Abstract
OBJECTIVE
To examine the effect of different patterns of durable glycemic control on the development of comorbidities among youth with type 2 diabetes (T2D) and to assess the impact of fasting glucose (FG) variability on the clinical course of T2D.
RESEARCH DESIGN AND METHODS
From the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) study, 457 participants (mean age, 14 years) with mean diabetes duration <2 years at entry and a minimum study follow-up of 10 years were included in these analyses. HbA1c, FG concentrations, and β-cell function estimates from oral glucose tolerance tests were measured longitudinally. Prevalence of comorbidities by glycemic control status after 10 years in the TODAY study was assessed.
RESULTS
Higher baseline HbA1c concentration, lower β-cell function, and maternal history of diabetes were strongly associated with loss of glycemic control in youth with T2D. Higher cumulative HbA1c concentration over 4 years and greater FG variability over a year within 3 years of diagnosis were related to higher prevalence of dyslipidemia, nephropathy, and retinopathy progression over the subsequent 10 years. A coefficient of variability in FG ≥8.3% predicted future loss of glycemic control and development of comorbidities.
CONCLUSIONS
Higher baseline HbA1c concentration and FG variability during year 1 accurately predicted youth with T2D who will experience metabolic decompensation and comorbidities. These values may be useful tools for clinicians when considering early intensification of therapy.
Maintaining blood glucose concentrations that approximate those of people without diabetes confers protection against long-term complications for those with diabetes (1–3). Even a transient period of glycemic control in the target range has long-lasting benefits via the so-called metabolic memory effect observed in the Diabetes Control and Complications Trial and other studies (4). More recent data indicate the magnitude of fasting blood glucose fluctuations or, more generally, glycemic variability may also influence the course of diabetes over and above that predicted by measures of average blood glucose concentration (5).
The Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) study population consists of >500 individuals with youth-onset type 2 diabetes (T2D) monitored for over a decade. Approximately 35% of participants maintained HbA1c <8.0% over the first 4 years while receiving metformin with or without rosiglitazone or intensive lifestyle intervention. This situation offered the opportunity to examine distinctions between those who maintained glycemic control over time and those who did not in a well-characterized cohort of young individuals with T2D (6). In a prior report, researchers found baseline measures of HbA1c and β-cell function were key predictors of durable glycemic control over 4 years (6).
The objective of the present analysis was to examine the development of long-term comorbidities in those exhibiting different patterns of glycemic control and to assess the impact of fasting glucose (FG) variability on the long-term clinical course of T2D in those diagnosed during youth. We hypothesized that the pattern of glycemic control over a 4-year period, based on HbA1c concentrations, would correlate with the prevalence of comorbidities and complications at 10 years. In addition, we posited that lower FG variability over the first year of the study would predict long-term durable control and lower incidence of complications.
Research Design and Methods
Study Design
The TODAY protocol (clinical trial reg. no. NCT00081328) and primary outcome results have been published (7–9). In brief, 699 participants with T2D diagnosed before the age of 18 years, with a duration of diabetes <2 years, BMI >85th percentile for age and sex, negative for islet cell antibodies, and C-peptide concentration >0.6 ng/mL were randomized at 15 participating diabetes centers to receive metformin alone, metformin plus rosiglitazone, or metformin plus an intensive lifestyle intervention program. TODAY participants were recruited over a 4-year period (2004–2009) and followed for a minimum of 2 years. The primary goal of the TODAY study (2004–2011 or study years 0–6) was to evaluate the effects of the three treatment arms on time to treatment failure, defined as loss of glycemic control (i.e., HbA1c ≥8% for six consecutive months or failure to wean from temporary insulin after acute metabolic decompensation).
In 2011, 572 TODAY participants (82%) enrolled in the TODAY2 postintervention follow-up study. Between 2011 and 2014 (study years 6–9), participants no longer received randomized treatment but continued to receive protocolized diabetes-related care from the TODAY study with visits at 3-month intervals. From 2014 to 2020 (study years 9–15), 518 TODAY participants (74% of original cohort) transitioned to community care and continued to be followed by the TODAY study group for annual observational visits. TODAY and TODAY2 were approved by institutional review boards at all 15 centers and all participants and guardians provided written informed assent and/or consent as appropriate for age and local guidelines.
Study Measures
Demographic data were collected at randomization (7). At each study visit, participants self-reported medical history and prescribed medication use (including antihypertensive and lipid-lowering medications); a physical examination was conducted and blood pressure, weight, height, and calculated BMI were obtained (7). Blood and spot urine samples were obtained after a 10- to 14-h overnight fast, were processed immediately according to standardized procedures, and shipped on dry ice for analysis at the TODAY central biochemical laboratory. HbA1c levels were assessed at every visit and FG, insulin, lipids, and serum cystatin C and creatinine levels, as well as urine albumin to creatinine ratio (UACR), were measured at least once at annual visits, as previously described (7). Measures of inflammatory markers (namely, concentrations of hs-CRP, interleukin-6, and tumor necrosis factor α) were collected annually through the end of study year 9 only. Estimated glomerular filtration rate was calculated using the Full Age Spectrum combined serum creatinine and cystatin C equation.
Oral glucose tolerance test (OGTT) results were obtained from all participants after a 10- to 14-h overnight fast at dedicated study visits, as previously described (10). Markers of insulin sensitivity (namely, 1/fasting insulin level [inverse insulin]), β-cell function (C-peptide index, defined as the ratio of the incremental C-peptide and glucose responses over the first 30 min of the OGTT test), and C-peptide oral disposition index (oDI) were calculated from OGTT data (11).
Standardized definitions were used for phenotyping throughout with longitudinal assessments of microalbuminuria, macroalbuminuria, hyperfiltration, hypertension, dyslipidemia, and neuropathy, as previously described (8,9). Fundus photography was performed twice (at study years 5–6 and 12,16) and graded according to the Early Treatment Diabetic Retinopathy Study (ETDRS) protocol by masked assessors at a centralized reading center (9). Retinopathy was defined as ETDRS grade ≥20 in either eye or clinically significant macular edema. A ≥3-step progression on the ETDRS scale was defined as retinopathy progression (9).
Samples for genetic analysis were genotyped on the Infinium genome-wide association study array by the Genetic Analysis Platform at the Broad Institute as part of the Progress in Diabetes Genetics in Youth consortium. Partnering tribal nations and the Indian Health Service elected not to participate in the genomics collection. Details on genotyping, imputation, and quality control steps have been previously reported (12). Polygenic risk scores were constructed for HOMA of β-cell function and fasting insulin by summing the number of risk alleles carried by each individual, weighted by the effect-size estimates from well-established genome-wide significant associations derived from the Meta-Analyses of Glucose and Insulin-Related Traits Consortium (MAGIC) Consortium (20081858, 22581228, 22885924) (13–15). Supplementary Table 1 lists the genetic variants, corresponding genes, and original genome-wide association study references for each score.
The occurrence of diabetes-related specific medical events was routinely documented during participant visits (in person or remote), and medical records were sought to verify all self-reported events. Medical records describing liver, pancreas, gallbladder, renal, kidney, eye, heart, vascular, or cerebrovascular disease, or reports of clinical neuropathy or nerve damage were obtained and centrally adjudicated by a review committee. Predetermined criteria were used to confirm the diagnosis of events (9).
Selection of Study Sample
Of the 699 participants originally enrolled in the TODAY study, 22 with monogenic diabetes mutations who were clinically diagnosed with T2D were excluded, along with five participants who chose to continue using insulin during TODAY and seven participants who started using insulin after the randomized treatment phase for a reason other than hyperglycemia (Supplementary Fig. 1). Beyond these exclusions, the study population was also restricted to participants followed for a minimum of 10 years to capture information on long-term outcomes. The duration of 10 years was chosen because it represents the average length of follow-up of TODAY participants in the entire study. The 457 participants included in the present study did not differ from the 242 excluded from the sample with respect to their baseline demographics.
Glycemic Control and Variability
For this analysis, glycemic control and variability were examined on the basis of two measures of glycemia: HbA1c and FG levels. First, the 457 participants were separated into three groups of glycemic control on the basis of HbA1c over the first 4 years in the study, as previously published (6). The first group remained in glycemic control (i.e., did not reach the primary outcome, defined as HbA1c ≥8% for six consecutive months) and had a stable HbA1c during that period (STABLE). Stable HbA1c was defined as a change in HbA1c from baseline to 4 years of <0.5%. The next group of participants also remained in glycemic control for at least 4 years but had an HbA1c value that increased ≥0.5% from baseline during that period (RISING). An increase of ≥0.5% in HbA1c is a predictor of glycemic failure (10). The final group reached glycemic failure within 4 years (Uncontrolled [UNC]). In additional analysis, the UNC group was further divided into Early-UNC, defined as reaching glycemic failure within year 1, and Late-UNC, defined as reaching glycemic failure after year 1.
Second, glycemic control was quantified on the basis of FG variability during the first year in the study, on the basis of recent literature describing FG variability as a more sensitive indicator of future comorbidity development than HbA1c in older adults with T2D (16,17). The FG coefficient of variation (FG-CV) was calculated as the ratio of the SD to the mean of the FG concentrations and is expressed as a percentage (i.e., the higher the percentage, the higher the variability around the mean). The mean number of FG measures (collected at baseline, month 6, and 12 during OGTTs, per study protocol) collected per participant in year 1 was 2.8.
Statistical Analysis
Baseline demographics and metabolic characteristics among the three glycemic control groups were compared using generalized linear models. Similar models were used to compare the clinical characteristics and diabetes-related complications and comorbidities among glycemic control groups (based on HbA1c or FG-CV) at year 10 in the study. For binary outcomes, the prevalence of the event (i.e., hypertension: yes or no) at the 10-year study visit is reported. For continuous variables, the mean or median value assessed at the 10-year visit is given, except for measures that were only collected through study year 9 (i.e., OGTT measures and levels of FG and inflammatory markers), in which case the year 9 value is presented. Variables not normally distributed were log transformed prior to testing. In addition to the HbA1c value at the 10-year visit, the time-weighted mean HbA1c (representing a measure of cumulative exposure) was computed by weighting each value by the time interval between measurements collected between randomization and study year 10. Separate generalized linear models were used to evaluate the association between the glycemic control groups and reported comorbidity medication use (i.e., antihypertensive and lipid-lowering medications) at select time points.
Receiver operating characteristic (ROC) curve analyses were performed to identify optimal cut points for FG-CV during study year 1 that predicted subsequent loss of glycemic control during study years 2–4 (18). For this analysis, participants who reached glycemic failure during the first year (Early-UNC) were excluded and those who experienced STABLE and RISING durable control were combined. The standard logistic regression model and the trapezoidal rule method were used to compute the total AUC and its associated 95% CI. The Youden index method (19) was used to select the optimal threshold point from the ROC curve. Similar ROC analysis was done using baseline HbA1c instead of FG-CV during year 1. All analyses were considered exploratory, and statistical significance was defined as P < 0.05.
Results
Baseline Participant Characteristics by Glycemic Control Status
Among the 457 participants, 153 (32.4%) were classified as having STABLE durable control (i.e., HbA1c change from baseline <0.5%), 71 (17.4%) as having RISING durable control (i.e., HbA1c change from baseline ≥0.5%), and 233 (50.2%) as UNC. At baseline, there were no differences by age, sex, race or ethnicity, birth weight, weight, or BMI among the three groups (Table 1). STABLE and RISING participants had a slightly shorter duration of diabetes (1.3 months) compared with the UNC group. STABLE participants were less likely to have a maternal history of diabetes compared with the other two groups (P < 0.007). UNC participants were more likely to have higher HbA1c, FG concentration and variability, and lower β-cell function (C-peptide index and C-peptide odI) compared with the two other groups at baseline (Table 1). Indices of insulin sensitivity (insulin inverse and fasting C-peptide level) did not differ across the three groups.
Table 1.
Baseline* demographics and metabolic characteristics of the TODAY participants by groups of glycemic control based on HbA1c (N = 457)
| Characteristic | Durable control: STABLE (n = 153; 32.4%) | Durable control: RISING (n = 71; 17.4%) | Glycemic failure/UNC (n = 233; 50.2%) | P value, STABLE vs. RISING | P value, STABLE vs. UNC | P value, RISING vs. UNC |
|---|---|---|---|---|---|---|
| Age at randomization (years) | 14.0 ± 1.9 | 13.7 ± 2.0 | 13.9 ± 2.1 | 0.36 | 0.71 | 0.50 |
| Duration of diabetes (months) | 7.2 ± 5.6 | 7.2 ± 6.1 | 8.5 ± 6.2 | 0.97 | 0.008 | 0.04 |
| Male sex (%) | 36.6 | 25.4 | 36.0 | 0.10 | 0.91 | 0.10 |
| Race/ethnicity (%) | ||||||
| Non-Hispanic Black | 33.3 | 25.4 | 40.8 | 0.42 | 0.61 | 0.62 |
| Hispanic | 37.9 | 46.5 | 36.9 | |||
| Non-Hispanic White | 20.9 | 18.3 | 15.0 | |||
| Other | 7.8 | 9.9 | 7.3 | |||
| Maternal history of diabetes (%) | 31.2 | 50.7 | 52.6 | 0.007 | <0.0001 | 0.79 |
| Birth weight category (%) | ||||||
| Small (<2,500 g) | 7.1 | 6.7 | 12.0 | 0.44 | 0.99 | 0.42 |
| Normal (2,500–4,000 g) | 74.3 | 82.2 | 65.1 | |||
| Large (>4,000 g) | 18.6 | 11.1 | 22.9 | |||
| Weight (kg) | 97.4 ± 23.1 | 94.8 ± 24.6 | 96.0 ± 26.4 | 0.46 | 0.59 | 0.71 |
| BMI (kg/m2) | 35.1 ± 7.8 | 35.2 ± 7.3 | 35.2 ± 7.9 | 0.99 | 0.90 | 0.93 |
| HbA1c (%) | 5.7 ± 0.6 | 5.7 ± 0.5 | 6.4 ± 0.8 | 0.73 | <0.0001 | <0.0001 |
| FG (mg/dL) | 99.1 ± 15.3 | 100.9 ± 15.9 | 120.7 ± 27.4 | 0.58 | <0.0001 | <0.0001 |
| FG-CV (%)† | 7.5 ± 7.8 | 8.7 ± 7.9 | 14.8 ± 12.2 | 0.45 | <0.0001 | <0.0001 |
| Fasting C-peptide (ng/mL)‡ | 3.5 [2.7–4.5] | 3.9 [2.7–4.8] | 3.7 [2.8–4.9] | 0.55 | 0.66 | 0.80 |
| Insulin inverse (× 102 mL/µU)‡ | 3.9 [2.5–5.7] | 3.9 [2.5–5.5] | 3.6 [2.6–5.3] | 0.94 | 0.56 | 0.52 |
| C-peptide index (× 102 ng/mL per mg/dL)‡ | 8.4 [5.5–14.0] | 7.9 [4.1–11.4] | 3.8 [2.5–6.8] | 0.44 | <0.0001 | <0.0001 |
| C-peptide oDI (× 102 mL/µU × ng/mL per mg/dL)‡ | 0.33 [0.20–0.52] | 0.31 [0.16–0.45] | 0.15 [0.08–0.30] | 0.59 | <0.0001 | <0.0001 |
Data are reported as mean ± SD, median [interquartile range], or percentage. The three groups of glycemic control are defined on the basis of HbA1c levels and HbA1c change within the first 4 years in the study. P values are from unadjusted generalized linear models examining pairwise differences in baseline characteristics across the three groups of glycemic control.
Baseline refers to the time of TODAY randomization.
The CV is defined as the ratio of the SD to the mean of the baseline FG values and is expressed as a percentage (i.e., the higher the percentage, the higher the variability around the mean).
Values log-transformed prior to testing to approximate normality.
Long-term Outcomes at Year 10 by Glycemic Control Status
At year 10, HbA1c and cumulative mean HbA1c (a measure of cumulative exposure) was significantly different across the three groups of glycemic control, with lower concentrations in the STABLE group, intermediate concentrations in the RISING group, and higher concentrations in the UNC group (Table 2). Additionally, mean FG concentration was lowest in the STABLE group compared with the other two groups, and indices of β-cell function were highest in STABLE and lowest in UNC. By year 10, the UNC group had lower levels of inverse insulin and fasting C-peptide compared with the other two groups. Neither HOMA of β-cell function nor insulin-resistance polygenic risk score was associated with differences in β-cell function or insulin sensitivity in TODAY (Supplementary Table 2).
Table 2.
Long-term outcomes of the TODAY participants at year 10 in the study by groups of glycemic control based on HbA1c
| Long-term outcomes at study year 10 | Durable control: STABLE (n = 153; 32.4%) | Durable control: RISING (n = 71; 17.4%) | Glycemic failure/UNC (n = 233; 50.2%) | P value, STABLE vs. RISING | P value, STABLE vs. UNC | P value, RISING vs. UNC |
|---|---|---|---|---|---|---|
| Obesity | ||||||
| BMI (kg/m2) | 37.7 ± 9.1 | 36.7 ± 8.0 | 34.8 ± 8.1 | 0.41 | 0.001 | 0.10 |
| Glycemic metabolism | ||||||
| HbA1c (%) | 7.8 ± 3.0 | 9.3 ± 2.5 | 10.8 ± 2.4 | 0.0001 | <0.0001 | <0.0001 |
| Cumulative mean HbA1c (%)* | 6.2 ± 1.1 | 7.6 ± 1.0 | 9.7 ± 1.5 | <0.0001 | <0.0001 | <0.0001 |
| FG (mg/dL) | 154.1 ± 78.3 | 210.3 ± 86.3 | 202.4 ± 84.5 | <0.0001 | <0.0001 | 0.48 |
| Fasting C-peptide (ng/mL)† | 3.1 [2.1–4.1] | 3.0 [1.7–3.8] | 1.6 [0.8–2.5] | 0.33 | <0.0001 | <0.0001 |
| Insulin inverse (× 102 mL/µU)† | 4.5 [3.0–6.6] | 4.4 [2.6–6.4] | 3.3 [1.8–6.4] | 0.61 | 0.0001 | 0.01 |
| C-peptide index (× 102 ng/mL per mg/dL)† | 3.8 [1.8–7.6] | 1.5 [0.6–2.9] | 0.9 [0.4–1.7] | <0.0001 | <0.0001 | 0.02 |
| C-peptide oDI (× 102 mL/µU × ng/mL per mg/dL)† | 0.17 [0.09–0.42] | 0.05 [0.02–0.10] | 0.03 [0.01–0.09] | <0.0001 | <0.0001 | 0.0008 |
| Comorbidities and complications | ||||||
| UACR (mg/g)† | 9 [4–20] | 10 [6–44] | 20 [9–57] | 0.02 | <0.0001 | 0.03 |
| UACR ≥30 mg/g (%) | 20.9 | 38.0 | 52.8 | 0.008 | <0.0001 | 0.03 |
| UACR ≥300 mg/g (%) | 2.6 | 7.0 | 12.0 | 0.13 | 0.003 | 0.24 |
| eGFR (mL/min/1.73 m2) | 121.6 ± 22.1 | 135.4 ± 29.8 | 138.0 ± 26.0 | 0.0002 | <0.0001 | 0.44 |
| Hyperfiltration (%) | 26.1 | 56.3 | 61.4 | <0.0001 | <0.0001 | 0.45 |
| SBP (mm Hg) | 120.5 ± 11.5 | 122.2 ± 15.5 | 120.1 ± 13.6 | 0.38 | 0.77 | 0.24 |
| DBP (mm Hg) | 74.4 ± 10.0 | 76.4 ± 11.3 | 75.4 ± 10.6 | 0.18 | 0.36 | 0.48 |
| Hypertension (%) | 53.6 | 64.8 | 63.1 | 0.12 | 0.06 | 0.79 |
| Total cholesterol (mg/dL) | 169.5 ± 40.3 | 177.5 ± 36.0 | 180.6 ± 39.8 | 0.15 | 0.007 | 0.57 |
| HDL cholesterol (mg/dL) | 45.0 ± 13.0 | 46.2 ± 15.7 | 45.9 ± 12.2 | 0.52 | 0.51 | 0.87 |
| LDL cholesterol (mg/dL) | 96.0 ± 32.5 | 101.0 ± 33.2 | 103.7 ± 30.7 | 0.27 | 0.02 | 0.52 |
| LDL dyslipidemia (%) | 17.0 | 21.1 | 30.0 | 0.46 | 0.004 | 0.15 |
| Triglycerides (mg/dL)† | 107 [73–167] | 139 [76–188] | 106 [74–187] | 0.15 | 0.43 | 0.35 |
| Triglyceride dyslipidemia (%) | 33.3 | 52.1 | 47.2 | 0.008 | 0.007 | 0.47 |
| Peripheral neuropathy (%) | 15.7 | 18.3 | 27.0 | 0.62 | 0.01 | 0.14 |
| Abnormal monofilament (%) | 1.3 | 1.4 | 6.9 | 0.95 | 0.02 | 0.11 |
| Any NPDR (%) | 18.8 | 23.5 | 65.5 | 0.42 | <0.0001 | <0.0001 |
| Retinopathy progression (3-step progression on the ETDRS scale) (%) | 4.5 | 9.3 | 44.5 | 0.24 | <0.0001 | <0.0001 |
| hs-CRP (mg/dL)† | 0.29 [0.14–0.66] | 0.42 [0.17–1.17] | 0.41 [0.18–0.81] | 0.08 | 0.03 | 0.84 |
| IL-6 (pg/mL)† | 1.9 [1.3–3.0] | 2.2 [1.5–3.4] | 2.2 [1.3–3.4] | 0.29 | 0.21 | 0.87 |
| TNF-α ≥5.6 pg/mL (%) | 18.3 | 27.1 | 21.6 | 0.13 | 0.43 | 0.34 |
Data are reported as mean ± SD, median [interquartile range], or percentage. P values are from unadjusted generalized linear models examining pairwise differences in the long-term outcomes at study year 10 across the three groups of glycemic control. DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; NPDR, nonproliferative diabetic retinopathy; SBP, systolic blood pressure.
Time-weighted mean HbA1c, a measure of cumulative exposure, was computed by weighting each value by the time interval between measurements collected between randomization and study year 10. Inflammatory markers, FG, and OGTT-derived measures (e.g., insulin sensitivity, C-peptide index) were collected through study year 9 (2014); for those measures, the last collected value was carried forward.
Values were log-transformed prior to testing to approximate normality.
Over 10 years of study participation, those in the STABLE group fared better than the UNC group for almost all measured comorbidities except inflammation markers, which were not different between groups (Table 2; Supplementary Table 3). In particular, the STABLE group had a significantly lower prevalence of nephropathy (UACR ≥300 mg/g: 2.6% in STABLE group vs. 12.0% in UNC group; P = 0.003), dyslipidemia, neuropathy, and retinopathy, where a three-step progression on the ETDRS scale was 4.5% vs. 44.5% in the STABLE and UNC groups, respectively (P < 0.0001). The RISING group had an intermediate prevalence between STABLE and UNC, with the exception of dyslipidemia and UACR ≥300 mg/g, where the RISING group was not significantly different from the UNC group. Significant differences across groups of glycemic control were unaffected when analyses were adjusted for the baseline value of the comorbidities, participant demographics (i.e., age, sex, race or ethnicity), and other traditional risk factors such as BMI, LDL cholesterol, blood pressure, and smoking (data not shown).
Differences in overall long-term comorbidity outcomes were reflected in the prescription of antihypertensive medication over time. The STABLE group always had significantly lower rates of antihypertensive medication prescription than the UNC group, and the RISING group was intermediate at all time points after baseline (Supplementary Fig. 2A). Similarly, those in the UNC group were more likely to be prescribed lipid-lowering agents by year 10 (Supplementary Fig. 2B).
Variability of FG and Risk of Glycemic Failure
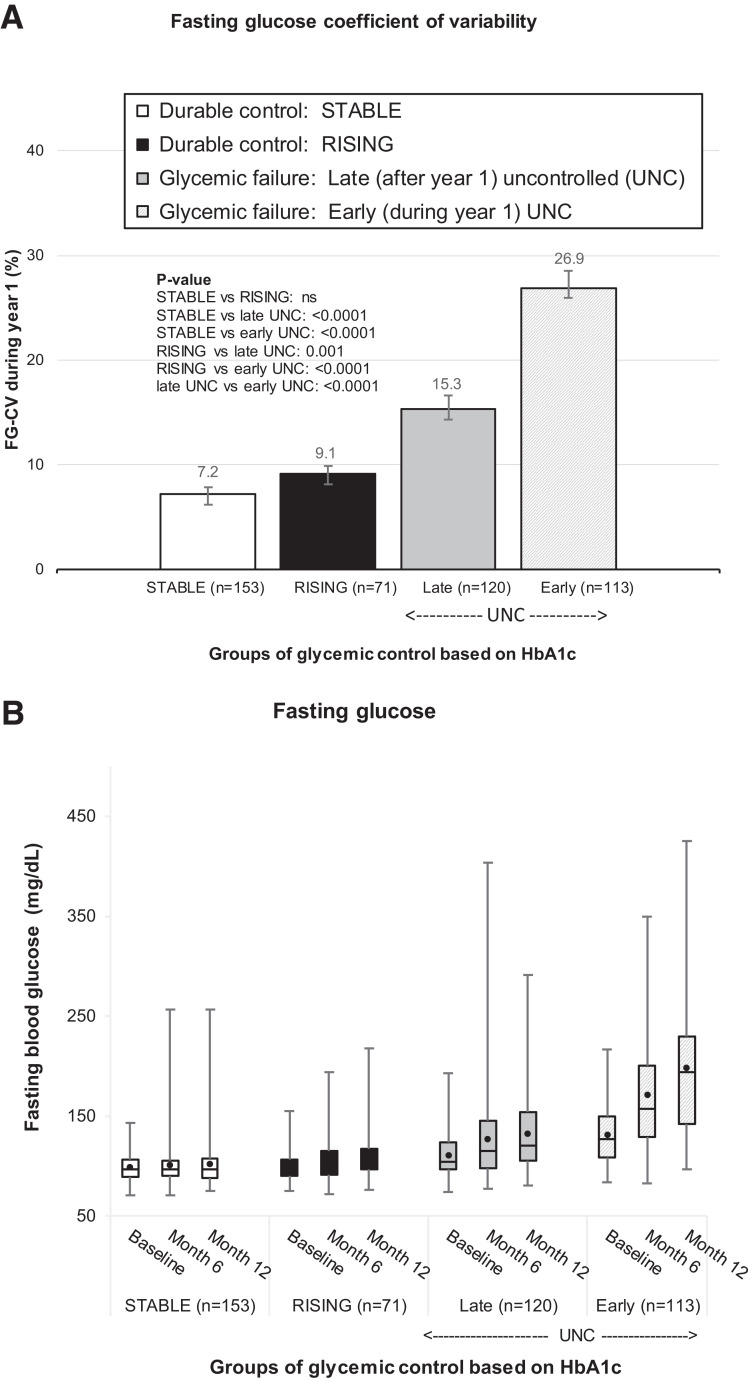
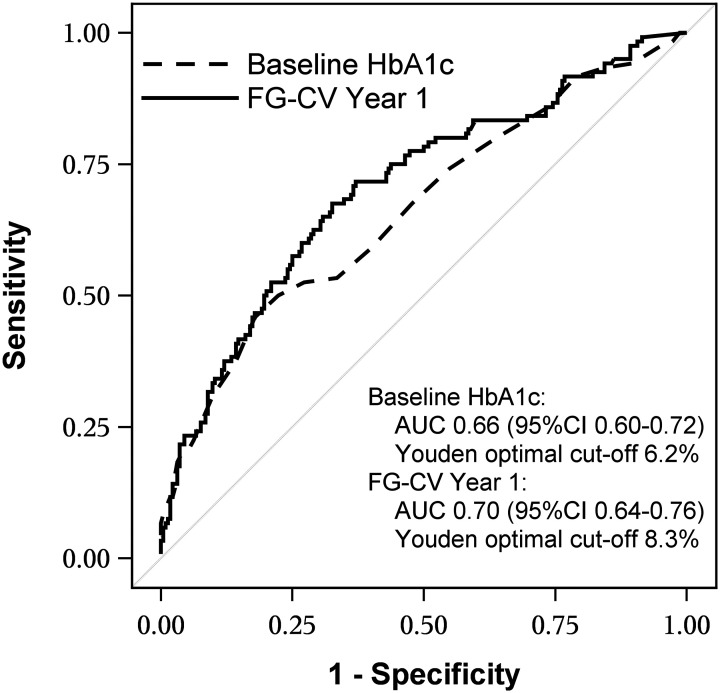
The variability in fasting blood glucose concentrations in year 1, as measured by FG-CV, was lowest in the STABLE and RISING groups and highest in the UNC group. STABLE and RISING FG-CV values were significantly different from UNC (all P < 0.001) but did not differ from each other (Fig. 1A). As expected, higher FG-CV was associated with increasing FG concentrations in the Early-UNC group (Fig. 1B), because these individuals, by definition, had HbA1c measures that exceeded 8.0% (Table 2). However, in STABLE, RISING, and Late-UNC, first year FG-CV was not a reflection of rising FG concentrations (Fig. 1B) but was strongly associated with predictive of loss of glycemic control in Late-UNC (P < 0.0001; AUC 0.70, 95% CI 0.64–0.76) (Fig. 2). The Youden index identified an FG-CV cutoff of 8.3% that maximized correct classification of participants, with sensitivity of 68% and specificity of 67%.
Figure 1.
FG-CV during year 1 ((A) and FG concentration levels at study baseline, month 6, and month 12 (B) by groups of glycemic control based on HbA1c values. The UNC group was further divided into Late-UNC) and Early-UNC. A: FG-CV (%) during year 1 (based on three FG values assessed at study baseline, month 6, and month 12) across the four groups of glycemic control based on HbA1c; P values for differences across the groups (STABLE [white bar], RISING [black bar], Late-UNC [solid grey bar], and Early-UNC [dashed grey bar]) are shown within panel A. ns, P > 0.05. B: A boxplot of FG levels at each visit during year 1 (study baseline, month 6, and month 12) when FG levels were assessed, across the four groups of glycemic control based on HbA1c.
Figure 2.
ROC curve for predicting glycemic failure as a function of baseline HbA1c and FG-CV during year 1. Durable control (RISING and STABLE) groups were combined, and the early UNC group that reached glycemic failure during year 1 was excluded. The AUC (95% CIs and Youden optimal cutoffs for baseline HbA1c and FG-CV for year 1 are shown in the figure. Baseline HbA1c, previously reported to be a strong predictor of glycemic failure (P < 0.0001), is shown for comparison (4).
Beyond prediction of glycemic failure, FG-CV during year 1, using the Youden index cutoff 8.3%, accurately and precisely predicted the likelihood of long-term comorbidities at study year 10, with high concordance to the HbA1c-based method that characterized the groups as STABLE, RISING, and UNC (Supplementary Table 4).
Conclusions
In this article, we provide the first evidence, to our knowledge, of a relationship between patterns of glycemic control with diabetic complications over a period of 10 years in a well-characterized sample of individuals with youth-onset T2D. Over 10 years of study participation, the UNC group had a high prevalence for all complications and comorbidities measured, particularly for nephropathy, dyslipidemia, neuropathy, and progressive retinopathy. Maintaining glycemic control over the first 4 years of the study (STABLE) was protective against these conditions at 10 years. These data are reflective of those found in adults with T2D (20), but the presence of complications was greater in our young cohort (Table 2). RISING glycemic control participants showed intermediate comorbidity prevalence between STABLE and UNC, affirming, after a longer follow-up period of 10 years, that glycemic control is a major determinant of long-term diabetic complications (1,21).
Progression to UNC has previously been attributed to β-cell failure rather than insulin resistance in this population (11). Interestingly, we show that distinctions in β-cell function categorized by glycemic control (STABLE, RISING, or UNC) at year 1 persist at 10 years, indicating that individual differences in β-cell function are maintained long term, even after diagnosis and treatment for T2D. Polygenic risk scores did not indicate a known genetic cause, because the aggregate genetic burden of known β-cell secretion and fasting insulin variants were not different among the groups. This is unsurprising given previous work in adults (22); however, it may be that this group of youth with T2D is too similar in phenotype to detect subtle differences in genetic burden.
A previous report in this study population showed that small increases in HbA1c concentration over time portend rapid decompensation and glycemic failure within 3–6 months (10). The rapid loss of glycemic control observed in the UNC group, accompanied by deteriorating β-cell function, led us to posit that FG variability, which should be lowest in those with highest β-cell function (23), would be associated with long-term glycemic control. By focusing on fasting blood glucose levels, these data primarily reflect the reduced ability of insulin to inhibit hepatic glucose output, either because of hepatic insulin resistance or, more likely based on our current data, β-cell dysfunction. Indeed, we demonstrate that greater FG variability in year 1, even with only two to three values, is highly predictive of glycemic failure in the subsequent 3 years. Additionally, our calculated Youden cutoff of 8.3% for the FG-CV concentration accurately predicts the incidence of future comorbidities in high concordance with glycemic control assessed by HbA1c concentration. After 10 years, based on HbA1c change over time, the most common comorbidities associated with loss of glycemic control are impaired renal function, triglyceride dyslipidemia, worsening retinopathy, and decreased quality of life. The same factors are highly significant when measured against FG variability, except for triglyceride dyslipidemia.
In adults with T2D, FG variability has been linked to the development of comorbidities and mortality, independent of mean HbA1c or glucose concentrations (16,17). When FG was examined over 5 years and comorbidities assessed at 10 years, higher FG variability was associated with a higher incidence of retinopathy and nephropathy (24,25). Our data in youth with T2D, using a similar study design, show analogous associations and also concur with data from a study in healthy young adults examined for cardiovascular complications over a 10-year interval (24). In the latter study, FG-CV for increased cardiovascular risk was 9.5%, which is comparable to the 8.3% cutoff in the present study.
Our analyses show that FG variability based on relatively few measures (2,3) is accurate and predictive of long-term complications as well as β-cell decompensation, with an estimated probability of glycemic failure and retinopathy progression for a year 1 FG-CV of 8.3% being approximately 31% and 20%, respectively. In the present study, FG variability was assessed only over year 1 of the study, so duration of diabetes and the period between FG assessments was short compared with all previous adult studies, which generally examined older adults with diabetes (16,26). As pointed out by Slieker et al. (24), these issues confound the relationship of FG variability with comorbidities because overall glucose variability increases over time.
Although HbA1c concentration and variability have been linked to development of comorbidities in adults (27–29) and children (10) with T2D, the extremely rapid decompensation in youth at a small change in HbA1c concentration (0.5%) may limit the clinical utility of this measure to intensify treatment before loss of glycemic control and the development of diabetes-related comorbidities. Diabetes clinical care visits are typically scheduled at 3-month intervals. Thus, using a modest increase in HbA1c concentration or variability to assess the risk of decompensation and future complications will likely fall short, because 84.4% of those who will reach glycemic failure will do so before their next clinical visit (10). FG variability may be less convenient to measure clinically than HbA1c. However, FG variability may be able to be detected before HbA1c increases and thus allow more time for clinical intervention to forestall the impending metabolic decompensation.
These analyses have important strengths, including the use of a well-characterized clinical cohort, use of standardized processes and techniques across sites, and a use of a central laboratory for all assays. A particular strength of this study is the well-defined duration of diagnosed diabetes in all participants at baseline and the short, defined interval for obtaining all FG measurements. In addition, we had a large sample size and a long follow-up period, sufficient to adequately assess the incidence of comorbidities. Moreover, we assessed a broad spectrum of diabetes-related comorbidities. However, assessment of medication was limited to prescribed medications only, without fulfillment or adherence data, and the calculated Youden cutoff for FG variability was not tested in an independent sample. Also, FG was measured in venous samples and the use of FG concentrations from clinic visits, blood glucose meters, or continuous glucose monitoring remains to be studied. Last, T2D is a complex metabolic disease affecting multiple systems involved in energy management and homeostasis. We have identified clear links between indices of glucose control early in the course of the disease and outcomes a decade later. However, the initiating events and conditions that may further define the course of disease remain to be determined.
These long-term data affirm previous reports, over shorter time periods, that higher baseline HbA1c concentration, lower β-cell function, and maternal history of diabetes are strongly associated with loss of glycemic control in youth with T2D (6,10). Additionally, both higher cumulative HbA1c concentration over 4 years and FG variability in year 1 are predictive of short- and long-term glycemic failure and development of a range of comorbidities, particularly dyslipidemia, nephropathy, and progressive retinopathy. Indeed, our study not only affirms the high rate of comorbidities observed in this population (9) but also shows that those at highest risk can be identified by assessment of β-cell function and glycemic variability. This suggests that more aggressive therapy is warranted for that group. SGLT-2 inhibitors and GLP-1 agonists have been shown to have organ-protective effects in adults with T2D (30–32); thus, these agents may play important therapeutic roles in youth-onset T2D in the future. Because determination of FG variability in the first year was predictive of a subsequent decline in glycemic control, this measure holds promise as a clinical decision-making tool for intensification of diabetes therapy for those at highest risk of rapid decompensation.
Article Information
Acknowledgments. The TODAY Study Group thanks the following companies for donations in support of the study’s efforts: Becton, Dickinson and Company; Bristol-Myers Squibb; Eli Lilly and Company; GlaxoSmithKline; LifeScan, Inc.; Pfizer; and Sanofi Aventis. The Group also gratefully acknowledges the participation and guidance of the American Indian partners associated with the clinical center located at the University of Oklahoma Health Sciences Center, including members of the Absentee Shawnee Tribe, Cherokee Nation, Chickasaw Nation, Choctaw Nation of Oklahoma, and Oklahoma City Area Indian Health Service. The opinions expressed in this article are those of the authors and do not necessarily reflect the views of the respective Tribes and the Indian Health Service.
Funding. This work was completed with funding from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and the NIH Office of the Director through grants U01-DK61212, U01-DK61230, U01-DK61239, U01-DK61242, and U01-DK61254.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The NIDDK project office was involved in all aspects of the study, including design and conduct; collection, management, analysis, and interpretation of the data; review and approval of the manuscript; and decision to submit the manuscript for publication.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. J.H. and S.D.C. designed the analyses and wrote the manuscript. L.E.G. conducted the statistical analyses, prepared the tables and figures, and wrote portions of the manuscript. E.I., M.M.K., M.D.M., S.M., M.S., S.S., and R.G.K. contributed to interpretation of data and reviewed, and edited the manuscript. L.E.G. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Data Availability. Data collected for the TODAY/TODAY2 Studies are available to the public through the NIDDK Repository (https://repository.niddk.nih.gov/studies/today/).
Appendix
TODAY Study Group Writing Committee. Janine Higgins (University of Colorado Anschutz Medical Campus, Aurora, CO), Steven D. Chernausek (University of Oklahoma Health Sciences Center, Oklahoma City, OK), Laure El ghormli (The Biostatistics Center, George Washington University, Rockville, MD), Elvira Isganaitis (Joslin Diabetes Center, Boston, MA), Megan M. Kelsey (University of Colorado Anschutz Medical Campus, Aurora, CO), Marsha D. Marcus (University of Pittsburgh School of Medicine, Pittsburgh, PA), Siripoom McKay (Baylor College of Medicine, Houston, TX), Maggie Siska (St. Louis University Health Sciences Center, St. Louis, MO), Shylaja Srinivasan (University of California, San Francisco, CA), and Rose Gubitosi-Klug (Case Western Reserve University, Cleveland, OH).
Footnotes
Clinical trial reg. no. NCT00081328, clinicaltrials.gov
This article contains supplementary material online at https://doi.org/10.2337/figshare.20732785.
Members of the TODAY Study Group Writing Committee are listed in the Appendix. A complete list of the TODAY Study Group members can be found in the supplementary material online.
Contributor Information
TODAY Study Group:
Janine Higgins, Steven D. Chernausek, Laure El Ghormli, Elvira Isganaitis, Megan M. Kelsey, Marsha D. Marcus, Siripoom McKay, Maggie Siska, Shylaja Srinivasan, and Rose Gubitosi-Klug
References
- 1. Nathan DM, Genuth S, Lachin J, et al.; Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 2. UK Prospective Diabetes Study (UKPDS) Group . Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 3. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–1589 [DOI] [PubMed] [Google Scholar]
- 4. Lachin JM, Nathan DM; DCCT/EDIC Research Group . Understanding metabolic memory: the prolonged influence of glycemia during the Diabetes Control and Complications Trial (DCCT) on future risks of complications during the Study of the Epidemiology of Diabetes Interventions and Complications (EDIC). Diabetes Care 2021;44:2216–2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ceriello A, Monnier L, Owens D. Glycaemic variability in diabetes: clinical and therapeutic implications. Lancet Diabetes Endocrinol 2019;7:221–230 [DOI] [PubMed] [Google Scholar]
- 6. Zeitler P, Hirst K, Copeland KC, et al.; TODAY Study Group . HbA1c after a short period of monotherapy with metformin identifies durable glycemic control among adolescents with type 2 diabetes. Diabetes Care 2015;38:2285–2292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zeitler P, Epstein L, Grey M, et al.; TODAY Study Group . Treatment options for type 2 diabetes in adolescents and youth: a study of the comparative efficacy of metformin alone or in combination with rosiglitazone or lifestyle intervention in adolescents with type 2 diabetes. Pediatr Diabetes 2007;8:74–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zeitler P, Hirst K, Pyle L, et al.; TODAY Study Group . A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med 2012;366:2247–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bjornstad P, Drews KL, Caprio S, et al.; TODAY Study Group . Long-term complications in youth-onset type 2 diabetes. N Engl J Med 2021;385:416–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zeitler P, El ghormli L, Arslanian S, et al. Deterioration of glycemic control in youth-onset type 2 diabetes: what are the early and late predictors? [published correction appears in J Clin Endocrinol Metab 2022:dgac465] J Clin Endocrinol Metab 2022;107:e3384–e3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. TODAY Study Group . Effects of metformin, metformin plus rosiglitazone, and metformin plus lifestyle on insulin sensitivity and β-cell function in TODAY. Diabetes Care 2013;36:1749–1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Srinivasan S, Chen L, Todd J, et al.; ProDiGY Consortium . The first genome-wide association study for type 2 diabetes in youth: the Progress in Diabetes Genetics in Youth (ProDiGY) Consortium. Diabetes 2021;70:996–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dupuis J, Langenberg C, Prokopenko I, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk [published correction appears in Nat Genet 2010;42:464]. Nat Genet 2010;4:105–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Manning AK, Hivert MF, Scott RA, et al. A genome-wide approach accounting for body mass index identifies genetic variants influencing fasting glycemic traits and insulin resistance. Nat Genet 2012;44:659–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Scott RA, Lagou V, Welch RP, et al. Large-scale association analyses identify new loci influencing glycemic traits and provide insight into the underlying biological pathways. Nat Genet 2012;44:991–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hirakawa Y, Arima H, Zoungas S, et al. Impact of visit-to-visit glycemic variability on the risks of macrovascular and microvascular events and all-cause mortality in type 2 diabetes: the ADVANCE trial. Diabetes Care 2014;37:2359–2365 [DOI] [PubMed] [Google Scholar]
- 17. Zinman B, Marso SP, Poulter NR, et al.; DEVOTE Study Group . Day-to-day fasting glycaemic variability in DEVOTE: associations with severe hypoglycaemia and cardiovascular outcomes (DEVOTE 2). Diabetologia 2018;61:48–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kumar R, Indrayan A. Receiver operating characteristic (ROC) curve for medical researchers. Indian Pediatr 2011;48:277–287 [DOI] [PubMed] [Google Scholar]
- 19. Youden WJ. Index for rating diagnostic tests. Cancer 1950;3:32–35 [DOI] [PubMed] [Google Scholar]
- 20. Laiteerapong N, Ham SA, Gao Y, et al. The legacy effect in type 2 diabetes: impact of early glycemic control on future complications (The Diabetes & Aging Study). Diabetes Care 2019;42:416–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. UK Prospective Diabetes Study (UKPDS) Group . Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854–865 [PubMed] [Google Scholar]
- 22. Hivert MF, Christophi CA, Franks PW, et al.; Diabetes Prevention Program Research Group . Lifestyle and metformin ameliorate insulin sensitivity independently of the genetic burden of established insulin resistance variants in diabetes prevention program participants. Diabetes 2016;65:520–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kramer CK, Choi H, Zinman B, Retnakaran R. Glycemic variability in patients with early type 2 diabetes: the impact of improvement in β-cell function. Diabetes Care 2014;37:1116–1123 [DOI] [PubMed] [Google Scholar]
- 24. Slieker RC, van der Heijden AAWH, Nijpels G, Elders PJM, ’t Hart LM, Beulens JWJ. Visit-to-visit variability of glycemia and vascular complications: the Hoorn Diabetes Care System cohort. Cardiovasc Diabetol 2019;18:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Feng W, Li Z, Guo W, et al. Association between fasting glucose variability in young adulthood and the progression of coronary artery calcification in middle age. Diabetes Care 2020;43:2574–2580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Muggeo M, Verlato G, Bonora E, et al. Long-term instability of fasting plasma glucose predicts mortality in elderly NIDDM patients: the Verona Diabetes Study. Diabetologia 1995;38:672–679 [DOI] [PubMed] [Google Scholar]
- 27. Orsi E, Solini A, Bonora E, et al.; Renal Insufficiency and Cardiovascular Events (RIACE) Study Group . Haemoglobin A1c variability is a strong, independent predictor of all-cause mortality in patients with type 2 diabetes. Diabetes Obes Metab 2018;20:1885–1893 [DOI] [PubMed] [Google Scholar]
- 28. Prentice JC, Pizer SD, Conlin PR. Identifying the independent effect of HbA1c variability on adverse health outcomes in patients with Type 2 diabetes. Diabet Med 2016;33:1640–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bonke FC, Donnachie E, Schneider A, Mehring M. Association of the average rate of change in HbA1c with severe adverse events: a longitudinal evaluation of audit data from the Bavarian Disease Management Program for patients with type 2 diabetes mellitus. Diabetologia 2016;59:286–293 [DOI] [PubMed] [Google Scholar]
- 30. Marso SP, Daniels GH, Brown-Frandsen K, et al.; LEADER Steering Committee; LEADER Trial Investigators . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gómez-Huelgas R, Gómez Peralta F, Rodríguez Mañas L, et al. Treatment of type 2 diabetes mellitus in elderly patients. Rev Clin Esp (Barc) 2018;218:74–88 [DOI] [PubMed] [Google Scholar]
- 32. Neal B, Perkovic V, Mahaffey KW, et al.; CANVAS Program Collaborative Group . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–657 [DOI] [PubMed] [Google Scholar]