Climate change-induced heat waves and environmental factors may contribute to diabetes-related morbidity and mortality. As climate change exacerbates the negative effects of extreme ambient temperature on health, there is urgency for personalized diabetes care to consider a person’s environment. This is especially true for disadvantaged populations such as Medicaid beneficiaries, who—due to structural racism and socioeconomic disparities—have a higher prevalence of type 2 diabetes (T2D) and may be particularly susceptible to extremes in outdoor temperature and their accompanying adverse health outcomes. Therefore, we sought to examine how extreme temperatures affect the occurrence of serious hypoglycemia, diabetic ketoacidosis (DKA), and sudden cardiac arrest/ventricular arrhythmia (SCA/VA) among Medicaid beneficiaries with T2D. The consideration of patient-experienced ambient temperature may be an essential missing piece to reducing disparities in diabetes-related adverse health outcomes.
We conducted an analytic epidemiologic study using 1999–2010 enrollment and health care claims data from Medicaid and Medicaid-Medicare (i.e., dual) enrollees from California, Florida, New York, Ohio, and Pennsylvania, linked to publicly available meteorologic data from the National Oceanic and Atmospheric Administration. We first identified people with T2D by using a validated International Classification of Diseases diagnosis-based algorithm (1). We then followed such individuals from the date of their first T2D diagnosis through either benefit disenrollment or death, whichever came first. Within this observation time, we calculated crude occurrence rates (and 95% CIs) for three health outcomes of high concern for people with T2D, namely, hospital presentation for serious hypoglycemia, hospital admission for DKA, and hospital presentation for SCA/VA, by prespecified strata of maximum daily ambient temperature (i.e., hour of highest temperature each day) in degrees Celsius, from which we generated trend lines with weighted 95% confidence bands. Health outcomes were identified using validated International Classification of Diseases diagnosis-based definitions. Our decision to study these outcomes is supported by an imperative to minimize hypoglycemic episodes and DKA, a federal action plan calling for hypoglycemia research, dramatic increases in DKA rates among people with T2D, and the fact that SCA/VA is responsible for half of all cardiovascular deaths in people with diabetes. Daily temperatures were mapped from geocoded weather stations to population-weighted centroids of zone improvement plan (ZIP) codes and then assigned to individuals based on their ZIP code of residence in the Medicaid enrollment file (2). We used Poisson regression conditioned on patient to assess the statistical significance of linear and quadratic temperature terms. This research was approved by the University of Pennsylvania’s institutional review board.
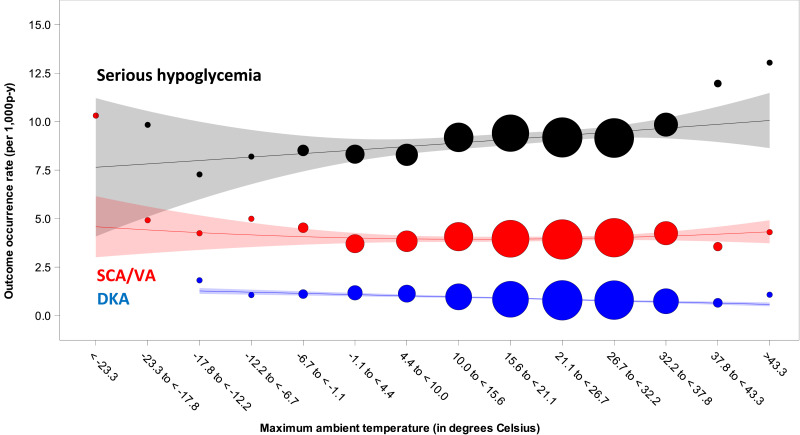
We identified 2,955,110 people with T2D contributing 9,588,861 person-years of observation. These people were predominantly White (41%), non-Hispanic (79%), female (62%), and California residents (42%), with a median follow-up time of 2.3 years. The median (1st, 99th percentile) maximum daily ambient temperature was 22.4°C (−3.6°C, 37.9°C). Occurrence rates per 1,000 person-years were 9.20 (95% CI 9.14–9.26) for serious hypoglycemia, 0.86 (0.85–0.88) for DKA, and 3.99 (3.95–4.03) for SCA/VA. Figure 1 depicts rates by prespecified temperature strata and trend lines.
Figure 1.
Outcome occurrence rates by prespecified strata of maximum daily ambient temperature, in degrees Celsius, among Medicaid and Medicaid-Medicare (i.e., dually eligible) beneficiaries with T2D. Black circles are occurrence rates of serious hypoglycemia. Red circles are occurrence rates of SCA/VA. Blue circles are occurrence rates of DKA. From these occurrence rates we generated trend lines with weighted 95% confidence bands. We used Poisson regression (conditioned on patient) to assess statistical significance for linear and quadratic terms for maximum daily ambient temperature. For serious hypoglycemia, P values for linear and quadratic terms for maximum daily ambient temperature, based on 116,894,339 observations, were 0.113 and <0.001, respectively. For SCA/VA, P values for linear and quadratic terms for maximum daily ambient temperature, based on 39,443,759 observations, were <0.001 and 0.031, respectively. For DKA, the P values for linear and quadratic terms for maximum daily ambient temperature, based on 9,170,990 observations, were 0.003 and 0.488, respectively. Note that we scaled the size of each data point to reflect its weight (using the inverse of the variance estimate). p-y, person-years.
We found differences in rates of health outcomes by daily ambient temperatures experienced by Medicaid and Medicaid-Medicare enrollees with T2D. For serious hypoglycemia (black curve), we found a J-shaped relationship in which rates were highest during cold and hot temperature extremes; the P value for the quadratic term for maximum ambient temperature was <0.001. This pattern is generally consistent with a prior study of ∼2,500 Germans with diabetes (68% with T2D), in which severe hypoglycemia was more common during temperature extremes (3). For DKA (blue trendline), we found a higher rate during cold (−17.8 to −12.2°C) vs. hot (>43.3°C) temperature extremes; the P value for the linear term for maximum ambient temperature was 0.003. This inverse linear relationship is consistent with a time series study of ∼40,000 Taiwanese with diabetes, in whom DKA was more likely during colder temperatures and winter months (4). For SCA/VA (red curve), we found a J-shaped relationship in which rates were highest during cold and hot temperature extremes; the P value for the quadratic term for maximum ambient temperature was 0.031. This pattern is consistent with an epidemiologic study of ∼50,000 Koreans in which out-of-hospital cardiac arrest was more common during temperature extremes, with effects being more pronounced for extreme cold than extreme heat (5). Mechanisms to explain these patterns may include the ability of ambient temperatures to affect glucose homeostasis; inflammation; thermoregulatory capacity; renal clearance of drugs; and arrhythmogenic, atherosclerotic, and/or coagulopathic states. This study is limited by the use of ZIP code as a proxy for individual exposure to the environment; no data on use of heating/ventilation/air conditioning; and concern for residual confounding, particularly by time-varying patient factors.
Article Information
Funding and Duality of Interest. This work was supported by a Quartet Pilot Research Award funded by the Population Aging Research Center at the University of Pennsylvania. C.E.L. and S.H. are further supported by grants from the National Institute of Mental Health (R01MH130435), National Institute on Aging (R01AG025152, R01AG060975, and R01AG064589), National Institute on Drug Abuse (R01DA048001), and Centers for Diseases Control and Prevention (R01CE003347). J.H.F. is supported by the following National Cancer Institute grant: P30CA008748. The effort of C.S. is supported by the University of Pennsylvania’s Undergraduate Clinical Scholars Program: Pathway to Clinical Research Careers, funded by the National Institute of Diabetes and Digestive Kidney Diseases (R25DK108711). M.L.B. is funded by the following grants or contracts paid to her institution: the Environmental Protection Agency (EPA), National Institutes of Health (NIH), High Tide Foundation, Yale Climate Change and Health Center, Robert Wood Johnson Foundation, Environmental Defense Fund, Health Effects Institute, and Wellcome Trust. M.L.B. receives consulting fees from the EPA. M.L.B. has recently received honoraria from Boston University, Harvard University, University of Montana, Korea University, The Organization of Teratology Information Specialists, NIH, Health Canada, Pacific-10 Conference, and UK Research and Innovation. M.L.B. recently received travel support from Boston University, Harvard University, and University of Illinois Urbana—Champaign. M.L.B. serves on the following data safety monitoring or advisory boards: EPA Clean Air Scientific Advisory Board, National Academies Panels, Lancet Countdown, Fifth National Climate Assessment, Johns Hopkins University Department of Environmental Health and Engineering, and the World Health Organization Global Air Pollution and Health Technical Advisory Group. C.E.L. is an Executive Committee Member of and S.H. directs the University of Pennsylvania’s Center for Real-World Effectiveness and Safety of Therapeutics. The Center for Real-World Effectiveness and Safety of Therapeutics receives funds from Pfizer and Sanofi to support pharmacoepidemiology education. S.H. reports grants from the U.S. Food and Drug Administration (FDA) and consulting for the Medullary Thyroid Cancer Consortium (Novo Nordisk, AstraZeneca, GlaxoSmithKline, and Eli Lilly), bluebird bio, and Amylyx Pharmaceuticals. S.H. is a special government employee of the FDA. C.E.L. recently received honoraria from the American College of Clinical Pharmacy Foundation, the University of Florida, the University of Massachusetts, the Scientific and Data Coordinating Center for the NIH-funded Chronic Renal Insufficiency Cohort Study, the Consortium for Medical Marijuana Outcomes Research, and Health Canada. C.E.L. is a special government employee of the FDA and consults for their Reagan-Udall Foundation. C.E.L. receives travel support from John Wiley & Sons. C.E.L.’s spouse is an employee of Merck. Neither C.E.L. nor his spouse owns stock in the company. No other potential conflicts of interest relevant to this article were reported.
The content herein is solely the responsibility of the authors and does not necessarily represent official views of the University of Pennsylvania, the NIH, or the Centers for Diseases Control and Prevention.
Author Contributions. C.M.B. and W.B.B. were responsible for the data analysis. K.B., C.M.B., and C.E.L. completed an initial draft of this work, and all authors contributed to subsequent drafts. C.E.L. was responsible for the study’s conception and design. C.E.L. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1. Klompas M, Eggleston E, McVetta J, Lazarus R, Li L, Platt R. Automated detection and classification of type 1 versus type 2 diabetes using electronic health record data. Diabetes Care 2013;36:914–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nam YH, Bilker WB, Leonard CE, Bell ML, Alexander LM, Hennessy S. Effect of statins on the association between high temperature and all-cause mortality in a socioeconomically disadvantaged population: a cohort study. Sci Rep 2019;9:4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hensel M, Stuhr M, Geppert D, Kersten JF, Lorenz J, Kerner T. Reduced frequency of severe hypoglycemia at mild ambient temperatures between 10 and 20 °C: a population-based study under marine west coast climate conditions. J Diabetes Complications 2017;31:1212–1214 [DOI] [PubMed] [Google Scholar]
- 4. Lu CL, Chang HH, Chen HF, et al. Inverse relationship between ambient temperature and admissions for diabetic ketoacidosis and hyperglycemic hyperosmolar state: a 14-year time-series analysis. Environ Int 2016;94:642–648 [DOI] [PubMed] [Google Scholar]
- 5. Kang SH, Oh IY, Heo J, et al. Heat, heat waves, and out-of-hospital cardiac arrest. Int J Cardiol 2016;221:232–237 [DOI] [PubMed] [Google Scholar]