Abstract
OBJECTIVE
Hyperglycemia can increase urinary zinc excretion. We evaluated the association of higher urinary zinc level with new diagnosis of incident type 2 diabetes mellitus (T2DM) in adult populations with a high burden of T2DM from AZ, OK, and ND and SD. We also assessed the cross-sectional association of urinary zinc levels with prevalent prediabetes.
RESEARCH DESIGN AND METHODS
We included 1,339 adults free of T2DM at baseline (1989–1991) followed through 1998–1999 in the Strong Heart Study (SHS) and 1,905 family members of SHS participants followed as part of the Strong Heart Family Study (SHFS) through 2006–2009.
RESULTS
T2DM incidence was 14.7% (mean follow-up 6.6 years) in the SHS and 13.5% (mean follow-up 5.6 years) in the SHFS. After adjustment for sex, site, education, smoking status, BMI, and estimated glomerular filtration rate, the hazard ratio of T2DM in comparing 75th vs. 25th percentiles of urinary zinc distribution was 1.21 (95% CI 1.08, 1.36) in the SHS and 1.12 (0.96, 1.31) in the SHFS. These associations were attenuated but significant in the SHS after adjustment for HOMA of insulin resistance (HOMA-IR) score. With exclusion of participants with prediabetes at baseline, urinary zinc remained significantly associated with T2DM in the SHS. In cross-sectional analyses, prediabetes was associated with higher urinary zinc levels.
CONCLUSIONS
Urinary zinc levels were associated with T2DM incidence and prediabetes prevalence even after adjustment for HOMA-IR in populations with a high burden of T2DM. These results highlight the importance of zinc metabolism in diabetes development.
Introduction
Zinc is an essential trace element that plays a direct role in insulin homeostasis by stimulating insulin secretion through a specific transporter (ZnT8) in pancreatic β-cells (1). In peripheral tissues, zinc inhibits protein tyrosine phosphatase 1B (PTP1B) leading to net phosphorylation of the insulin receptor and, consequently, activating insulin signaling (2). Zinc is also a major antioxidant, protecting pancreatic β-cells from reactive oxygen species (3). Zinc supplementation in an animal model attenuated diabetes complications, mainly mediated by its antioxidant effect (4). In a meta-analysis of eight epidemiological studies (seven prospective and one cross-sectional), dietary zinc was inversely related to the risk of type 2 diabetes mellitus (T2DM), suggesting that zinc intake could play a role in diabetes prevention (5). It is known that individuals with diabetes versus those without diabetes have blood zinc deficiency and urinary zinc excess (6,7). However, despite the potential role of zinc metabolism in the pathogenesis of insulin resistance, prospective studies investigating the role of zinc exposure in diabetes development are largely lacking.
The Strong Heart Study (SHS) is a community-based participatory cohort study aiming to improve the prevention of cardiovascular disease and its risk factors in American Indian communities, one of the populations most affected by diabetes in the U.S. (8). In 1989–1991, 13 American Indian communities from ND and SD, OK, and AZ were invited to participate. The prevalence of T2DM was ∼50%, and over the next 10 years, 23.4% participants were newly diagnosed with T2DM confirming an extremely high burden of T2DM in the SHS communities (9). In 1998–1999 and 2001–2004, family members (median age 36 years) of the original SHS participants were invited to an SHS expansion called the Strong Heart Family Study (SHFS).
In this study, we aimed to evaluate the prospective association of urinary zinc levels with incident T2DM among participants free of T2DM at baseline in the SHS and the SHFS. To evaluate whether the prospective association was related to the presence of prediabetes at baseline, we conducted an additional analysis in participants free of both T2DM and prediabetes at baseline. Further, we evaluated the association of dietary zinc at baseline with incident T2DM in SHS and SHFS participants. We also assessed the association of urinary zinc levels with the prevalence of prediabetes in cross-sectional analyses.
Research Design and Methods
Study Population
The SHS is a prospective population-based cohort funded by the U.S. National Heart, Lung, and Blood Institute. The study includes adults between 45 and 74 years of age from 13 tribal communities in AZ, OK, and ND and SD (10) (n = 4,549). Participants were initially recruited in 1989–1991 and followed during two subsequent visits between 1993 and 1995 and between 1998 and 1999 (10). For the family members recruited in 1998–1999 and 2001–2004 as part of the SHFS, follow-up visits were conducted in 2001–2004 (for those included in 1998–1999) and 2006–2009 (for all). In 2016, one of the tribes declined participating in further research, resulting in 3,516 participants available for this study. The SHS protocol was approved by institutional review boards, participating tribal communities, and the respective area Indian Health Service institutional review boards, and all participants provided informed consent. The present manuscript was reviewed and approved for submission by the participating tribes.
Among the 3,516 participants included in the SHS, we excluded 1,556 with T2DM at baseline, 99 missing glycated hemoglobin (HbA1c) and 245 urinary zinc, 111 participants who died before any follow-up visits, 130 participants missing T2DM status at follow-up, and participants missing educational level (n = 1), alcohol intake (n = 3), BMI (n = 1), estimated glomerular filtration rate (eGFR) (n = 29), and fasting plasma glucose (FPG) (n = 2), resulting in 1,339 participants from the SHS included in this analysis.
Among participants included in the SHFS (n = 2,919), we excluded 524 with T2DM at baseline, 447 missing urine zinc levels and 45 other variables of interest (9 missing education status, 2 smoking status, 2 alcohol intake, 1 eGFR, 7 BMI, and 24 insulin levels). Finally, 1,903 participants from SHFS were included in this analysis.
Urinary Zinc, Selenium, and Arsenic Determination
Spot urine samples at baseline in the SHS and the SHFS were collected in polypropylene tubes, frozen within 1–2 h of collection, shipped buried in dry ice, and stored at −70°C in freezers with strict quality control in the MedStar Health Research Institute, Washington, DC. In 2009–2010 for the SHS and 2012 for the SHFS, urine samples were transported to the Trace Element Laboratory of Graz University (Austria), under strict controls on the sampling, transport, and storage of urine (11). The analysis of zinc was performed with inductively coupled plasma mass spectrometry (ICPMS) (Agilent 7700x ICP-MS; Agilent Technologies, Waldbronn, Germany) using a multielement technology that also analyzed selenium. The detection limit was 10 μg/L for zinc and 2 μg/L for selenium, and no samples were below the limit.
We determined urinary arsenic species (inorganic arsenic [iAs], i.e., arsenite plus arsenate; monomethylarsonate [MMA]; dimethylarsinate [DMA]; and arsenobetaine) with high-performance liquid chromatography (HPLC) ICPMS. We used the sum of urine concentrations of iAs, MMA, and DMA as a biomarker of total exposure to iAs. The limits of detection were 0.1 μg/L for arsenic species. We imputed samples below the limit of detection (96 for iAs, 13 for MMA, 0 for DMA), dividing the limit of detection by the square root of 2. The interassay coefficients of variation were 6.0% for iAs, 3.5% for MMA, and 4.4% for DMA.
Urinary arsenic, selenium, and zinc levels in μg/L were corrected by urinary creatinine (g/L) and reported in μg/g creatinine to account for urine dilution.
Type 2 Diabetes Status
Fasting blood for 12 h was collected and stored at −70°C (12). Plasma glucose concentrations were determined through enzymatic methods with reagent kits from Boehringer Mannheim (Indianapolis, IN) on a chemistry analyzer, and plasma insulin concentrations were measured by radioimmunoassay (10). HOMA of insulin resistance (HOMA-IR) score was calculated from insulin and FPG concentrations, determined with Accu-Chek II (Baxter Healthcare, Grand Prairie, TX), with the computed solved model for HOMA-IR.
In SHS participants with FPG <225 mg/dL a 75-g oral glucose tolerance test was performed. Participants with diabetes on insulin, or with oral medication reporting two prior random glucoses >250 mg/dL, were also excluded, independently of fasting glucose level for safety reasons. HbA1c was measured with high-performance liquid chromatography at the laboratory of the National Institute of Diabetes and Digestive and Kidney Diseases Epidemiology and Clinical Research Branch, Phoenix, AZ (13).
In the SHS, incident T2DM was defined according to FPG ≥126 mg/dL or a 2-h postload plasma glucose level ≥200 mg/dL or HbA1c ≥6.5% or self-reported use of insulin or oral diabetes treatment. Prediabetes was defined according to FPG level between 100 and 126 mg/dL or HbA1c level between 5.7 and 6.4%.
For the SHFS participants, HbA1c was not measured in participants without diabetes and therefore T2DM was defined according to FPG ≥126 mg/dL, self-reported physician diagnosis, or self-reported use of insulin or oral diabetes treatment. Prediabetes was defined according to FPG concentrations between 100 and 126 mg/dL.
Follow-up time for incident T2DM was estimated considering the date of diagnosis obtained under the assumption that glucose levels increased at a linear rate between study visits for participants based on glucose levels in participants from the SHS and SHFS.
Other Variables
Trained staff performed a standardized questionnaire to participants asking for sociodemographic (age, sex, site), lifestyle (smoking status, alcohol intake and dietary patterns), and clinical data; a physical exam; and collection of blood and urine samples. Smoking status was defined as never smoking (<100 cigarettes in their lifetime), former smoking (≥100 cigarettes in their lifetime but not smoking at the time of the interview), and current smoking (≥100 cigarettes in their lifetime and smoking at the time of the interview). Cigarette pack-years was calculated by multiplying the average number of cigarettes smoked per day reported by the participants by the total years of smoking, divided by 20. Alcohol intake was grouped in three categories, never (no alcohol consumption in their lifetime), former (alcohol consumer in the past but not in recent time), and current (alcohol consumer in the past and in recent time).
In the SHS, dietary data were reported by the participants during the second visit through a 24-h dietary recall with all food and beverages, including frequency and sizes, consumed the previous day to estimate the mean intake of nutrients. In the SHFS, dietary intake was collected at baseline with a Block 119-item food-frequency questionnaire administered by trained staff including questions on usual food intake, frequency, and sizes during the previous year with the assessment of photographs (14). Participants also completed supplemental questionnaires about the frequency and size of common foods consumed among American Indians. The Block database (Block Dietary Data Systems) was used to obtain the average daily energy and macronutrient intakes. The frequency response for each food was multiplied by the nutrient content of the documented portion size of the food and consequently summed for all foods (15).
BMI was calculated as weight in kilograms divided by the square of height in meters. Urinary creatinine concentrations were measured with an automated alkaline picrate reagent method. Kidney function was determined with the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula to calculate the eGFR (16).
Statistical Analysis
We provide median and interquartile range (IQR) (for continuous variables) and frequencies (for categorical variables) of baseline participant characteristics by incident T2DM status. We also provide median and IQR of urinary zinc levels by sociodemographic, lifestyle, clinical data, and dietary zinc intake according to Recommended Dietary Allowances, 8 mg for women and 11 mg for men (17).
For answering the question of whether in adults without impaired glucose tolerance or T2DM increased urinary zinc levels (categorized as having the highest 25% of urinary zinc concentrations in comparison with those with the lowest 25% of urinary zinc concentrations) predict prediabetes or T2DM, over the follow-up (average 6.6 years in the SHS and 5.6 years in the SHFS), we used multiadjusted Cox proportional regression models for the SHS and the mixed-effects Cox proportional hazards models for the SHFS to account for the lack of independence among family members. We used age as the timescale and individual entry times (age at baseline) treated as staggered entries among participants who were free of T2DM at baseline. To answer the main study question, we included zinc levels as quartiles, comparing each quartile 2, 3, and 4 with the 1st quartile, which was set as the reference. In additional models, we log transformed urinary zinc, comparing zinc distribution in the 75th vs. the 25th percentile. For model building and adjustment for potential confounders measured at baseline, the following progressive adjustments were conducted: model 1 was adjusted for sex and stratified by site (AZ, OK, and ND and SD); model 2 included model 1 adjustments and was additionally adjusted for educational level (<12 years completed, ≥12 years completed), smoking status (never, current, former), BMI (kg/m2) and eGFR (mL/min/1.73 m2); model 3 was included model 2 adjustments and was additionally adjusted for HOMA-IR score. In sensitivity analyses, we adjusted for urinary arsenic (μg/g) and urinary selenium (μg/g) levels, in separate models, as some studies suggest a potential role of these trace elements in diabetes development (9,18,19).
We conducted an additional analysis excluding participants with prediabetes at baseline, as impaired FPG could influence urinary zinc excretion. For this analysis, we excluded the 417 participants with prediabetes at baseline leaving 922 participants in the SHS and 484 participants with prediabetes at baseline leaving 1,419 participants in the SHFS. Moreover, we calculated the Spearman correlation between dietary and urinary zinc and evaluated the association between dietary zinc (milligrams per day) and incident T2DM in SHS and SHFS participants.
We ran a cross-sectional analysis for the association of urinary zinc levels—as quartiles (comparing zinc distribution in each quartile with zinc distribution in the first quartile, the reference), and log transformed (continuous), comparing the zinc distribution in the 75th vs. the 25th percentile—with prevalent prediabetes using logistic regression models for the SHS and using logistic regression with generalized estimated effect models for the SHFS to account for the lack of independence among family members.
The statistical significance level was set at α = 0.05. All statistical analyses were conducted with R software (version 4.1.0).
Results
Participant Characteristics
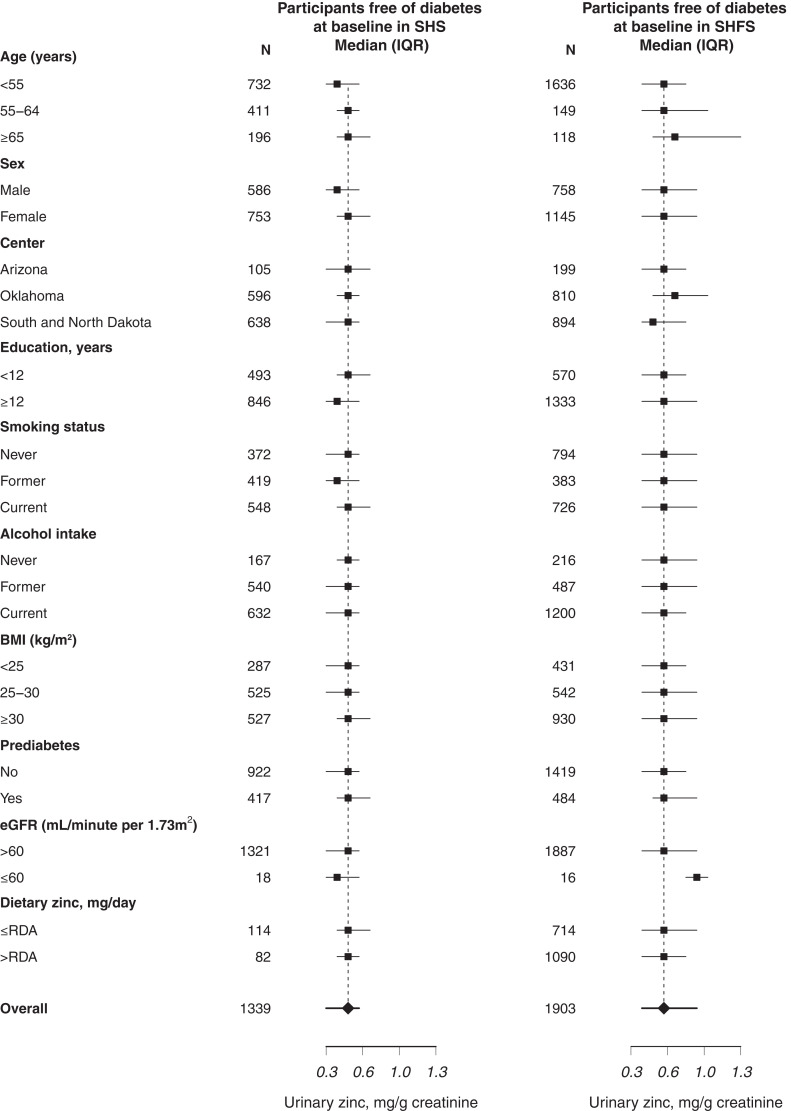
Median participant age at baseline was 53.9 years in the SHS and 35.9 years in the SHFS. Participants with incident T2DM in the SHS and the SHFS were more likely to be female and have higher BMI and higher levels of FPG and insulin, had greater prediabetes prevalence, and were more likely to have higher HOMA-IR score compared with participants without T2DM through the follow-up (Table 1). SHFS participants had higher urinary zinc levels compared with SHS participants. In the SHS, but not in the SHFS, urinary zinc levels were lower in men compared with women. Zinc levels were similar by smoking status and alcohol consumption, particularly among participants free of T2DM at baseline (Fig. 1).
Table 1.
Participant characteristics by diabetes status in the SHS (N = 1,339) and the SHFS (N = 1,903)
| No diabetes in the SHS | Incident diabetes in the SHS | No diabetes in the SHFS | Incident diabetes in the SHFS | |
|---|---|---|---|---|
| N (%) | 938 (70.1) | 401 (29.9) | 1,645 (86.4) | 258 (13.6) |
| Age, years, median (IQR) | 54.2 (48.9, 61.0) | 53.0 (48.3, 60.5) | 35.8 (23.1, 47.0) | 37.1 (27.7, 48.1) |
| Sex, n (%) | ||||
| Female | 514 (55) | 239 (60) | 999 (61) | 146 (57) |
| Male | 424 (45) | 162 (40) | 646 (39) | 112 (43) |
| Location, n (%) | ||||
| AZ | 72 (8) | 33 (8) | 147 (9) | 52 (20) |
| OK | 420 (45) | 176 (44) | 720 (44) | 90 (35) |
| ND and SD | 446 (48) | 192 (48) | 778 (47) | 116 (45) |
| Education, years, n (%) | ||||
| <12 | 338 (36) | 155 (39) | 498 (30) | 72 (28) |
| ≥12 | 600 (64) | 246 (61) | 1,147 (70) | 186 (72) |
| Smoking status, n (%) | ||||
| Never | 258 (28) | 114 (28) | 688 (42) | 106 (41) |
| Former | 283 (30) | 136 (34) | 335 (20) | 48 (19) |
| Current | 397 (42) | 151 (38) | 622 (38) | 104 (40) |
| Alcohol intake, n (%) | ||||
| Never | 110 (12) | 57 (14) | 184 (11) | 32 (12) |
| Former | 367 (39) | 173 (43) | 412 (25) | 75 (29) |
| Current | 461 (49) | 171 (43) | 1,049 (64) | 151 (59) |
| BMI, kg/m2, median (IQR) | 27.8 (24.8, 31.2) | 30.5 (27.5, 34.5) | 29.0 (25.0, 33.7) | 34.4 (30.0, 40.0) |
| eGFR, mL/min/1.73 m2, median (IQR) | 100.3 (92.6, 106.8) | 100.9 (91.3,107.5) | 118.7 (105.9, 130.7) | 118.7 (109.1, 131.4) |
| FPG, mg/dL, median (IQR) | 99 (93, 106) | 105 (97, 112) | 92 (86, 98) | 100 (92, 110) |
| HbA1c, %, median (IQR) | 5.0 (4.7, 5.4) | 5.2 (4.9, 5.6) | 5.2 (5.1, 5.5) | 5.7 (5.5,6.0) |
| HbA1c, mmol/mol, median (IQR) | 31 (28, 36) | 33 (30, 38) | 33 (32, 37) | 39 (37, 42) |
| Insulin levels, mIU/L, median (IQR) | 10.1 (6.6, 15.5) | 15.6 (10.1, 23.2) | 10.5 (7.2, 16.7) | 20.6 (13.1, 31.5) |
| HOMA-IR score, median (IQR) | 2.5 (1.5, 3.8) | 4.0 (2.6, 6.2) | 2.4 (1.6, 4.0) | 5.0 (3.3, 8.2) |
| Urinary zinc levels, mg/g creatinine, median (IQR) | 0.46 (0.33, 0.62) | 0.50 (0.36, 0.66) | 0.59 (0.40, 0.85) | 0.65 (0.45, 0.90) |
| Dietary zinc, mg/day, median (IQR) | 8.46 (6.35, 12.12) | 8.39 (6.32, 12.15) | 10.70 (6.90, 16.90) | 11.16 (7.45, 16.87) |
Figure 1.
Median and interquartile range (IQR) of urinary zinc levels (mg/g) by participants’ characteristics. eGFR, estimated glomerular filtration rate; RDA, recommended dietary allowance.
Urinary Zinc and Incident T2DM in the SHS
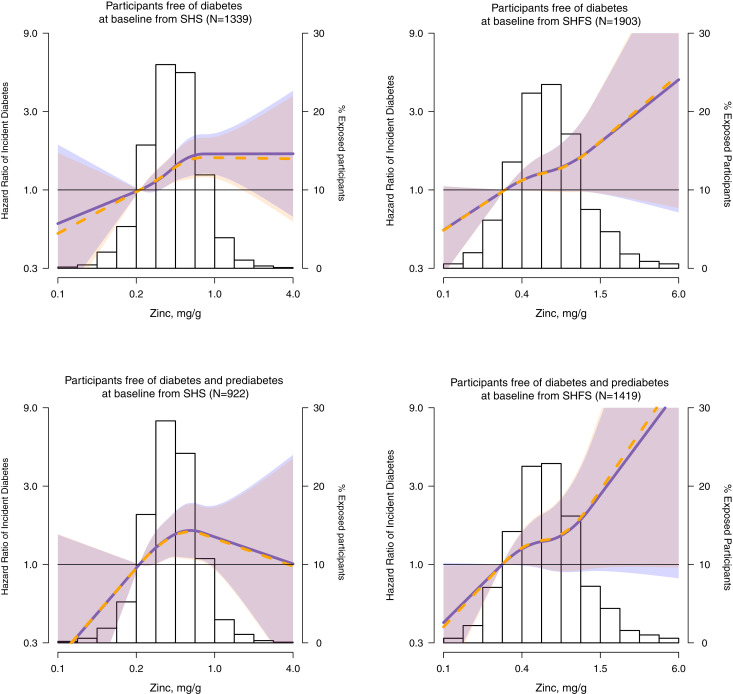
Among SHS participants free of T2DM at baseline (n = 1,339), 401 (29.9%) developed T2DM over 8871.1 person-years of follow-up (incidence of 45.2 per 1,000 person-years). The hazard ratio of T2DM incidence in comparing 75th vs. 25th percentiles of zinc distribution was 1.24 (95% CI 1.10, 1.40) and in comparing the 4th vs. 1st quartile of zinc 1.62 (1.21, 2.17) after adjustment for sex, site, education, smoking status, BMI, and eGFR (model 2). In progressive adjustments with HOMA-IR (model 3) the association of urinary zinc with incident T2DM was slightly attenuated but remained significant (Table 2). Models adjusted for fasting glucose or insulin levels separately instead of HOMA-IR resulted in similar findings (not shown). Dose-response models using quadratic splines confirmed these findings (Fig. 2). Similar results were detected in sensitivity analysis including urinary iAs and selenium levels. We found no effect modification of the association between zinc concentrations and incident T2DM by participant characteristics (Supplementary Table 1).
Table 2.
Hazard ratio (95% CI) of incident diabetes by urinary zinc levels in comparing 75th with 25th percentile for participants free of diabetes at baseline in the SHS and SHFS
| SHS (N = 1,339) | Q1 (<0.34 mg/g) | Q2 (0.34–0.47 mg/g) | Q3 (0.47–0.63 mg/g) | Q4 (>0.63 mg/g) | 0.63 vs. 0.34 mg/g | P | Nonlinear* | P |
|---|---|---|---|---|---|---|---|---|
| Case subjects/noncase subjects | 83/252 | 83/249 | 116/221 | 119/216 | 401/938 | 401/938 | ||
| Model 1 | 1.00 (Ref) | 0.97 (0.72, 1.32) | 1.51 (1.13, 2.01) | 1.61 (1.21, 2.13) | 1.22 (1.08, 1.37) | 0.001 | 1.45 (1.13, 1.87) | 0.004 |
| Model 2 | 1.00 (Ref) | 0.96 (0.71, 1.31) | 1.52 (1.14, 2.03) | 1.62 (1.21, 2.17) | 1.24 (1.10, 1.40) | 0.001 | 1.42 (1.10, 1.83) | 0.007 |
| Model 3 | 1.00 (Ref) | 0.99 (0.73, 1.35) | 1.47 (1.10, 1.97) | 1.55 (1.16, 2.08) | 1.21 (1.07, 1.37) | 0.002 | 1.34 (1.04, 1.74) | 0.02 |
| Model 2 + arsenic | 1.00 (Ref) | 0.96 (0.71, 1.31) | 1.52 (1.14, 2.03) | 1.61 (1.20, 2.15) | 1.23 (1.09, 1.39) | 0.001 | 1.41 (1.10, 1.82) | 0.008 |
| Model 2 + selenium | 1.00 (Ref) | 0.95 (0.70, 1.30) | 1.49 (1.11, 1.99) | 1.61 (1.21, 2.15) | 1.23 (1.09, 1.39) | 0.001 | 1.41 (1.09, 1.82) | 0.008 |
| SHFS (N = 1,903) | Q1 (<0.41 mg/g) | Q2 (0.41–0.60 mg/g) | Q3 (0.60–0.86 mg/g) | Q4 (>0.86 mg/g) | 0.86 vs. 0.41 mg/g | P | Nonlinear* | P |
| Case subjects/noncase subjects | 51/425 | 64/411 | 73/403 | 70/406 | 258/1645 | 258/1645 | ||
| Model 1 | 1.00 (Ref) | 1.28 (0.87, 1.87) | 1.52 (1.04, 2.22) | 1.44 (0.98, 2.11) | 1.17 (1.01, 1.36) | 0.04 | 1.32 (1.06, 1.65) | 0.01 |
| Model 2 | 1.00 (Ref) | 1.22 (0.83, 1.79) | 1.44 (0.99, 2.10) | 1.23 (0.84, 1.82) | 1.11 (0.95, 1.30) | 0.21 | 1.23 (0.98, 1.54) | 0.07 |
| Model 3 | 1.00 (Ref) | 1.26 (0.86, 1.86) | 1.44 (0.98, 2.11) | 1.23 (0.83, 1.81) | 1.10 (0.94, 1.29) | 0.23 | 1.22 (0.97, 1.53) | 0.09 |
| Model 2 + arsenic | 1.00 (Ref) | 1.22 (0.83, 1.79) | 1.44 (0.98, 2.11) | 1.23 (0.84, 1.82) | 1.11 (0.94, 1.30) | 0.21 | 1.23 (0.98, 1.54) | 0.07 |
| Model 2 + selenium | 1.00 (Ref) | 1.22 (0.83, 1.78) | 1.43 (0.98, 2.09) | 1.21 (0.82, 1.79) | 1.09 (0.93, 1.28) | 0.27 | 1.22 (0.97, 1.53) | 0.08 |
Model 1 was adjusted for sex (female, male), and stratified by site. Model 2 was further adjusted for educational level (<12 years completed, ≥12 years completed), smoking status (never, former, current), BMI (kg/m2), and eGFR (mL/min/1.73 m2). Model 3 included model 2 adjustments and was further adjusted for HOMA-IR score. In sensitivity analyses, we also adjusted for urinary arsenic (μg/g) and urinary selenium (μg/g) levels, in separate models. Ref, reference; Q, quartile.
Association obtained from zinc modeled as restricted quadratic splines for hazard regression models (SHS) and cubic splines for mixed-effects hazard regression models (SHFS) with knots at the 10th, 50th, and 90th percentiles. The P value of nonlinearity was obtained from a Wald test of the spline terms.
Figure 2.
Hazard ratios of incident diabetes by urinary zinc levels (mg/g) in participants free of diabetes at baseline in the SHS (N = 1,339) and in the SHFS (N = 1,903) and participants free of diabetes and pre-diabetes at baseline in the SHS (N = 922) and in the SHFS (N = 1,419). Lines with shaded areas represent the hazard ratios (95% CI) of incident diabetes, based on restricted quadratic splines for hazard regression models (SHS) and cubic splines for mixed effects hazard regression models (SHFS) for log-transformed zinc distribution with knots at the 10th, 50th, and 90th percentiles (0.26, 0.47, and 0.82 mg/g in the SHS and 0.29, 0.60, and 1.32 mg/g in the SHFS, respectively, in participants free of diabetes at baseline, 0.26, 0.46, and 0.78 mg/g in the SHS and 0.28, 0.58, and 1.30 mg/g in the SHFS in participants free of diabetes and prediabetes at baseline). The reference value was set at the 10th percentile of zinc distribution. Blue lines (blue-shaded areas) represent the estimated hazard ratios in models stratified by study region and adjusted for sex, baseline education (<12 years, ≥12 years), smoking status (never, former, and current), BMI (kg/m2), and eGFR (mL/min/1.73 m2). Orange lines (orange-shaded areas) represent the estimated hazard ratios in the same model used previously but adjusted by HOMA-IR score. The histogram represents the frequency distribution of zinc in the study sample. The extreme tail of the histograms in the SHS is truncated because 2 participants among 1,339 participants free of diabetes at baseline and one participant among 922 free of diabetes and prediabetes at baseline had levels higher than 4 mg/g. The extreme tails in the SHFS are truncated because 8 participants in both populations (free of diabetes at baseline and free of diabetes and pre-diabetes at baseline) presented lower levels than 0.1 mg/g and 3 among them had levels higher than 6 mg/g.
Dietary Zinc, Urinary Zinc, and Incident T2DM in the SHS
Median estimated dietary zinc in the SHS was 8.41 g/day (IQR 6.32, 12.14). We observed an inverse correlation between dietary and urinary zinc (r = −0.15; P = 0.03) in participants from the SHS with estimated dietary zinc data available (n = 196). The hazard ratio of incident T2DM in comparing 75th vs. 25th percentiles of dietary zinc distribution was 0.53 (95% CI 0.26, 1.09) in models adjusted for sex, stratified by site, education at baseline, smoking status, BMI, eGFR and HOMA-IR score at baseline (model 3).
Urinary Zinc and Incident T2DM in the SHFS
Among SHFS participants free of T2DM at baseline (n = 1,903), 258 (13.5%) developed T2DM over 10,676.6 person-years of follow-up (incidence of 24.2 per 1,000 person-years). The hazard ratio of T2DM incidence in comparing 75th vs. 25th percentiles of zinc distribution was 1.11 (95% CI 0.95, 1.30) and in comparing 4th vs. 1st quartile of zinc 1.23 (0.84, 1.82) after adjustment for sex, site, education, smoking status, BMI, and eGFR (model 2). In progressive adjustments with HOMA-IR (model 3) the association of urinary zinc with incident T2DM remained consistent (Table 2). Models adjusted for fasting glucose or insulin levels separately instead of for HOMA-IR resulted in similar findings (not shown). Dose-response models confirmed these findings (Fig. 2). Additionally, we adjusted for HOMA2-IR instead of HOMA-IR in sensitivity analysis and the associations remained similar (data not shown). We found effect measure modification of the association between zinc and incident T2DM by BMI levels, with a stronger association in participants with lower BMI (Supplementary Table 3).
Dietary Zinc, Urinary Zinc, and Incident T2DM in the SHFS
Median estimated dietary zinc in the SHFS was 10.8 g/day (IQR 6.9, 16.9). We observed an inverse correlation between dietary zinc and urinary zinc in participants from the SHFS (r = −0.04; P = 0.12) with estimated dietary zinc data available (n = 1,804). The hazard ratio of incident T2DM in comparing 75th vs. 25th percentiles of dietary zinc distribution was 0.98 (95% CI 0.82, 1.17) in models adjusted for sex, stratified by site, education at baseline, smoking status, BMI, eGFR, and HOMA-IR score at baseline (model 3).
Analysis Excluding Participants With Prediabetes at Baseline
After exclusion of participants with prediabetes at baseline, the association of baseline urinary zinc levels with incident T2DM remained significant in the SHS, while the association was not statistically significant in the SHFS, although the magnitude of the association was similar in both studies (Supplementary Table 2) compared with the models that included participants with prediabetes (Table 2). For instance, the adjusted HRs in comparing the 75th with the 25th percentile of urinary zinc with incident T2DM (model 2) excluding and not excluding prediabetes at baseline was 1.21 and 1.24, respectively, in the SHS and 1.04 and 1.11 in the SHFS.
Cross-sectional Association With Prediabetes
We ran a cross-sectional analysis of the association between urinary zinc and the prevalence of prediabetes. In the SHS, 417 (31.1%) participants had prediabetes at baseline. The odds ratio of prediabetes prevalence in comparing 75th vs. 25th percentiles of zinc distribution was 1.26 (95% CI 1.08, 1.47) and in comparing the 4th vs. 1st quartile of zinc 1.55 (1.10, 2.19) after adjustment for age, sex, site, education, smoking status, BMI, and eGFR (model 2).
In the SHFS, 484 (25.4%) participants had prediabetes at baseline. The odds ratio of prediabetes prevalence in comparing 75th vs. 25th percentiles of zinc distribution was 1.30 (95% CI 1.05, 1.60) and in comparing 4th vs. 1st quartile of zinc 1.11 (0.99, 1.25) after adjustment for age, sex, site, education, smoking status, BMI, and eGFR (model 2) (Supplementary Table 3). Dose-response models using splines confirmed the association of prediabetes with higher urinary zinc levels (Supplementary Fig. 1).
Conclusions
In this study, higher urinary zinc levels at baseline were associated with T2DM incidence in American Indian adult populations from AZ, OK, and ND and SD including both the original SHS cohort and the younger family expansion. In the SHS, this association was attenuated but remained significant in models adjusted for HOMA-IR score and even after exclusion of participants with prediabetes at baseline. In the SHFS, the association was not significant. Higher urinary zinc levels were associated with prevalence of prediabetes at baseline.
These findings suggest that changes in zinc metabolism are detected in early stages of T2DM pathogenesis and could even play a key role in its progression. The association between zinc levels and T2DM could be partly explained by the role of zinc in insulin homeostasis. Zinc is an essential element whose levels are critical for insulin secretion, signaling, and regulation. In pancreatic β-cells, where high zinc levels are stored, a specific transporter (ZnT8) allows the entrance of zinc into the insulin secretory granules triggering insulin release (1). In a clinical trial investigators observed that insulin response to zinc supplementation depended on genotype of SLC30A8, which is the gene that encodes ZnT8, suggesting a dependence on insulin metabolism by this transporter (20). Insulin receptors are regulated by multiple enzymes, including tyrosine phosphatase 1B (PTP1B), which dephosphorylates the subunit β of the insulin receptor blocking insulin-dependent signals, allowing the phosphorylation of the insulin receptor and consequently activating its functionality (2). Moreover, the insulin-mimetic activity of zinc can promote glycolysis, lipogenesis, and protein synthesis and inhibit gluconeogenesis (21) and has been explained by the activation of protein kinase B (PKB), which ultimately leads to increase in the availability of GLUT-4 transport increasing glucose uptake in cells (22).
There is mounting evidence from evaluations of the role of zinc concentrations, measured in different biospecimens, in T2DM status but mainly from cross-sectional studies (23–26). For instance, increased urinary excretion of zinc (hyperzincuria) and decreased zinc blood levels (hypozincemia) are well-known in individuals with diabetes (6,7). In participants from Wuhan, China, urinary zinc levels >41 μg/dL were positively associated with FPG levels (26). In postmenopausal women with T2DM from Moscow, Russia, serum zinc (mean ∼100 μg/dL) was negatively corrected with FPG (r = −0.32) and HbA1c (r = −0.34) (27). In individuals with T2DM from Sao Paulo, Brazil, HbA1c levels were inversely associated with plasma zinc (mean 83.3 μg/dL) and positively associated with urinary zinc (mean 899 μg zinc/24 h) (25). Few epidemiological studies, however, have assessed urinary zinc levels in diabetes status prospectively or in individuals with prediabetes. Our results are consistent with the positive association of urinary zinc (median ∼37 μg/dL) with incident T2DM in a multiethnic study of women across the U.S. (28).
Some studies have suggested that hyperglycemia influences zinc status in diabetes pathogenesis by hindering renal zinc reabsorption and, in turn, raising urinary zinc excretion and decreasing blood zinc (29). In our prospective study, we excluded participants with diabetes at baseline. However, it is possible that even the hyperglycemia levels observed during prediabetes might be sufficient to alter zinc metabolism and its urinary excretion. A meta-analysis documented that blood zinc concentrations in patients with T2DM drop progressively over time compared with healthy participants (30). Although it is clear that zinc plays a main role in insulin metabolism and therefore in diabetes pathogenesis, the precise mechanisms involved remain a major gap in current knowledge. Another relevant role of zinc in diabetes may be related to its antioxidant properties, which may protect the pancreatic β-cells from reactive oxygen species (2).
Low blood zinc levels due to hyperzincuria could aggravate insulin resistance status in diabetes development, and it is possible that zinc could delay the development of diabetes by preventing early continuous hyperglycemic status. This could provide potential new targets for treatment and diabetes prevention. Some studies propose ZnT8 transporter and tyrosine phosphatase as diabetes therapeutic targets underlining the influence of zinc on insulin metabolism (31). In the SHS, dietary zinc was negatively correlated with urinary zinc, suggesting that zinc excretion does not properly reflect zinc intake, but zinc metabolism and dysfunctional loss could be exacerbated by low zinc intake. Dietary zinc could indicate a protective role in insulin resistance. Several experimental studies in humans show the potential benefit of zinc supplementation improving glycemic control and reducing diabetes complications (32,33). Two meta-analyses of clinical trials in patients with diabetes found that zinc supplementation alone (32) or as cosupplement decreased FPG levels compared with placebo. In a randomized clinical trial of 200 participants with prediabetes, investigators found that those treated with zinc supplementation were less likely to develop incident diabetes than those untreated (11% vs. 25%, respectively) after a year of follow-up (34). Another randomized clinical trial with 20 participants with prediabetes showed statistically significant reductions of FPG levels after 6 months of zinc supplementation, compared with baseline and with a control group untreated (35).
Regarding what could be considered a healthy urinary zinc level, that is not related to increased T2DM risk and that could be used as a reference point clinically, more research across diverse populations is needed. Our results, together with findings from other studies, however, suggest that zinc homeostasis is relevant for diabetes development and that controlling the loss of zinc through the urine could contribute to diabetes prevention, which could expand current preventative targets and further add to the clinical relevance of our findings.
Strengths of the study include the evaluation of two adult population-based cohorts with different ages; the prospective follow-up and highly standardized protocols for recruitment, interviewing, and examining study participants; the community-based participatory approach; and the laboratory methods for zinc analyses. Among the limitations, we had no data on blood zinc, which would allow us to better study zinc homeostasis, and information on dietary zinc was available only in a small sample of participants from the SHS. Also, we cannot discard misclassification of some participants with diabetes at baseline among the SHFS participants due to the lack of HbA1c measurement.
In conclusion, in the original population of the SHS and the family expansion in AZ, OK, and ND and SD, urinary zinc levels were associated with incident diabetes after exclusion of participants with diabetes at baseline and after adjustment for HOMA-IR. Higher urinary zinc levels were associated with prevalence of prediabetes. These results suggest that urinary zinc can serve as a biomarkers for diabetes risk, independently of HOMA-IR. Research is needed for an understanding of the role of zinc metabolism in diabetes physiopathology and evaluation of the potential of zinc-related therapeutic targets for diabetes prevention, in particular in individuals with prediabetes.
Article Information
Acknowledgments. The authors thank all the SHS participants and Tribal Nations who made this research possible.
Funding. The SHS was supported by grants from National Heart, Lung, and Blood Institute contracts 75N92019D00027, 75N92019D00028, 75N92019D00029, and 75N92019D00030, previous grants R01HL090863, R01HL109315, R01HL109301, R01HL109284, R01HL109282, and R01HL109319, and cooperative agreements U01HL41642, U01HL41652, U01HL41654, U01HL65520, and U01HL65521 and by National Institute of Environmental Health Sciences grants R01ES021367, R01ES025216, R01ES032638, P42ES033719, and P30ES009089.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. M.G.-F., M.P., M.G.-P., A.D.-R., N.L., N.M., and A.N.-A. contributed to the preparation of the research data, statistical analysis, and writing of the manuscript. W.G. contributed to the zinc, selenium, and arsenic measurements in the SHS and SHFS participants. Y.Z., A.F., and J.G.U. contributed to the research data as primary investigations on the SHS. A.N.-A. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 34th Annual Conference of the International Society for Environmental Epidemiology, 18–21 September 2022, Athens, Greece, and the XVIII Congress of Biostatistics CEBMADRID, Madrid, Spain, 25–27 May 2022.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.20610612.
References
- 1. Fukunaka A, Fujitani Y. Role of zinc homeostasis in the pathogenesis of diabetes and obesity. Int J Mol Sci 2018;19:476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Myers SA. Zinc transporters and zinc signaling: new insights into their role in type 2 diabetes. Int J Endocrinol 2015;2015:167503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chabosseau P, Rutter GA. Zinc and diabetes. Arch Biochem Biophys 2016;611:79–85 [DOI] [PubMed] [Google Scholar]
- 4. Barman S, Srinivasan K. Zinc supplementation ameliorates diabetic cataract through modulation of crystallin proteins and polyol pathway in experimental rats. Biol Trace Elem Res 2019;187:212–223 [DOI] [PubMed] [Google Scholar]
- 5. Fernández-Cao JC, Warthon-Medina M, H Moran V, et al. Zinc intake and status and risk of type 2 diabetes mellitus: a systematic review and meta-analysis. Nutrients 2019;11:E1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Afridi HI, Kazi TG, Kazi N, et al. Status of essential trace metals in biological samples of diabetic mother and their neonates. Arch Gynecol Obstet 2009;280:415–423 [DOI] [PubMed] [Google Scholar]
- 7. Xu J, Zhou Q, Liu G, Tan Y, Cai L. Analysis of serum and urinal copper and zinc in Chinese northeast population with the prediabetes or diabetes with and without complications. Oxid Med Cell Longev 2013;2013:63521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Centers for Disease Control and Prevention . National Diabetes Statistics Report. U.S. Department of Health and Human Services, 2020 [Google Scholar]
- 9. Kuo CC, Howard BV, Umans JG, et al. Arsenic exposure, arsenic metabolism, and incident diabetes in the Strong Heart Study. Diabetes Care 2015;38:620–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee ET, Welty TK, Fabsitz R, et al. The Strong Heart Study. A study of cardiovascular disease in American Indians: design and methods. Am J Epidemiol 1990;132:1141–1155 [DOI] [PubMed] [Google Scholar]
- 11. Scheer J, Findenig S, Goessler W, et al. Arsenic species and selected metals in human urine: validation of HPLC/ICPMS and ICPMS procedures for a long-term population-based epidemiological study. Anal Methods 2012;4:406–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Howard BV, Welty TK, Fabsitz RR, et al. Risk factors for coronary heart disease in diabetic and nondiabetic Native Americans: the Strong Heart Study. Diabetes 1992;41(Suppl. 2):4–11 [DOI] [PubMed] [Google Scholar]
- 13. Wang W, Lee ET, Howard BV, Fabsitz RR, Devereux RB, Welty TK. Fasting plasma glucose and hemoglobin A1c in identifying and predicting diabetes: the strong heart study. Diabetes Care 2011;34:363–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. North KE, Howard BV, Welty TK, et al. Genetic and environmental contributions to cardiovascular disease risk in American Indians: the strong heart family study. Am J Epidemiol 2003;157:303–314 [DOI] [PubMed] [Google Scholar]
- 15. Block G, Wakimoto P, Block T. Revision of the Block Dietary Questionnaire and database, based on NHANES III Data, 1998. Available from https://www.nutritionquest.com/products/B98_DEV.pdf
- 16. Levey AS, Stevens LA, Schmid CH, et al.; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Institute of Medicine (US) Panel on Micronutrients . Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington, DC, National Academies Press, 2001 [PubMed] [Google Scholar]
- 18. Grau-Perez M, Kuo CC, Gribble MO, et al. Association of low-moderate arsenic exposure and arsenic metabolism with incident diabetes and insulin resistance in the Strong Heart Family Study. Environ Health Perspect 2017;125:127004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kohler LN, Foote J, Kelley CP, et al. Selenium and type 2 diabetes: systematic review. Nutrients 2018;10:1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maruthur NM, Clark JM, Fu M, Linda Kao WH, Shuldiner AR. Effect of zinc supplementation on insulin secretion: interaction between zinc and SLC30A8 genotype in Old Order Amish. Diabetologia 2015;58:295–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cruz KJC, de Oliveira ARS, Morais JBS, et al. Zinc and insulin resistance: biochemical and molecular aspects. Biol Trace Elem Res 2018;186:407–412 [DOI] [PubMed] [Google Scholar]
- 22. Vardatsikos G, Pandey NR, Srivastava AK. Insulino-mimetic and anti-diabetic effects of zinc. J Inorg Biochem 2013;120:8–17 [DOI] [PubMed] [Google Scholar]
- 23. Velmurugan G, Swaminathan K, Veerasekar G, et al. Metals in urine in relation to the prevalence of pre-diabetes, diabetes and atherosclerosis in rural India. Occup Environ Med 2018;75:661–667 [DOI] [PubMed] [Google Scholar]
- 24. Zhang J, Hu J, Zhao J, Li J, Cai X. Serum zinc concentrations and prediabetes and diabetes in the general population. Biol Trace Elem Res 2022;200:1071–1077 [DOI] [PubMed] [Google Scholar]
- 25. Bandeira VDS, Pires LV, Hashimoto LL, et al. Association of reduced zinc status with poor glycemic control in individuals with type 2 diabetes mellitus. J Trace Elem Med Biol 2017;44:132–136 [DOI] [PubMed] [Google Scholar]
- 26. Feng W, Cui X, Liu B, et al. Association of urinary metal profiles with altered glucose levels and diabetes risk: a population-based study in China. PLoS One 2015;10:e0123742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Skalnaya MG, Skalny AV, Yurasov VV, et al. Serum trace elements and electrolytes are associated with fasting plasma glucose and HbA1c in postmenopausal women with type 2 diabetes mellitus. Biol Trace Elem Res 2017;177:25–32 [DOI] [PubMed] [Google Scholar]
- 28. Wang X, Karvonen-Gutierrez CA, Herman WH, Mukherjee B, Harlow SD, Park SK. Urinary metals and incident diabetes in midlife women: Study of Women’s Health Across the Nation (SWAN). BMJ Open Diabetes Res Care 2020;8:e001233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chausmer AB. Zinc, insulin and diabetes. J Am Coll Nutr 1998;17:109–115 [DOI] [PubMed] [Google Scholar]
- 30. Fernández-Cao JC, Warthon-Medina M, Hall Moran V, Arija V, Doepking C, Lowe NM. Dietary zinc intake and whole blood zinc concentration in subjects with type 2 diabetes versus healthy subjects: a systematic review, meta-analysis and meta-regression. J Trace Elem Med Biol 2018;49:241–251 [DOI] [PubMed] [Google Scholar]
- 31. Norouzi S, Adulcikas J, Sohal SS, Myers S. Zinc transporters and insulin resistance: therapeutic implications for type 2 diabetes and metabolic disease. J Biomed Sci 2017;24:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jayawardena R, Ranasinghe P, Galappatthy P, Malkanthi R, Constantine G, Katulanda P. Effects of zinc supplementation on diabetes mellitus: a systematic review and meta-analysis. Diabetol Metab Syndr 2012;4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang X, Wu W, Zheng W, et al. Zinc supplementation improves glycemic control for diabetes prevention and management: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr 2019;110:76–90 [DOI] [PubMed] [Google Scholar]
- 34. Ranasinghe P, Wathurapatha WS, Galappatthy P, Katulanda P, Jayawardena R, Constantine GR. Zinc supplementation in prediabetes: A randomized double-blind placebo-controlled clinical trial. J Diabetes 2018;10:386–397 [DOI] [PubMed] [Google Scholar]
- 35. Islam MR, Attia J, Ali L, et al. Zinc supplementation for improving glucose handling in pre-diabetes: A double blind randomized placebo controlled pilot study. Diabetes Res Clin Pract 2016;115:39–46 [DOI] [PubMed] [Google Scholar]