul M, Chabbi I, Dammak R, Amdouni R, Medhioub K, Azri C. Geochemical behaviour of PM10 aerosol constituents under the influence of succeeding anticyclonic/cyclonic situations: case of Sfax City, southern Tunisia. Environ Monit Assess. 2015;187:1–17. doi: 10.1007/s10661-015-4980-x. [DOI] [PubMed] [Google Scholar]
- 31.Bahloul M, Baati H, Amdouni R, Azri C. Assessment of heavy metals contamination and their potential toxicity in the surface sediments of Sfax Solar Saltern, Tunisia. Environ Earth Sci. 2018;77:1–27. [Google Scholar]
- 32.Baati H, Bahloul M, Amdouni R, Azri C. Metal contamination and resistance of superficial sediment’s prokaryotic flora in extreme environments: case of Sfax solar saltern (Tunisia) Geomicrobiol J. 2020;37:345–354. [Google Scholar]
- 33.DasSarma S, Fleischmann EM, Rodriguez-Valera F. Appendix 2. Media for halophiles. In: Robb FT, editor. archaea: a laboratory manual. New York: Cold Spring Harbor Laboratory Press; 1995. pp. 225–230. [Google Scholar]
- 34.Dussault HP. An improved technique for staining red halophilic bacteria. J Bacteriol. 1955;70:484–485. doi: 10.1128/jb.70.4.484-485.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oren A, Ventosa A, Grant WD. Proposed minimal standards for description of new taxa in the order Halobacteriales. Int J Syst Bacteriol. 1997;47:233–238. [Google Scholar]
- 36.Gerhardt P, Murray RGE, Wood WA, Krieg NR. Methods for general and molecular bacteriology. Washington: American Society for Microbiology; 1994. [Google Scholar]
- 37.Baati H, Siala M, Azri C, Ammar E, Dunlap C, Trigui M. Resistance of a Halobacterium salinarum isolate from a solar saltern to cadmium, lead, nickel, zinc, and copper. Antonie Van Leeuwenhoek. 2020;113:1699–1711. doi: 10.1007/s10482-020-01475-6. [DOI] [PubMed] [Google Scholar]
- 38.DeLong EF. Archaea in coastal marine environments. Proc Nalt Acad Sci USA. 1992;89:5685–5690. doi: 10.1073/pnas.89.12.5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng A, Alm EW, Stahl DA, Raskin L. Characterization of universal small-subunit rRNA hybridization probes for quantitative molecular microbial ecology studies. Appl Environ Microbiol. 1996;62:4504–4513. doi: 10.1128/aem.62.12.4504-4513.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoon SH, Ha SM, Kwon S, Lim J, Kim Y, Seo H, Chun J. Introducing EzBioCloud: a taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int J Syst Evol Microbiol. 2017;67:1613–1617. doi: 10.1099/ijsem.0.001755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumar S, Stecher G, Li M, Knyaz C, Tamur K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gonzalez C, Gutierre C, Ramirez C. Halobacterium vallismortis sp. nov. An amylolytic and carbohydrate-metabolizing: extremely halophilic bacterium. Can J Microbiol. 1978;24:710–715. doi: 10.1139/m78-119. [DOI] [PubMed] [Google Scholar]
- 43.Montalvo RR, Vreeland RH, Oren A, Kessel M, Betancourt C, Lopez GJ. Halogeometricum borinquense gen. nov. sp. nov., a novel halophilic archaeon from Puerto Rico. Int J Syst Bacteriol. 1998;48:1305–1312. doi: 10.1099/00207713-48-4-1305. [DOI] [PubMed] [Google Scholar]
- 44.Bhatnagar T, Boutaiba S, Hacene H, Cayol JL, Fardeau ML, Ollivier B, Baratti J. Lipolytic activity from Halobacteria: screening and hydrolase production. FEMS Microbiol Lett. 2005;248:133–140. doi: 10.1016/j.femsle.2005.05.044. [DOI] [PubMed] [Google Scholar]
- 45.Jeffries CD, Holtman DF, Guse DG. Rapid method for determining the activity of microorganisms on nucleic acids. J Bacteriol. 1957;73:590–591. doi: 10.1128/jb.73.4.590-591.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soares MMCN, Silva D, Gomez E. Screening of bacterial strains for pectinolytic activity: characterization of the polygalacturonase produced by Bacillus sp. Rev Microbiol. 1999;30:299–303. [Google Scholar]
- 47.Ghio S, Sabarís D, Lorenzo GJ, Lia V, Talia P, Cataldi A, Grasso D, Campos E. Isolation of Paenibacillus sp. and Variovorax sp. strains from decaying woods and characterization of their potential for cellulose deconstruction. Int J Biochem Mol Biol. 2012;3:352–364. [PMC free article] [PubMed] [Google Scholar]
- 48.Kasana RC, Salwan R, Dhar H, Dutt S, Gulati A. A rapid and easy method for the detection of microbial cellulases on agar plates using gram’s iodine. Curr Microbiol. 2008;57:503–507. doi: 10.1007/s00284-008-9276-8. [DOI] [PubMed] [Google Scholar]
- 49.Frazier WC. A method for the detection of changes in gelatin due to bacteria. J Infect Dis. 1926;39:302–306. [Google Scholar]
- 50.Sahli K, Gomri MA, Esclapez J, Villegas PG, Bonete MJ, León R, Kharroub K. Characterization and biological activities of carotenoids produced by three haloarchaeal strains isolated from Algerian salt lakes. Arch Microbiol. 2022;204:6. doi: 10.1007/s00203-021-02611-0. [DOI] [PubMed] [Google Scholar]
- 51.Quadri I, Hassania II, Haridon S, Chalopin M, Hacène H, Jebbar M. Characterization and antimicrobial potential of extremely halophilic archaea isolated from hypersaline environments of the Algerian Sahara. Microbiol Res. 2016;187:119–131. doi: 10.1016/j.micres.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 52.Ghanmi F, Carré-Mlouka A, Vandervennet M, Boujelben I, Frikha D, Ayadi H, Peduzzi J, Rebuffat S, Maalej S. Antagonistic interactions and production of halocin antimicrobial peptides among extremely halophilic prokaryotes isolated from the solar saltern of Sfax, Tunisia. Extremophiles. 2016;20:363–374. doi: 10.1007/s00792-016-0827-9. [DOI] [PubMed] [Google Scholar]
- 53.Berney M, Weilenmann HU, BassinC IhssenJ, Egli T. Specific growth rate determines the sensitivity of Escherichia coli to thermal, UVA, and solar disinfection. Appl Environ Microbiol. 2006;72:2586–2593. doi: 10.1128/AEM.72.4.2586-2593.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abd Elrazak A, Ward AC, Glassey J. Polyunsaturated fatty acid production by marine bacteria. Bioprocess Biosyst Eng. 2013;36:1641–1652. doi: 10.1007/s00449-013-0936-0. [DOI] [PubMed] [Google Scholar]
- 55.Baati H, Siala M, Azri C, Ammar E, Dunlap C, Trigui M. Genomic analysis of heavy metal-resistant Halobacterium salinarum isolated from Sfax solar saltern sediments. Extremophiles. 2022;26:25. doi: 10.1007/s00792-022-01273-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pfeiffer F, Weidinger G, Goebel W. Genetic variability in Halobacterium halobium. J Bacteriol. 1981;145:375–381. doi: 10.1128/jb.145.1.375-381.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.DasSarma S. Mechanisms of genetic variability in Halobacterium halobium: the purple membrane and gas vesicle mutations. Can J Microbiol. 1989;35:65–72. doi: 10.1139/m89-010. [DOI] [PubMed] [Google Scholar]
- 58.Charlebois RL. Evolutionary origins of the haloarchaeal genome. In: Oren A, editor. Microbiology and biogeochemistry of hypersaline environments. Boca Raton: CRC Press; 1999. pp. 309–317. [Google Scholar]
- 59.Fileé J, Siguier P, Chandler M. Insertion sequence diversity in archaea. Microbiol Mol Biol Rev. 2007;71:121–157. doi: 10.1128/MMBR.00031-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pfeiffer F, Schuster SC, Broicher A, Falb M, Palm P, Rodewald K, Ruepp A, Soppa J, Tittor T, Oesterhelt D. Evolution in the laboratory: the genome of Halobacterium salinarum strain R1 compared to that of strain NRC-1. Genomics. 2008;91:335–346. doi: 10.1016/j.ygeno.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 61.Kunka KS, Griffith JM, Holdener C, Bischof KM, Li H, DasSarma P, DasSarma S, Slonczewski JL. Acid experimental evolution of the haloarchaeon Halobacterium sp. NRC-1 selects mutations affecting arginine transport and catabolism. Front Microbiol. 2020;11:1–14. doi: 10.3389/fmicb.2020.00535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Akmoussi TS, Khemili TS, Kebbouche GS, Lenchi INe, Khelfaoui MA, Sayah A, Bouarab G, Ferrioune I, Mokhtari W, Najjari A. Diversity of culturable halophilic archaea and Bacteria from chott Tinsilt and El Malah Salt-Lake in Algeria. Curr Res Bioinform. 2020;8:46–54. [Google Scholar]
- 63.Andrei AS, Banciu HL, Oren A. Living with salt: metabolic and phylogenetic diversity of archaea inhabiting saline ecosystems. FEMS Microbiol Lett. 2012;330:1–9. doi: 10.1111/j.1574-6968.2012.02526.x. [DOI] [PubMed] [Google Scholar]
- 64.Rodrigo-Baños M, Montero Z, CrespoJT, Garbayo I, Vílchez C, Martínez-Espinosa RM (2021) Haloarchaea: a promising biosource for carotenoid production. N. Misawa (ed.), carotenoids: biosynthetic and biofunctional approaches, Advances in Experimental Medicine and Biology 1261. 10.1007/978-981-15-7360-6_13 Springer Nature Singapore Pte Ltd [DOI] [PubMed]
- 65.Jehlička J, Edwards HGM, Oren A. Bacterioruberin and salinixanthin carotenoids of extremely halophilic archaea and bacteria: a Raman spectroscopic study. Spectrochim Acta A Mol Biomol Spectrosc. 2013;106:99–103. doi: 10.1016/j.saa.2012.12.081. [DOI] [PubMed] [Google Scholar]
- 66.Fard AK, Deldar AA, Sedaghat S. The effect of bacterioruberin deletion on production of bacteriorhodopsin in Halobacterium salinarum R1. J Pure App Chem Res. 2018;7:39–44. [Google Scholar]
- 67.Yatsunami R, Ando A, YangY TS, Kohno M, Matsumura Y, Ikeda H, Fukui T, Nakasone K, Fujita N, Sekine M, Takashina T, Nakamura S. Identification of carotenoids from the extremely halophilic archaeon Haloarcula japonica. Front Microbiol. 2014;5:1–5. doi: 10.3389/fmicb.2014.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vázquez-Madrigal AS, Barbachano-Torres A, Arellano-Plaza M, Kirchmayr MR, Finore I, Poli A, Nicolaus B, Zavala SDT, Camacho-Ruiz RM. Effect of carbon sources in carotenoid production from Haloarcula sp M1, Halolamina sp M3 and Halorubrum sp M5, halophilic archaea isolated from Sonora Saltern Mexico. Microorganisms. 2021;9:1096. doi: 10.3390/microorganisms9051096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fang CJ, Ku KL, Lee MH, Su NW. Influence of nutritive factors on C50 carotenoids production by Haloferax mediterranei ATCC 33500 with two-stage cultivation. Biores Technol. 2010;101:6487–6493. doi: 10.1016/j.biortech.2010.03.044. [DOI] [PubMed] [Google Scholar]
- 70.Hamidi M, Abdin MZ, Nazemyieh H, Hejazi MA, Hejazi MS. Optimization of total carotenoid production by Halorubrum sp. TBZ126 using Response surface methodology. J Microb Biochem Technol. 2014;6:286–294. [Google Scholar]
- 71.De la Vega M, Sayago A, Ariza J, Barneto AG, León R. Characterization of a bacterioruberin-producing haloarchaea isolated from the marshlands of the Odiel river in the southwest of Spain. Biotechnol Prog. 2016;32:592–600. doi: 10.1002/btpr.2248. [DOI] [PubMed] [Google Scholar]
- 72.Okmen G, Arslan A. The effects of environmental conditions on growths of halophilic archaea isolated from Lake Tuz. Int J Environ Sci Technol. 2019;16:5155–5162. [Google Scholar]
- 73.Giani M, Lobato ZM, Garbayo I, Vílchez C, Vega JM, Martínez-Espinosa RM. Haloferax mediterranei cells as C50 carotenoid factories. Mar Drugs. 2021;19:100–110. doi: 10.3390/md19020100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bhosale P. Environmental and cultural stimulants in the production of carotenoid from microorganisms. Appl Microbiol Biotechnol. 2004;63:351–361. doi: 10.1007/s00253-003-1441-1. [DOI] [PubMed] [Google Scholar]
- 75.Raghavan T, Furtado I. Occurrence of extremely halophilic archaea in sediments from the continental shelf of west coast of India. Curr Sci. 2004;86:1065–1067. [Google Scholar]
- 76.Schneegurt MA (2012) Media and conditions for the growth of halophilic and halotolerant bacteria and archaea. In Advances in Understanding the Biology of Halophilic Microorganisms; Vreeland, R.H., Ed.; Springer: Dordrecht, The Netherlands, ISBN 9789400755390
- 77.Gochnauer M, Kushwaha SC, Kate M, Kushner D. Nutritional control of pigment and isoprenoid compound formation in extremely halophilic bacteria. Arch Microbiol. 1972;84:339–349. [Google Scholar]
- 78.Hallsworth JE, Yakimov MM, Golyshin PN, Gillion JLM, D’Auria G, Alves FL, La Cono V, Genovese M, Mckew BA, Hayes SL, Harris G, Giuliano L, Timmis K, Mcgenity TJ. Limits of life in MgCl2-containing environments: chaotropicity defines the window. Environ Microbiol. 2007;9:801–813. doi: 10.1111/j.1462-2920.2006.01212.x. [DOI] [PubMed] [Google Scholar]