Abstract
OBJECTIVE
N-glycosylation is a functional posttranslational modification of immunoglobulins (Igs). We hypothesized that specific IgG N-glycans are associated with incident type 2 diabetes and cardiovascular disease (CVD).
RESEARCH DESIGN AND METHODS
We performed case-cohort studies within the population-based European Prospective Investigation into Cancer and Nutrition (EPIC)–Potsdam cohort (2,127 in the type 2 diabetes subcohort [741 incident cases]; 2,175 in the CVD subcohort [417 myocardial infarction and stroke cases]). Relative abundances of 24 IgG N-glycan peaks (IgG-GPs) were measured by ultraperformance liquid chromatography, and eight glycosylation traits were derived based on structural similarity. End point–associated IgG-GPs were preselected with fractional polynomials, and prospective associations were estimated in confounder-adjusted Cox models. Diabetes risk associations were validated in three independent studies.
RESULTS
After adjustment for confounders and multiple testing correction, IgG-GP7, IgG-GP8, IgG-GP9, IgG-GP11, and IgG-GP19 were associated with type 2 diabetes risk. A score based on these IgG-GPs was associated with a higher diabetes risk in EPIC-Potsdam and independent validation studies (843 total cases, 3,149 total non-cases, pooled estimate per SD increase 1.50 [95% CI 1.37–1.64]). Associations of IgG-GPs with CVD risk differed between men and women. In women, IgG-GP9 was inversely associated with CVD risk (hazard ratio [HR] per SD 0.80 [95% CI 0.65–0.98]). In men, a weighted score based on IgG-GP19 and IgG-GP23 was associated with higher CVD risk (HR per SD 1.47 [95% CI 1.20–1.80]). In addition, several derived traits were associated with cardiometabolic disease incidence.
CONCLUSIONS
Selected IgG N-glycans are associated with cardiometabolic risk beyond classic risk factors, including clinical biomarkers.
Introduction
N-glycans are complexly regulated, posttranslational protein modifications that participate in essential molecular processes, including protein folding and stability, cell-cell recognition, and signal transduction (1,2). Recent mechanistic (3–5) and human cross-sectional studies (6–10) implicated protein N-glycosylation in insulin resistance, diabetes, inflammation, and cardiovascular disease (CVD) pathogenesis. We recently reported that total plasma N-glycan profiles improve prediction of incident CVD and type 2 diabetes beyond clinical prediction models (11,12). However, whole-plasma N-glycan profiles are unspecific with regard to the source proteins of the N-glycans.
Antibodies of the immunoglobulin class G (IgG) are a major fraction of circulating N-glycoproteins. IgG glycosylation determines structure and immunological function of the IgG, including modulation of pro- and anti-inflammatory signaling and cellular immune response (13,14). For instance, fully sialylated and galactosylated IgG glycoforms exhibit anti-inflammatory properties (13–15), which are harnessed in pharmacological interventions (14,16). The links between low-grade systemic inflammation, type 2 diabetes, and CVD are well established (17–19). Proinflammatory cytokines like interleukins and tumor necrosis factor-α induce oxidative stress, promote insulin resistance, and oxidize LDLs, leading to endothelial dysfunction and proatherogenic and prothrombotic milieus (17–19). In addition, proinflammatory alterations in the IgG N-glycome encompassing decreased sialylation and galactosylation and increased levels of bisecting N-acetylglucosamine (GlcNAc) were cross-sectionally associated with cardiometabolic risk factors, including age, BMI, smoking, and markers of inflammation and dyslipidemia (20–22).
However, prospective evidence of altered IgG N-glycosylation in cardiometabolic disease etiology is scarce. Our primary aim was to examine the association of baseline IgG N-glycan peaks (IgG-GPs) with the risk of incident type 2 diabetes and CVD in the population-based European Prospective Investigation into Cancer and Nutrition (EPIC)–Potsdam cohort, summarizing significant associations of end point–specific IgG glycan scores. Prospective type 2 diabetes risk associations were externally validated in three independent study samples within the Finland Cardiovascular Risk Study (Finrisk); the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) trial; and the Treating to New Targets (TNT) trial. In secondary analyses, we examined the associations of the derived glycan traits with type 2 diabetes and CVD incidence and assessed whether IgG glycan data may further improve our previously published total plasma N-glycan–based cardiometabolic risk prediction models.
Research Design and Methods
Study Population of EPIC-Potsdam
The current study is embedded in the EPIC-Potsdam cohort consisting of 27,548 individuals (16,644 women aged 35–65 years and 10,904 men aged 40–65 years), of whom 26,437 provided blood samples at baseline (23). Participants were recruited between 1994 and 1998 from the Potsdam area of Germany. At baseline, anthropometric and blood pressure measurements were taken, followed by a personal interview and a questionnaire on prevalent medical conditions and sociodemographic and lifestyle characteristics, including diet. Follow-up on incident diseases and lifestyle factors was conducted every 2–3 years, with response rates ranging between 90 and 96% per follow-up round (24). The study was conducted according to the Declaration of Helsinki and approved by the ethics committee of the State of Brandenburg, Germany. All participants provided written informed consent.
Analyses on incident type 2 diabetes and CVD were performed in a nested case-cohort setting for efficient molecular phenotyping. From all participants who provided blood at baseline (n = 26,437), a random sample (subcohort) (n = 2,500) was drawn, which served as a common reference population for both end points. For each end point, all incident cases that occurred in the full EPIC-Potsdam cohort until a specified censoring date (31 August 2005 for type 2 diabetes [n = 820, of which 74 cases were part of the subcohort] and 30 November 2006 for CVD [n = 508, of which 60 cases were part of the subcohort]) were included in the analysis. After exclusion of participants with prevalent conditions, who missed a follow-up, with insufficient plasma samples, or with missing glycan data, the case cohort for type 2 diabetes analyses comprised 2,804 participants, including 741 with diabetes (Supplementary Fig. 1), and the case cohort for CVD analyses consisted of 2,548 participants, including 417 with myocardial infarction or stroke (Supplementary Fig. 2). The median follow-up time was 6.5 (interquartile range [IQR] 6.0–8.6) years for diabetes and 8.3 (IQR 7.5–9.2) years for CVD. The Supplementary Methods provide case ascertainment procedures in EPIC-Potsdam.
Validation Studies
To externally validate the associations with type 2 diabetes, data from three nested case-control studies were used: 1) a study nested within the Finrisk cohort, including 38 participants with incident type 2 diabetes and 38 age- and sex-matched control subjects (6); 2) a study nested within the JUPITER trial (25), including 13 participants with incident type 2 diabetes and 52 matched control subjects (one-to-four matching on age, sex, and plate); and 3) a study nested within the TNT trial (26), including 51 participants with incident type 2 diabetes and 153 control subjects (one-to-three matching on age, sex, and plate). More details on the validation study populations are given in the Supplementary Methods.
Laboratory Analyses
All samples were randomized throughout the multiwell plates, and laboratory personnel were blinded to case status. IgG was isolated from individual plasma samples using 96-well protein G monolithic plates, eluted with 0.1 mol/L formic acid, and neutralized with 1 mol/L ammonium bicarbonate as previously described in detail (27). Prepared samples were stored at −20°C until ultraperformance liquid chromatography analysis on a Waters ACQUITY UPLC H-Class instrument (27). All chromatograms were separated in the same manner into 24 IgG-GPs, and the amount of glycans in each peak was expressed as the percentage of the total integrated area (Supplementary Fig. 3 and Supplementary Table 1). The Supplementary Methods provide a more detailed description of IgG glycoprofiling.
Plasma adiponectin was measured with a commercially available sandwich ELISA (LINCO Research). HDL and total cholesterol, triglycerides, hemoglobin A1c (HbA1c), and hs-CRP were measured using an automatic ADIVA 1650 analyzer (Siemens Medical Solutions) at the University of Tübingen.
Statistical Analyses
Participant characteristics are reported as median and IQR or percentages. Correlation analyses were performed using Spearman rank correlation. For prospective analyses, we z-standardized all glycans (mean 0, SD 1) to allow for comparison of the effect estimates.
We selected IgG-GPs significantly associated with each end point in mutually adjusted models with multiple fractional polynomials (MFPs), applying false discovery rate (FDR) correction. MFP-selected glycans were subsequently tested in confounder-adjusted Prentice-weighted Cox proportional hazards models. We combined all significantly associated MFP-selected glycans into end point–specific weighted IgG glycan scores for type 2 diabetes and CVD risk, using the standardized regression coefficients for the mutually adjusted IgG glycans from Cox model 1 as weights.
For type 2 diabetes and CVD end points, Cox model 1 was adjusted for age (strata variable) and sex, and model 2 was additionally adjusted for lifestyle and anthropometry (BMI, waist circumference, education [vocational training or lower, technical college, university], smoking status [never, former, current <20 cigarettes/day, current ≥20 cigarettes/day], alcohol intake [<6.0, 6.1–12.0, 12.1–24.0, 24.1–60.0, 60.1–96.0, >96.0 g/day], biking [<2.5, 2.5–4.9, ≥5 h/week], sports [≤4, >4 h/week], prevalent hypertension, and intake of aspirin, antihypertensive, or lipid-lowering drugs). Diabetes-specific main model 3 was model 2 additionally adjusted for estimated glomerular filtration rate calculated using the Chronic Kidney Disease Epidemiology Collaboration equation, total and HDL cholesterol, triglycerides, hs-CRP, and adiponectin. CVD-specific main model 3 was adjusted for the same confounder set as diabetes-specific main model 3 but additionally included HbA1c. In the secondary diabetes-specific analyses, we additionally adjusted the main model 3 for HbA1c and tested interactions on a multiplicative scale by creating cross-product terms with age, sex, BMI, and waist circumference applied on model 1. In the interaction analyses, a two-sided P < 0.05 denoted statistical significance. Moreover, we excluded participants with baseline HbA1c ≥6.5% (undiagnosed diabetes) in a sensitivity analysis and assessed potential nonlinearity between IgG glycans and cardiometabolic diseases with cubic splines in secondary analyses.
In addition to 24 directly measured IgG-GPs, 8 IgG glycosylation traits were derived and standardized (Supplementary Table 2): agalactosylation (G0), monogalactosylation (G1), digalactosylation (G2), asialylation (S0), monosialylation (S1), disialylation (S2), bisecting GlcNAc, and core fucosylation (CF). Derived traits were calculated as sums of glycan residuals with specific structural features. The associations between derived glycosylation traits and diabetes and CVD risk were tested individually in Cox models, correcting for confounders (as described above) and applying FDR correction. Finally, we compared the predictive performances of end point–specific IgG N-glycan–based and total plasma N-glycan–based scores (11).
For the external validation, the significant findings from EPIC-Potsdam, including diabetes-associated individual IgG-GPs, the diabetes-specific weighted IgG sum score, and derived IgG glycosylation traits, were tested in the Finrisk, TNT, and JUPITER studies. We applied conditional logistic regression to account for the matched case-control design, using age at recruitment and case-control pair matching ID as strata variables and type 2 diabetes case status as outcome. The TNT and JUPITER trials were additionally adjusted for the intervention arm. The weighted IgG glycan scores for type 2 diabetes risk in Finrisk, TNT, and JUPITER were created with weights from EPIC-Potsdam. Subsequently, we pooled risk estimates across four studies using a random-effects model, restricting analyses to external cohorts in a sensitivity analysis. Between-study heterogeneity was explored by τ2 and I2 statistics.
All statistical analyses were performed using SAS 9.4, Enterprise Guide 7.1 (SAS Institute) and R version 4.1.0 software. The Supplementary Methods provide a detailed list of SAS macros and R packages used.
Results
Participant Characteristics
EPIC-Potsdam participants with incident type 2 diabetes or CVD were, on average, more likely to be men, obese, and older; be on antihypertensive or lipid-lowering treatment; and have higher plasma total cholesterol, triglycerides, hs-CRP, and HbA1c concentrations at recruitment, while HDL cholesterol and adiponectin concentrations were lower compared with participants without incident diabetes or CVD (Table 1). Baseline characteristics of external validation samples are listed in Supplementary Table 3.
Table 1.
EPIC-Potsdam participant baseline characteristics
| Characteristic | Subcohort at T2D risk* (n = 2,127) | Incident T2D (n = 741) | Subcohort at CVD risk† (n = 2,175) | Incident CVD (n = 417) |
|---|---|---|---|---|
| Sociodemographic | ||||
| Age at recruitment, years | 49.1 (42.1–57.6) | 56.6 (50.0–60.9) | 49.3 (42.1–57.6) | 57.6 (51.8–61.8) |
| Women, % | 61 | 41 | 61 | 36 |
| BMI, kg/m2 | 25.4 (23.0–28.1) | 29.8 (27.2–32.8) | 25.6 (23.1–28.3) | 27.0 (24.7–29.9) |
| Obesity, % | 14 | 48 | 16 | 23 |
| Education, % | ||||
| Vocational training or lower | 37 | 45 | 37 | 38 |
| Technical college | 24 | 24 | 24 | 26 |
| University | 39 | 31 | 39 | 36 |
| Sports, h/week | 0 (0–1.5) | 0 (0–1.0) | 0 (0–1.5) | 0 (0–1.0) |
| Biking, h/week | 0.5 (0–2.5) | 0 (0–2.0) | 0.5 (0–2.5) | 0 (0–2.0) |
| Alcohol consumption, g/day | 8.61 (3.02–20.2) | 8.37 (2.94–20.9) | 8.65 (2.97–20.4) | 9.28 (2.47–24.1) |
| Smoking, % | ||||
| Never | 47 | 35 | 47 | 34 |
| Former | 32 | 44 | 32 | 32 |
| Current <20 cigarettes/day | 15 | 12 | 15 | 20 |
| Current ≥20 cigarettes/day | 6 | 9 | 6 | 14 |
| Prevalent hypertension, % | 47 | 79 | 47 | 73 |
| Prevalent hyperlipidemia, % | 5 | 10 | 4 | 7 |
| Antihypertensive treatment, % | 18 | 39 | 17 | 35 |
| Aspirin treatment, % | 9 | 13 | 8 | 10 |
| eGFR, mL/min/1.73 m2 | 91.4 (79.7–101) | 88.5 (77.1–99.0) | 91.6 (79.9–101) | 86.5 (75.7–97.6) |
| Biomarkers | ||||
| HbA1c | ||||
| % | 5.39 (5.12–5.73) | 6.12 (5.7–6.7) | 5.41 (5.12–5.76) | 5.65 (5.36–6.08) |
| mmol/mol | 35.4 (32.5–39.1) | 43.4 (38.8–49.7) | 35.6 (32.5–39.5) | 38.3 (35.1–43.0) |
| Total cholesterol, mg/dL | 203 (177–230) | 213 (187–241) | 203 (177–230) | 216 (190–243) |
| HDL, mg/dL | 54.7 (46.0–64.7) | 45.7 (39.0–52.8) | 54.6 (45.8–64.5) | 47.7 (40.7–59.5) |
| Triglycerides, mg/dL | 107 (75.6–159) | 170 (128–242) | 107 (76.3–161) | 140 (93.0–214) |
| hs-CRP, mg/dL | 0.07 (0.02–0.21) | 0.19 (0.07–0.42) | 0.07 (0.02–0.22) | 0.14 (0.05–0.34) |
| Adiponectin, μg/mL | 7.76 (5.55–11.0) | 5.47 (3.99–7.43) | 7.68 (5.51–11.0) | 6.80 (5.09–9.90) |
Data are median (IQR) unless otherwise indicated. eGFR, estimated glomerular filtration rate; T2D, type 2 diabetes.
Random subcohort at risk for T2D contains 64 incident diabetes cases, which are also included in the column with all incident T2D cases.
Random subcohort at risk for CVD contains 44 incident CVD cases, which are also included in the column with all incident CVD cases.
Supplementary Fig. 4 depicts correlation matrices of IgG glycans with cardiometabolic traits in the EPIC-Potsdam subcohort. Considering the mutually adjusted partial correlation structure of all individually measured IgG-GPs, IgG-GP2 and IgG-GP7, IgG-GP7 and IgG-GP12, IgG-GP9 and IgG-GP16, IgG-GP16 and IgG-GP18, and IgG-GP19 with IgG-GP24 showed the strongest positive pairwise correlations (Supplementary Fig. 5). The strongest negative correlations were between IgG-GP4 and IgG-GP14, IgG-GP9 and IgG-GP18, and IgG-GP14 and IgG-GP16.
Associations of IgG-GPs With Type 2 Diabetes Risk
The MFP selection yielded five independently diabetes-associated IgG-GPs in a mutually adjusted model. IgG-GP7, IgG-GP8, IgG-GP9, and IgG-GP19 were associated with lower and IgG-GP11 with higher type 2 diabetes risk (Table 2). The associations of these MFP-selected IgG-GPs with diabetes were attenuated after confounder adjustment in the mutual model (Table 2).
Table 2.
Associations between MFP-selected, mutually adjusted IgG glycans and incident type 2 diabetes in the EPIC-Potsdam cohort
| Type 2 diabetes in EPIC-Potsdam per SD, HR (95% CI) | |||
|---|---|---|---|
| Model 1 (n = 2,804) | Model 2 (n = 2,801) | Model 3 (n = 2,678) | |
| IgG-GP | |||
| IgG-GP7 | 0.84 (0.77–0.93) | 0.96 (0.87–1.07) | 0.99 (0.88–1.11) |
| IgG-GP8 | 0.81 (0.73–0.89) | 0.88 (0.78–1.00) | 0.88 (0.77–1.01) |
| IgG-GP9 | 0.83 (0.75–0.91) | 0.86 (0.77–0.96) | 0.89 (0.79–1.00) |
| IgG-GP11 | 1.24 (1.11–1.39) | 1.21 (1.08–1.36) | 1.13 (1.00–1.28) |
| IgG-GP19 | 0.85 (0.77–0.93) | 0.98 (0.88–1.09) | 1.04 (0.92–1.17) |
| IgG glycan score for type 2 diabetes risk | 1.49 (1.35–1.65) | 1.29 (1.15–1.44) | 1.21 (1.08–1.35) |
The shown IgG-GPs were independently associated with type 2 diabetes risk in MFP models (MFP selection based on model 1). The HRs of single IgG-GPs are mutually adjusted. The IgG glycan score for type 2 diabetes risk is a linear combination of the selected glycans, weighted by the regression coefficient from the mutually adjusted Cox model. Model 1 is adjusted for age (strata variable) and sex. Model 2 is additionally adjusted for education (three categories), smoking (four categories), alcohol intake (six categories), physical activity (sports, biking h/week), BMI, waist circumference, prevalent hypertension, antihypertensive and lipid-lowering drugs, and use of aspirin. Model 3 is model 2 adjusted for estimated glomerular filtration rate, total cholesterol, HDL, triglycerides, hs-CRP, and adiponectin. Boldface indicates significance after FDR correction.
We combined the MFP-selected IgG-GPs in a sum score for type 2 diabetes risk, using the mutually adjusted β-coefficients from Cox model 1 as weights: IgG glycan score for type 2 diabetes risk = −0.19 ∗ IgG-GP7 + 0.23 ∗ IgG-GP11 − 0.18 ∗ IgG-GP9 − 0.20 ∗ IgG-GP8 − 0.18 ∗ IgG-GP19.
This IgG glycan score for type 2 diabetes risk was associated with a higher relative diabetes risk per SD higher score in the fully adjusted model in EPIC-Potsdam (hazard ratio [HR] 1.21, 95% CI 1.08–1.36) (Table 2). In addition, after sex and age adjustment, the IgG glycan score for type 2 diabetes risk was cross-sectionally associated with adverse levels of anthropometric measures, blood pressure, hs-CRP, HbA1c, triglycerides, adiponectin, and HDL cholesterol (Supplementary Fig. 6A).
In sensitivity analyses, exclusion of participants with baseline HbA1c ≥6.5% (Supplementary Table 4) or additional adjustment in model 3 for HbA1c (Supplementary Table 5) did not substantially alter the IgG-GP-type 2 diabetes associations. No statistically significant effect modification by sex, BMI, abdominal adiposity, or age was detected for the association between the IgG glycan score for type 2 diabetes risk and diabetes risk (P > 0.05 for all interaction terms). The glycan score-type 2 diabetes association was robust against dropping single IgG-GPs from the score; leaving IgG-GP8 out resulted in the strongest attenuation of the diabetes risk association (Supplementary Fig. 6B). The associations of each individual IgG-GP (not adjusted for the other IgG-GPs) with type 2 diabetes risk are shown in Supplementary Fig. 7.
Associations of the Derived IgG Glycosylation Traits With Type 2 Diabetes
In secondary analyses, we examined the association of derived IgG glycan traits with type 2 diabetes risk. These glycan traits are based on the same structural features contributing to different glycan peaks and are, therefore, assumed to capture general characteristics of glycan biosynthesis and degradation. Among these traits, G0, S0, and bisecting GlcNAc were positively associated with diabetes incidence, while G1, G2, and S1 were inversely associated with diabetes risk after extensive adjustment and FDR correction in EPIC-Potsdam (Supplementary Table 6). After additional adjustment for HbA1c, only bisecting GlcNAc remained significantly associated with type 2 diabetes risk (Supplementary Table 6). Interaction analyses are provided in Supplementary Table 7.
External Validation of IgG-GP and Derived Traits Associations With Type 2 Diabetes Risk
We examined the external validity of the results of the EPIC-Potsdam discovery analyses. To this end, we replicated the statistically significant type 2 diabetes associations of the IgG-GPs, IgG glycan score for type 2 diabetes risk, and derived IgG glycan traits in subsamples of three independent studies (Finrisk, JUPITER, and TNT) with similar IgG glycan profiles and meta-analyzed the single-study estimates. The relative median abundances of IgG glycans in the EPIC-Potsdam, Finrisk, JUPITER, and TNT studies were comparable across all studies (Supplementary Fig. 8).
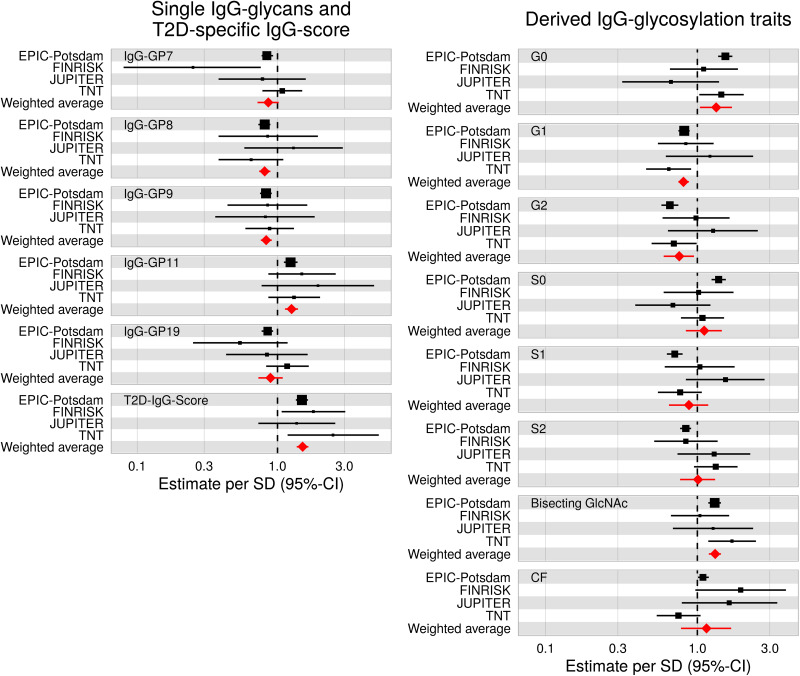
The point estimates for the diabetes risk association of EPIC-Potsdam–derived IgG glycan score for type 2 diabetes risk were as follows: odds ratio per SD 1.80 (95% CI 1.07–3.04) in Finrisk, 1.37 (0.73–2.58) in JUPITER, and 2.49 (1.18–5.26) in TNT. A meta-analysis of these single-study estimates resulted in a pooled estimate of 1.50 (1.37–1.64) (Fig. 1 and Supplementary Table 8). Among individual IgG-GPs, type 2 diabetes risk associations were generally consistent across all studies for IgG-GP8, IgG-GP9, and IgG-GP11, resulting in significant pooled risk estimates (Fig. 1 and Supplementary Table 8). Among the derived IgG glycan traits, bisecting GlcNAc (1.31 [1.19–1.43]), G0 (1.33 [1.04–1.69]), G1 (0.81 [0.75–0.88]), and G2 (0.76 [0.60–0.95]) were significantly associated with diabetes risk in the meta-analysis of the four underlying studies (Fig. 1 and Supplementary Table 8).
Figure 1.
External replication and meta-analyses of significantly type 2 diabetes–associated IgG N-glycans and derived IgG N-glycosylation traits. Type 2 diabetes–specific IgG N-glycans were first selected in the EPIC-Potsdam study using MFPs and combined into a weighted IgG glycan score for type 2 diabetes risk (T2D-IgG-Score) in EPIC-Potsdam. Subsequently, the glycans, traits, and T2D-IgG-Score were validated in Finrisk (38 incident type 2 diabetes cases, 38 normoglycemia cases), JUPITER (13 incident type 2 diabetes cases, 52 normoglycemia cases), and TNT (51 incident type 2 diabetes cases, 153 normoglycemia cases).
Associations of IgG-GPs and IgG Glycosylation Traits With CVD Risk
Our previous analysis of total plasma protein N-glycans revealed substantial sex differences of N-glycan-CVD risk associations (11). Therefore, we analyzed the associations between IgG N-glycans and CVD separately in men and women. For men, the MFP procedure selected IgG-GP19 and IgG-GP23 as CVD-associated IgG-GPs in the mutually adjusted, FDR-corrected model (Table 3). We combined IgG-GP19 and IgG-GP23 into a score weighted with the estimates from the Cox model 1: IgG glycan score for CVD risk = −0.49 ∗ IgG-GP23 + 0.24 ∗ IgG-GP19.
Table 3.
Associations between MFP-selected, mutually adjusted IgG N-glycans and incident CVD in the EPIC-Potsdam cohort
| CVD in EPIC-Potsdam per SD, HR (95% CI) | |||
|---|---|---|---|
| IgG-GP | Model 1 | Model 2 | Model 3 |
| Men, n | 1,074 | 1,070 | 981 |
| IgG-GP19 | 1.28 (1.09–1.49) | 1.20 (1.01–1.44) | 1.25 (1.04–1.51) |
| IgG-GP23 | 0.61 (0.51–0.74) | 0.70 (0.57–0.85) | 0.67 (0.55–0.83) |
| IgG glycan score for CVD risk | 1.60 (1.34–1.91) | 1.41 (1.17–1.71) | 1.47 (1.20–1.80) |
| Women, n | 1,474 | 1,473 | 1,369 |
| IgG-GP9 | 0.74 (0.61–0.89) | 0.79 (0.65–0.96) | 0.80 (0.65–0.98) |
The shown IgG-GPs were independently associated with CVD risk in sex-stratified MFP models (MFP selection based on model 1). The HRs of single IgG-GPs are mutually adjusted. The IgG glycan score for CVD risk in men is a linear combination of the selected glycans, weighted by the regression coefficient from the mutually adjusted Cox model. Model 1 is adjusted for age (strata variable) and sex. Model 2 is additionally adjusted for education (three categories), smoking (four categories), alcohol intake (six categories), physical activity (sports, biking h/week), BMI, waist circumference, prevalent hypertension, antihypertensive and lipid-lowering drugs, and use of aspirin. Model 3 is model 2 adjusted for estimated glomerular filtration rate, total cholesterol, HDL, triglycerides, hs-CRP, adiponectin, and HbA1c. Boldface indicates significance after FDR correction.
The IgG glycan score for CVD risk in men was associated with a 47% higher risk for incident CVD after full adjustment (HR per SD 1.47 [95% CI 1.20–1.80]) (Table 3). In women, IgG-GP9 was selected in the MFP procedure and was significantly inversely associated in the extensively confounder-adjusted model 3 (0.80 [0.65–0.98]) (Table 3). The sex-stratified associations of single IgG-GPs (not adjusted for the other IgG-GPs) are provided in Supplementary Fig. 9).
We also examined the sex-stratified CVD associations of the derived glycan traits. In men, G0, S0, and bisecting GlcNAc were positively and G2 and S1 inversely associated with CVD risk after extensive adjustment (FDR P < 0.05) (Supplementary Table 9). In women, G1 was nominally inversely associated with CVD incidence in model 3 (HR 0.82 [95% CI 0.68–0.99]) (Supplementary Table 9). The association was rendered statistically nonsignificant after multiple testing correction.
Type 2 Diabetes and CVD Risk Prediction With IgG N-Glycan Scores
In a previous study on plasma N-glycan–based cardiometabolic risk prediction (11), we constructed total plasma protein N-glycan–based risk sores to assess diabetes and CVD risk. Total plasma N-glycan profiles capture limited IgG glycosylation–derived signals but in a less specific and comprehensive manner than targeted IgG glycan profiles. We observed a weak correlation between the previously published total plasma N-glycan–based type 2 diabetes risk score and the herein-derived diabetes-related IgG N-glycan score (age-adjusted Spearman r = 0.20). Addition of the selected IgG N-glycans did not add to type 2 diabetes prediction with a clinical score or improve the accurate type 2 diabetes prediction with the total plasma N-glycans (Supplementary Table 10). The correlation between the previously published total plasma N-glycan–based CVD risk predictors and herein-selected CVD-related IgG-GPs was stronger (r = 0.50 in men, r = 0.70 in women), corresponding to a prominent role of IgG glycosylation–related total plasma GPs in CVD prediction (11). We found no indication that combination of IgG glycosylation with a clinical risk score and total plasma N-glycan data may improve CVD prediction (Supplementary Table 10).
Conclusions
In the prospective EPIC-Potsdam study, we derived a weighted score consisting of five diabetes-related IgG N-glycans (IgG-GP7, IgG-GP8, IgG-GP9, IgG-GP11, and IgG-GP19). The association of this IgG glycan score with higher type 2 diabetes risk was externally validated in three independent studies. In addition, we detected sex-specific IgG glycan associations with CVD incidence. A glycan score consisting of IgG-GP19 and IgG-GP23 was associated with higher CVD risk in men. In women, IgG-GP9 was inversely associated with CVD incidence. The type 2 diabetes– and CVD-specific risk associations were robust against confounder and clinical biomarker adjustment.
Previous cross-sectional studies on the associations between IgG N-glycosylation and type 2 diabetes provided heterogenous results (7,28–30), and large prospective analyses are lacking. We investigated the associations on different levels (individual IgG-GPs, weighted sum IgG glycan score, and calculated glycosylation traits). While the IgG glycan score for type 2 diabetes risk was robustly associated with diabetes incidence, even after exclusion of EPIC-Potsdam from the pooled analyses, individual glycans and derived traits showed more heterogeneity and lack of power. In addition to the lack of power in some of the studies, another possible explanation for this increased heterogeneity could be the different composition of the validation samples. While the EPIC-Potsdam and Finrisk samples were derived from population-based cohorts, JUPITER and TNT samples represented primary and secondary prevention trials in participants at high risk for CVD or with previous CVD.
In mice, hyposialylation of IgG was implicated in obesity-induced insulin resistance through activation of the endothelial FcγRIIB receptor, leading to impaired skeletal muscle glucose uptake caused by attenuated insulin transcytosis (5). In line with our findings, one cross-sectional study reported S0 and decreased galactosylation (IgG-GP8 [FA2[6]G1] and IgG-GP9 [FA2[3]G1]) and increased bisection of fucosylated IgG glycans (IgG-GP11 [FA2[3]BG1]) in patients with type 2 diabetes (7), which correspond to a proinflammatory IgG glycan profile. In our study, higher abundance of the corresponding IgG N-glycosylation traits (S0, G0, and bisecting GlcNAc) was associated with higher diabetes risk. Adjustment for baseline HbA1c levels attenuated these associations. However, HbA1c is a diagnostic marker for diabetes, and this attenuation may suggest a mediation of the potential link between these IgG glycosylation traits and diabetes risk by blood glucose.
For cardiovascular end points, previous prospective studies have focused on the relationships of total plasma protein N-glycans (11) or nuclear magnetic resonance–measured GlcNAc (31,32) with CVD risk. To our knowledge, we are the first to associate a comprehensive panel of 24 individual IgG-GPs and 8 derived IgG glycosylation traits with CVD incidence. A cross-sectional study demonstrated that a higher abundance of bisecting GlcNAc in the IgG N-glycome was positively associated with the presence of atherosclerotic plaques in carotid and femoral arteries, while sialylated glycans without a bisecting GlcNAc were negatively associated (9). Despite differences in the selected IgG glycans and distinct end points, both studies (EPIC-Potsdam and TwinsUK) consistently suggest similar underlying structures (e.g., galactosylated and sialylated fucosylated glycans without bisecting GlcNAc and of CF glycans with bisecting GlcNAc) as potential CVD risk factors.
Our sex-specific CVD analysis was informed by the evidence of sexual dimorphism in CVD (33) and our own observation of sex-specific associations of total plasma N-glycans with CVD risk (11). Several studies reported sex-specific modulation of glycosylation, possibly through sex hormones. For instance, IgG glycan sialylation appeared to be greater in women than in men (34), and increased G0 was associated with transition to menopause, whereas estrogens promoted galactosylation in both women and men (35).
Similar to the diabetes associations, S0, G0, and bisecting GlcNAc were positively associated with CVD risk, mainly in men. In addition, IgG-GP9 was inversely associated with CVD risk in women and with diabetes risk in both sexes. These findings suggest potential common etiological pathways that implicate IgG N-glycosylation in cardiometabolic disease development. One of these processes might be the activation of the endothelial IgG receptor FcγRIIB through Fc hyposialylation. In addition to its role in insulin resistance, IgG hyposialylation–sensitive endothelial FcγRIIB signaling may be critical for obesity-induced hypertension (5,34). Furthermore, increased IgG galactosylation promotes cooperative FcγRIIB signaling with dectin-1, suppressing the proinflammatory signaling of the C5aR and CXCR2 pathways (36), and IgG acquires anti-inflammatory properties upon Fc sialylation (13,15). Hence, experimental evidence suggests a potential direct involvement of IgG glycosylation in CVD development through atherosclerosis, inflammatory pathways, and cytotoxicity (9,13,34,37).
Addition of the selected IgG N-glycans did not further improve type 2 diabetes or CVD prediction beyond the already strong performance of the total N-glycan–based scores (11). Total plasma N-glycan profiling information appears to be sufficient to capture relevant glycan profiling information for cardiometabolic risk prediction. Therefore, our current findings are primarily of etiological interest, pointing toward a possible independent role of IgG glycosylation in the preclinical development of cardiometabolic diseases.
This study has several strengths and limitations. The associations between the IgG glycan score for type 2 diabetes risk and diabetes risk were validated in three independent studies. The associations in the smaller Finrisk and JUPITER studies were not statistically significant because of lack of statistical power. However, the meta-analysis of all four studies yielded a statistically significant pooled estimate of 1.5-fold higher relative risk per SD higher IgG glycan score for type 2 diabetes risk, with no indication of substantial between-study heterogeneity. The EPIC-Potsdam cohort consisted almost exclusively of Caucasian participants. Studies to examine the IgG glycosylation–related cardiometabolic risk in other ethnicities are warranted. Compared with the diabetes analyses, the power to detect CVD-associated IgG N-glycans was lower because of sex stratification and fewer incident cases, and external validation samples were unavailable. Additional studies on CVD-associated IgG N-glycans are needed to establish the generalizability of our results. Some of the IgG N-glycans were highly intercorrelated so that the selection of other, closely related IgG N-glycans as risk markers may have produced similar results. Because of the observational nature of our study, the etiological interpretation of our findings remains speculative.
In conclusion, our study suggests that IgG N-glycosylation may play a role in cardiometabolic disease etiology, possibly through its potent immunomodulatory functions. Modification of IgG N-glycosylation may alter the risk of incident type 2 diabetes and CVD. Further research into IgG glycosylation as a potential target for pharmacological or lifestyle-based cardiometabolic disease prevention is encouraged.
Article Information
Acknowledgments. The authors thank the Human Study Centre of the German Institute of Human Nutrition Potsdam-Rehbrücke, namely the trustee and the data hub for the processing, the participants for providing data, the biobank for the processing of the biological samples, and the head of the Human Study Centre Manuela Bergmann for contributing to the study design and leading the underlying processes of data generation.
Funding. The recruitment phase of the EPIC-Potsdam study was supported by the Federal Ministry of Science, Germany (EA 9401), and the European Union (05F02 SOC 95201408). The follow-up of the EPIC-Potsdam study was supported by German Cancer Aid (70-2488-Ha) and the European Community (05F02 SOC 98200769). This work was also supported by the German Ministry of Education and Research (BMBF) and the State of Brandenburg (grants 82DZD00302 and 82DZD03D03). C.W. was supported by the German Research Foundation (DFG) (individual fellowship WI5132/1-1) and the SciLifeLab & Wallenberg Data Driven Life Science Program (grant KAW 2020.0239). A.B. was supported by the German Research Foundation (DFG) (individual fellowship BI 2427/1-1). C.W. was supported with grant WI 5132/1-1, and A.B. was supported with grant BI 2427/1-1). S.M. was supported by the National Heart, Lung, and Blood Institute (grants R01 HL134811, HL117861, and K24 HL136852) and National Institute of Diabetes and Digestive and Kidney Diseases (grant DK112940). R.A.H. was funded by the Lemann Foundation. O.D. was supported by grant K01 HL135342 (NHLBI). IgG N-glycome analysis was performed in the Genos Glycoscience Research Laboratory and partly supported by the European Union’s Horizon 2020 (IM for FUTURE) (grant agreement 721815) and Horizon 2020 (GlySign) (grant agreement 722095), as well as by the European Structural and Investment Funds: Research and Development (IRI) (grant KK.01.2.1.01.0003); Centre of Competence (CEKOM) (grant KK.01.2.2.03.0006); and Croatian National Centre of Research Excellence in Personalized Healthcare (grant KK.01.1.1.01.0010).
Duality of Interest. G.L. is the founder and chief executive officer of Genos Ltd., a private research organization that specializes in high-throughput glycomics analysis and has several patents in this field. T.Š., I.T.-A., M.M., and A.C. are employees of Genos Ltd. M.P. is partly funded by the study FinnGen (https://www.finngen.fi), which is jointly funded by a Finnish governmental agency, Business Finland, and 13 international pharmaceutical companies: AbbVie; AstraZeneca; Biogen; Boehringer Ingelheim; Bristol-Myers Squibb; Genentech, a member of the Roche Group; GlaxoSmithKline; Janssen; Maze Therapeutics; MSD; Novartis; Pfizer; and Sanofi. S.M. has served as a consultant to Pfizer. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. A.B. performed the statistical analyses, interpreted the data, and drafted the manuscript. B.P., N.R., T.Š., I.T.-A., M.M., and A.C. were involved in laboratory measurements and processing of the glycomics data. F.E., O.K., C.S., and J.M. contributed to the statistical analyses, creation of graphs, and interpretation of data. R.A.H. performed replication analyses in JUPITER and TNT. Y.L. and O.D. contributed to the replication analyses. M.P. and S.M. provided data for validation cohorts. M.B.S., G.L., and C.W. designed the glycomics profiling study in the EPIC-Potsdam cohort and interpreted the data. All authors critically revised the manuscript. A.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the analyses.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.20670123.
G.L. and C.W. share senior authorship.
References
- 1. Freeze HH. Genetic defects in the human glycome. Nat Rev Genet 2006;7:537–551 [DOI] [PubMed] [Google Scholar]
- 2. Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell 2006;126:855–867 [DOI] [PubMed] [Google Scholar]
- 3. Ohtsubo K, Takamatsu S, Minowa MT, Yoshida A, Takeuchi M, Marth JD. Dietary and genetic control of glucose transporter 2 glycosylation promotes insulin secretion in suppressing diabetes. Cell 2005;123:1307–1321 [DOI] [PubMed] [Google Scholar]
- 4. Ohtsubo K, Chen MZ, Olefsky JM, Marth JD. Pathway to diabetes through attenuation of pancreatic beta cell glycosylation and glucose transport. Nat Med 2011;17:1067–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tanigaki K, Sacharidou A, Peng J, et al. Hyposialylated IgG activates endothelial IgG receptor FcγRIIB to promote obesity-induced insulin resistance. J Clin Invest 2018;128:309–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Keser T, Gornik I, Vučković F, et al. Increased plasma N-glycome complexity is associated with higher risk of type 2 diabetes. Diabetologia 2017;60:2352–2360 [DOI] [PubMed] [Google Scholar]
- 7. Lemmers RFH, Vilaj M, Urda D, et al. IgG glycan patterns are associated with type 2 diabetes in independent European populations. Biochim Biophys Acta, Gen Subj 2017;1861:2240–2249 [DOI] [PubMed] [Google Scholar]
- 8. Dotz V, Lemmers RFH, Reiding KR, et al. Plasma protein N-glycan signatures of type 2 diabetes. Biochim Biophys Acta, Gen Subj 2018;1862:2613–2622 [DOI] [PubMed] [Google Scholar]
- 9. Menni C, Gudelj I, Macdonald-Dunlop E, et al. Glycosylation profile of immunoglobulin G is cross-sectionally associated with cardiovascular disease risk score and subclinical atherosclerosis in two independent cohorts. Circ Res 2018;122:1555–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Joshi AA, Lerman JB, Aberra TM, et al. GlycA is a novel biomarker of inflammation and subclinical cardiovascular disease in psoriasis. Circ Res 2016;119:1242–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wittenbecher C, Štambuk T, Kuxhaus O, et al. Plasma N-glycans as emerging biomarkers of cardiometabolic risk: a prospective investigation in the EPIC-Potsdam cohort study. Diabetes Care 2020;43:661–668 [DOI] [PubMed] [Google Scholar]
- 12. Cvetko A, Mangino M, Tijardović M, et al. Plasma N-glycome shows continuous deterioration as the diagnosis of insulin resistance approaches. BMJ Open Diabetes Res Care 2021;9:e002263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science 2006;313:670–673 [DOI] [PubMed] [Google Scholar]
- 14. Anthony RM, Kobayashi T, Wermeling F, Ravetch JV. Intravenous gammaglobulin suppresses inflammation through a novel T(H)2 pathway. Nature 2011;475:110–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Quast I, Lünemann JD. Fc glycan-modulated immunoglobulin G effector functions. J Clin Immunol 2014;34(Suppl. 1):S51–S55 [DOI] [PubMed] [Google Scholar]
- 16. Sjögren J, Lood R, Nägeli A. On enzymatic remodeling of IgG glycosylation; unique tools with broad applications. Glycobiology 2020;30:254–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 1993;259:87–91 [DOI] [PubMed] [Google Scholar]
- 18. Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature 1997;389:610–614 [DOI] [PubMed] [Google Scholar]
- 19. Libby P. Inflammation in atherosclerosis. Nature 2002;420:868–874 [DOI] [PubMed] [Google Scholar]
- 20. Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol 2018;14:576–590 [DOI] [PubMed] [Google Scholar]
- 21. Greto VL, Cvetko A, Štambuk T, et al. Extensive weight loss reduces glycan age by altering IgG N-glycosylation. Int J Obes 2021;45:1521–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Plomp R, Ruhaak LR, Uh HW, et al. Subclass-specific IgG glycosylation is associated with markers of inflammation and metabolic health. Sci Rep 2017;7:12325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boeing H, Korfmann A, Bergmann MM. Recruitment procedures of EPIC-Germany. European Investigation into Cancer and Nutrition. Ann Nutr Metab 1999;43:205–215 [DOI] [PubMed] [Google Scholar]
- 24. Schienkiewitz A, Schulze MB, Hoffmann K, Kroke A, Boeing H. Body mass index history and risk of type 2 diabetes: results from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Am J Clin Nutr 2006;84:427–433 [DOI] [PubMed] [Google Scholar]
- 25. Ridker PM; JUPITER Study Group . Rosuvastatin in the primary prevention of cardiovascular disease among patients with low levels of low-density lipoprotein cholesterol and elevated high-sensitivity C-reactive protein: rationale and design of the JUPITER trial. Circulation 2003;108:2292–2297 [DOI] [PubMed] [Google Scholar]
- 26. LaRosa JC, Grundy SM, Waters DD, et al.; Treating to New Targets (TNT) Investigators . Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005;352:1425–1435 [DOI] [PubMed] [Google Scholar]
- 27. Pucić M, Knezević A, Vidic J, et al. High throughput isolation and glycosylation analysis of IgG-variability and heritability of the IgG glycome in three isolated human populations. Mol Cell Proteomics 2011;10:M111 010090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li X, Wang H, Russell A, et al. Type 2 diabetes mellitus is associated with the immunoglobulin G N-glycome through putative proinflammatory mechanisms in an Australian population. OMICS 2019;23:631–639 [DOI] [PubMed] [Google Scholar]
- 29. Wu Z, Li H, Liu D, et al. IgG glycosylation profile and the glycan score are associated with type 2 diabetes in independent Chinese populations: a case-control study. J Diabetes Res 2020;2020:5041346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ge S, Wang Y, Song M, et al. Type 2 diabetes mellitus: integrative analysis of multiomics data for biomarker discovery. OMICS 2018;22:514–523 [DOI] [PubMed] [Google Scholar]
- 31. Lawler PR, Akinkuolie AO, Chandler PD, et al. Circulating N-linked glycoprotein acetyls and longitudinal mortality risk. Circ Res 2016;118:1106–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Akinkuolie AO, Buring JE, Ridker PM, Mora S. A novel protein glycan biomarker and future cardiovascular disease events. J Am Heart Assoc 2014;3:e001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Colafella KMM, Denton KM. Sex-specific differences in hypertension and associated cardiovascular disease. Nat Rev Nephrol 2018;14:185–201 [DOI] [PubMed] [Google Scholar]
- 34. Peng J, Vongpatanasin W, Sacharidou A, et al. Supplementation with the sialic acid precursor N-acetyl-D-mannosamine breaks the link between obesity and hypertension. Circulation 2019;140:2005–2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ercan A, Kohrt WM, Cui J, et al. Estrogens regulate glycosylation of IgG in women and men. JCI Insight 2017;2:e89703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Karsten CM, Pandey MK, Figge J, et al. Anti-inflammatory activity of IgG1 mediated by Fc galactosylation and association of FcγRIIB and dectin-1. Nat Med 2012;18:1401–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Umaña P, Jean-Mairet J, Moudry R, Amstutz H, Bailey JE. Engineered glycoforms of an antineuroblastoma IgG1 with optimized antibody-dependent cellular cytotoxic activity. Nat Biotechnol 1999;17:176–180 [DOI] [PubMed] [Google Scholar]