Abstract
OBJECTIVE
To evaluate associations between a broad range of approaches to classifying diet and incident type 2 diabetes in the REasons for Geographic And Racial Differences in Stroke (REGARDS) study.
RESEARCH DESIGN AND METHODS
This study included 8,750 Black and White adults without diabetes at baseline. Diabetes was defined according to fasting glucose ≥70 mmol/L, random glucose ≥111 mmol/L, or use of diabetes medications. The exposures were diet scores for Mediterranean and Dietary Approaches to Stop Hypertension (DASH) diets and Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND), dietary inflammatory index (DII), dietary inflammation score (DIS), and empirical dietary patterns (plant-based and Southern) determined using data collected with use of the Block98 food-frequency questionnaire. Modified Poisson regression was used to assess association of dietary measures with risk of incident type 2 diabetes, with models adjusted for total energy intake, demographics, lifestyle factors, and waist circumference.
RESULTS
There were 1,026 cases of incident type 2 diabetes during follow-up (11.7%). Adherence to the Southern dietary pattern was most strongly associated with risk of incident type 2 diabetes after adjustment for demographics and lifestyle (quintile [Q]5 vs. lowest Q1: risk ratio [RR] 1.95; 95% CI 1.57, 2.41). Of the diet scores, DIS (Q5 vs. Q1 RR 1.41) and MIND (Q1 vs. Q5 RR 1.33), demonstrated anti-inflammatory diets, had strongest associations with lower diabetes incidence.
CONCLUSIONS
We found associations of several dietary approaches with incident type 2 diabetes. Investigation into mechanisms driving the association with the Southern dietary pattern is warranted. Further research into use of DIS, DII, and MIND diet score should be considered for dietary recommendations for diabetes prevention.
Introduction
Although diabetes incidence has been decreasing over the past several years, approximately 1 out of 12 twelve American adults has a diagnosis of type 2 diabetes and will experience the increased morbidity and mortality risk associated with the disease, highlighting the continued need for intensified diabetes prevention efforts (1–3). Dietary intake is a modifiable risk factor that may be a promising target for diabetes prevention. Unfortunately, few randomized controlled trials have investigated the role of dietary pattern interventions in diabetes incidence. Further, in the trials that do exist individual foods or nutrients are emphasized rather than overall dietary patterns, which recent literature suggests might be more predictive of chronic disease (4,5). The PREvención con DIeta MEDiterránea (PREDIMED) trial is an example of a randomized trial that did include investigation of the impact of a dietary pattern and demonstrated a 30% reduction in diabetes incidence in the Mediterranean diet groups compared with a low-fat diet comparison group (6). Although these results are encouraging, the scarcity of interventions in this area limits the confidence with which practitioners can make diet-related recommendations (7). Observational research comparing associations of a variety of dietary pattern approaches with diabetes incidence may yield further insight into future opportunities for dietary intervention for diabetes prevention. Findings of prospective cohort studies exploring the association of diet with incident type 2 diabetes have shown a reduced risk associated with healthy diet scores, such as the Mediterranean diet score and Dietary Approaches to Stop Hypertension (DASH) diet score (8,9). These a priori diet scores are calculated based on adherence to recommended intakes of specific food groups. In contrast, data-driven methodology, referred to as an empirical approach, derives dietary patterns directly from the data based on reported food consumption. In a 2017 systematic review and meta-analysis Jannasch et al. (10) compared the association for the highest versus lowest intake for a priori diet scores representing the Mediterranean diet, DASH diet, and the Alternate Healthy Eating Index (AHEI) and empirical dietary patterns with risk of incident type 2 diabetes, finding that the DASH diet score was associated with a 5% greater decrease in risk of incident type 2 diabetes as compared with the Mediterranean diet score for the highest versus lowest intakes. However, this meta-analysis was limited in that it did not include direct comparison of the diet scores and dietary patterns in the same racially diverse cohort.
Although similar studies have been conducted in other chronic diseases like cardiovascular disease and colorectal cancer (11,12), no studies to our knowledge have compared different approaches to classify diet, including both a priori and empirical approaches in the same diverse population, in evaluating incident type 2 diabetes. Knowing which approaches to classifying diet are most strongly associated with incident type 2 diabetes would help inform diabetes prevention efforts. Therefore, in this study we compared approaches to classifying diet and risk of diabetes in a cohort of Black and White adults in the U.S. using data from the REasons for Geographic And Racial Differences in Stroke (REGARDS) study. Due to plausible associations with incident type 2 diabetes, in this study we investigated dietary patterns created through assigning of higher scores to foods a priori hypothesized to be associated with health outcomes (i.e., Mediterranean diet score, DASH diet score, Mediterranean-DASH Intervention for Neurodegenerative Delay [MIND] diet score, dietary inflammation score [DIS], and dietary inflammatory index [DII]) and empirical dietary scores created based on associations observed in the data of foods that cluster in diets of individuals with specific eating patterns (i.e., plant-based and Southern dietary patterns).
Research Design and Methods
Study Design and Participants
The primary objective of the REGARDS study is to assess potential contributors to the excess stroke mortality throughout the southeastern “stroke belt” of U.S. and in the Black population (13). The study is an ongoing national prospective cohort study of Black and White Americans at least 45 years of age, with 30,239 enrolled at baseline (first in-home visit) from 2003 to 2007 and 14,448 examined at a second in-home visit ∼10 years after baseline assessment (from 2013 to 2016) (14). At both baseline and follow-up contacts, demographics and cardiovascular risk profile were assessed by telephone interview, with a subsequent in-home assessment to collect information including blood pressure, fasting blood and urine samples, electrocardiogram, and medication inventory. Written informed consent was obtained from all participants, and all study protocols were approved by the institutional review boards at all participating institutions.
Measures
Dietary Assessment
On completion of the baseline in-home visit, the participant was given dietary assessment forms for self-administration for return to the REGARDS study coordinating center. Dietary assessment included the Block98 food-frequency questionnaire (FFQ), which was developed by Block Dietary Data Systems (Berkeley, CA) and distributed by NutritionQuest. The FFQ inquired about food intake over the previous year based on 107 food items.
The Block98 FFQ has been validated in populations similar to that of REGARDS (15). The REGARDS coordinating center checked the received forms for completeness, and the scanned files were sent to NutritionQuest for processing. The NutritionQuest scoring resulted in the grams per day for each food line item. The 107 individual FFQ line items were condensed into 56 food groups as previously described, and total energy intake (TEI) in kilocalories was calculated. At baseline, there were 21,636 (72%) participants with usable dietary data (for reasons for unusable data see eMethods in Supplementary Material) (16).
Dietary Pattern and Score Derivation
The empirical dietary pattern scores have previously been described for REGARDS (16), and details are provided in eMethods. Due to previous associations with health outcomes, this study included evaluation of the association of incident type 2 diabetes with only the Southern and plant-based dietary patterns (14,17,18). The Southern dietary pattern reflected a culinary pattern common in the southeastern U.S. and is characterized by fried foods, organ meats, processed meats, eggs and egg dishes, added fats, high-fat dairy foods, sugar-sweetened beverages, and bread (16). The plant-based dietary pattern is characterized by high factor loadings for vegetables, as well as fruits, beans, poultry, and fish.
The dietary scores based on hypothesized a priori relationships of specific foods with health outcomes included Mediterranean diet, DASH diet, and MIND diet scores; DIS; and DII. The Mediterranean diet score was defined by summing of scores of nine food groups: vegetables, fruits, legumes, cereals (including bread, pasta, and rice), fish, meat, dairy products, fat intake, and alcohol intake (19,20). A score of 1 was given for each component that was at or above the median intake for healthy components of vegetables, fruits, legumes, cereals, and fish and below the median intake for unhealthy components of meat and dairy products. For fat intake, assessed as the ratio of daily consumption (in grams) of monounsaturated lipids to that of saturated lipids, individuals with ratios at or above the sex-specific median were assigned a score of 1. For moderate alcohol consumption, defined according to >0 and ≤7 drinks per week for women and >0 and ≤14 drinks per week for men, individuals were assigned a score of 1. DASH diet score was defined by eight components: high intake of fruits, vegetables, nuts and legumes, low-fat dairy products, and whole grains and low intake of sodium, sweetened beverages, and red and processed meats (21,22). The MIND diet score was derived based on a combination of the Mediterranean and DASH diet scores and components demonstrated to be neuroprotective from cognitive decline, such as green leafy vegetables and berries (23).
DIS and DII are relatively recently proposed dietary scores that may be useful with respect to incident type 2 diabetes, as inflammation has been hypothesized to be a part of the pathway of diabetes development (24–26). The DIS was constructed from selecting a priori 19 food groups, and based on the association with inflammatory biomarkers weights were created for each food group (26). The DII was created based on selected dietary factors associated with inflammation, which were primarily nutrient based (27). Higher scores of the DIS and DII have been associated with poor health outcomes (24).
For the Southern dietary pattern, plant-based dietary pattern, DII, and DIS, the quintiles contained an equal number of individuals, with 1,750 in each quintile. The quintiles are not equal for the other scores due to the discrete nature of the dietary scores, with tied values binned in the lower quintile.
Incident Diabetes
The primary outcome was incident type 2 diabetes defined according to fasting glucose ≥70 mmol/L, or a random glucose ≥111 mmol/L among those failing to fast, or self-reported use of diabetes medications at the second in-home examination.
Demographic and Lifestyle Risk factors
Age, sex, race, education, income, smoking status, alcohol use, and physical activity were self-reported. Trained staff measured waist circumference (in centimeters). Moderate alcohol consumption was defined as >0 and ≤7 drinks per week for women and >0 and ≤14 drinks per week for men; individuals were assigned a score of 1 and otherwise a score of 0. Region was determined from the residential address of each participant, and the areas were classified as stroke belt with higher stroke mortality (includes LA, AR, MI, AL, TN, GA, NC, and SC), “stroke buckle” with highest stroke mortality (includes the coastal plains of NC, SC, and GA), or “nonbelt/buckle” (remaining continental U.S.). The stroke belt has substantial overlap with diabetes belt due to shared risk factors including hypertension (28).
Statistical Analysis
Dietary patterns and scores were divided into quintiles with tied values binned into the lower quantile. Baseline characteristics were assessed overall and by type 2 diabetes status at the second visit, which was statistically tested. Study population baseline characteristics were reported by quintile of dietary pattern measure with statistical tests of dietary pattern detailed as follows. Demographic characteristics of sex (male, female), race (Black, White), region (stroke buckle, stroke belt, nonbelt/buckle), education (less than high school, high school graduate, some college, college graduate and above), and annual income (<$20,000, $20,000–$34,000, $35,000–$74,000, ≥$75,000, refused) and lifestyle risk factors of smoking status (current, past, never), alcohol use (moderate, never/heavy), elevated waist circumference (dichotomized according to sex-specific cutoffs of 88 cm for females and 102 cm for males), and frequency of physical activity sufficient to sweat (none, 1–3 times/week, ≥4 times/week) were analyzed with Cochran-Mantel-Haenszel test. Continuous measures of age (years) and TEI (kilocalories) were analyzed with ANOVA if distributional assumptions were satisfied and Kruskal-Wallis otherwise.
The risk ratio (RR) of incident type 2 diabetes by diet quintile for each dietary pattern and score was calculated with use of Poisson regression with robust variance estimation (29). The RR of incident type 2 diabetes was calculated by diet score quintiles with the anticipated most healthy quintile as the reference group for crude and adjusted models. For Mediterranean diet score, DASH diet score, MIND diet score, and plant-based pattern, where higher scores were healthier, the highest quintile (Q5) was used as the reference group. For DII, DIS, and Southern pattern, where lower scores were healthier, the lowest quintile (Q1) was used as the reference group. Model 1 included adjustment for TEI and demographics (age, race, sex, income, education, and region). Model 2 included adjustment for model 1 variables with additional adjustment for lifestyle risk factors (smoking, physical activity, alcohol). Model 3 included additional adjustment for waist circumference potentially in the causal pathway. The associations of the defined dietary measures with incident type 2 diabetes were compared for magnitude of association and statistical significance. SEs of the quintile slopes for each diet were calculated with bootstrap methods with 1,000 replications. The P values were calculated for linear trend of each diet slope and for the differences in slopes between the diet approaches with use of the Wald statistic.
For evaluation of the robustness of the diet-diabetes association to potential unmeasured confounding, the E-value was estimated for the point estimate and 95% CI with use of the EValue R package (30,31). The E-value is defined as the minimum strength of association that an unmeasured confounder would need to have with both the exposure and the outcome to potentially explain away the exposure-outcome association, conditioned on the measured covariates (see eMethods). For associations that are not statistically significant, the E-values for the 95% CI are 1, since no additional unmeasured confounder is needed to completely attenuate significance of the association.
All statistical tests were two sided, and P values were considered statistically significant at the <0.05 α-level. Analyses were conducted with SAS, version 9.4 (SAS Institute, Cary, NC), and R, version 4.1.0.
Results
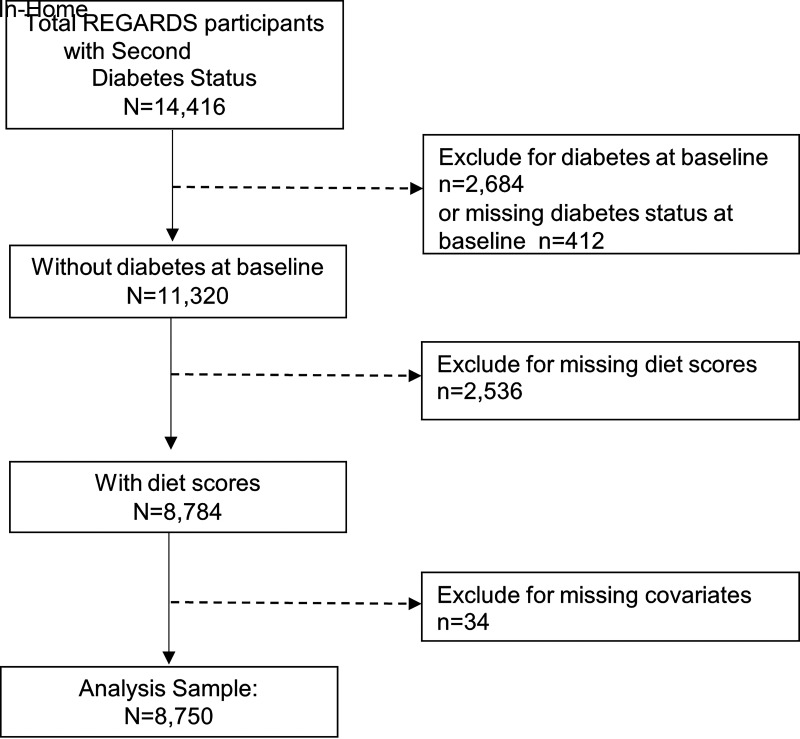
Of 14,416 REGARDS participants who were examined at the second in-home visit, participants were excluded for prevalent diabetes at baseline (n = 2,684), missing diabetes status at baseline (n = 4,12), missing diet data (n = 2,536), and missing data on risk factors of interest (n = 34), resulting in 8,750 participants included in this analysis (Fig. 1).
Figure 1.
Participant selection.
Demographic and lifestyle characteristics for the participants overall and by diabetes status at the second visit are presented in Table 1. Mean (SD) age was 63.2 (8.5) years at baseline, 27.1% were Black, 43.8% were male, 33.3% lived in the stroke belt, and 21.8% lived in the stroke buckle (Table 1). Participants with diabetes were more likely to be Black, male, have less education, have lower income, live in the stroke buckle, be current smokers, have no or heavy alcohol use, and have elevated waist circumference.
Table 1.
Baseline characteristics, overall and by incident type 2 diabetes status
| Overall (N = 8,750) | Incident diabetes | P | ||
|---|---|---|---|---|
| No (N = 7,724) | Yes (N = 1,026) | |||
| TEI (kcal), median (IQR) | 1,615 (1,237–2,092) | 1,610 (1,231–2,085) | 1,659 (1,284–2,130) | 0.04 |
| Age (years), mean (SD) | 63.2 (8.5) | 63.3 (8.5) | 62.3 (7.7) | 0.04 |
| Race | ||||
| Black | 2,370 (27.1) | 1,954 (25.3) | 416 (40.5) | <0.0001 |
| White | 6,380 (72.9) | 5,770 (74.7) | 610 (59.5) | |
| Sex | ||||
| Female | 4,916 (56.2) | 4,367 (56.5) | 549 (53.5) | 0.06 |
| Male | 3,834 (43.8) | 3,357 (43.5) | 477 (46.5) | |
| Education | ||||
| Less than high school | 475 (5.4) | 385 (5.0) | 90 (8.8) | <0.0001 |
| High school graduate | 1,965 (22.5) | 1,700 (22.0) | 265 (25.8) | |
| Some college | 2,302 (26.3) | 1,983 (25.7) | 319 (31.1) | |
| College graduate and above | 4,008 (45.8) | 3,656 (47.3) | 352 (34.3) | |
| Income | ||||
| <$20,000 | 874 (10.0) | 724 (9.4) | 150 (14.6) | <0.0001 |
| $20,000–$34,000 | 1,855 (21.2) | 1,607 (20.8) | 248 (24.2) | |
| $35,000–$74,000 | 3,059 (35.0) | 2,683 (34.7) | 376 (36.6) | |
| ≥$75,000 | 2,053 (23.5) | 1,888 (24.4) | 165 (16.1) | |
| Did not answer | 909 (10.4) | 822 (10.6) | 87 (8.5) | |
| Geographic region | ||||
| Stroke buckle | 1,906 (21.8) | 1,668 (21.6) | 238 (23.2) | 0.16 |
| Stroke belt | 2,911 (33.3) | 2,556 (33.1) | 355 (34.6) | |
| Nonbelt/buckle | 3,933 (45.0) | 3,500 (45.3) | 433 (42.2) | |
| Smoking | ||||
| Current | 917 (10.5) | 758 (9.8) | 159 (15.5) | <0.0001 |
| Past | 3,515 (40.2) | 3,102 (40.2) | 413 (40.3) | |
| Never | 4,318 (49.4) | 3,864 (50.0) | 454 (44.2) | |
| Alcohol | ||||
| None and heavy | 3,623 (41.4) | 3,276 (42.4) | 679 (66.2) | <0.0001 |
| Moderate | 5,127 (58.6) | 4,448 (57.6) | 347 (33.8) | |
| Physical activity | ||||
| None | 2,426 (27.7) | 2,105 (27.3) | 321 (31.3) | 0.02 |
| 1–3 times per week | 3,511 (40.1) | 3,115 (40.3) | 396 (38.6) | |
| ≥4 times per week | 2,813 (32.2) | 2,504 (32.4) | 309 (30.1) | |
| Waist circumference | ||||
| Normal | 5,131 (58.6) | 4,768 (61.7) | 363 (35.4) | <0.0001 |
| Elevated (men ≥120 cm, women ≥88 cm) | 3,619 (41.4) | 2,956 (38.3) | 663 (64.6) | |
Data are n (%) unless otherwise indicated. IQR, interquartile range.
Baseline characteristics are reported by quintile of dietary pattern adherence/diet score with bivariate statistical tests in Supplementary Table 1. Generally, people with more unhealthy diet tended to be older, be male, be Black, have lower income, have lower education, be current smokers, have less physical activity, and have higher waist circumference and TEI (Supplementary Table 1).
Models for the association of diet with incident type 2 diabetes and unadjusted estimates of the incidence of diabetes are presented in Table 2 with model 2 of primary interest, since it is the most fully adjusted model without factors that may be in the causal pathway. The range of the dietary score for each of the quintiles is provided in Supplementary Table 2. Figure 2 presents the results for model 2 with diet measures ordered by increasing magnitude of association. As shown in Fig. 2, the Southern pattern was statistically significantly different from all other dietary approaches in the slope of the quintile RRs and showed strong linear trend for all models (P ≤ 0.0001). Association of plant-based diet with incident type 2 diabetes was weaker than for any other dietary pattern (Ptrend = 0.16). The strength of the association based on testing trend with risk of incident type 2 diabetes was not significantly different compared among DIS and MIND and DASH diet scores (P > 0.05). The strength of the association with incident type 2 diabetes also did not differ compared among the MIND diet score, DASH diet score, and DII that were not significantly different. The strengths of association for DII with Mediterranean diet and with Mediterranean and plant-based diet were not significantly different.
Table 2.
Association of dietary patterns and scores with incident type 2 diabetes
| N | Incidence of diabetes | Crude | Model 1 | Model 2 | Model 3 | |
|---|---|---|---|---|---|---|
| Mediterranean diet score | ||||||
| Q5 | 1,083 | 10.3 | Ref | Ref | Ref | Ref |
| Q4 | 1,440 | 10.0 | 0.98 (0.77, 1.23) | 0.93 (0.74, 1.18) | 0.90 (0.71, 1.14) | 0.85 (0.68, 1.07) |
| Q3 | 1,867 | 11.1 | 1.08 (0.87, 1.35) | 1.02 (0.82, 1.27) | 0.96 (0.77, 1.19) | 0.90 (0.72, 1.11) |
| Q2 | 1,818 | 12.0 | 1.17 (0.94, 1.45) | 1.06 (0.85, 1.31) | 0.96 (0.77, 1.20) | 0.89 (0.71, 1.10) |
| Q1 | 2,542 | 13.6 | 1.33 (1.09, 1.62) | 1.11 (0.90, 1.36) | 0.97 (0.79, 1.20) | 0.87 (0.71, 1.07) |
| Ptrend | 0.005 | 0.20 | 0.99 | 0.35 | ||
| DASH diet score | ||||||
| Q5 | 1,604 | 8.7 | Ref | Ref | Ref | Ref |
| Q4 | 1,224 | 9.2 | 1.06 (0.84, 1.34) | 1.01 (0.80, 1.28) | 0.99 (0.79, 1.26) | 0.94 (0.74, 1.18) |
| Q3 | 2,150 | 11.5 | 1.32 (1.09, 1.61) | 1.20 (0.98, 1.46) | 1.18 (0.97, 1.44) | 1.09 (0.89, 1.32) |
| Q2 | 2,001 | 12.9 | 1.48 (1.22, 1.80) | 1.25 (1.02, 1.52) | 1.20 (0.98, 1.46) | 1.08 (0.88, 1.31) |
| Q1 | 1,771 | 15.1 | 1.73 (1.42, 2.10) | 1.36 (1.11, 1.65) | 1.26 (1.03, 1.54) | 1.11 (0.91, 1.36) |
| Ptrend | <0.0001 | 0.003 | 0.02 | 0.23 | ||
| MIND diet score | ||||||
| Q5 | 1,424 | 8.0 | Ref | Ref | Ref | Ref |
| Q4 | 1,333 | 10.2 | 1.27 (1.01, 1.62) | 1.22 (0.97, 1.55) | 1.21 (0.96, 1.53) | 1.16 (0.92, 1.47) |
| Q3 | 1,737 | 10.8 | 1.35 (1.08, 1.69) | 1.23 (0.99, 1.54) | 1.20 (0.96, 1.50) | 1.08 (0.86, 1.34) |
| Q2 | 2,474 | 13.1 | 1.64 (1.34, 2.01) | 1.41 (1.15, 1.73) | 1.35 (1.10, 1.66) | 1.20 (0.98, 1.47) |
| Q1 | 1,782 | 14.8 | 1.85 (1.50, 2.28) | 1.43 (1.15, 1.77) | 1.33 (1.07, 1.65) | 1.18 (0.95, 1.46) |
| Ptrend | <0.0001 | 0.005 | 0.02 | 0.26 | ||
| DII | ||||||
| Q5 | 1,750 | 10.0 | Ref | Ref | Ref | Ref |
| Q4 | 1,750 | 10.7 | 1.07 (0.88, 1.30) | 1.08 (0.89, 1.32) | 1.07 (0.87, 1.30) | 1.03 (0.85, 1.26) |
| Q3 | 1,750 | 12.4 | 1.24 (1.03, 1.50) | 1.23 (1.01, 1.50) | 1.19 (0.98, 1.45) | 1.11 (0.91, 1.35) |
| Q2 | 1,750 | 11.5 | 1.15 (0.95, 1.39) | 1.13 (0.91, 1.39) | 1.07 (0.87, 1.32) | 0.99 (0.80, 1.21) |
| Q1 | 1,750 | 14.1 | 1.41 (1.17, 1.69) | 1.32 (1.05, 1.64) | 1.21 (0.96, 1.51) | 1.07 (0.86, 1.34) |
| Ptrend | 0.003 | 0.048 | 0.19 | 0.75 | ||
| DIS | ||||||
| Q5 | 1,750 | 8.1 | Ref | Ref | Ref | Ref |
| Q4 | 1,750 | 9.1 | 1.13 (0.91, 1.40) | 1.08 (0.87, 1.34) | 1.07 (0.87, 1.33) | 1.03 (0.84, 1.28) |
| Q3 | 1,750 | 11.7 | 1.44 (1.17, 1.76) | 1.32 (1.07, 1.61) | 1.29 (1.05, 1.58) | 1.20 (0.98, 1.46) |
| Q2 | 1,750 | 13.7 | 1.68 (1.38, 2.05) | 1.44 (1.18, 1.76) | 1.38 (1.13, 1.69) | 1.29 (1.05, 1.57) |
| Q1 | 1,750 | 16.1 | 1.98 (1.64, 2.39) | 1.51 (1.24, 1.85) | 1.41 (1.15, 1.73) | 1.28 (1.04, 1.56) |
| Ptrend | <0.0001 | 0.0002 | 0.001 | 0.02 | ||
| Plant-based pattern | ||||||
| Q5 | 1,750 | 10.7 | Ref | Ref | Ref | Ref |
| Q4 | 1,750 | 12.6 | 1.18 (0.98, 1.42) | 1.19 (0.99, 1.44) | 1.17 (0.98, 1.41) | 1.12 (0.93, 1.35) |
| Q3 | 1,750 | 11.1 | 1.04 (0.86, 1.26) | 1.01 (0.83, 1.22) | 0.99 (0.81, 1.20) | 0.94 (0.77, 1.14) |
| Q2 | 1,750 | 11.3 | 1.06 (0.88, 1.28) | 0.98 (0.81, 1.20) | 0.95 (0.78, 1.15) | 0.90 (0.74, 1.10) |
| Q1 | 1,750 | 12.9 | 1.20 (1.003, 1.44) | 1.04 (0.86, 1.26) | 0.97 (0.79, 1.18) | 0.91 (0.75, 1.11) |
| Ptrend | 0.24 | 0.57 | 0.16 | 0.06 | ||
| Southern pattern | ||||||
| Q5 | 1,750 | 6.9 | Ref | Ref | Ref | Ref |
| Q4 | 1,750 | 9.5 | 1.37 (1.10, 1.72) | 1.33 (1.07, 1.67) | 1.31 (1.05, 1.64) | 1.27 (1.02, 1.59) |
| Q3 | 1,750 | 11.4 | 1.64 (1.33, 2.04) | 1.53 (1.23, 1.90) | 1.49 (1.20, 1.85) | 1.37 (1.11, 1.70) |
| Q2 | 1,750 | 13.2 | 1.91 (1.55, 2.35) | 1.66 (1.35, 2.05) | 1.60 (1.30, 1.98) | 1.42 (1.15, 1.75) |
| Q1 | 1,750 | 17.7 | 2.55 (2.09, 3.12) | 2.05 (1.66, 2.54) | 1.95 (1.57, 2.41) | 1.66 (1.35, 2.06) |
| Ptrend | <0.0001 | <0.0001 | <0.0001 | 0.003 |
Data are RR (95% CI) or % unless otherwise indicated. Crude model: unadjusted estimates. Model 1: adjustment for TEI and demographics of age, race, sex, region, income, and education. Model 2: further adjustment for lifestyle factors of smoking, physical activity, and alcohol. Ref, reference.
CI estimated through modified Poisson model. Boldface type indicates CI does not include 1.00 or statistical significance at P < 0.05 level.
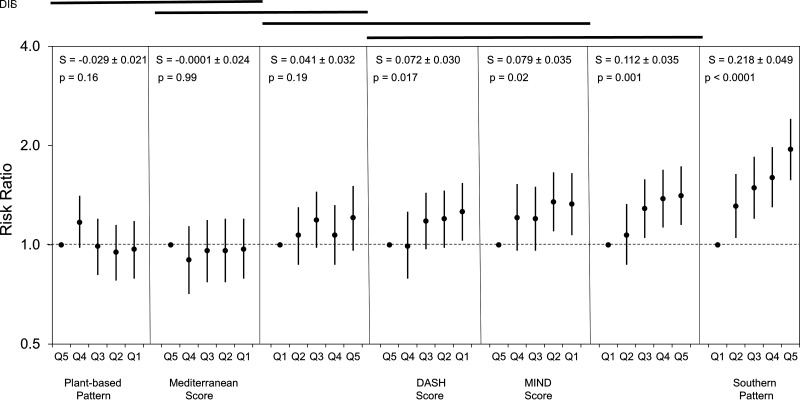
Figure 2.
Association of dietary scores and incident type 2 diabetes with adjustment for demographic and lifestyle factors. Model 2 presented here includes adjustment for TEI; demographics of age, race, sex, region, income, and education; and lifestyle factors of smoking, physical activity, and alcohol. RR for incident type 2 diabetes as a function of quintile (ordered from the quintile with the lowest expected risk of incident type 2 diabetes to the quintile with the highest expected risk of incident type 2 diabetes), where dietary measures have been ordered by the slope of increase of the RR (S ±SE). RRs are plotted on logarithmic scale for clarity of interpretation. P values in each panel are for the significance of the trend test for a relationship of the dietary measure with risk of incident type 2 diabetes. The lines at the top of the graph are over dietary measures where the differences in slope are not significant different (i.e., for any pair of dietary measures, the two measures are significantly different at P < 0.05 if there is not a line connecting the two measures).
Of the diet scores, DIS had the largest slope with a statistically significant linear trend (P = 0.001) and also showed the strongest association with incident type 2 diabetes, with RR of 1.41 (95% CI 1.15, 1.73) for the highest quintile after adjustment for TEI, sociodemographic, and lifestyle risk factors in model 2. The estimated association for the DIS retained statistical significance in all models for linear trend and the relative risk of the two highest quintiles. After DIS, the MIND diet score showed the next strongest association with incident type 2 diabetes with statistically significant linear trend for all models except after adjustment for a measure of obesity potentially in the causal pathway. The DII showed statistically significant associations for the linear trend for the crude model and model 1, but not models 2 and 3. The magnitude of the associations for the DII was less than that for both MIND diet score and DASH diet score but greater than that for Mediterranean diet score.
The Mediterranean diet score was statistically significant for only the most unhealthy quintile of the crude model and the test for linear trend was statistically significant, but this was attenuated and there was no longer significant association for the adjusted models. The most unhealthy quintile of both the DASH diet score and the MIND diet score was statistically significantly associated with increased risk of type 2 diabetes for model 2 and the test for linear trend was statistically significant for each. The most unhealthy quartile of the MIND diet score showed a slightly greater (7% greater) association with incident type 2 diabetes compared with the DASH diet score.
In comparing all dietary classification approaches, the magnitude of association with incident type 2 diabetes was greatest for the Southern dietary pattern, with an RR of 1.95 (95% CI 1.57, 2.41; P for trend <0.0001) for the most unhealthy quintile in model 2 (Table 2). The Southern dietary pattern association with incident type 2 diabetes was statistically significantly associated based on linear trend and for the RR of all models and all quintiles, with increasing RR with increasing adherence to this pattern. Even after consideration of a measure of obesity hypothesized to be in causal pathway, RR for the highest quintile was 1.66 (95% CI 1.35, 2.06; Ptrend = 0.003) and all quintiles retained statistical significance. For the plant-based dietary pattern, none of the associations for the adjusted models were statistically significant.
In evaluating the potential for unmeasured confounding, the Southern dietary pattern for model 2, with adjustment for TEI, demographic, and lifestyle factors, had an E-value of 3.31 for the highest quintile and an E-value of 2.58 for the second quintile compared with the lowest quintile used as reference (Supplementary Table 3). Based on the E-value, an unmeasured confounder would need to have an association of 3.31 with the exposure, Southern dietary pattern, and the outcome, incident type 2 diabetes, to completely explain away the diet-diabetes association. For DIS, the E-value was 2.17 for model 2 with adjustment for demographic and lifestyle factors.
Conclusions
In comparing various diet scores and dietary patterns in the REGARDS cohort, the Southern dietary pattern showed the strongest association with incident type 2 diabetes of all the dietary patterns/diet scores followed by DIS, MIND diet score, and DASH diet score. The Southern dietary pattern showed an approximately twofold higher risk in adjusted models and strong linear trend for all models. The Southern dietary pattern has been associated with a 39% increased hazard of stroke, 56% increased hazard of acute coronary heart disease, and 57% increased hazard of all-cause mortality and has been shown to be the most powerful mediator of the racial disparity in hypertension (14,17,18,32). Individuals with higher adherence to the Southern diet are more likely to be Black, be men, have lower education and income, and be current smokers and physically inactive (33). Race has been shown to be the strongest factor in Southern diet adherence. After accounting for these factors associated with adherence, the Southern diet remained strongly positively associated with incident type 2 diabetes. However, the negative outcomes associated with the Southern dietary pattern are based nearly completely on the REGARDS cohort, underscoring the need for confirmation in independent populations. As such, the strong association between the empirical Southern dietary pattern and incident type 2 diabetes provides rationale for investigating the creation of a diet score based on the strongest components of the Southern dietary pattern such as fried foods, organ meat, processed meat, high-fat dairy foods, and sugar-sweetened beverages that could be applied and evaluated in other cohort studies. In our analysis, the plant-based dietary pattern was not associated with incident type 2 diabetes. Increased adherence to plant-based dietary pattern has been shown to be associated with lower odds for high fasting insulin in REGARDS, and healthy plant-based diets have been associated with decreased incidence of diabetes in previous cohort studies (34,35).
Among the diet scores, the DIS reflecting inflammatory foods was the most strongly associated with incident type 2 diabetes as compared with more commonly studied Mediterranean and DASH diet scores. The food-based inflammation score of the DIS was consistently more strongly associated with incident type 2 diabetes for all models than the nutrient-based inflammation score of the DII, which was attenuated after the adjustment for lifestyle factors in model 2. These results strengthen the evidence that dietary inflammation is one of the potential pathways for incident type 2 diabetes. Diet scores reflecting the potential of the diet to influence inflammation should be further explored for use in dietary recommendations for type 2 diabetes prevention.
After DIS, the MIND and DASH diet scores showed the next strongest associations with significant trend for incident type 2 diabetes with the models adjusted for lifestyle and demographic factors, but association for the Mediterranean diet score was not significant past the crude model. In the systematic review and meta-analysis of diet scores and incident type 2 diabetes by Jannasch et al. (10), the DASH diet score was found to be more strongly associated with incident type 2 diabetes than the Mediterranean diet score, which was consistent with our analysis. The lack of association with the Mediterranean diet score could be because the Mediterranean diet is scaled according to adherence and not a full measure of compliance as in the PREDIMED trial. As compared with the Mediterranean and DASH diet scores, the MIND diet score was the most strongly associated with incident diabetes for the lowest quintile. Since the MIND diet score is composed of a hybrid of the Mediterranean diet and DASH diet, it is reasonable that the combination yields a stronger association than either Mediterranean or DASH diet score. Lower adherence to the MIND diet score has been shown to be associated with cognitive decline and mortality but not has not previously been explored for association with incident type 2 diabetes (23,36). Type 2 diabetes is an independent risk factor for cognitive decline, and it has been suggested that diabetes could be in the causal pathway of cognitive impairment through vascular pathogenesis and chronic exposure to hyperglycemia (37,38).
This study has both strengths and limitations to consider. While other studies have compared dietary patterns in association with incident type 2 diabetes, in this study we compare both empirical dietary patterns and diet scores based on hypothesized associations with food intake in a large biracial sample of adults across the continental U.S. DIS, DII, and MIND diet score have not previously been studied in relation to incident type 2 diabetes. While REGARDS is more diverse than many cohort studies, it is limited to Black and White individuals of older age, and so results may not be generalizable to more diverse and younger populations. Another limitation is that comparisons could not be made for AHEI and dietary information was based on FFQ data, which could lead to measurement error and misclassification of dietary intake. In addition, the FFQ was not returned by all individuals, which could result in selection bias. Overall, those who did not return the FFQ were more likely to be Black, have lower income, and not have graduated from high school. Another potential source of selection bias is that those who did not participate in the second visit were more likely to be less educated and have lower income. While confounding due to unmeasured factors is possible, the E-value provides context for the strength of an unmeasured confounder needed to explain away the associations observed. The association of incident type 2 diabetes with the Southern dietary pattern was the most robust to the potential for unmeasured confounding in that an unmeasured confounder would need to have an association of at least 3.31 with both incident type 2 diabetes and Southern dietary pattern to explain away the observed association and thus the association would be unlikely to be explained away by confounding.
In conclusion, high adherence to the Southern dietary pattern demonstrated the overall strongest association with incident type 2 diabetes and the DIS showed the strongest association of the diet scores. Further investigation is warranted into the mechanisms driving the robust association between incident type 2 diabetes and the Southern dietary pattern. The MIND diet demonstrated more utility for incident type 2 diabetes risk reduction as compared with the more researched Mediterranean and DASH diets. Further investigation and implementation of the dietary inflammation scores should be considered in dietary recommendations for diabetes prevention.
Article Information
Acknowledgments. The authors thank the other investigators, the staff, and the participants of the REGARDS study for their contributions. A full list of participating REGARDS investigators and institutions can be found at https://www.uab.edu/soph/regardsstudy/.
Funding. This research project is supported by a cooperative agreement co-funded by the National Institute of Neurological Disorders and Stroke (NINDS) and the National Institute on Aging (NIA), National Institutes of Health, Department of Health and Human Services (U01 NS041588).
Representatives of NINDS were involved in the review of the manuscript but were not directly involved in the collection, management, analysis, or interpretation of the data.
Duality of Interest. A.P.C. has received research support from Amgen. No other potential conflicts of interest relevant to this article were reported.
The research support from received from Amgen by A.P.C. is unrelated to this work.
Author Contributions. S.E.T. analyzed data and wrote the manuscript. S.E.J. acquired data and contributed to the writing and reviewing of the manuscript. J.M.S., D.L.L, A.P.C., S.S.C., K.E.P., and G.H. contributed to the writing and reviewing of the manuscript. S.E.T. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the American Heart Association Epidemiology Lifestyles Conference 5 March 2020.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.20642556.
References
- 1. Cho NH, Shaw JE, Karuranga S, et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract 2018;138:271–281 [DOI] [PubMed] [Google Scholar]
- 2. Lin J, Thompson TJ, Cheng YJ, et al. Projection of the future diabetes burden in the United States through 2060. Popul Health Metr 2018;16:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. CDC . National Diabetes Statistics Report. Atlanta, GA, Centers for Disease Control and Prevention, 2022 [Google Scholar]
- 4. Mayer-Davis EJ, Sparks KC, Hirst K, et al.; Diabetes Prevention Program Research Group . Dietary intake in the diabetes prevention program cohort: baseline and 1-year post randomization. Ann Epidemiol 2004;14:763–772 [DOI] [PubMed] [Google Scholar]
- 5. Tinker LF, Bonds DE, Margolis KL, et al.; Women’s Health Initiative . Low-fat dietary pattern and risk of treated diabetes mellitus in postmenopausal women: the Women’s Health Initiative randomized controlled dietary modification trial. Arch Intern Med 2008;168:1500–1511 [DOI] [PubMed] [Google Scholar]
- 6. Salas-Salvadó J, Bulló M, Estruch R, et al. Prevention of diabetes with Mediterranean diets: a subgroup analysis of a randomized trial. Ann Intern Med 2014;160:1–10 [DOI] [PubMed] [Google Scholar]
- 7. Evert AB, Dennison M, Gardner CD, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care 2019;42:731–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jacobs S, Harmon BE, Boushey CJ, et al. A priori-defined diet quality indexes and risk of type 2 diabetes: the Multiethnic Cohort. Diabetologia 2015;58:98–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Koning L, Chiuve SE, Fung TT, Willett WC, Rimm EB, Hu FB. Diet-quality scores and the risk of type 2 diabetes in men. Diabetes Care 2011;34:1150–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jannasch F, Kröger J, Schulze MB. Dietary patterns and type 2 diabetes: a systematic literature review and meta-analysis of prospective studies. J Nutr 2017;147:1174–1182 [DOI] [PubMed] [Google Scholar]
- 11. Menotti A, Puddu PE. Comparison of four dietary scores as determinants of coronary heart disease mortality. Sci Rep 2018;8:15001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sharma I, Roebothan B, Zhu Y, et al. Hypothesis and data-driven dietary patterns and colorectal Cancer survival: findings from Newfoundland and Labrador colorectal Cancer cohort. Nutr J 2018;17:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Howard VJ, Cushman M, Pulley L, et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology 2005;25:135–143 [DOI] [PubMed] [Google Scholar]
- 14. Howard G, Cushman M, Moy CS, et al. Association of clinical and social factors with excess hypertension risk in black compared with white US adults. JAMA 2018;320:1338–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Block G, Woods M, Potosky A, Clifford C. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol 1990;43:1327–1335 [DOI] [PubMed] [Google Scholar]
- 16. Judd SE, Letter AJ, Shikany JM, Roth DL, Newby PK. Dietary patterns derived using exploratory and confirmatory factor analysis are stable and generalizable across race, region, and gender subgroups in the REGARDS study. Front Nutr 2015;1:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Judd SE, Gutiérrez OM, Newby PK, et al. Dietary patterns are associated with incident stroke and contribute to excess risk of stroke in black Americans. Stroke 2013;44:3305–3311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shikany JM, Safford MM, Newby PK, Durant RW, Brown TM, Judd SE. Southern dietary pattern is associated with hazard of acute coronary heart disease in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. Circulation 2015;132:804–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tsivgoulis G, Judd S, Letter AJ, et al. Adherence to a Mediterranean diet and risk of incident cognitive impairment. Neurology 2013;80:1684–1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tsivgoulis G, Psaltopoulou T, Wadley VG, et al. Adherence to a Mediterranean diet and prediction of incident stroke. Stroke 2015;46:780–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med 2008;168:713–720 [DOI] [PubMed] [Google Scholar]
- 22. Shimbo D, Levitan EB, Booth JN 3rd, et al. The contributions of unhealthy lifestyle factors to apparent resistant hypertension: findings from the Reasons for Geographic And Racial Differences in Stroke (REGARDS) study. J Hypertens 2013;31:370–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Morris MC, Tangney CC, Wang Y, Sacks FM, Bennett DA, Aggarwal NT. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement 2015;11:1007–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li Z, Gao Y, Byrd DA, et al. Novel dietary and lifestyle inflammation scores directly associated with all-cause, all-cancer, and all-cardiovascular disease mortality risks among women. J Nutr 2021;151:930–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van Woudenbergh GJ, Theofylaktopoulou D, Kuijsten A, et al. Adapted dietary inflammatory index and its association with a summary score for low-grade inflammation and markers of glucose metabolism: the Cohort study on Diabetes and Atherosclerosis Maastricht (CODAM) and the Hoorn study. Am J Clin Nutr 2013;98:1533–1542 [DOI] [PubMed] [Google Scholar]
- 26. Byrd DA, Judd SE, Flanders WD, Hartman TJ, Fedirko V, Bostick RM. Development and validation of novel dietary and lifestyle inflammation scores. J Nutr 2019;149:2206–2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shivappa N, Steck SE, Hurley TG, Hussey JR, Hébert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr 2014;17:1689–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Myers CA, Slack T, Broyles ST, Heymsfield SB, Church TS, Martin CK. Diabetes prevalence is associated with different community factors in the diabetes belt versus the rest of the United States. Obesity (Silver Spring) 2017;25:452–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004;159:702–706 [DOI] [PubMed] [Google Scholar]
- 30. Mathur MB, Ding P, Riddell CA, VanderWeele TJ. Web site and R package for computing E-values. Epidemiology 2018;29:e45–e47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med 2017;167:268–274 [DOI] [PubMed] [Google Scholar]
- 32. Shikany JM, Safford MM, Bryan J, et al. Dietary patterns and Mediterranean diet score and hazard of recurrent coronary heart disease events and all-cause mortality in the REGARDS study. J Am Heart Assoc 2018;7:e008078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Couch CA, Gray MS, Shikany JM, et al. Correlates of a southern diet pattern in a national cohort study of blacks and whites: the REasons for Geographic And Racial Differences in Stroke (REGARDS) study. Br J Nutr 2021;126:1904–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gower BA, Pearson K, Bush N, et al. Diet pattern may affect fasting insulin in a large sample of black and white adults. Eur J Clin Nutr 2021;75:628–635 [DOI] [PubMed] [Google Scholar]
- 35. Satija A, Bhupathiraju SN, Rimm EB, et al. Plant-based dietary patterns and incidence of type 2 diabetes in US men and women: results from three prospective cohort studies. PLoS Med 2016;13:e1002039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Corley J. Adherence to the MIND diet is associated with 12-year all-cause mortality in older adults. Public Health Nutr 2022;25:358–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Teixeira MM, Passos VMA, Barreto SM, et al. Association between diabetes and cognitive function at baseline in the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Sci Rep 2020;10:1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Papunen S, Mustakallio-Könönen A, Auvinen J, Timonen M, Keinänen-Kiukaanniemi S, Sebert S. The association between diabetes and cognitive changes during aging. Scand J Prim Health Care 2020;38:281–290 [DOI] [PMC free article] [PubMed] [Google Scholar]