Abstract
OBJECTIVE
Diabetes and the outpatient diabetes treatment regimen have been identified as risk factors for poor outcomes in patients with sepsis. However, little is known about the effect of tight inpatient glycemic control in the setting of coronavirus disease 2019 (COVID-19). Therefore, we examined the effect of hyperglycemia in patients with diabetes hospitalized because of COVID-19.
RESEARCH DESIGN AND METHODS
We analyzed data from 1,938 COVID-19 patients with diabetes hospitalized for COVID-19 from March to May 2020 at a large academic medical center in New York City. Patients were divided into two groups based on their inpatient glycemic values, and a Cox proportional hazards regression model was used to assess the independent association of inpatient glucose levels with mortality (primary outcome) and the risk of requiring mechanical ventilation (MV) (secondary outcome).
RESULTS
In our analysis, 32% of the patients were normoglycemic and 68% hyperglycemic. Moreover, 31% of the study subjects died during hospitalization, and 14% required MV, with inpatient hyperglycemia being significantly associated with both mortality and the requirement for MV. Additionally, in the Cox regression analysis, after adjustment for potential confounders, including age, sex, race, BMI, HbA1c, comorbidities, inflammatory markers, and corticosteroid therapy, patients with uncontrolled hyperglycemia had a higher risk of dying (hazard ratio [HR] 1.54, 95% CI 1.00–2.36, P = 0.049) and of requiring MV (HR 4.41, 95% CI 1.52–2.81, P = 0.006) than those with normoglycemia.
CONCLUSIONS
A tight control of inpatient hyperglycemia may be an effective method for improving outcomes in patients with diabetes hospitalized for COVID-19.
Introduction
Higher rates of mechanical ventilation (MV) and death from coronavirus disease 2019 (COVID-19) have been documented in patients with diabetes compared with those without diabetes (1–4). Moreover, hyperglycemia before and after hospitalization and diabetes duration have been significantly associated with worse prognosis in patients with COVID-19, most likely through alterations of the immune and coagulation systems (4–10). While the presence of diabetes is more commonly associated with severe COVID-19, the mechanisms underlying this association remain to be elucidated (11–13). Interestingly, glycemic control in the inpatient environment has been linked to outcomes in critically ill patients, regardless of diabetes status (14–17). Therefore, hyperglycemia may represent a risk factor for COVID-19 outcomes that could be modifiable with an improved glucose control. However, data evaluating the impact of inpatient glycemic control in patients with diabetes and COVID-19 on prognosis remain scarce (18,19), and there is an urgent need for research to help to guide the management of these patients. Thus, the aim of this study was to assess whether uncontrolled inpatient hyperglycemia was associated with mortality and MV in our study population enrolled at a large academic medical center in New York City.
Research Design and Methods
Setting and Patients
Our analysis was conducted in patients with COVID-19 (confirmed by PCR testing) admitted to Montefiore Medical Center in New York City from March to May 2020. The study population included patients with COVID-19 and with documented diabetes (defined by HbA1c ≥6.5%) before or during the first week of hospitalization. Participants who were still hospitalized at the end of the data collection period were excluded from the analysis because the end points of hospital discharge or death were not known. The investigation conformed with the principles outlined in the Declaration of Helsinki, and the study protocol was approved by the institutional ethics committee. Written informed consent was obtained from all patients or their legal representatives.
Demographic and Clinical Characteristics
Information on age, sex, race, ethnicity, insurance status, and BMI was collected for all participants. Race and ethnicity were self-reported. The most recent HbA1c level within 3 years before or during the first week of admission was used for analysis. C-reactive protein (CRP) and D-dimer levels were measured from blood collected in EDTA-coated tubes immediately after admission. Comorbidities, including hypertension, cerebrovascular events, coronary and peripheral artery diseases, chronic kidney disease, and chronic obstructive pulmonary disease were evaluated using ICD-10-CM codes from outpatient visits for 2 years before admission or admission diagnoses (20–22). Patients (independently of their fasting state) were stratified according to their glycemic values during hospitalization; those with blood glucose levels at a target glucose range of 80–180 mg/dL for the entire hospital stay were considered normoglycemic (23).
Study Outcomes
The primary outcome was mortality at any point during hospitalization in the study time frame or live discharge; the secondary outcome was the requirement of MV during hospitalization.
Statistical Analysis
Descriptive data were reported as mean ± SD for approximately normally distributed continuous variables, median (interquartile range) for severely skewed continuous variables, and numerical values (percentages) for categorical variables. Normal distribution of the data was assessed through normal probability plots and confirmed with the skewness/kurtosis test for normality. Bivariate tests were used to assess the association between the inpatients’ glycemic values and their baseline characteristics. Statistical significance was determined by a two-tailed P < 0.05. In the statistical analysis, differences for continuous variables were evaluated using two-sample t test for approximately normally distributed variables and Mann-Whitney U test for severely skewed variables. The χ2 test or Fisher exact test was used to measure associations between dichotomous and categorical variables. A Cox proportional hazards regression analysis was used to relate inpatient glycemic values to the primary and the secondary outcomes, adjusting for likely confounders (selected a priori on the basis of their clinical significance and possible confounding effect). A backward selection method was used to create the final model, including age, sex, race, BMI, HbA1c, comorbidities, inflammatory markers, and corticosteroid therapy. Hazard ratios (HRs) from both the bivariate (unadjusted) and the multivariate (adjusted) analyses are reported along with their 95% CIs. Statistical analysis was done using Stata 16.1 for Macintosh software (StataCorp, College Station, TX).
Results
Demographic, anthropometric, and clinical characteristics of the population studied in this analysis (total and by glycemic status) are shown in Table 1. The cohort included 1,938 patients; the mean age was 68.08 ± 13.7 years, 50% were male, 76% were Black, and 40% were Hispanic. In addition, the mean BMI of the enrolled patients was 30 kg/m2; 98% of participants had type 2 diabetes, 2% had type 1 diabetes, 32% received systemic corticosteroids in the hospital (Table 1), 31% died during hospitalization, and 14% required MV (Fig. 1A and B). Participants were divided into two groups based on their inpatient glycemic values (32% normoglycemic, 68% hyperglycemic). Bivariate analysis comparing characteristics between patients with normoglycemia and those with hyperglycemia revealed that the two groups did not differ in terms of age, sex, race, ethnicity, and insurance type (Table 1).
Table 1.
Characteristics of study participants: total and stratified by inpatient glycemic values
| Variable | Total (n = 1,938) | Normoglycemia (glucose ≤180 mg/dL) (n = 622) | Hyperglycemia (glucose ≥180 mg/dL) (n = 1,316) | P for trend |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age (years) | 68.08 ± 13.7 | 67.27 ± 14.71 | 68.46 ± 13.18 | 0.09 |
| Sex | ||||
| Male | 963 (49.69) | 291 (46.78) | 672 (51.06) | |
| Female | 975 (50.31) | 331 (53.22) | 644 (48.94) | 0.08 |
| Race | ||||
| White | 195 (18.77) | 76 (21.47) | 119 (17.37) | |
| Black | 789 (75.94) | 266 (75.14) | 523 (76.35) | |
| Other | 55 (5.29) | 12 (3.39) | 43 (6.28) | 0.06 |
| Ethnicity | ||||
| Hispanic | 691 (39.64) | 207 (36.77) | 484 (41.02) | |
| Not Hispanic | 1,052 (60.36) | 356 (63.23) | 696 (58.98) | 0.09 |
| Insurance | ||||
| Medicaid | 827 (42.78) | 260 (41.80) | 260 (41.80) | |
| Medicare | 915 (47.34) | 290 (46.62) | 625 (47.67) | |
| Commercial | 191 (9.88) | 72 (11.58) | 119 (9.08) | 0.23 |
| Clinical characteristics and laboratory parameters | ||||
| BMI (kg/m2) | 29.92 ± 7.25 | 30.48 ± 0.3 | 29.65 ± 0.2 | 0.02 |
| HbA1c (%) | 7.26 ± 1.95 | 6.05 ± 0.76 | 7.85 ± 2.07 | <0.001 |
| CRP (mg/L) | 10.9 (4.9–19.2) | 7.6 (3.7–14.7) | 12.7 (5.8–21) | <0.001 |
| D-dimer (μg/mL) | 1.74 (0.89–3.34) | 1.31 (0.7–2.5) | 1.74 (1.01–3.62) | <0.001 |
| Comorbidities | ||||
| Hypertension | ||||
| No | 318 (16.41) | 122 (19.61) | 196 (14.89) | |
| Yes | 1,620 (83.59) | 500 (80.39) | 1,120 (85.11) | 0.009 |
| Previous stroke | ||||
| No | 1,355 (79.19) | 429 (80.94) | 926 (78.41) | |
| Yes | 356 (20.81) | 101 (19.06) | 255 (21.59) | 0.23 |
| Coronary artery disease | ||||
| No | 1,206 (62.23) | 416 (66.88) | 790 (60.03) | |
| Yes | 732 (37.77) | 206 (33.12) | 526 (39.97) | 0.004 |
| Peripheral artery disease | ||||
| No | 1,757 (90.66) | 594 (95.50) | 1,163 (88.37) | |
| Yes | 181 (9.34) | 28 (4.50) | 153 (11.63) | <0.001 |
| Chronic kidney disease | ||||
| No | 1,305 (67.34) | 452 (72.67) | 853 (64.82) | |
| Yes | 633 (32.66) | 170 (27.33) | 463 (35.18) | <0.001 |
| Chronic obstructive pulmonary disease | ||||
| No | 1,493 (87.26) | 451 (85.09) | 1,042 (88.23) | |
| Yes | 218 (12.74) | 79 (14.91) | 139 (11.77) | 0.07 |
Data are mean ± SD, n (%) or median (IQR). Laboratory results were obtained on admission to the hospital.
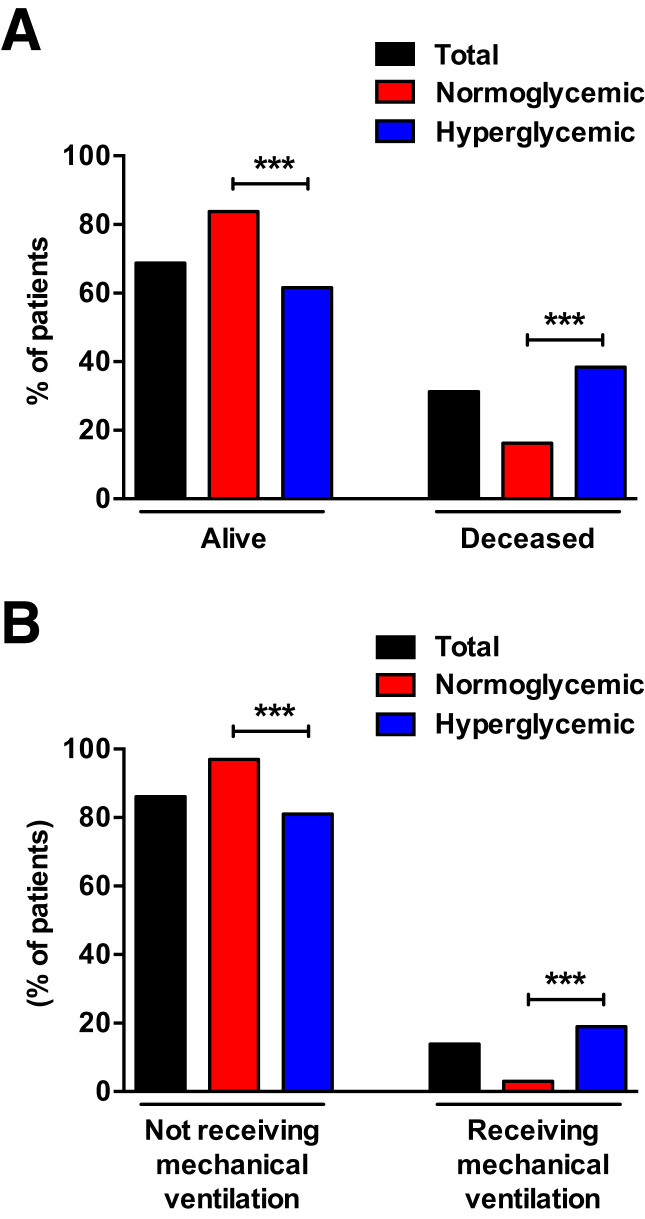
Figure 1.
Primary (A) and secondary (B) outcomes by inpatient glycemic values in patients with diabetes hospitalized for COVID-19. ***P < 0.001.
Significant differences were found, however, between the two cohorts in some of their clinical characteristics, laboratory parameters, and comorbidities. Specifically, patients with higher HbA1c, CRP, and D-dimer had uncontrolled hyperglycemia during hospitalization (P < 0.001). In addition, cardiovascular diseases (including hypertension [P = 0.009], coronary artery disease [P = 0.004], and peripheral artery disease [P < 0.001]) and chronic kidney disease (P < 0.001) were significantly associated with inpatient hyperglycemia (Table 1). We then evaluated the percentages of patients with hyperglycemia and normoglycemia who died (38% and 16%, respectively) or required MV (19% and 3%, respectively) during hospitalization (Fig. 1), and we observed a significant difference between the two groups (P < 0.001).
Moreover, we detected a significant association between inpatient glycemic control and the primary outcome (HR 1.62, 95% CI 1.31–2.00, P < 0.001). This association remained significant (HR 1.54, 95% CI 1.00–2.36, P = 0.049) in a Cox regression analysis (Table 2) using the fully adjusted multivariable model containing potential confounders of age, sex, race, BMI, HbA1c, comorbidities, inflammatory markers, and corticosteroid therapy. The variables used in the regression analyses were selected according to the available literature on COVID-19 (24,25). Thus, our data indicate that patients with diabetes and an uncontrolled hyperglycemic status had a 54% greater risk of dying during hospitalization for COVID-19 compared with patients with normoglycemia.
Table 2.
Cox proportional hazards model assessing the effect of inpatient hyperglycemia on mortality, adjusting for potential confounders
| HR | 95% CI | P for trend | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Hyperglycemia | 1.54 | 1.00 | 2.36 | 0.049 |
| Age | 1.03 | 0.97 | 1.02 | 1.05 |
| Sex | 0.95 | 0.69 | 1.31 | 0.77 |
| Race | 0.83 | 0.63 | 1.09 | 0.17 |
| BMI | 0.98 | 0.001 | 0.95 | 1.02 |
| HbA1c | 0.97 | 0.38 | 1.04 | 0.786 |
| Hypertension | 0.70 | 0.37 | 1.34 | 0.28 |
| Chronic kidney disease | 1.23 | 0.93 | 1.62 | 0.14 |
| Chronic obstructive pulmonary disease | 1.54 | 1.08 | 2.19 | 0.02 |
| CRP | 1.03 | 1.01 | 1.04 | 0.001 |
| D-dimer | 0.99 | 0.96 | 1.03 | 0.66 |
| Corticosteroid therapy | 1.20 | 0.89 | 1.61 | 0.23 |
Our secondary outcome of need for MV also showed a significant association with inpatient glycemia. Patients with hyperglycemia had a significantly higher risk of being ventilated during hospitalization (HR 4.11, 95% CI 2.58–3.56, P < 0.001). After adjustment for age, sex, race, BMI, HbA1c, comorbidities, inflammatory markers, and corticosteroid therapy, as shown in Table 3, patients with diabetes and an uncontrolled hyperglycemic status had a 4.4-times greater risk of receiving MV during hospitalization for COVID-19 compared with those with normoglycemia (HR 4.41, 95% CI 1.52–2.81, P = 0.006). Taken together, our data indicate that optimal glycemic control during hospitalization is associated with a lower risk of severe disease and death in patients with COVID-19.
Table 3.
Cox proportional hazards model assessing the effect of inpatient hyperglycemia on MV, adjusting for potential confounders
| HR | 95% CI | P for trend | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Hyperglycemia | 4.41 | 1.52 | 2.81 | 0.006 |
| Age | 0.99 | 0.97 | 1.01 | 0.238 |
| Sex | 1.54 | 0.94 | 2.53 | 0.09 |
| Race | 0.77 | 0.50 | 1.16 | 0.21 |
| BMI | 0.94 | 0.89 | 1.00 | 0.04 |
| HbA1c | 1.00 | 0.89 | 1.12 | 0.21 |
| Hypertension | 0.71 | 0.25 | 1.97 | 0.51 |
| Chronic kidney disease | 1.25 | 0.81 | 1.93 | 0.31 |
| Chronic obstructive pulmonary disease | 1.55 | 0.86 | 2.79 | 0.15 |
| CRP | 1.03 | 1.01 | 1.04 | 0.001 |
| D-dimer | 0.98 | 0.93 | 1.04 | 0.46 |
| Corticosteroid therapy | 1.71 | 1.06 | 2.78 | 0.03 |
Conclusions
The recent pandemic of COVID-19 has proven a reciprocal, detrimental interaction between the immune and endocrine systems in the context of diabetes, confirming that immune-mediated changes in systemic metabolism upon infection may aggravate glycemic control in patients with diabetes (26–28). Mounting evidence indicates that diabetes or hyperglycemia at admission are associated with poor prognosis in patients with sepsis (29–31) and that COVID-19 itself can cause stress hyperglycemia, worsening the outcome in hospitalized patients (32). Interestingly, in critically ill patients with COVID-19 who had similar glucose levels at admission, those with diabetes had a better prognosis than those without (33), suggesting that the magnitude of acute glycemic rise from chronic levels (rather than the glycemic level per se) is an important and underestimated prognostic factor. Indeed, in-hospital factors (including interventions inducing hyperglycemia, e.g., corticosteroid therapy) may affect in-hospital mortality and need to be further investigated.
In this study, we examined the role of inpatient glycemic control on COVID-19 severity and prognosis in patients with diabetes, and our main finding is that inpatient hyperglycemia is associated with an increased risk of in-hospital mortality and need for MV. However, our patients were enrolled in the study up to May 2020, months before COVID-19 vaccines were available; thus, our results are applicable only to unvaccinated patients, and it is unknown whether hyperglycemia will have the same impact in vaccinated subjects. Consistent with data in the literature (10), we observed that patients with hyperglycemia who progressed to severe disease presented with higher inflammatory markers (including CRP) and coagulation abnormalities (elevated D-dimer). Hence, inpatient hyperglycemia is a strong prognostic predictor of outcome in hospitalized patients with COVID-19. Nonetheless, in patients with severe pneumonia, an overactivated inflammatory response could drive stress hyperglycemia and a severe disease course (34); thus, there is a possibility that a high blood glucose level might simply represent a biomarker of more severe disease. Moreover, glucose management in patients with COVID-19 is complicated by the disease itself and by the current standards of care; thus, the use of corticosteroids as standard treatment for patients with COVID-19 adds more complexity to optimal glycemic control in hospitalized patients with diabetes (35–37). Nevertheless, in our analysis, the HRs for both primary and secondary outcomes remained consistent and statistically significant after rigorous adjustment, indicating that the association was independent of baseline confounders, including markers of inflammation and corticosteroid therapy.
Our study has several important strengths. First, to our knowledge, even if this is a single-center study, it is the largest observational study of patients hospitalized for COVID-19 with type 1 and type 2 diabetes. Moreover, our hospital has had unified COVID-19 treatment protocols from the start of the pandemic, and all patients were treated using the same protocols, reducing the likelihood that treatment variation is a confounder. Nevertheless, our analysis is not exempt from limitations, including the retrospective single-center observational design of the study; therefore, causality cannot be assessed, and our results may not be applicable to other geographic regions of the U.S. Additionally, we focused on hospital COVID-19 cases, and our results cannot be generalized to all people with COVID-19 and diabetes, especially those with a less severe form of the disease. Furthermore, this study was conducted in the early phases of the COVID-19 pandemic and may not apply to patients admitted now who are treated with new management strategies not available in the spring of 2020. Finally, since only the most recent HbA1c value was used in the analysis, it may not wholly reflect the relationship between glycemic control and mortality.
In summary, while we cannot establish causality, our data strongly suggest that tight glycemic control has a beneficial effect on outcomes in patients with diabetes hospitalized because of COVID-19. Hence, in the critical care setting, insulin infusion may be an effective method for achieving glycemic targets and reducing mortality and/or the need of MV in these patients. Additional studies are needed to better understand the interrelationship between diabetes and COVID-19 and to examine prospectively whether tight glycemic control improves outcomes. As a future direction, it would be useful to understand the safety profile of recently introduced glucose-lowering agents in patients with symptoms of COVID-19 (38–40).
Article Information
Funding. This research was supported in part by the Center for Scientific Review, National Institutes of Health (grants UL1TR002556, KL2TR002558, and part of 2P30DK020541-45 to A.L. and DK067555 to Y.T.), as well as by the Diabetes Action and Education Foundation (to A.L.).
None of the sources of funding have an interest in the subject matter or materials discussed in the article.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. A.L. developed the research idea, designed the study, analyzed the data, interpreted the results, and wrote the manuscript. S.A. and Y.T. researched the data and review and edited the manuscript. C.S. contributed to the study design, data analysis, and discussion. A.L. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article is part of a special article collection available at diabetesjournals.org/journals/collection/52/Diabetes-and-COVID-19.
References
- 1. Khunti K, Del Prato S, Mathieu C, Kahn SE, Gabbay RA, Buse JB. COVID-19, hyperglycemia, and new-onset diabetes. Diabetes Care 2021;44:2645–2655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Richardson S, Hirsch JS, Narasimhan M, et al.; the Northwell COVID-19 Research Consortium . Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020;323:2052–2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guan WJ, Ni ZY, Hu Y, et al.; China Medical Treatment Expert Group for COVID-19 . Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhu L, She ZG, Cheng X, et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab 2020;31:1068–1077.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Agarwal S, Schechter C, Southern W, Crandall JP, Tomer Y. Preadmission diabetes-specific risk factors for mortality in hospitalized patients with diabetes and coronavirus disease 2019. Diabetes Care 2020;43:2339–2344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gambardella J, Kansakar U, Sardu C, et al. Exosomal miR-145 and miR-885 regulate thrombosis in COVID-19. J Pharmacol Exp Ther 30 June 2022 [Epub ahead of print]. DOI: 10.1124/jpet.122.001209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bornstein SR, Rubino F, Khunti K, et al. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol 2020;8:546–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS; HLH Across Speciality Collaboration, UK . COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020;395:1033–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and antiphospholipid antibodies in patients with COVID-19. N Engl J Med 2020;382:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sardu C, D’Onofrio N, Balestrieri ML, et al. Outcomes in patients with hyperglycemia affected by COVID-19: can we do more on glycemic control? Diabetes Care 2020;43:1408–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sardu C, Gambardella J, Morelli MB, Wang X, Marfella R, Santulli G. Hypertension, thrombosis, kidney failure, and diabetes: is COVID-19 an endothelial disease? A comprehensive evaluation of clinical and basic evidence. J Clin Med 2020;9:1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Apicella M, Campopiano MC, Mantuano M, Mazoni L, Coppelli A, Del Prato S. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol 2020;8:782–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lim S, Bae JH, Kwon HS, Nauck MA. COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Nat Rev Endocrinol 2021;17:11–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dungan KM, Braithwaite SS, Preiser JC. Stress hyperglycaemia. Lancet 2009;373:1798–1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Finfer S, Chittock DR, Su SY, et al.; NICE-SUGAR Investigators . Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009;360:1283–1297 [DOI] [PubMed] [Google Scholar]
- 16. Bellaver P, Schaeffer AF, Dullius DP, Viana MV, Leitão CB, Rech TH. Association of multiple glycemic parameters at intensive care unit admission with mortality and clinical outcomes in critically ill patients. Sci Rep 2019;9:18498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang S, Ma P, Zhang S, et al. Fasting blood glucose at admission is an independent predictor for 28-day mortality in patients with COVID-19 without previous diagnosis of diabetes: a multi-centre retrospective study. Diabetologia 2020;63:2102–2111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wargny M, Potier L, Gourdy P, et al.; CORONADO Investigators . Predictors of hospital discharge and mortality in patients with diabetes and COVID-19: updated results from the nationwide CORONADO study. Diabetologia 2021;64:778–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Holman N, Knighton P, Kar P, et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol 2020;8:823–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shu J, Santulli G. Update on peripheral artery disease: epidemiology and evidence-based facts. Atherosclerosis 2018;275:379–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wilson S, Mone P, Jankauskas SS, Gambardella J, Santulli G. Chronic kidney disease: definition, updated epidemiology, staging, and mechanisms of increased cardiovascular risk. J Clin Hypertens (Greenwich) 2021;23:831–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lainscak M, Anker SD. Heart failure, chronic obstructive pulmonary disease, and asthma: numbers, facts, and challenges. ESC Heart Fail 2015;2:103–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Draznin B, Aroda VR, Bakris G, et al.; American Diabetes Association Professional Practice Committee . 6. Glycemic targets: Standards of Medical Care in Diabetes–2022. Diabetes Care 2022;45:S83–S96 [DOI] [PubMed] [Google Scholar]
- 24. Fiorentino G, Coppola A, Izzo R, et al. Effects of adding L-arginine orally to standard therapy in patients with COVID-19: a randomized, double-blind, placebo-controlled, parallel-group trial. Results of the first interim analysis. EClinicalMedicine 2021;40:101125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA 2020;323:1824–1836 [DOI] [PubMed] [Google Scholar]
- 26. Blackard JT, Kong L, Lombardi A, Homann D, Hammerstad SS, Tomer Y. A preliminary analysis of hepatitis C virus in pancreatic islet cells. Virol J 2017;14:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lombardi A, Tomer Y. Interferon alpha impairs insulin production in human beta cells via endoplasmic reticulum stress. J Autoimmun 2017;80:48–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lombardi A, Tsomos E, Hammerstad SS, Tomer Y. Interferon alpha: the key trigger of type 1 diabetes. J Autoimmun 2018;94:7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lin S, Ge S, He W, Zeng M. Association between comorbid diabetes mellitus and prognosis of patients with sepsis in the intensive care unit: a retrospective cohort study. Ann Transl Med 2021;9:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Clain J, Ramar K, Surani SR. Glucose control in critical care. World J Diabetes 2015;6:1082–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tiwari S, Pratyush DD, Gahlot A, Singh SK. Sepsis in diabetes: a bad duo. Diabetes Metab Syndr 2011;5:222–227 [DOI] [PubMed] [Google Scholar]
- 32. Cromer SJ, Colling C, Schatoff D, et al. Newly diagnosed diabetes vs. pre-existing diabetes upon admission for COVID-19: associated factors, short-term outcomes, and long-term glycemic phenotypes. J Diabetes Complications 2022;36:108145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bode B, Garrett V, Messler J, et al. Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States. J Diabetes Sci Technol 2020;14:813–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schuetz P, Friedli N, Grolimund E, et al.; ProHOSP Study Group . Effect of hyperglycaemia on inflammatory and stress responses and clinical outcome of pneumonia in non-critical-care inpatients: results from an observational cohort study. Diabetologia 2014;57:275–284 [DOI] [PubMed] [Google Scholar]
- 35. Horby P, Lim WS, Emberson JR, et al.; RECOVERY Collaborative Group . Dexamethasone in hospitalized patients with COVID-19. N Engl J Med 2021;384:693–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Qi D, Pulinilkunnil T, An D, et al. Single-dose dexamethasone induces whole-body insulin resistance and alters both cardiac fatty acid and carbohydrate metabolism. Diabetes 2004;53:1790–1797 [DOI] [PubMed] [Google Scholar]
- 37. Cheung NW. Steroid-induced hyperglycaemia in hospitalised patients: does it matter? Diabetologia 2016;59:2507–2509 [DOI] [PubMed] [Google Scholar]
- 38. Bai Y, Lian P, Li J, Zhang Z, Qiao J. The active GLP-1 analogue liraglutide alleviates H9N2 influenza virus-induced acute lung injury in mice. Microb Pathog 2021;150:104645. [DOI] [PubMed] [Google Scholar]
- 39. Mone P, Lombardi A, Gambardella J, et al. Empagliflozin improves cognitive impairment in frail older adults with type 2 diabetes and heart failure with preserved ejection fraction. Diabetes Care 2022;45:1247–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sato T, Shimizu T, Fujita H, et al. GLP-1 receptor signaling differentially modifies the outcomes of sterile vs viral pulmonary inflammation in male mice. Endocrinology 2020;161:bqaa201. [DOI] [PMC free article] [PubMed] [Google Scholar]