Abstract
OBJECTIVE
We examined longitudinal associations of air pollution exposure, including fine particulate matter (PM2.5), nitrogen dioxide (NO2), and ozone (O3), with weight, BMI, waist circumference, fat mass, lean mass, and proportion fat mass in midlife women.
RESEARCH DESIGN AND METHODS
The study population included 1,654 White, Black, Chinese, and Japanese women from the Study of Women’s Health Across the Nation, with the baseline median age of 49.6 years, followed from 2000 to 2008. Annual air pollution exposures were assigned by linking residential addresses with hybrid estimates of air pollutant concentrations at 1-km2 resolution. Body size was measured, and body composition was measured using DXA at approximately annual visits. Linear mixed effects models were used to examine the associations between air pollution and body size and composition measures and whether these associations differed by physical activity.
RESULTS
After adjusting for potential confounders, an interquartile range increase in PM2.5 concentration (4.5 μg/m3) was associated with 4.53% (95% CI 3.85%, 5.22%) higher fat mass, 1.10% (95% CI 0.95%, 1.25%) higher proportion fat mass, and 0.39% (95% CI −0.77%, −0.01%) lower lean mass. Similar associations were also observed for NO2 and O3. Weaker associations of PM2.5 and NO2 with body composition were observed in participants who engaged in more physical activity.
CONCLUSIONS
Our analyses provide evidence that exposure to PM2.5, NO2, and O3, is adversely associated with body composition, including higher fat mass, higher proportional fat mass, and lower lean mass, highlighting their potential contribution to obesity.
Introduction
Obesity has tripled in prevalence over the previous few decades, making it a global public health problem (1). The health consequences of obesity are not trivial, including type 2 diabetes, cardiovascular disease, certain cancers, and mortality, so it is of great importance to identify modifiable risk factors for obesity. This information will help characterize the population at high risk and identify targets and strategies for prevention. Unhealthful diets and physical inactivity are well-established risk factors for obesity (2). The fast rise in obesity prevalence has paralleled the increasing exposure to environmental factors, emphasizing a plausible and potentially critical role for obesity risk factors with environmental origins (3,4).
Exposure to air pollution, including fine particulate matter (PM2.5), nitrogen dioxide (NO2), and ozone (O3), increases oxidative stress and adipose tissue inflammation, induces brown adipose tissue dysfunction, disrupts the hypothalamic-pituitary-adrenal axis, and activates peroxisome proliferator-activated receptors, all of which are tightly linked to obesity (5–10). Recent epidemiologic studies have reported associations between exposure to air pollution and obesity, although most evidence is limited to children (7). Notably, a majority of epidemiologic studies so far on this topic have focused on BMI. BMI does not differentiate between lean and fat body mass; however, body composition measurements obtained using DXA reveal more information on adiposity and better predict cardiometabolic disorders (11–13). Nevertheless, no study of which we are aware has examined the association between air pollution and body composition, except for two recent studies in the United Kingdom and Taiwan, which did examine the relationships between air pollution and body composition (fat percentage) (14,15). Importantly, the midlife is a period of rapid changes in body composition for women; gains in fat mass and declines in lean mass accelerate during this life stage (16). Therefore, the midlife may represent a period of vulnerability to obesity. However, to our knowledge, the associations between air pollution and body size and composition have never been explored in midlife women.
To inform the hypothesis that higher exposure to air pollution may adversely affect body size and composition during the critical midlife stage, we examined the associations of long-term exposure to air pollution, specifically estimates of annual concentrations of PM2.5, NO2, and O3, with repeated measures of body weight, waist circumference, BMI, and DXA-derived fat mass, lean mass, and proportion fat mass in the Study of Women’s Health Across the Nation (SWAN). We hypothesized that higher exposure to PM2.5, NO2, and O3 is adversely associated with body size and composition measures. Because physical activity is a well-established protective factor against adverse body size and composition, we additionally assessed effect modification by physical activity on the associations of air pollution with body size and composition.
Research Design and Methods
Study Population
SWAN is an ongoing prospective cohort study of midlife women, designed to investigate physiologic and psychosocial changes during the menopausal transition and their long-term health impacts (17). Between 1996 and 1997, 3,302 women age 42 to 52 years were enrolled in the study, all of whom had an intact uterus, at least one ovary, and at least one menstrual cycle and had not used hormone therapy in the previous 3 months. Participants were recruited from seven sites; each site enrolled White women and one site-specific racial/ethnic group, including Black women in Boston, Massachusetts, Pittsburgh, Pennsylvania, southeast Michigan, and Chicago, Illinois; Hispanic women in Newark, New Jersey; Chinese women in Oakland, California; and Japanese women in Los Angeles, California.
Air pollution data were available from 2000 (analysis baseline) to 2008 among 2,452 participants recruited from six sites, excluding the Boston site because of a lack of residential history to generate exposure data. Additionally, the Chicago and Newark sites did not assess body composition using the DXA instruments; therefore, 1,675 participants from the remaining four sites were eligible for the current analysis. Of these, we further excluded 21 participants who had no information on key covariates, leaving a final analytic sample of 1,654 women with 10,370 observations between 2000 and 2008. A flowchart of the analytic sample is shown in Supplementary Fig. 1. The institutional review board at each participating site approved the study protocol, and all participants provided written, signed informed consent at each study visit.
Air Pollution Exposure Measures
For each SWAN participant included in the current analysis, we assigned an annual average of the 24-h average PM2.5 concentration, annual average of the daily 1-h maximum NO2 concentration, and annual average of the daily 8-h maximum O3 concentration for each calendar year according to their residential history from 2000 to 2008. Residential addresses were geocoded to the latitude and longitude coordinates and then linked to the air pollution data. Daily ambient PM2.5, NO2, and O3 concentrations were estimated at a 1-km2 spatial resolution based on the ensemble of three machine learning models (neural network, random forest, and gradient boosting) with >100 predictor variables, including satellite variables, land-use variables, meteorologic variables, chemical transport model–stimulated variables, and other ancillary variables. The ensemble models were shown to have outstanding predictive performance, with an average 10-fold cross-validated coefficient of determination (R2) for annual predictions of 0.89 for PM2.5, 0.84 for NO2, and 0.86 for O3. More information about exposure models can be found elsewhere in detail (18–20).
Body Size and Composition
Body size outcomes, including weight (kg), waist circumference (cm), and BMI (kg/m2), and body composition outcomes, including fat mass (kg), lean mass (kg), and proportion fat mass (%), were measured at each SWAN visit using a standardized protocol across all study sites. Body size was measured in light clothing and without shoes. Height was measured to the nearest 0.01 m, and weight was measured to the nearest 0.1 kg using calibrated scales and stadiometers. BMI was calculated as weight (kg) divided by the square of height (m). Waist circumference was measured to the nearest 0.1 cm using inelastic tape at the narrowest part of the torso. Fat and lean mass were measured by DXA using Hologic instruments (Hologic, Inc.) following strict quality assurance and calibration methods that have been described previously (16). Proportion fat mass was calculated as fat mass divided by the sum of fat and lean mass as a percentage.
Covariates
Demographic characteristics, including age, self-defined race/ethnicity (White, Black, Chinese, or Japanese), education (high school or less, some college, or college degree or higher), and financial hardship, were assessed through a self-administered questionnaire. Financial hardship was categorized as very hard, somewhat hard, or not hard at all based on the response to the following question: “How hard is it for you to pay for the very basics like food, housing, medical care, and heating?” Smoking status (never smoker, former smoker, or current smoker), exposure to secondhand smoke (0, <5, or ≥5 h per week), alcohol drinking (fewer than one drink per month, more than one drink per month, one or fewer drinks per week, or more than one drink per week), physical activity, and menopausal status (premenopausal, early perimenopausal, late perimenopausal, postmenopausal, or unknown because of hormone therapy use) were assessed through standardized interviews. Exposure to secondhand smoking was based on total person-hours of exposure at home, work, and other social settings (21). A modified version of the Kaiser Physical Activity Survey was used to quantify physical activity during the past year by two domains, including sports/exercise and daily living (22). Domain-specific indices were derived by averaging the ordinal responses to questions in each domain, resulting in values from 1 to 5. Thus, the physical activity score ranged from 2 to 10, with 10 indicating the highest level of activity. The physical activity score was not measured at two follow-up visits, so we imputed the value by computing the mean score for the visits preceding and following the unmeasured visit. Premenopausal was defined as having menstruation in the past 3 months with no change in bleeding regularity, early perimenopausal was having menstruation in the past 3 months but with decreasing regularity between menses, late perimenopausal was no menstruation in past 3 to 11 months, and postmenopausal was no menstruation in past 12 or more months. Total energy intake (kCal/day) was determined based on information collected from a thorough semiquantitative food frequency questionnaire adapted from the Block Food Frequency Questionnaire (23).
Statistical Analysis
Distributions of participants’ characteristics were examined at the study baseline (2000). Distributions of annual air pollution concentrations were examined by year from 2000 to 2008. Correlations between PM2.5, NO2, and O3 were also calculated.
We used linear mixed effects models to examine the associations of annual air pollution concentrations with repeated measures of body size and composition. Weight, waist circumference, BMI, fat mass, and lean mass were log-transformed, given their right-skewed distributions, and effect estimates were then back-transformed and interpreted as percent change in each outcome associated with an interquartile range (IQR) increase in air pollution concentration. We did not apply log-transformation to proportion fat mass, because it was normally distributed. The covariates adjusted in the models were selected based on a priori knowledge and included the non–time-varying variables of age (baseline), race/ethnicity, study site, and education; time-varying variables included follow-up time, financial hardship, smoking status, exposure to secondhand smoke, alcohol drinking, physical activity, total energy intake, and menopausal status. Random intercepts were included to account for intraparticipant correlations of repeated outcome measures.
To examine the effect modification of physical activity on the associations of air pollution with body size and composition, we incorporated multiplicative interaction terms between air pollutants and physical activity score in the linear mixed effects models. The physical activity score was treated as a continuous variable, and the effects of air pollution were calculated at the 25th percentile, median, and 75th percentile of the physical activity score.
In secondary analyses, we examined whether exposure to air pollution affected the longitudinal trajectories of body size and composition. We first used generalized additive mixed models with penalized splines to model the trajectories of body size and composition during follow-up. The smoothing curves (Supplementary Fig. 2) suggested linear trajectories for all body size and composition measures. We then fitted the linear mixed effects models with interaction terms between follow-up time and air pollution to explore whether air pollution exposures were associated with the rate of longitudinal changes in body size and composition. We also examined the associations between air pollution and body size and composition stratified by racial/ethnic group. To control for the potential confounding by study site in the stratification analysis by racial/ethnic group, we further examined the associations between air pollution and body size and composition among White women who were available in all sites and stratified by site.
Two sensitivity analyses were performed to test the robustness of our results. First, we examined the health effects of exposure to O3 during warm seasons by averaging daily maximum 8-h concentrations from May to October (24). Second, we additionally adjusted for quadratic term of age at baseline, country of birth (United States vs. foreign born), occupation, marital status, parity, stress, and sleep disturbance symptoms. All analyses were conducted using R (version 4.0.3) (www.R-project.org).
Results
The study population of 1,654 women had a median age of 49.6 years; most were never smokers, not exposed to secondhand smoke, and premenopausal at baseline (Table 1). Approximately half of the women were White (47.8%), 23.2% were Black, 13.9% were Chinese, and 15.1% were Japanese. The median (IQR) body size and composition measures at baseline were 69.7 (58.4, 85.0) kg for weight, 26.3 (22.7, 32.0) kg/m2 for BMI, 83.0 (74.1, 96.6) cm for waist circumference, 25.7 (19.0, 35.4) kg for fat mass, 37.5 (33.4, 43.1) kg for lean mass, and 40.9% (36.0%, 45.8%) for proportion fat mass. The median (IQR) annual PM2.5 concentration ranged from 12.3 (11.3, 14.0) μg/m3 (2008) to 15.9 (12.1, 17.6) μg/m3 (2001), the median (IQR) NO2 ranged from 28.0 (27.1, 32.1) parts per billion (ppb) (2007) to 37.3 (30.7, 43.3) ppb (2000), and the median (IQR) O3 ranged from 33.3 (28.4, 34.7) ppb (2000) to 37.2 (34.9, 38.2) ppb (2007) (Supplementary Table 1 and Supplementary Fig. 3). Japanese women in Los Angeles had the highest air pollution exposures, and Chinese women in Oakland had the lowest concentrations (Supplementary Table 2). PM2.5, NO2, and O3 were positively correlated, with the strongest correlation observed between PM2.5 and NO2 (r = 0.79) (Supplementary Fig. 4).
Table 1.
Characteristics of the study population at the baseline analysis (N = 1,654)
| Characteristic | Median (IQR) or n (%) |
|---|---|
| Age, years | 49.6 (47.4, 51.7) |
| Race/ethnicity | |
| White | 791 (47.8) |
| Black | 384 (23.2) |
| Chinese | 230 (13.9) |
| Japanese | 249 (15.1) |
| Study site | |
| Michigan | 430 (26.0) |
| Oakland | 408 (24.7) |
| Los Angeles | 436 (26.4) |
| Pittsburgh | 380 (23.0) |
| Education | |
| High school or less | 364 (22.0) |
| Some college | 579 (35.0) |
| College and above | 711 (43.0) |
| Financial hardship | |
| Not hard | 1,115 (67.4) |
| Somewhat hard | 432 (26.1) |
| Very hard | 107 (6.5) |
| Smoking status | |
| Never smoker | 1,004 (60.7) |
| Former smoker | 434 (26.2) |
| Current smoker | 216 (13.1) |
| Secondhand smoking, h/week | |
| 0 | 959 (58.0) |
| <5 | 367 (22.2) |
| ≥5 | 328 (19.8) |
| Alcohol drinking | |
| ≤1 drink/month | 917 (55.4) |
| >1 drink/month and ≤1/week | 395 (23.9) |
| >1 drink/week | 342 (20.7) |
| Menopausal status | |
| Premenopausal | 181 (10.9) |
| Early perimenopausal | 797 (48.2) |
| Late perimenopausal | 143 (8.7) |
| Postmenopausal | 272 (16.4) |
| Unknown* | 261 (15.8) |
| Physical activity score | 5.0 (4.0, 6.0) |
| Total energy intake, kCal/day | 1,703 (1,331, 2,210) |
| Weight, kg | 69.7 (58.4, 85.0) |
| BMI, kg/m2 | 26.3 (22.7, 32.0) |
| Waist circumference, cm | 83.0 (74.1, 96.6) |
| Fat mass, kg | 25.7 (19.0, 35.4) |
| Lean mass, kg | 37.5 (33.4, 43.1) |
| Proportion fat mass, % | 40.9 (36.0, 45.8) |
Menopausal status unknown because of hormone therapy or hysterectomy.
Associations between air pollution concentration and body size and composition measures are presented in Table 2. Effect estimates are summarized as percent changes in outcome measures per IQR increase in PM2.5 (4.5 μg/m3), NO2 (9.5 ppb), and O3 (5.0 ppb) concentrations. After adjusting for confounders, PM2.5 and NO2 were positively associated with fat mass and proportional fat mass and inversely associated with lean mass. Higher O3 was associated with higher proportional fat mass and lower lean mass. Except for waist circumference, which was inversely associated with O3, none of the other body size or composition measures changed significantly relative to changes in all pollutant concentrations.
Table 2.
Percent change in body size and composition measures per IQR increase in air pollution concentration
| PM2.5 (4.5 μg/m3) | NO2 (9.5 ppb) | O3 (5.0 ppb) | |
|---|---|---|---|
| Weight | 0.08 (−0.23, 0.39) | −0.30 (−0.62, 0.03) | −0.10 (−0.35, 0.15) |
| BMI | 0.06 (−0.24, 0.37) | −0.27 (−0.60, 0.05) | −0.18 (−0.43, 0.07) |
| Waist circumference | 0.08 (−0.20, 0.36) | −0.05 (−0.34, 0.25) | −0.28 (−0.51, −0.05) |
| Fat mass | 4.53 (3.85, 5.22) | 3.39 (2.68, 4.10) | 0.05 (−0.47, 0.58) |
| Lean mass | −0.39 (−0.77, −0.01) | −0.86 (−1.26, −0.45) | −0.95 (−1.25, −0.64) |
| Proportion fat mass | 1.10 (0.95, 1.25) | 0.94 (0.78, 1.10) | 0.25 (0.13, 0.37) |
Effect estimates are summarized as percent changes in outcome measures per IQR increase in PM2.5 (4.5 μg/m3), NO2 (9.5 ppb), and O3 (5.0 ppb) concentrations. All models were adjusted for age at baseline, follow-up time, race/ethnicity, study site, education, financial hardship, smoking, exposure to secondhand smoke, alcohol drinking, physical activity, total energy intake, and menopausal status. Bold font indicates P < 0.05.
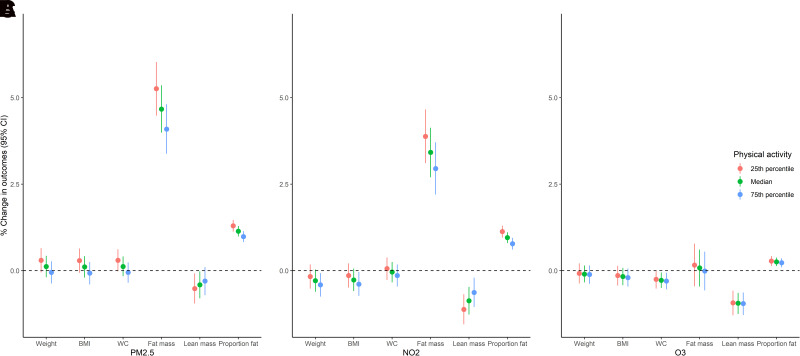
Figure 1 and Supplementary Table 3 show the effect modification by physical activity on the associations between air pollution and body size and composition. There was significant effect modification by physical activity in the associations of PM2.5 with weight (P for interaction = 0.008), BMI (P for interaction = 0.006), waist circumference (P for interaction = 0.004), fat mass (P for interaction <0.0001), and proportion fat mass (P for interaction <0.0001). The effect of PM2.5 was stronger among women with low levels of physical activity as compared with women with high levels. The positive associations of NO2 with fat mass (P for interaction = 0.001) and proportion fat mass (P for interaction <0.0001) and inverse association between NO2 and lean mass (P for interaction = 0.003) were also stronger in participants with lower physical activity scores. No significant differences in the associations between O3 and body size and composition measures were observed by physical activity.
Figure 1.
Associations between air pollution and body size and composition measures at the 25th percentile, median, and 75th percentile of physical activity score. Effect estimates are summarized as percent changes in outcome measures per IQR increase in PM2.5 (4.5 μg/m3) (A), NO2 (9.5 ppb) (B), and O3 (5.0 ppb) (C) concentrations. All models were adjusted for age at baseline, follow-up time, race/ethnicity, study site, education, financial hardship, smoking, exposure to secondhand smoke, alcohol drinking, physical activity score, interaction terms between study site and physical activity score, total energy intake, and menopausal status. WC, waist circumference.
In the secondary analysis examining the rate of change in body size and composition, we observed that BMI, waist circumference, fat mass, and proportion fat mass increased with time, whereas lean mass decreased (Supplementary Fig. 2). Higher levels of PM2.5 and NO2 were associated with slightly faster increases in waist circumference and slower increases in fat mass and proportion fat mass. O3 was associated with slightly faster increases in body size measures and a slower increase in fat mass and proportion fat mass (Supplementary Table 4).
In the secondary analysis stratifying the associations by racial/ethnic group, we found stronger positive associations of PM2.5 and NO2 with fat mass and proportion fat mass in Japanese women in Los Angeles (Supplementary Table 5). We also observed stronger positive associations of PM2.5 and NO2 with waist circumference and inverse associations with lean mass in Black women. Additionally, PM2.5 was positively associated with weight and waist circumference in Chinese women in Oakland, and O3 was inversely associated with fat mass in Japanese women in Los Angeles. We further evaluated whether the stratified associations by racial/ethnic group were confounded by study site by examining the associations among White women at each site (Supplementary Table 6). Stronger associations of PM2.5 and NO2 with waist circumference, fat mass, lean mass, and proportion fat mass were observed in White women at the Michigan site.
In sensitivity analyses, warm-season O3 was positively associated with proportional fat mass and inversely associated with lean mass, as observed in the primary analysis, whereas the effect estimates were attenuated (Supplementary Table 7). Additional adjustments for quadratic term of age at baseline, country of birth, occupation, marital status, parity, stress, and sleep disturbance symptoms did not alter the associations (Supplementary Table 8).
Conclusions
In this prospective cohort study of 1,654 midlife women representing diverse racial/ethnic groups, exposure to air pollution was associated with adverse changes in body composition measures. In particular, PM2.5 and NO2 were positively associated with fat mass and proportion fat mass and inversely associated with lean mass. In addition, O3 was positively associated with proportion fat mass and inversely associated with lean mass. Associations of PM2.5 and NO2 with body size and composition were modified by physical activity; associations were attenuated among participants with higher physical activity levels.
The adverse changes in body composition may have substantial health consequences, even in women within the normal BMI range. For example, a recent study using data from the Women’s Health Initiative reported a positive association between fat mass and cardiovascular disease risk among postmenopausal women with normal BMI (25). In the National Health and Nutrition Examination Survey, a higher ratio of fat to lean mass was associated with increased prevalence of abnormal blood glucose in adults age ≥40 years who were in the normal BMI range (26). The associations detected between air pollution and the DXA-based body composition measures provide insight into the potential role of air pollution in cardiometabolic disorders that may not be reflected by body size changes like weight and BMI. Associations of air pollution with fat and lean mass found in opposite directions might also shed light on why no associations were found for body size measures.
Only a few studies have examined the association between air pollution and body composition. Furlong and Klimentidis (14) recently observed positive but not statistically significant associations of PM2.5 and NO2 with proportion body fat in a large U.K. prospective cohort study of ∼20,000 participants age 40 to 69 years during an average of 4.4 years of follow-up. Most participants in this study were obese or overweight at the time of enrollment; therefore, the associations could have been underestimated, because they were mainly captured among those who were less vulnerable to the effects of air pollution (i.e., selective survival). A cross-sectional study of 429 overweight and obese Black and Latino youth from the Childhood Obesity Research Center Air Study reported no relationship between PM2.5 or NO2 and body fat mass (27). Like our study, a recent cross-sectional study of 530 elderly individuals age ≥65 years in Taiwan also found that an increase in PM2.5 was associated with higher body fat mass and lower skeletal muscle mass (15).
The association between air pollution and body composition is biologically plausible and supported by evidence from animal and mechanistic studies. In animal models, exposure to PM2.5 enhanced the expression of genes involved in adipocyte differentiation, lipogenesis, and lipolysis, resulting in larger adipocytes and higher visceral fat mass (5,6). In the same mouse models, PM2.5 boosted macrophage infiltration into adipose tissue, increased production of proinflammatory cytokines, disrupted adipokine profiles, and induced insulin resistance (5,6), all of which may enhance adipose disposition and further limit muscle synthesis and exacerbate muscle atrophy (28,29). Air pollution also impairs brown adipose tissue function through disruption of mitochondrial uncoupling protein 1 and affects brown adipose development through tumor necrosis factor-α–mediated apoptosis and inflammation, and brown adipose tissue function is essential in fatty acid metabolism and energy expenditure (30–32). Recent animal studies showed that exposure to PM2.5 and O3 induced hypothalamic-pituitary-adrenal axis dysfunction, leading to elevated glucocorticoid levels, and adipocyte glucocorticoid receptor activation contributes to obesity by promoting the expression of adipogenic transcription factors (8). Finally, some components of PM, such as polyaromatic hydrocarbons and peroxisome proliferator-activated receptors, are known to be involved in adipogenesis, inflammation, and lipid metabolism (10,33). Future studies examining the associations between air pollution and intermediate metabolic traits and biomarkers in human populations are required to elucidate the underlying biologic mechanisms.
Physical inactivity is a major factor accounting for the obesity epidemic, and it is a modifiable factor frequently targeted for prevention and intervention (34). Nevertheless, it should be noted that greater physical activity, when undertaken outdoors, can lead to higher exposure to air pollution. Therefore, the interaction between air pollution and physical activity on obesity is less well understood. In our study, the associations between PM2.5 and body size measures were attenuated in participants with higher physical activity levels, which is consistent with the recent findings of a large cross-sectional study in China (35). Our study also observed weaker adverse effects of PM2.5 and NO2 on body composition in participants with more intense physical activity, which was in accordance with the findings from two other studies. In a cross-sectional study in Taiwan, both a greater loss of muscle mass and an increase in fat mass associated with PM2.5 were observed in elderly individuals who were less active (15). Another cross-sectional study using data from the Behavioral Risk Factor Surveillance System and the Environmental Quality Index across all U.S. counties revealed that the relationship between air pollution and obesity was exacerbated among more physically inactive populations (36). Similar effect modifications by physical activity have also been reported between air pollution and obesity-related health outcomes, such as diabetes (37) and cardiovascular disease (38). These findings support the current view on air pollution, physical activity, and health that the benefits of physical exercise, particularly in terms of obesity and related health outcomes, may exceed the risks associated with air pollution exposure. Existing evidence indicates that physical activity reduces systemic inflammation, especially in obese individuals with high levels of inflammatory biomarkers (39). Nonetheless, to confirm our findings, additional prospective cohort studies in different populations with a broader range of air pollution concentrations or already performed physical activity intervention trials with obesity and air pollution data are required.
This study had several major strengths, including a large cohort of midlife women followed for up to 9 years with repeated measures of body size and composition and high-resolution air pollution data, which provided good statistical power to detect differences and permitted assessment of temporal relation of exposure and outcomes. The DXA-quantified body composition also offered additional insights into the potential effects of air pollution on adiposity that are difficult to capture by summary measures, such as weight or BMI. However, because SWAN is a community-based study and therefore not population based, the findings may not be generalizable to women in the general population of the United States or other Asian ethnic groups (e.g., Vietnamese, Indian, Korean, and Filipino). Additionally, although we adjusted for several known confounders, we cannot rule out unknown residual confounding, such as family history of obesity. Finally, only total body fat mass was measured using DXA, and information on regional body fat was not available. Recent studies suggested that regional body fat may better predict cardiovascular disease risk (25). Therefore, it is critically important to examine the effects of air pollution on regional fat in future research.
In summary, our analysis provides evidence that exposure to air pollution, including PM2.5, NO2, and O3, is adversely associated with body composition, including higher fat mass, higher proportional fat mass, and lower lean mass. The opposite effects on fat and lean mass may explain the nonassociation between air pollution and weight or BMI in the current study. BMI as a proxy for adiposity is frequently challenged for its inadequate ability to discriminate between fat and lean mass, because weight includes fat and lean mass, both of which can vary and contribute to distinct elements of cardiometabolic risk (11). Therefore, our findings suggest that air pollution may contribute to midlife women’s adiposity through its impact on fat and lean mass changes. Finally, we found that the adverse effects of air pollution on body composition could be mitigated by higher physical activity levels. More large prospective studies are required to confirm our findings.
Article Information
Acknowledgments. The authors thank the study staff at each site and all the women who participated in SWAN.
Funding. SWAN has grant support from the National Institutes of Health (NIH), Department of Health and Human Services, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR), and the NIH Office of Research on Women’s Health (ORWH) (grants U01NR004061, U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495, and U19AG063720). This study was also supported by grants from the National Institute of Environmental Health Sciences (R01-ES026578 and P30-ES017885) and the Centers for Disease Control and Prevention/National Institute for Occupational Safety and Health (T42-OH008455).
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH, or NIH.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. X.Wa. designed the study, conducted data analysis, and wrote the manuscript. C.A.K.-G., E.B.G., C.D., G.G., X.Wu, J.S., and S.K.P. were involved in the design of the analysis plan, contributed to interpretation of the data, and critically revised the manuscript. All authors read and approved the final version of the manuscript. X.Wa. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Appendix
Clinical Centers. University of Michigan, Ann Arbor, MI: Carrie A. Karvonen-Gutierrez, principal investigator (PI) 2021 to present; Siobán Harlow, PI 2011 to 2021; and MaryFran Sowers, PI 1994 to 2011. Massachusetts General Hospital, Boston, MA: Sherri-Ann Burnett-Bowie, PI 2020 to present; Joel Finkelstein, PI 1999 to 2020; and Robert Neer, PI 1994 to 1999. Rush University, Rush University Medical Center, Chicago, IL: Imke Janssen, PI 2020 to present, Howard Kravitz, PI 2009 to 2020; and Lynda Powell, PI 1994 to 2009. University of California Davis/Kaiser, Davis, CA: Elaine Waetjen and Monique Hedderson, PIs 2020 to present, and Ellen Gold, PI 1994 to 2020. University of California Los Angeles, Los Angeles, CA: Arun Karlamangla, PI 2020 to present; Gail Greendale, PI 1994 to 2020. Albert Einstein College of Medicine, Bronx, NY: Carol Derby, PI 2011 to present; Rachel Wildman, PI 2010 to 2011; and Nanette Santoro, PI 2004 to 2010. University of Medicine and Dentistry–New Jersey Medical School, Newark, NJ: Gerson Weiss, PI 1994 to 2004. University of Pittsburgh, Pittsburgh, PA: Rebecca Thurston, PI 2020 to present, and Karen Matthews, PI 1994 to 2020.
NIH Program Office. National Institute on Aging, Bethesda, MD: Rosaly Correa-de-Araujo, 2020 to present; Chhanda Dutta, 2016 to present; Winifred Rossi, 2012 to 2016; Sherry Sherman, 1994 to 2012; and Marcia Ory, 1994 to 2001. National Institute of Nursing Research, Bethesda, MD: program officers.
Central Laboratory. University of Michigan, Ann Arbor, MI: Daniel McConnell (Central Ligand Assay Satellite Services).
SWAN Repository. University of Michigan, Ann Arbor, MI: Siobán Harlow, 2013 to present; Dan McConnell, 2011 to 2013; and MaryFran Sowers, 2000 to 2011.
Coordinating Center. University of Pittsburgh, Pittsburgh, PA: Maria Mori Brooks, PI 2012 to present, and Kim Sutton-Tyrrell, PI 2001 to 2012. New England Research Institutes, Watertown, MA: Sonja McKinlay, PI 1995 to 2001.
Steering Committee. Susan Johnson, current chair, and Chris Gallagher, former chair.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.20633310.
References
- 1. NCD Risk Factor Collaboration (NCD-RisC) . Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017;390:2627–2642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology 2007;132:2087–2102 [DOI] [PubMed] [Google Scholar]
- 3. Wang X, Mukherjee B, Park SK. Associations of cumulative exposure to heavy metal mixtures with obesity and its comorbidities among U.S. adults in NHANES 2003–2014. Environ Int 2018;121:683–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ding N, Karvonen-Gutierrez CA, Herman WH, Calafat AM, Mukherjee B, Park SK. Perfluoroalkyl and polyfluoroalkyl substances and body size and composition trajectories in midlife women: the study of women’s health across the nation 1999–2018. Int J Obes (Lond) 2021;45:1937–1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sun Q, Yue P, Deiuliis JA, et al. Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation 2009;119:538–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mendez R, Zheng Z, Fan Z, Rajagopalan S, Sun Q, Zhang K. Exposure to fine airborne particulate matter induces macrophage infiltration, unfolded protein response, and lipid deposition in white adipose tissue. Am J Transl Res 2013;5:224–234 [PMC free article] [PubMed] [Google Scholar]
- 7. Seo MY, Kim SH, Park MJ. Air pollution and childhood obesity. Clin Exp Pediatr 2020;63:382–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thomson EM. Neurobehavioral and metabolic impacts of inhaled pollutants: a role for the hypothalamic-pituitary-adrenal axis? Endocr Disruptors (Austin) 2014;1:e27489 [Google Scholar]
- 9. Di Gregorio I, Busiello RA, Burgos Aceves MA, Lepretti M, Paolella G, Lionetti L. Environmental pollutants effect on brown adipose tissue. Front Physiol 2019;9:1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sone H, Ito T, Win-Shwe TT, Miki M, Fujitani Y, Nakajima D. Hazard evaluation of air pollution by using the key characteristics approach. In IOP Conference Series: Earth and Environmental Science, 2020, Bristol, United Kingdom, IOP Publishing, 2020 [Google Scholar]
- 11. Wells JC. Commentary: The paradox of body mass index in obesity assessment: not a good index of adiposity, but not a bad index of cardio-metabolic risk. Int J Epidemiol 2014;43:672–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clifton PM. Relationship between changes in fat and lean depots following weight loss and changes in cardiovascular disease risk markers. J Am Heart Assoc 2018;7:e008675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kalyani RR, Metter EJ, Xue Q-L, et al. The relationship of lean body mass with aging to the development of diabetes. J Endocr Soc 2020;4:bvaa043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Furlong MA, Klimentidis YC. Associations of air pollution with obesity and body fat percentage, and modification by polygenic risk score for BMI in the UK Biobank. Environ Res 2020;185:109364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen CH, Huang LY, Lee KY, et al. Effects of PM2.5 on skeletal muscle mass and body fat mass of the elderly in Taipei, Taiwan. Sci Rep 2019;9:11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Greendale GA, Sternfeld B, Huang M, et al. Changes in body composition and weight during the menopause transition. JCI Insight 2019;4:e124865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sowers MR, Crawford SL, Sternfeld B, et al. SWAN: a multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In Menopause: Biology and Pathology. Lobo RA, Kelsey J, Marcus R, Eds. San Diego, CA, Academic Press, 2000, pp. 175–188 [Google Scholar]
- 18. Di Q, Amini H, Shi L, et al. An ensemble-based model of PM2.5 concentration across the contiguous United States with high spatiotemporal resolution. Environ Int 2019;130:104909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Di Q, Amini H, Shi L, et al. Assessing no2 concentration and model uncertainty with high spatiotemporal resolution across the contiguous United States using ensemble model averaging. Environ Sci Technol 2020;54:1372–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Requia WJ, Di Q, Silvern R, et al. An ensemble learning approach for estimating high spatiotemporal resolution of ground-level ozone in the contiguous United States. Environ Sci Technol 2020;54:11037–11047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Coghlin J, Hammond SK, Gann PH. Development of epidemiologic tools for measuring environmental tobacco smoke exposure. Am J Epidemiol 1989;130:696–704 [DOI] [PubMed] [Google Scholar]
- 22. Sternfeld B, Cauley J, Harlow S, Liu G, Lee M. Assessment of physical activity with a single global question in a large, multiethnic sample of midlife women. Am J Epidemiol 2000;152:678–687 [DOI] [PubMed] [Google Scholar]
- 23. Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol 1986;124:453–469 [DOI] [PubMed] [Google Scholar]
- 24. Shi L, Steenland K, Li H, et al. A national cohort study (2000–2018) of long-term air pollution exposure and incident dementia in older adults in the United States. Nat Commun 2021;12:6754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen GC, Arthur R, Iyengar NM, et al. Association between regional body fat and cardiovascular disease risk among postmenopausal women with normal body mass index. Eur Heart J 2019;40:2849–2855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jo A, Mainous AG 3rd. Informational value of percent body fat with body mass index for the risk of abnormal blood glucose: a nationally representative cross-sectional study. BMJ Open 2018;8:e019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Toledo-Corral CM, Alderete TL, Habre R, et al. Effects of air pollution exposure on glucose metabolism in Los Angeles minority children. Pediatr Obes 2018;13:54–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schaap LA, Pluijm SMF, Deeg DJH, Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med 2006;119:526.e9–526.e17 [DOI] [PubMed] [Google Scholar]
- 29. Choi KM. Sarcopenia and sarcopenic obesity. Korean J Intern Med (Korean Assoc Intern Med) 2016;31:1054–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xu Z, Xu X, Zhong M, et al. Ambient particulate air pollution induces oxidative stress and alterations of mitochondria and gene expression in brown and white adipose tissues. Part Fibre Toxicol 2011;8:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xu X, Yavar Z, Verdin M, et al. Effect of early particulate air pollution exposure on obesity in mice: role of p47phox. Arterioscler Thromb Vasc Biol 2010;30:2518–2527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang G, Sun Q, Liu C. Influencing factors of thermogenic adipose tissue activity. Front Physiol 2016;7:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schoonjans K, Staels B, Auwerx J. The peroxisome proliferator activated receptors (PPARS) and their effects on lipid metabolism and adipocyte differentiation. Biochim Biophys Acta 1996;1302:93–109 [DOI] [PubMed] [Google Scholar]
- 34. Wiklund P. The role of physical activity and exercise in obesity and weight management: time for critical appraisal. J Sport Health Sci 2016;5:151–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Guo Q, Xue T, Wang B, et al. Effects of physical activity intensity on adulthood obesity as a function of long-term exposure to ambient PM2.5: Observations from a Chinese nationwide representative sample. Sci Total Environ 2022;823:153417. [DOI] [PubMed] [Google Scholar]
- 36. Gray CL, Messer LC, Rappazzo KM, Jagai JS, Grabich SC, Lobdell DT. The association between physical inactivity and obesity is modified by five domains of environmental quality in U.S. adults: a cross-sectional study. PLoS One 2018;13:e0203301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim SR, Choi D, Choi S, et al. Association of combined effects of physical activity and air pollution with diabetes in older adults. Environ Int 2020;145:106161. [DOI] [PubMed] [Google Scholar]
- 38. Giorgini P, Rubenfire M, Bard RL, Jackson EA, Ferri C, Brook RD. Air pollution and exercise: a review of the cardiovascular implications for health care professionals. J Cardiopulm Rehabil Prev 2016;36:84–95 [DOI] [PubMed] [Google Scholar]
- 39. You T, Arsenis NC, Disanzo BL, Lamonte MJ. Effects of exercise training on chronic inflammation in obesity. Sports Med 2013;43:243–256 [DOI] [PubMed] [Google Scholar]