Abstract
Background and Aims
From 2019 till the present, infections induced by the novel coronavirus and its mutations have posed a new challenge for healthcare. However, comparative studies on pediatric infections throughout waves are few. During four different pandemic waves, we intended to investigate the clinical and epidemiological characteristic of the pediatric population hospitalized for severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) virus infection.
Methods
Between March 2020 and December 2021, we performed our retrospective research on children infected with the SARS‐CoV‐2 virus at the University of Szeged. We analyzed the data of all patients who required hospitalization due to positive results of SARS‐CoV‐2 tests (Nucleic Acid Amplification Test or rapid antigen test). Data analysis included demographic data, medical history, clinical findings, length of hospitalization, and complications, using medical records.
Results
In this study, data from 358 coronavirus‐infected children were analyzed. The most affected age group was children over 1 month and under 1 year (30.2%). The highest number of cases was recorded in the fourth wave (53.6%). Fever (65.6%), cough (51.4%), nasal discharge (35.3%), nausea and vomiting (31.3%), and decreased oral intake (28.9%) were the most common symptoms. The most common complications were dehydration (50.5%), pneumonia (14.9%), and bronchitis/bronchiolitis (14.5%). Based on RR values, there are considerable differences in the prevalence of the symptoms and complications between the different age groups and waves. Cox proportional hazard model analyzes showed that fever and tachypnoea had a relevant effect on days to recovery.
Conclusions
We found trends similar to those previously published, overall statistics. The proportion of children requiring hospitalization varied from wave to wave, with the fourth wave affecting the Hungarian child population the most. Our findings suggest that hospitalization time is unrelated to age, but that certain symptoms (fever and tachypnoea) are associated with longer hospitalization. The onset of certain symptoms may differ by age group.
Keywords: children, COVID‐19, hospitalization wave, SARS‐CoV‐2 infection
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) infection caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is the first worldwide pandemic of the twenty‐first century. Statistics from the World Health Organization (WHO, March 2022) show that since it started, there have been more than 452 million confirmed cases and more than 6 million deaths. 1
Compared with adults, SARS‐CoV‐2 infections in children and adolescents usually result in less severe illness and lower mortality rate. 2 Asymptomatic infection in children has been documented in proportions ranging from 4.4% to 39%. 3 Fever (53%–59%) and cough (48%–56%) are the most common clinical symptoms in infants and neonates, whereas the upper and lower respiratory, gastrointestinal, and neurological symptoms are less common. 3 , 4 Infection with SARS‐CoV‐2 has also been linked to a serious consequence known as pediatric inflammatory syndrome with multisystem involvement (MIS‐C). 5 Deaths due to SARS‐CoV‐2 were uncommon and mostly occurred in children with underlying diseases. 4
According to the Hungarian database, 1,256,415 cases of COVID‐19 have been confirmed in Hungary by the end of December 2021. In contrast to the high adult mortality rate (n = 39,186), only 14 children died from coronavirus infection in Hungary by December 2021. 6
The Department of Pediatrics at the University of Szeged in Hungary is the Regional Healthcare Center for the treatment of 245,000 children, including pediatric cases of SARS‐CoV‐2 virus, according to National Public Health Center data. 7
It also provides high‐level medical care for children with underlying diseases, including intensive care, surgical, onco‐hematological, nephrological, cardiological, gastroenterological, and pulmonary management.
Although the different waves of the COVID‐19 pandemic have been the subject of extensive research, few studies have compared the symptoms and complications of the successive four waves, especially in hospitalized children belonging to different age groups. Moreover, there is a remarkable lack of studies assessing the condition of hospitalized children in East‐Central Europe.
The aim of our study is to expand our knowledge of COVID‐19 in childhood infections by comparing four waves among hospitalized patients in the underreported East‐Central Europe.
2. METHODS
2.1. Patients
Our retrospective survey was performed between March 2020 and December 2021. According to the appearance of SARS‐CoV2 variations in Hungary, the four COVID‐19 waves we examined in our study were as follows: The first wave, started in March 2020 caused by 2019‐nCoV; the second wave due to the Beta variant (B.1.351) started in June 2020; the third wave began in February 2021 due to the alpha variant (B.1.1.7); and the Delta variant‐caused (B.1.617) fourth wave started on July 2021.
In the Department of Pediatrics at the University of Szeged in Hungary, we analyzed data of all patients who required hospitalization due to positive results of SARS‐CoV‐2 Nucleic Acid Amplification Test or rapid antigen test. SARS‐CoV‐2 variants were not identified in every patient.
Hospital admission is recommended in cases of moderate to severe COVID‐19 disease, febrile infants younger than 3 months of age, severe underlying chronic disease (immunodeficiency, pulmonary, cardiovascular, neuromuscular, oncological, trauma, or surgical) and poor family compliance.
The study included not only COVID‐19 patients who were hospitalized because of more severe manifestations of SARS‐CoV‐2 infection, but also patients who required hospital treatment for other reasons (such as surgical management, poisoned children, trauma, etc.) and tested positive for coronavirus. Children who were admitted to hospital for late complications of COVID‐19 and had tested negative already were not included in our study.
We used the following definition of age groups: newborn refers to a baby from birth to 1 month old. Infants between the ages of 1 month and 1 year old. A toddler is between the ages of 1 and 3 years. Children aged 3–6 years can be classified as preschoolers, school‐aged children are those aged 6–12 years, and adolescents are those aged 12 years and over. Data analysis included demographic data, past medical history, clinical findings upon admission, length of hospitalization and complications, using medical records.
In Hungary, the National Centre for Public Health regulates the conditions of discharge for patients with confirmed SARS‐Cov‐2 infection who have been hospitalized. The recommendation primarily applies to adults and does not include children. In light of the foregoing, the discharge of children is primarily determined by the clinical picture, which applies to all waves. Normal body temperature for 24 h, no need for oxygen or oral medication, adequate fluid intake, no vomiting or diarrhea, manageable home care, and 10 days of quarantine from the onset of clinical symptoms.
At the time of admission, all the patients were between the ages of 0 and 18. WHO's COVID‐19 case definition was applied. 8
2.2. Microbiological investigations
For SARS‐CoV‐2 nucleic acid detection, nasopharyngeal swabs and/or throat swabs were collected using UTM‐RT (Copan). QIAamp 96 Virus QIAcube HT Kit (Qiagen) was used for RNA isolation; for amplification and detection, IDTM SARS‐CoV‐2 Fast Essential Triplex (ID Solutions) and Allplex SARS‐CoV‐2 Assay (Seegene) were applied. Evaluation of real‐time PCR results was performed according to the manufacturers' instructions.
For the quick detection of the nucleocapsid protein from SARS‐CoV‐2 in nasal or oropharyngeal swab specimens, we employed the VivaDiagTM Pro SARS‐CoV‐2 Ag Rapid Test (VivaChek Biotech) and PanbioTM COVID‐19 Ag Rapid Test Device (Abbott). We evaluated test results according to the manufacturer's instructions. 9 , 10
2.3. Statistical analysis
Relative risk (RR) values and confidence intervals (CIs) were calculated by χ 2 test with Koopman's asymptotic method.
Cox proportional‐hazard model with enter method was used for investigating the effect of the symptoms, complications (with indicator contrast), and age groups (with Helmert and reverse Helmert contrast) on the hospitalization time hazard ratio (HR) values obtained by Cox proportional‐hazard models with 95% CIs indicate an increase or decrease in hospitalization time.
Kaplan–Meier analysis with log‐rank test was used to assess the effect of the immunocompromised status on the hospitalization time.
The prevalence of symptoms and complications across age groups was plotted as a heatmap. The z‐scored prevalences were used to implement principal component analysis (PCA).
The statistical analyses were carried out with IBM SPSS Statistics 25 and R programming language. The graphs were created using GraphPad Prism 8.4.3 and R Studio 4.1.2 (survival, ggplot2 packages)
Then, p < 0.05 were considered statistically significant. The statistical tests were two‐sided.
The investigation was approved by the local ethics committee (number of ethical permission: 222/2021).
3. RESULTS
3.1. Characteristics of COVID‐19 patients
We analyzed data about 358 SARS‐CoV‐2‐infected children who required hospitalization between March 2020 and December 2021 based on medical records. Rapid antigen test confirmed SARS‐CoV‐2 infection in 55.3% of 358 patients. In all, 53.35% of the study's participants were female. The proportion of males among the patients decreases steadily with age until the age of 12 years (Table 1).
Table 1.
Characteristics of SARS‐CoV‐2‐infected children in Szeged, Hungary, between March 2020 and December 2021 (n = 358)
| Age groups | Total number of patients (%) | Male (%) | Female (%) | Age | Number of patients in different waves (% of column total) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (±SD) | Median | First | Second | Third | Fourth | ||||
| <1 Month | 13 (3.6) | 8 (61.5) | 5 (38.5) | 0.05 (±0.01) | 0.06 | 1 (100) | 3 (3.2) | 0 (0) | 9 (4.7) |
| 1 Month–1 year | 108 (30.2) | 50 (46.3) | 58 (53.7) | 0.43 (±0.27) | 0.36 | 0 | 32 (34.0) | 17 (24.0) | 59 (30.7) |
| 1–3 Years | 78 (21.8) | 34 (43.6) | 44 (56.4) | 1.98 (±0.56) | 2.06 | 0 | 18 (19.1) | 18 (25.4) | 42 (21.9) |
| 3–6 Years | 32 (8.9) | 11 (34.4) | 21 (65.6) | 4.26 (±0.89) | 4.18 | 0 | 6 (6.4) | 5 (7.0) | 21 (10.9) |
| 6–12 Years | 50 (14) | 15 (30) | 35 (70) | 8.94 (±1.76) | 9.05 | 0 | 15 (16.0) | 12 (17.0) | 23 (12.0) |
| >12 Years | 77 (21.5) | 49 (63.6) | 28 (36.4) | 15.02 (±1.67) | 14.96 | 0 | 20 (21.3) | 19 (26.8) | 38 (19.8) |
| ∑ | 358 (100) | 167 (46.7) | 191 (53.3) | 5.42 (±5.84) | 2.35 | 1 (0.3) | 94 (26.3) | 71 (19.8) | 192 (53.6) |
Abbreviations: SARS‐Cov‐2, severe acute respiratory syndrome coronavirus 2.
More than half of the patients (55.6%, 199 of 358) are under the age of 3 years and one‐third (33.8%, 121 of 358) are under the age of 1 year (Table 1).
During the first wave (starting in March 2020), only one child required in‐patient treatment (Figure 1A). In the second wave (starting in June 2020)—which was dominated mainly by the Beta variant (B.1.351)—94 patients needed in‐patient treatment, of which 13 patients were not hospitalized due to the infection. In the second wave, the number of patients peaked in December 2020 (n = 34). In the third wave (starting in February 2021), caused by the Alpha variant (B.1.1.7), 71 patients were registered, of which 10 patients were hospitalized for other reasons. During the third wave, the highest number of patients (n = 35) was registered in March 2021. During the fourth wave (starting in July 2021), caused by the Delta variant (B.1.617.2), 192 patients were hospitalized, 12 of whom were hospitalized for reasons other than infection. The number of patients in the fourth wave peaked in November 2021 (n = 107; Table 1 and Figure 1A).
Figure 1.
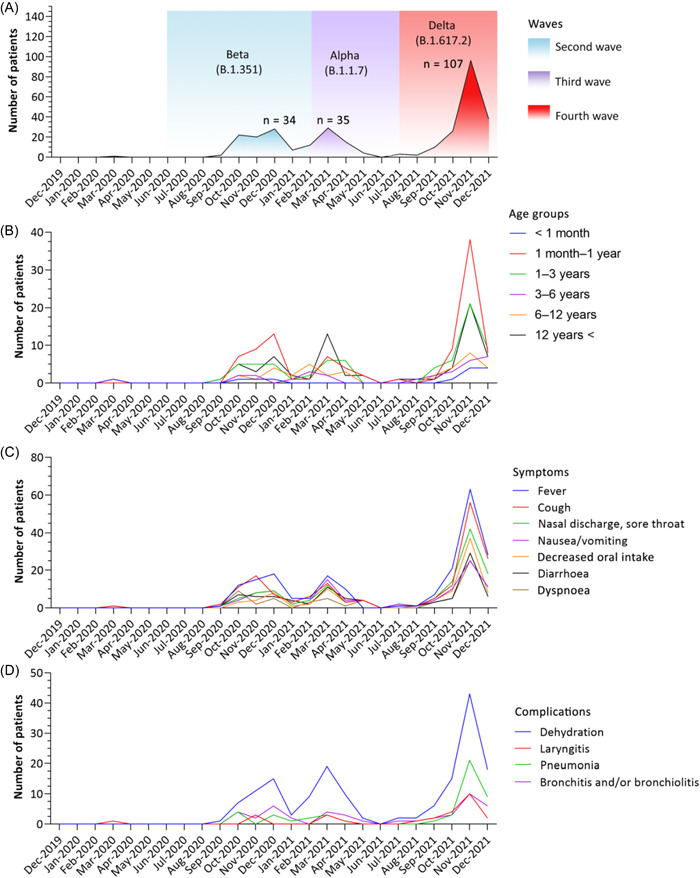
Time series data. (A) Patient number distribution over timer (the highest number of patients in a given wave is denoted by n; colors represent the period of each wave based on Hungarian database 6 ; first wave was not present due to the low patient number), (B) age distribution of hospitalized patients over time, (C) dominating symptoms in coronavirus disease 2019 (COVID‐19) patients over time, (D) complications in COVID‐19 patients over time.
3.2. COVID‐19 patient with underlying diseases
Comorbidity data from 358 patients was analyzed. Seventy‐nine children (22.06%) had underlying diseases, such as chronic lung diseases (n = 35), hematological malignancy (n = 10), neurologic disorders (n = 9), renal comorbidities (n = 7), gastrointestinal abnormalities (n = 4), congenital heart defects (n = 3), serious immunologic abnormalities (n = 2) and others, including obesity, hypertonia, metabolic disorders, and so on (n = 9) (Supporting Information: Table 1).
3.3. Recorded clinical symptoms
Almost 10% of cases (9.7%, 35 of 358) required in‐patient treatment for reasons other than COVID‐19 (non‐COVID‐19‐related hospitalization), including appendicitis (n = 8), traumatic injury (n = 5), diabetic ketoacidosis (n = 3), psychiatric disorder (n = 2), intoxication (n = 2), onco‐hematological treatment (n = 2), nephrotic syndrome relapse (n = 1), pyelonephritis (n = 1), urinary tract obstruction (n = 1), oral bleeding (n = 1), and observation for other cases (n = 3). Six asymptomatic patients required isolation due to COVID‐19.
The study included 323 COVID‐19 patients who were hospitalized due to more severe manifestations of SARS‐CoV‐2 infection (COVID‐19‐related hospitalization).
Among the recorded symptoms, we observed a prevalence of over 10% for 10 symptoms: fever, cough, nasal discharge, nausea/vomiting, decreased oral intake, diarrhea and dyspnea, weakness and fatigue, abdominal pain, and headache. In addition, 13 respiratory, systemic, neurological, and other symptoms were observed with a prevalence of <10%. The prevalence of symptoms is summarized in Figure 2A.
Figure 2.
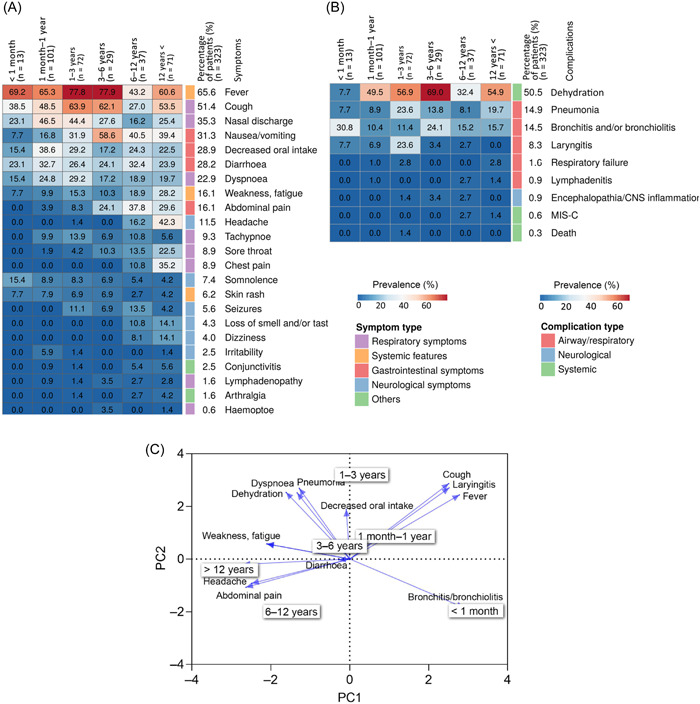
Characteristics of different age groups. (A) The heatmap show the prevalence of the symptoms in the investigated age groups. The columns and rows represent the different age groups and symptoms, respectively. (B) The heatmap show the prevalence of the complications in the different age groups. (C) The biplot from principal component analysis revealing the relationship between the symptoms, complications, and different age groups. Arrows indicate symptoms and complications, pointing to age groups in two‐dimensional space. Arrow length and direction are informative: longer arrows are more specific, whereas shorter arrows indicate more general symptoms and complications. Symptoms and complications that are close to each other at right angles are not correlated. Angles smaller than right angles indicate a positive correlation, whereas angles larger than right angles indicate a negative correlation.
The prevalence of the most common symptoms among 18 immunocompromised showed slight difference (fever, 67.4%; cough, 47.3%; nasal discharge, 39.1%; nausea/vomiting, 38.6%; decreased oral intake, 26.2%; diarrhea, 29.5%; dyspnea, 27.2%; weakness and fatigue, 22.4%; abdominal pain, 17.3%; headache, 19.7%). However, as the number of observations in the two groups differs remarkably, we cannot draw any firm conclusions and obtain reliable p.
3.4. Recorded complications
Of the nine complications investigated, four had a prevalence above 8%: dehydration, pneumonia, bronchitis, and laryngitis. Additional respiratory, neurological, and other symptoms were observed with a lower prevalence.
During the four waves, the infection was fatal in one case in the 1–3 year age group. The prevalence of symptoms is summarized in Figure 2B.
The prevalence of the most common complications among the 18 immunocompromised showed slight difference (dehydration, 62.4%; pneumonia, 18.3%; bronchitis, 17.7%; laryngitis, 14.7%). However, as the number of observations in the two groups differs remarkably, we cannot draw any firm conclusions and obtain reliable p.
3.5. Characteristic symptoms and complications of the age groups
The prevalence of the recorded 23 symptoms differed by age groups. All 23 symptoms manifested in children over 12 years of age. In contrast, only 43.5% of symptoms (10 of 23) occurred among the neonates. Most complications occurred in the 6–12‐year‐old age group, whereas only 44.4% were found in neonates (4 of 9).
PCA biplot (Figure 2C) showed a trend in the prevalence of symptoms with increasing age. Over 3 years old, patients experienced more frequent symptoms such as headache, abdominal pain, weakness, and fatigue with a correlation in prevalence. However, this may be explained by the fact that the perception of these symptoms is not objective and older children are better able to express themselves.
The PCA biplot shows that cough and fever, as well as laryngitis as a complication, are more characteristic among children under 12 years of age. In addition to this, bronchitis and bronchiolitis have been found to be typical complications in children under 1 month of age.
3.6. Characteristic symptoms and complications of the four waves
The age distribution of patients differs in each wave. In the second and fourth waves, children aged over 1 month and under 1 year were the most exposed (34%, 32 of 94, and 30.7%, 59 of 192), but in the third wave, the age distribution was even (Table 1 and Figure 1B).
Evaluating the symptoms over time, fever was found to be the most frequent symptom in every wave (Figures 1C and 3A) and it dominates the clinical scenario. Cough proved to be the second most common symptom in the fall waves of 2020 and 2021, whereas nausea/vomiting was seen in the spring wave of 2021, associated with the Alpha variant (B1.1.7).
Figure 3.
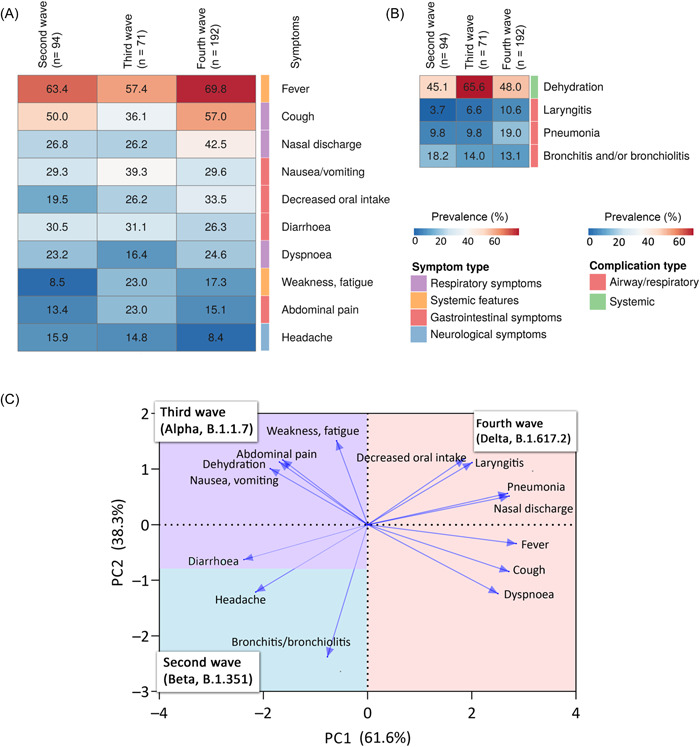
Characteristics of different waves. (A) The heatmap show the prevalence of the symptoms in the different waves. The columns and rows represent the different waves and symptoms, respectively. The heatmap represents the symptoms with prevalence over 10%. (B) The heatmap show the prevalence of the complications in the different waves. The heatmap represents the complications with prevalence over 8.3%. (C) The biplot from principal component analysis revealing the relationship between the symptoms, complications, and the different waves. Arrows indicate symptoms and complications, pointing to each wave in two‐dimensional space. Arrow length and direction are informative: longer arrows are more specific, whereas shorter arrows indicate more general symptoms and complications. Symptoms and complications that are close to each other at right angles are not correlated. Angles smaller than right angles indicate a positive correlation, whereas angles larger than right angles indicate a negative correlation. The different colors in the plot represent the waves. Their area is scaled according to which wave the arrows are most associated with.
Compared with other waves, the fourth wave showed the highest prevalence of symptoms, followed by the third and second waves.
The PCA biplot points out that the fourth wave had characteristic symptoms and complications such as reduced oral intake, nasal discharge, fever, cough, dyspnea, pneumonia, and laryngitis (Figure 3C). Based on RR values, cough was significantly 29.3% more common in the fourth wave than in the second and third waves combined (RR = 1.29, 95% CI = 1.03–1.63, p = 0.022). At the same time, the prevalence of nasal discharge was also 56.2% higher than in second and third waves combined (RR = 1.56, 95% CI = 1.17–2.22, p = 0.009).
The prevalence of weakness and fatigue were proven to be characteristic symptoms for the fourth and third waves, as the second wave was 54% lower than in the third and fourth waves combined (RR = 0.46, 95% CI = 0.21–0.73, p = 0.017) (Figure 3A–C).
The third wave was characterized by abdominal pain, dehydration, and nausea/vomiting, which were correlated. Dehydration was the most characteristic complication, as the prevalence of dehydration was 40.0% higher than in the second and fourth waves combined (RR = 1.40, 95% CI = 1.10–1.71, p = 0.023) (Figure 3A–C).
In the second wave, the highest percentages of diarrhea, headache, bronchitis, and bronchiolitis are observed, but RR values are not significantly different from the other waves (Figure 3A–C).
Sixteen children received remdesivir antiviral therapy. In the second wave, 2.13% (2 of 94) of patients and in the fourth wave 7.23% (14 of 192). This treatment was indicated in 11 patients for pneumonia, in 1 patient for obstructive bronchitis, and in 1 case for bronchiolitis due to RSV coinfection. A further 1 case of encephalopathy and 1 case of multisystem inflammatory syndrome in children (MIS‐C) led to the initiation of the drug. In one patient, the indication for treatment was not identified.
In total, 25 people received oxygen during the 4 waves (with oxygen saturation below 94%, according to WHO). In the second wave, 5.32% (5 of 94) of patients, in the third wave 5.63% (4 of 71) of children, and in the fourth wave a slightly higher proportion of 8.33% (16 of 192). One patient required mechanical ventilation, one needed noninvasive ventilation, and one had both. Twenty‐two individuals were given oxygen using a nasal cannula or mask.
3.7. Hospitalization time among children
The overall mean and median hospitalization time were estimated as 5 and 4 days, respectively. The worst results were seen in the age group <1 month, in which >50% of the cases required in‐patient care for more than 5 days and in 15% more than 7 days. The longest hospitalization time was 39 days (37 days at intensive care unit [ICU]) in a patient with severe COVID‐19 pneumonia.
To investigate the effect of the patients' age group on the hospitalization time, Cox proportional‐hazards model was used, where HR values indicate the increase or decrease in hospitalization time. The worst HR value was measured in the <1 month group, in which the patients spend 20% more time in clinical care compared with other age groups (HR = 0.80, 95% CI: 0.49–1.40, p = 0.21). However, this 20% increase does not prove to be significant, as the CI, as for all other groups, includes 0.
Analyzing the symptoms and complications, the Cox proportional‐hazard model results showed that fever has a significant negative effect on recovery time, causing a 21% increase in days needed to recover among the patients with fever compared with patients without fever (HR = 0.79, 95% CI: 0.6–0.97; p = 0.03). Patients with tachypnoea recovered 44% slower than patients without tachypnoea (HR = 0.56, 95% CI: 0.38–0.82; p = 0.003).
Using Kaplan–Meier analysis, we found that immunocompromised status did not significantly affect hospitalization time (3.5 for immunocompromised vs. 4 for nonimmunocompromised patients). However, as the number of observations in the two groups differs remarkably, we cannot draw any firm conclusions (HR = 0.93, 95% CI = 0.59–1.48, p = 0.69).
3.8. Vaccination among the hospitalized children
In Hungary, the COVID‐19 vaccines became available for children aged 12–17 years from 14 June 2021 and for children aged 5–11 years from 15 December 2021. This study covers the period from March 2020 to December 2021. This partly explains the fact that only 1.55% of the children in the study (5 of 323) were vaccinated with messenger RNA vaccines.
It is important to emphasize that 78% of the children in the study were under 12 years of age and had access to the vaccine by mid‐December 2021. Due to the small number of children who were vaccinated, it was not possible to use statistics to test how vaccination affected different clinical parameters.
4. DISCUSSION
In this research, we analyzed data collected at the University of Szeged, South Hungary, between March 2020 and December 2021. Unlike most of the original research articles published so far, 11 , 12 , 13 , 14 we had the opportunity to examine data from four waves.
During the study period, 358 SARS‐CoV‐2‐positive children aged 0–18 years were hospitalized in the Department of Paediatrics, University of Szeged.
Compared with our time series data, the distribution of the number of patients over time is consistent with the WHO European coronavirus data. 15 Children in the age groups studied were most exposed to infection in the fourth wave caused by the Delta variant (B.1.617.2), which had the highest number of cases compared with the second and third waves. In the fourth wave, only five children were vaccinated even though a vaccine against the virus was available.
In the remainder of the discussion, we compare the results of our study with findings from research that have examined the same waves.
Our results on the occurrence of underlying disease are consistent with the published literature. Based on the results of Garazzino et al., 11 19.6% of infected children in Italy suffered from underlying disease. Garazzino et al. 11 and Bialek et al. 16 also observed a higher rate of hospitalized children under the age of 1 year (62–78.8%), suggesting increased susceptibility of this age group of patients to symptomatic SARS‐CoV‐2 infection. However, the higher rate of hospitalization may also be explained by the fact that parents may seek medical advice for these children more frequently than with older children. The age distribution we describe is consistent with these results.
In our study, the most common COVID‐19 symptoms were fever, cough, and nasal discharge. These symptoms dominated all waves with a high prevalence. A particularly high proportion of patients experienced coughing and nasal discharge during the fourth wave. These findings are also supported by various international studies from the early COVID era. 11 , 12 , 13 , 14 , 17 , 18 In contrast with these data, in Chinese and US American children, fever was less common (36%–56%) compared with cough. 16 , 19 , 20
Based on our time series data, these gastrointestinal symptoms were more frequent during the third wave of the pandemic, compared with previously described. 21 During the second and fourth waves, the presence of respiratory symptoms was prominent; these results are consistent with other studies. 18 , 22 , 23
A previously published study highlighted those neurologic symptoms can occur in patients with severe COVID‐19, up to 22% of 1695 hospitalized children and adolescents. 22 Consistent with these findings, our results also suggest a low incidence of neurological symptoms, with the majority of cases occurring over the age of 12 years. This might be explained by the fact that several subjective symptoms—such as dizziness, loss of smell and taste, and headache—are difficult to measure, in younger children who are less capable of self‐expression. In agreement with a previous study, 24 headache was also found to be the most common neurological symptom in our research.
In acute COVID‐19 infection, status epilepticus, encephalopathy, encephalitis, Guillain‐Barré syndrome, and acute demyelinating occur around 4% of hospitalized children and are particularly frequent in children with pre‐existing neurological disorders. 24 In our study, only three children suffered from neurological complications and none of them had underlying neurological disease.
In contrast to an international study on neonates, 25 which found that gastrointestinal symptoms and poor oral intake are more common, we found that there is no difference in the distribution of typical symptoms in neonates. Rather, a higher proportion of reduced oral intake and diarrhea is observed up to 1 month to 1 year of age.
In our study, dehydration was the most common complication ranking as one of the top reasons for hospitalization. Dehydration can be caused by virus‐induced gastroenteritis, a lack of oral intake due to fever and sore throat, as well as cough‐related vomiting.
In our study, laryngitis is the fourth most common complication, affecting 8.3% patients, most commonly in toddlers (23.6%). One patient, similar to study of Linn et al., 26 requires intubation, mechanical ventilation due to COVID‐induced severe upper airway obstruction.
A study of 186 cases from the United States discovered that a minority of children were symptomatic before the development of MIS‐C, with a median gap of 25 days between the onset of COVID‐19 symptoms and the commencement of MIS‐C. 22 This explains our low number of MIS‐C cases during acute infection (n = 2) (Figure 3). Both patients presented during the second COVID wave. One of them required intensive care with cardiac support for four days. Lower respiratory tract inflammation (bronchitis, bronchiolitis) was the most common complication in neonates, whereas dehydration was more common in infancy.
The majority of the patients in the meta‐analysis of Toba et al. 27 were admitted to the hospital, with 10.8% (4.2%–25.3%) receiving treatment in the ICU. This proportion was lower in our study. In our study, only 3% of the patients were admitted to the ICU. The higher number of cases during the fourth wave was not associated with a higher rate of ICU admission. Although symptoms were most manifested in the fourth wave with the highest prevalence on average, it did not cause an increase in the proportion of patients requiring ICU.
The observed mortality rate was consistent with the results of other studies. According to a case series review of studies published until March 25, 2021, the mortality rate among children with COVID‐19 was 0‐0.69. 22 The proportion of deaths was higher, 2.4% according to Toba et al. 27
We identified symptoms and complications that may have a significant impact on hospitalization time, such as fever and tachypnoea. However, the negative impact of fever and tachypnoea on hospitalization time in the present study is smaller. These finding are consistent with previous published research, in which fever was identified as one of the most important negative prognostic factors for time to recovery, 28 and tachypnoea as negative predictor of survival and recovery time. 29
Our clinical research has several limitations. First, there are limitations from retrospective data collection. In the absence of detailed studies spanning multiple waves and a longer COVID‐19 period, comparisons and drawing conclusions are difficult. Furthermore, we only processed data from acutely SARS‐CoV‐2‐positive patients, possible subsequent COVID‐associated complications are not included in this study. Furthermore, SARS‐CoV‐2 variants were not identified in every patient.
Our 22‐month retrospective study provides detailed data for a better understanding of childhood COVID‐19 disease. Newer and newer variants of the virus continue to produce disease of varying severity. With more than 1.8 million COVID‐infected citizens, Hungary is highly severely affected by the pandemic. Based on our regional data from South‐Eastern Hungary presented, childhood is characterized by a milder course, lower hospitalization rates, shorter hospital stays, and lower mortality rates, which will hopefully remain the same in the next waves.
5. CONCLUSION/SUMMARY
The emergence of various clinical symptoms is age‐related. The third wave had a larger proportion of gastrointestinal problems, whereas respiratory symptoms dominated in the fourth wave. Dehydration was the most prevalent infectious consequence due to hospitalization. Virus strains that caused infection in the early periods of the COVID era were associated with lower mortality and complication rates in the pediatric population compared to adults.
AUTHOR CONTRIBUTIONS
Andrea T. Takács: Conceptualization; Data curation; formal analysis; investigation; visualization; writing – original draft; writing – review & editing. Mátyás Bukva: Formal analysis; software; supervision; validation; visualization; writing – original draft; writing – review & editing. Gabriella Gavallér: Conceptualization; data curation; formal analysis; investigation. Katalin Kapus: Conceptualization; data curation; formal analysis; investigation. Mária Rózsa: Data curation; investigation. Boglárka Bán‐Gagyi: Data curation; investigation. Mária Sinkó: Data curation; investigation. Dániel Szűcs: Data curation; investigation. Gabriella Terhes: Conceptualization; formal analysis; funding acquisition; investigation; supervision; writing – review & editing. Csaba Bereczki: Conceptualization; software; supervision; writing – review & editing.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
TRANSPARENCY STATEMENT
The lead author Andrea T. Takács affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Supporting information
Supporting information.
ACKNOWLEDGMENT
The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Takács AT, Bukva M, Gavallér G, et al. Epidemiology and clinical features of SARS‐CoV‐2 infection in hospitalized children across four waves in Hungary: a retrospective, comparative study from March 2020 to December 2021. Health Sci Rep. 2022;5:e937. 10.1002/hsr2.937
REFERENCES
- 1. World Health Organization . 2022. Coronavirus Disease (COVID‐19) Dashboard, Accessed March 15, 2022. https://covid19.who.int/
- 2. World Health Organization . 2021. COVID‐19 disease in children and adolescents: scientific brief. September 29, 2021. Accessed March 16, 2022: https://apps.who.int/iris/handle/10665/345575
- 3. Borrelli M, Corcione A, Castellano F, Fiori Nastro F, Santamaria F. Coronavirus disease 2019 in children. Front Pediatr. 2021;9:668484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jutzeler CR, Bourguignon L, Weis CV, et al. Comorbidities, clinical signs and symptoms, laboratory findings, imaging features, treatment strategies, and outcomes in adult and pediatric patients with COVID‐19: A systematic review and meta‐analysis. Travel Med Infect Dis. 2020;37:101825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hoste L, Van Paemel R, Haerynck F. Multisystem inflammatory syndrome in children related to COVID‐19: a systematic review. Eur J Pediatr. 2021;180(7):2019‐2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Coronamonitor . 2022. Accessed April 24, 2022. https://atlo.team/koronamonitor
- 7. National Public Health Center, Hungary . 2022. Ellátási területek nyilvántartásai. Accessed March 25, 2022. https://www.nnk.gov.hu/index.php/egeszsegugyi-igazgatasi-foosztaly/nyilvantartasok/ellatasi-teruletek-nyilvantartasai
- 8. World Health Organization . 2020. WHO COVID‐19 case definition. Accessed March 31, 2022. https://apps.who.int/iris/handle/10665/333912
- 9. Viva Diag Pro . 2022. SARS‐CoV‐2 rapid antigen test. Accessed March 31, 2022. https://www.tga.gov.au/sites/default/files/covid-19-rapid-antigen-self-tests-are-approved-australia-ifu-348890.pdf
- 10. Abbot. COVID‐19 Ag Rapid Test Device . 2022. Accessed March 31, 2022. https://dam.abbott.com/en-gb/panbio/120007883-v1-Panbio-COVID-19-Ag-Nasal-AsymptomaticSe.pdf
- 11. Garazzino S, Montagnani C, Donà D, et al. Multicentre Italian study of SARS‐CoV‐2 infection in children and adolescents, preliminary data as at 10 April 2020. Euro Surveill. 2020;25(18):2000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tagarro A, Epalza C, Santos M, et al. Screening and severity of coronavirus disease 2019 (COVID‐19) in children in Madrid, Spain. JAMA Pediatr. 2021;175(3):316‐317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kinross P, Suetens C, Gomes Dias J, et al. Rapidly increasing cumulative incidence of coronavirus disease (COVID‐19) in the European Union/European Economic Area and the United Kingdom, 1 January to 15 March 2020. Euro Surveill. 2020;25(11):2000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Götzinger F, Santiago‐García B, Noguera‐Julián A, et al. COVID‐19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020;4(9):653‐661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. (2021). Tracking SARS‐CoV‐2 variants. Accessed May 1, 2022. https://www.who.int/en/activities/tracking-SARS-CoV-2-variantsWorld
- 16. Bialek S, Gierke R, Hughes M, McNamara LA, Pilishvili T, Skoff T, CDC COVID‐19 Response Team . Coronavirus disease 2019 in children ‐ United States, February 12‐April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(14):422‐426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Esposito S, Marchetti F, Lanari M, et al. COVID‐19 management in the pediatric age: consensus document of the COVID‐19 Working Group in Paediatrics of the Emilia‐Romagna Region (RE‐CO‐Ped), Italy. Int J Environ Res Public Health. 2021;18(8):3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guo CX, He L, Yin JY, et al. Epidemiological and clinical features of pediatric COVID‐19. BMC Med. 2020;18(1):250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. She J, Liu L, Liu W. COVID‐19 epidemic: disease characteristics in children. J Med Virol. 2020;92(7):747‐754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lu X, Zhang L, Du H, et al. SARS‐CoV‐2 infection in children. N Engl J Med. 2020;382(17):1663‐1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Meyer M, Holfter A, Ruebsteck E, et al. The alpha variant (B.1.1.7) of SARS‐CoV‐2 in children: first experience from 3544 nucleic acid amplification tests in a cohort of children in Germany. Viruses. 2021;13(8):1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nikolopoulou GB, Maltezou HC. COVID‐19 in children: where do we stand? Arch Med Res. 2022;53(1):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Howard‐Jones AR, Burgner DP, Crawford NW, et al. COVID‐19 in children. II: pathogenesis, disease spectrum and management. J Paediatr Child Health. 2022;58(1):46‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lin JE, Asfour A, Sewell TB, et al. Neurological issues in children with COVID‐19. Neurosci Lett. 2021;743:135567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ryan L, Plötz FB, van den Hoogen A, et al. Neonates and COVID‐19: state of the art: neonatal sepsis series. Pediatr Res. 2022;91(2):432‐439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lim CC, Saniasiaya J, Kulasegarah J. Croup and COVID‐19 in a child: a case report and literature review. BMJ Case Rep. 2021;14(9):e244769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Toba N, Gupta S, Ali AY, et al. COVID‐19 under 19: a meta‐analysis. Pediatr Pulmonol. 2021;56(6):1332‐1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tolossa T, Wakuma B, Seyoum Gebre D, et al. Time to recovery from COVID‐19 and its predictors among patients admitted to treatment center of Wollega University Referral Hospital (WURH) Western Ethiopia. Survival analysis of retrospective cohort study. PLoS One. 2021;16(6):e0252389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mejía F, Medina C, Cornejo E, et al. Oxygen saturation as a predictor of mortality in hospitalized adult patients with COVID‐19 in a public hospital in Lima, Peru. PLoS One. 2020;15(1512):e0244171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.


