Abstract
Aims
We aimed to evaluate the effects of maternal diabetes on neonatal iron status, measuring erythrocyte indices including hemoglobin, hematocrit, reticulocytes, mean corpuscular volume (MCV), percent (%) hypochromia, ferritin, and additionally mean reticulocyte hemoglobin content (MCHr) as an early marker of iron deficiency, and examine the association between neonatal MCHr, red cell indices, and ferritin.
Materials and Methods
We conducted a hospital-based prospective cohort study in a tertiary neonatal unit of a University Hospital from 2018 to 2020. We enrolled 126 maternal-infant pairs of mothers whose pregnancy was associated with diabetes and 74 maternal-infant pairs from uncomplicated pregnancies. Erythrocyte indices were analyzed within the first twelve hours after birth. Erythrocyte parameters were compared between infants of the diabetes and the non-diabetic group. We examined the correlation of the neonatal MCHr with perinatal characteristics, including gestation, birth weight, maternal body mass index, the erythrocytic indices, maternal diabetes, maternal obesity, prematurity, small-for-gestational-age status, maternal preeclampsia, and maternal anemia. Finally, we evaluated the discordance between neonatal MCHr and neonatal ferritin.
Results
Infants of the diabetes group had a significantly lower MCHr (32.6 pg vs. 34.2 pg, p=0.003) compared with infants of uncomplicated pregnancies. Neonatal MCHr was significantly correlated with maternal hypochromia (r=-0.237, p=0.004) and neonatal MCV (r=0.674, p<0.001). Neonatal MCHr was significantly associated with maternal diabetes [standardized coefficients 0.21, 95% confidence interval (CI) 0.05-0.58, p=0.003) and maternal preeclampsia (standardized coefficients 0.17, 95% CI 0.02-0.92, p=0.019), after adjusting for maternal anemia, maternal obesity, prematurity, and small-for-gestational-age status. Those results were consistent also when analyzing maternal-infant pairs with pre-existing diabetes, and maternal-infant pairs with gestational diabetes. There was significant discordance between neonatal MCHr and neonatal ferritin (p=0.001).
Conclusions
MCHr was significantly lower in infants of mothers whose pregnancy was associated with diabetes compared with infants of non-diabetic mothers and correlated with neonatal and maternal red cell indices of iron deficiency. Since there was significant discordance between neonatal MCHr and ferritin during the first postnatal day, it is possible that MCHr could be used as a screening test for iron deficiency, especially in infants.
Keywords: anemia, gestational diabetes, hypochromia, iron deficiency, reticulocytes
Introduction
Iron forms an important co-factor of enzymes involved in cell replication, myelination, neurotransmitter synthesis, and cellular energy metabolism, playing a key role in brain development (1, 2). Several factors influence iron status at birth, including maternal nutrition and morbidities. Prematurity impacts iron stores since these are mainly accrued during the third trimester of pregnancy (3). Also, maternal diabetes mellitus is associated with depleted fetal iron stores, and this is proportionate to the degree of maternal glycemic control (4, 5). Previous research has described the impact of altered glucose handling in utero on fetal iron deficiency through chronic hypoxia and increased erythropoiesis (6). Current evidence has suggested that infants of mothers whose pregnancy was complicated by diabetes (infants of diabetic mothers, IDM) are at increased risk for developing iron deficiency resulting in neurocognitive impairment later in life (4–6).
Of note, iron deficiency, even before the manifestation of anemia, may contribute to impaired psychomotor development with potential permanent deficits (1, 7, 8); therefore, early recognition of iron deficiency in high-risk individuals is essential. Iron deficiency is characterized by microcytic, hypochromic erythrocytes and low iron stores. Microcytosis is revealed by the low mean corpuscular volume (MCV), which measures the average red blood cell volume, whereas hypochromia by a low mean corpuscular hemoglobin concentration, which is the measure of the concentration of hemoglobin in a given volume of packed red blood cells (9). Previous studies have suggested that ferritin, soluble transferrin receptor, transferrin saturation, and zinc protoporphyrin were poor predictors of iron deficiency in children (9). The ferritin concentrations may be misleading in the presence of acute or chronic inflammation as ferritin is also an acute phase reactant. In contrast, mean reticulocyte hemoglobin content (MCHr), which reflects the availability of iron for bone marrow hemoglobin production during the prior 24-48 h, has been proposed as a tool for evaluating the iron status and a sensitive predictor for later anemia (10). Previous studies have evaluated MCHr concentrations in term and preterm infants, suggesting that MCHr indicates iron deficiency with a better consistency compared with other indices, such as ferritin (3, 10, 11); however, evidence regarding the impact of maternal diabetes on those specific erythrocytic parameters is limited.
Given the significance of the early detection of iron deficiency in high-risk neonates such as the IDMs for the long-term neurodevelopment, this study aimed to examine the effects of maternal diabetes on neonatal iron status after birth, measuring erythrocyte indices including hemoglobin, hematocrit, reticulocytes, MCV, percent (%) hypochromia, and additionally neonatal MCHr. Moreover, the study aimed to examine the possible discordance between neonatal MCHr and neonatal ferritin among IDMs and infants from uncomplicated pregnancies.
Materials and methods
Study population
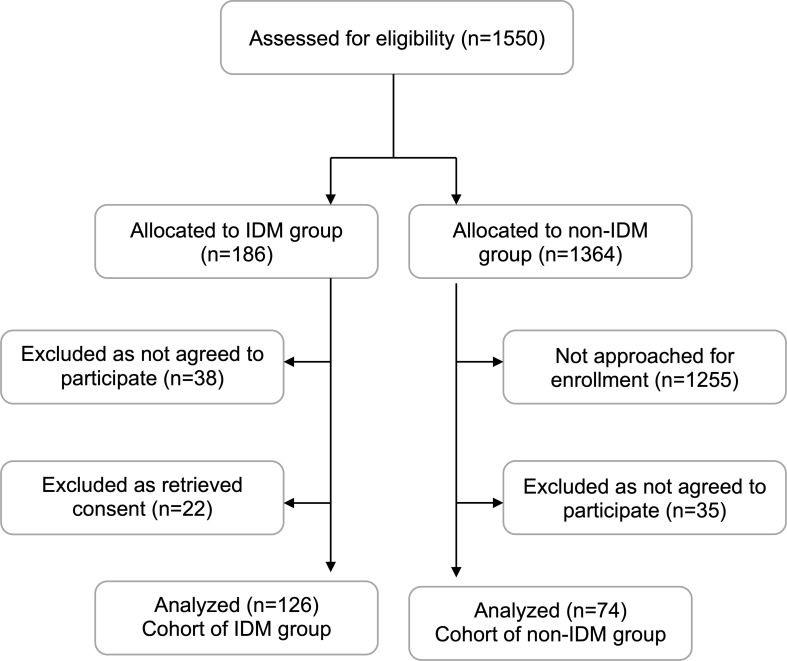
We conducted a hospital-based prospective cohort study in a tertiary neonatal unit of a University Hospital from 2018 to 2020. During the study period, we approached all mothers whose pregnancies were complicated by diabetes (IDM group) and mothers whose pregnancies were not complicated by diabetes (non-IDM group), for a potential enrollment; we consecutively enrolled those mothers who agreed to participate, including maternal-infant pairs of the IDM group, and maternal-infant pairs of the non-IDM group, matched for gestation (± one week) and birth weight (± 100 g) in a 2:1 ratio ( Figure 1 ). Maternal diabetes was diagnosed according to the guidelines of the American Diabetes Association, including pre-existing diabetes and gestational diabetes mellitus (12). The study was approved by the institutional ethics committee (29285/20-06-2018), whereas informed parental consent was obtained as appropriately.
Figure 1.
Flowchart of the study population. IDM, infant of diabetic mother.
For every infant, we recorded the perinatal characteristics, including gestational age, birth weight, gender, small-for-gestational-age status, delivery mode, maternal preeclampsia, and maternal anthropometrics, including weight, height, and body mass index (BMI) at the beginning and the end of the pregnancy. Prematurity was defined as any pregnancy delivered before 37 weeks of gestation. Maternal weight and height were measured at the first prenatal visit and birth. BMI was calculated by the formula BMI = weight/height2 , while a BMI ≥ 30 kg/m2 was utilized to define obesity (13).
Blood sampling and analyses
Erythrocyte parameters were measured on routine blood analysis for every maternal-infant pair. A peripheral arterial blood sample was collected from each infant within the first twelve hours after birth and erythrocyte parameters [hemoglobin, hematocrit, reticulocytes, MCHr, MCV, percent (%) hypochromia, ferritin] were analyzed with an automated hematology analyzer (Alinity Hq, Abbott, Abbott Park, Illinois, USA). The percentage of hypochromic red blood cells (%hypochromia) can be derived from the hemoglobin concentration distribution curve and is usually defined as the percentage of red blood cells with a cellular hemoglobin content below 28.0 g/dL. In older children and adults, values above 1% are highly suggestive of iron deficiency (3). C-reactive protein, with a cut-off value of 1.0 mg/dL, was evaluated simultaneously as ferritin to exclude underlying infection. A venous blood sample for evaluating the complete set of the same erythrocyte parameters was also collected for each infant’s mother within the first twelve hours after birth.
Neonatal anemia during the first 24 hours of age was defined by hemoglobin lower than 13 g/dL based on standard criteria (14). Ferritin values below 75 μg/L were considered “low” and above 250 μg/L “high” (15); MCHr values below 29 pg were considered “low” and above 38 pg “high” (11).
Statistical analysis
Descriptive statistics were calculated for perinatal data and the maternal and neonatal erythrocytic parameters. Continuous variables were expressed as mean ± standard deviation or median (interquartile range), as appropriate. The normality of the distributions of continuous variables was assessed by the Kolmogorov-Smirnov test. Continuous variables were compared using the student’s unpaired t-test or the non-parametric Mann-Whitney test. Categorical variables were expressed as n (%) and compared with the chi-square (x2) test or Fisher’s exact test.
The correlation of the neonatal MCHr value (continuous variable) with continuous perinatal characteristics that have been previously reported to affect the neonatal iron status (5, 16–18), including gestation, birth weight, maternal BMI, and the erythrocytic indices (i.e., hemoglobin, hematocrit, MCV, %hypochromia, reticulocytes, ferritin) was examined with Spearman’s rho, with a subgroup analysis between IDMs and non-IDMs. Given the differences in hemopoiesis in preterm compared to term infants, namely low plasma erythropoietin levels, low circulating blood volumes, and insufficient erythropoiesis (18), we also performed a stratified analysis between term and preterm infants. Finally, a linear regression analysis was utilized to evaluate the effect of maternal diabetes on neonatal MCHr, adjusted for factors known to influence neonatal iron status (5, 16–18), namely, maternal obesity at the beginning and the end of the pregnancy, prematurity, small-for-gestational-age status, maternal preeclampsia, and maternal anemia (categorical variables). Amongst the above perinatal factors, only those with a significant effect in univariate analysis, with a p-value cut-off value <0.05, were included in the multivariate model. To examine the possible heterogeneity of the association between maternal diabetes and neonatal MCHr and conduct sensitivity analyses, we also performed a linear regression analysis to evaluate the effect of maternal diabetes on neonatal MCHr, adjusted for factors known to influence neonatal iron status as previously reported, in the subgroups of mothers with preexisting diabetes and mothers with gestational diabetes.
The discordance between neonatal MCHr and neonatal ferritin was evaluated with Kendall’s Coefficient of Concordance W test. Discordance was defined when one metric (MCHr or ferritin) exceeded the high value of reference intervals for infants, and the other fell below the low reference interval (11, 15).
All tests were two-sided, and a p-value less than 0.05 was considered statistically significant (alpha 0.05). A power analysis revealed that a sample size of 126 infants in the IDM group and 74 non-IDM infants would be sufficient to detect a difference of at least 5% in the erythrocytic parameters between groups (based on the mean SD of the first ten measurements), with a power of 0.8 and a type-I error of 0.05. The data were analyzed using SPSS Statistics Version 25.0 (IBM, Chicago, Illinois, USA).
Results
We initially enrolled 222 maternal-infant pairs. Of them, 22 subjects retrieved consent, therefore, 200 maternal-infant pairs were included in the analysis: 126 IDMs, and 74 non-IDMs ( Figure 1 ). The perinatal characteristics of the two groups are depicted in Table 1 . Of the total, 58 (29%) infants were born preterm, 34 (27%) of the IDM, and 24 (32%) of the non-IDM group. Maternal weight gain and BMI increase during pregnancy were significantly higher in non-IDM compared with the IDM group (12 kg compared to 9 kg, p<0.001, and 4.1 kg/cm2 compared to 3.1 kg/cm2, p<0.001, respectively). No differences were recorded between the IDM and the non-IDM group in the rate of maternal obesity either at the beginning or the end of the pregnancy ( Table 1 ).
Table 1.
Perinatal characteristics and hematological indices of the study population.
| IDM group (n = 126) | Non-IDM group (n = 74) | p | |
|---|---|---|---|
| Gestational age, weeks | 37.0 ± 2.5 | 37.1 ± 2.8 | 0.727 |
| Preterm neonates | 34 (27%) | 24 (32%) | 0.520 |
| Birth weight, g | 2923 ± 715 | 2803 ± 709 | 0.253 |
| Gender, male | 63 (50%) | 28 (37%) | 0.080 |
| Small-for-gestational-age status | 18 (14%) | 14 (19%) | 0.430 |
| Preeclampsia | 10 (8%) | 4 (5%) | 0.572 |
| Delivery mode, cesarean section | 91 (73%) | 49 (65%) | 0.339 |
| Maternal weight (beginning of pregnancy), kg | 71.9 ± 16.9 | 67.7 ± 16.1 | 0.116 |
| Maternal weight (end of pregnancy), kg | 79.6 ± 16.1 | 79.5 ± 15.8 | 0.962 |
| Maternal weight difference, kg | 9 (4-13)§ | 12 (8-15)§ | 0.001† |
| Maternal BMI (beginning of pregnancy), kg/cm2 | 26.4 ± 6.1 | 24.9 ± 6.7 | 0.147 |
| Maternal obesity (beginning of pregnancy) | 21 (16%) | 10 (14%) | 0.539 |
| Maternal BMI (end of pregnancy), kg/cm2 | 29.3 ± 5.7 | 29.3 ± 6.3 | 0.992 |
| Maternal obesity (end of pregnancy) | 42 (33%) | 22 (30%) | 0.412 |
| Maternal BMI difference, kg/cm2 | 3.1 (1.4-4.6)§ | 4.1 (2.9-5.6)§ | 0.001† |
| Maternal anemia (Hb <11 g/dL) | 33 (31%) | 14 (22%) | 0.217 |
| Maternal ferritin, μg/L | 39 (17-75)§ | 35 (18-66)§ | 0.676 |
| Maternal CRP, mg/dL | 0.70 (0.47-0.96)§ | 0.60 (0.45-0.86)§ | 0.333 |
| Maternal hematocrit, % | 34.5 ± 3.6 | 35.4 ± 3.6 | 0.162 |
| Maternal hemoglobin, g/dL | 11.6 ± 1.4 | 12.1 ± 1.3 | 0.030† |
| Maternal MCV, fL | 101.4 ± 7.2 | 100.2 ± 9.6 | 0.392 |
| Maternal hypochromia, % | 4 (2-8)§ | 4 (2-8)§ | 0.831 |
| Maternal MCHr, pg | 29.8 ± 3.0 | 29.9 ± 3.3 | 0.957 |
| Maternal reticulocytes, % | 2.1 ± 0.8 | 2.3 ± 1.3 | 0.175 |
| Maternal reticulocytes, absolute, cells/μL | 1.6 ± 0.6 | 1.8 ± 1.0 | 0.114 |
| Neonatal anemia (hemoglobin <13 g/dL) | 4 (3%) | 4 (6%) | 0.472 |
| Neonatal ferritin μg/L | 199 (127-309)§ | 171 (114-278)§ | 0.346 |
| Neonatal CRP, mg/dL | 0.18 (0.11-0.29)§ | 0.20 (0.13-0.31)§ | 0.437 |
| Neonatal hematocrit, % | 49.6 ± 6.2 | 50.4 ± 7.4 | 0.473 |
| Neonatal hemoglobin, g/dL | 16.4 ± 1.8 | 16.8 ± 2.2 | 0.137 |
| Neonatal MCV, fL | 121.3 ± 9.4 | 124.6 ± 10.0 | 0.049† |
| Neonatal hypochromia, % | 12 (7-18)§ | 9 (6-16)§ | 0.117 |
| Neonatal MCHr, pg | 32.6 ± 3.6 | 34.2 ± 2.8 | 0.003† |
| Neonatal reticulocytes, % | 4.4 ± 1.3 | 4.4 ± 1.4 | 0.925 |
| Neonatal reticulocytes, absolute, cells/μL | 4.8 ± 1.4 | 4.9 ± 1.6 | 0.872 |
Continuous variables are expressed as mean ± SD or median (IQR). P-values of student’s t-test or Mann–Whitney test. Categorical variables are expressed as n (%). P-values of chi-square test or Fisher’s exact test.
†, statistically significant.
§, non-parametric variables.
IDM, infant of diabetic mother; BMI, body mass index; CRP, C-reactive protein; MCV, mean corpuscular volume; MCHr, mean reticulocyte hemoglobin content.
Most maternal red cell indices of the IDM group were not different compared to the non-diabetic mothers; maternal hemoglobin in the IDM group was significantly lower compared with non-diabetic mothers ( Table 1 ). Regarding the neonatal red cell indices, the IDM group had a significantly lower neonatal MCV compared with the non-IDM group (121.3 ± 9.4 fL vs. 124.6 ± 10 fL, p=0.049). Also, the average MCHr of infants in the IDM was significantly lower compared to the non-IDM group (32.6 pg vs. 34.2 pg, p=0.003) ( Table 1 ).
Neonatal MCHr was significantly correlated with maternal hypochromia (r=-0.237, p=0.004) and neonatal MCV (r=0.674, p<0.001) ( Table 2 ). Within the non-IDM group, neonatal MCHr was not correlated with maternal hypochromia but was significantly correlated with maternal BMI change (-0.475, p<0.001) ( Table 2 ). In the stratified analysis, in term infants, MCHr was significantly correlated with maternal ferritin (r=0.206, p=0.022), in addition to maternal hypochromia (r=-0.202, p<0.039), and neonatal MCV (r=0.788, p<0.001) ( Table 2 ). In preterm infants, MCHr was significantly correlated with maternal hemoglobin (r=0.313, p=0.046), maternal hypochromia (r=-0.311, 0.048), maternal reticulocytes (0.330, p=0.046), neonatal MCV (0.402, p=0.006) and neonatal hypochromia (r=-0.312, p=0.044) ( Table 2 ).
Table 2.
Correlation between neonatal MCHr and continuous variables of interest in the total cohort, in IDM in comparison to non-IDM infants, and in term in comparison to preterm infants.
| Total cohort | IDMs versus non-IDMs | Term versus preterm infants | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IDM group | Non-IDM group | Term infants | Preterm infants | |||||||
| rho | p | rho | p | rho | p | rho | p | rho | p | |
| Neonatal MCHr | ||||||||||
| Gestational age | 0.020 | 0.791 | -0.002 | 0.987 | -0.006 | 0.960 | -0.072 | 0.428 | 0.002 | 0.990 |
| Birth weight | 0.019 | 0.800 | -0.025 | 0.795 | 0.107 | 0.395 | -0.043 | 0.637 | -0.041 | 0.768 |
| Maternal BMI (beginning of pregnancy) | -0.075 | 0.370 | -0.135 | 0.202 | 0.076 | 0.583 | -0.058 | 0.556 | -0.084 | 0.615 |
| Maternal BMI (end of pregnancy) | -0.117 | 0.161 | -0.135 | 0.203 | -0.101 | 0.465 | -0.100 | 0.305 | -0.140 | 0.401 |
| Maternal BMI difference | -0.130 | 0.120 | -0.025 | 0.814 | -0.475 | <0.001† | -0.165 | 0.089 | -0.026 | 0.879 |
| Maternal ferritin | 0.104 | 0.171 | 0.189 | 0.057 | -0.048 | 0.707 | 0.206 | 0.022† | -0.076 | 0.591 |
| Maternal hematocrit | -0.122 | 0.134 | -0.111 | 0.280 | -0.173 | 0.197 | -0.160 | 0.090 | 0.011 | 0.949 |
| Maternal hemoglobin | 0.002 | 0.983 | -0.061 | 0.550 | 0.046 | 0.734 | -0.133 | 0.234 | 0.313 | 0.046† |
| Maternal MCV | 0.019 | 0.813 | -0.026 | 0.801 | 0.130 | 0.365 | 0.007 | 0.944 | 0.073 | 0.643 |
| Maternal hypochromia | -0.237 | 0.004† | -0.269 | 0.008† | -0.196 | 0.169 | -0.202 | 0.039† | -0.311 | 0.048† |
| Maternal MCHr | 0.147 | 0.112 | 0.083 | 0.476 | 0.268 | 0.083 | 0.146 | 0.170 | 0.150 | 0.439 |
| Maternal reticulocytes | 0.135 | 0.110 | 0.120 | 0.267 | 0.131 | 0.344 | 0.061 | 0.537 | 0.330 | 0.046† |
| Neonatal ferritin | -0.035 | 0.638 | -0.029 | 0.756 | -0.089 | 0.480 | -0.046 | 0.613 | -0.013 | 0.926 |
| Neonatal hematocrit | -0.016 | 0.839 | 0.001 | 0.993 | -0.085 | 0.513 | -0.050 | 0.586 | 0.091 | 0.522 |
| Neonatal hemoglobin | 0.003 | 0.972 | -0.068 | 0.473 | 0.046 | 0.714 | 0.023 | 0.802 | -0.057 | 0.532 |
| Neonatal MCV | 0.674 | <0.001† | 0.648 | <0.001† | 0.706 | <0.001† | 0.788 | <0.001† | 0.402 | 0.006† |
| Neonatal hypochromia | -0.078 | 0.349 | -0.103 | 0.320 | 0.022 | 0.878 | 0.029 | 0.767 | -0.312 | 0.044† |
| Neonatal reticulocytes | 0.136 | 0.075 | 0.072 | 0.450 | 0.212 | 0.102 | 0.166 | 0.069 | 0.158 | 0.264 |
Spearman’s rho coefficient.
†, statistically significant.
IDM, infant of diabetic mother; MCHr, mean reticulocyte hemoglobin content; BMI, body mass index; MCV, mean corpuscular volume.
In regression analysis, neonatal MCHr was significantly associated with maternal diabetes [standardized coefficients 0.21, 95% confidence interval (CI) 0.05-0.58, p=0.003] and maternal preeclampsia (standardized coefficients 0.17, 95% CI 0.02-0.92, p=0.019), after adjusting for maternal BMI difference, maternal obesity at the beginning and the end of the pregnancy, prematurity, small-for-gestational-age status, and maternal anemia ( Table 3 ). Those results were consistent also when analyzing maternal-infant pairs with pre-existing diabetes [maternal pre-existing diabetes (standardized coefficients 0.27, 95% CI 0.20-0.71, p=0.016) and maternal preeclampsia (standardized coefficients 0.28, 95% CI 0.12-0.98, p=0.047)], and maternal-infant pairs with gestational diabetes [maternal gestational diabetes (standardized coefficients 0.20, 95% CI 0.05-0.62, p=0.007) and maternal preeclampsia (standardized coefficients 0.19, 95% CI 0.10-0.79, p=0.015)], as depicted in Supplemental Tables 1 , 2 .
Table 3.
Linear regression analysis (univariate and multivariate) of the association of neonatal MCHr with categorical factors of interest.
| Standardized Coefficients | 95% CI | p | |
|---|---|---|---|
| Univariate | |||
| Neonatal MCHr | |||
| Maternal diabetes | 0.22 | 0.06-0.61 | 0.003† |
| Maternal obesity (beginning of pregnancy) | 0.95 | 0.44-2.36 | 0.180 |
| Maternal obesity (end of pregnancy) | 0.75 | 0.36-1.87 | 0.183 |
| Prematurity | 0.42 | 0.02-1.42 | 0.566 |
| Small-for-gestational-age status | 0.40 | 0.18-1.88 | 0.598 |
| Preeclampsia | 0.18 | 0.06-0.90 | 0.015† |
| Maternal anemia | 0.17 | 0.07-2.03 | 0.837 |
| Multivariate | |||
| Neonatal MCHr | |||
| Maternal diabetes | 0.21 | 0.05-0.58 | 0.003† |
| Preeclampsia | 0.17 | 0.02-0.92 | 0.019† |
MCHr, mean reticulocyte hemoglobin content; CI, confidence intervals; BMI, body mass index.
Amongst the perinatal factors, only those with a significant effect in univariate analysis, with a p-value cut-off value <0.05, were included in the multivariate model.
†, statistically significant.
A discordance was recorded between neonatal MCHr and neonatal ferritin in 12/200 (6%) infants that were statistically significant when evaluated in the whole study group (Kendall’s W=0.155, p=0.001) ( Table 4A ). Of note, when a stratified analysis was performed in the IDM and the non-IDM group, a discordance was recorded in the IDM group that did not reach statistical significance (10/126 infants, 8%, Kendall’s W=0.413, p=0.064) ( Table 4B ). Finally, between term and preterm infants, the discordance remained significant only in term infants (9/152 infants, 6%, Kendall’s W=0.257, p=0.001) ( Table 4C ).
Table 4.
Discordance between neonatal MCHr and ferritin, with a subgroup analysis in IDM group and non-IDM group, and term and preterm infants.
| A. Total cohort | |||||
| MCHr, pg | |||||
| Low (<29) | Normal (29–38) | High (>38) | p | ||
| Ferritin, μg/L | Low (<75) | 5 | 11 | 4 | 0.001† |
| Normal (75–250) | 8 | 79 | 5 | ||
| High (>250) | 8 | 54 | 5 | ||
| B. IDM group | |||||
| MCHr, pg | |||||
| Low (<29) | Normal (29–38) | High (>38) | p | ||
| Ferritin, μg/L | Low (<75) | 5 | 7 | 8 | 0.064 |
| Normal (75–250) | 9 | 43 | 38 | ||
| High (>250) | 2 | 2 | 0 | ||
| Non-IDM group | |||||
| MCHr, pg | |||||
| Low (<29) | Normal (29–38) | High (>38) | p | ||
| Ferritin, μg/L | Low (<75) | 0 | 2 | 2 | 0.106 |
| Normal (75–250) | 1 | 36 | 3 | ||
| High (>250) | 0 | 16 | 5 | ||
| C. Term infants | |||||
| MCHr, pg | |||||
| Low (<29) | Normal (29–38) | High (>38) | p | ||
| Ferritin, μg/L | Low (<75) | 2 | 3 | 3 | 0.001† |
| Normal (75–250) | 5 | 55 | 3 | ||
| High (>250) | 6 | 43 | 5 | ||
| Preterm infants | |||||
| MCHr, pg | |||||
| Low (<29) | Normal (29–38) | High (>38) | p | ||
| Ferritin, μg/L | Low (<75) | 3 | 8 | 1 | 0.442 |
| Normal (75–250) | 3 | 24 | 2 | ||
| High (>250) | 2 | 11 | 0 | ||
Categorical variables are expressed as n (%). P-values of Kendall’s Coefficient of Concordance W test.
†, statistically significant.
MCHr, mean reticulocyte hemoglobin content; IDM, infant of diabetic mother.
Discussion
In a cohort of 200 infants, we found significantly lower neonatal MCHr and MCV in IDMs compared to non-IDMs. Even though there were significantly more mothers with anemia in the IDM compared to the non-IDM group, neonatal MCHr was significantly associated with maternal diabetes and preeclampsia after adjusting for maternal anemia, maternal obesity, prematurity, and small-for-gestational-age status. Neonatal MCHr during the first postnatal day was significantly correlated with maternal hypochromia and neonatal MCV. Among infants from uncomplicated pregnancies, neonatal MCHr was also strongly correlated with maternal BMI change during pregnancy. Finally, a significant discordance was noted between neonatal MCHr and neonatal ferritin, specifically among full-term infants, whereas the discordance between these two hematologic indices in IDMs was not statistically significant.
Our findings are in line with current evidence suggesting that during the first postnatal day, IDMs have evidence of iron deficiency based on hematological indices, specifically MCV and MCHr. Moreover, maternal diabetes and preeclampsia were independently associated with reduced neonatal MCHr in our cohort. Maternal diabetes is associated with depleted fetal iron stores (4–6, 19). The level of maternal glycaemic control and the associated vasculopathy are thought to lead to chronic intrauterine hypoxia and increased erythropoiesis with resultant polycythemia and increased iron demands for the fetus (6). This increased iron demand may exceed placental iron transport capacity because the transferrin binding capacity in the placentae of diabetic mothers is reduced (6), and placental vascular disease further limits iron transport across the placenta (17). Maternal preeclampsia has also been associated with placental vasculopathy and a further decrease in the transplacental iron transport.
Previous studies suggested that infants born to diabetic mothers synthesized significantly higher levels of fetal hemoglobin (20), and authors have found a significant delay in the switch from δ-globin to β-globin in full-term infants born to diabetic mothers, compared with the infants of non-diabetic mothers (21). Furthermore, MCHr has been found significantly lower in cases of a reduced β-globin synthesis, such as beta thalassemia trait, compared to healthy controls (22, 23). The above mechanism, although our study could not test this hypothesis, might also explain the decreased MCHr levels that were recorded in IDMs.
In infants, several clinically used iron status parameters such as ferritin, soluble transferrin receptor, transferrin saturation, and zinc protoporphyrin were poor predictors of iron deficiency (24–26). On the other hand, several studies in children have suggested that MCHr, could be a significant predictor of iron deficiency before the manifestation of anemia (9, 10, 27–31). The neonatal MCHr mean values in the present study were between 32.6 ± 3.6 pg to 34.2 ± 2.8 pg and these are comparable to prior large cohort studies in which MCHr values in term and preterm infants were between 30.3 ± 1.1 pg (27) to 35.9 ± 3.1 pg (11, 26, 29, 32). An MCHr cutoff threshold of 26.9 pg (10) to 28.1 pg (26) has been proposed to indicate iron deficiency and predict anemia. However, since anemia is a late sign of iron deficiency, the optimal MCHr threshold for predicting iron deficiency in infants may be higher, especially during the first week of age (10). Given that iron is essential to neural myelination and neurotransmitter function, playing an important role in the development of the central nervous system (1, 7, 14, 33, 34), the early detection of iron deficiency would be crucial in high-risk infants (34).
In the present study, neonatal MCHr was strongly correlated with maternal hypochromia and neonatal MCV. When analyzed separately, in the preterm cohort, we found a strong correlation of neonatal MCHr with maternal hemoglobin, hypochromia, and reticulocytes. Hypochromic maternal erythrocytes reflect deranged maternal iron status. It is, therefore, not surprising that this finding was associated with a lower neonatal MCHr and MCV, likely due to decreased transplacental iron transport. In line with our findings, other studies have reported a strong independent association between MCHr and erythrocyte indices in infants (10, 29, 32). An interesting finding of the present study is that, although neonatal MCHr was not associated with BMI or maternal obesity either at the beginning or the end of the pregnancy, it was strongly correlated with maternal BMI change during pregnancy among infants of the group of uncomplicated pregnancies. Potential explanations for this novel finding include the expansion of maternal blood volume and a substantial increase in iron demand (35) in the setting of excessive maternal weight gain during pregnancy. In addition, overweight status has been associated with an elevation of hepcidin and serum ferritin and, thus, a decreased iron absorption from the diet (16).
Our findings do not support any strong correlation between neonatal MCHr and neonatal ferritin. On the contrary, a significant discordance was noted between neonatal MCHr and neonatal ferritin levels. As previously noted, this discordance was significant among term infants regardless of maternal diabetes status. In line with our findings, Bahr et al., who evaluated 190 paired ferritin and MCHr measurements, reported a marked discordance in 8% of the samples in a neonatal population (3). When ferritin and MCHr were discordant, other erythrocytic indices, such as erythrocyte microcytosis and hypochromasia, suggested that MCHr gave a more accurate interpretation of iron status (3). Along the same line, German et al. examined the trends of MCHr and ferritin in critically ill infants and concluded that there was a poor correlation between them (26). Factors that influence neonatal ferritin concentration at birth include duration of gestation, fetal sex, or nutrition (36). Furthermore, serum ferritin concentrations are also elevated during periods of infection, or inflammation, when serum ferritin behaves as an acute-phase reactant (37). The pathogenesis of hyperferritinemia is thought to be cytokine-mediated, with interleukin (IL)1a, IL1b, IL6, IL18, tumor necrosis factor-a, c-interferon, and macrophage-colony stimulating factor all implicated. Other inflammatory infectious conditions also produce elevations in ferritin, usually with elevated levels of C-reactive protein (37). In our study, as per study design, we excluded subjects with elevated CRP, however, the impact of the other non-infectious factors on neonatal ferritin levels could not be excluded. In contrast, MCHr reflects the current iron availability for hemoglobin synthesis due to the very short lifespan of reticulocytes (1–2 days in circulation) compared to erythrocytes (life span of 120 days) and is not influenced by inflammation (38, 39). Following an erythropoietic stimulus, iron begins to be incorporated into developing red cells with the appearance of reticulocytes at 48–96 h (38, 39). Besides, in infants with low ferritin but normal MCHr, it might be indicated that normal erythropoiesis is maintained at the expense of diminishing iron stores (3). Thus, MCHr concentrations may be more reflective of changes in iron bioavailability (34). In summary, our findings support that in high-risk infants, such as infants of mothers whose pregnancy was complicated by diabetes, MCHr could be a useful biomarker of iron bioavailability which has implications for hematopoiesis and neurodevelopment.
The findings of the present study should be interpreted in the context of certain limitations. We acknowledge that this was a single-center study, and the population was relatively homogeneous (predominantly Caucasian). Thus, the results may not be generalizable in other populations, especially in resource-poor settings, in racially/ethnically diverse populations, and in the setting of concurrent additional nutritional deficiencies. Furthermore, our sample size was relatively small, while we could not exclude a possible selection bias; however, we included a significant proportion of the available for enrolment maternal-infant pairs of the IDM group during the study duration, whereas the power analysis suggested that our study sample was sufficient to detect a difference of at least 5% in the erythrocytic parameters between groups. We did not measure other markers of iron status, such as serum transferrin or zinc protoporphyrin; therefore, the evaluation of the correlation between neonatal MCHr and other erythrocytic indices and ferritin was limited. Moreover, since we measured the erythrocyte parameters only within the first twelve hours after birth, we could not evaluate whether the lower neonatal MCHr values of IDMs compared to non-IDMs were transient or long-term. Previous studies have reported that MCHr values normally drop over the first few days, while factors such as prematurity or the stress of delivery might be implicated in a higher fall (11, 26, 27, 29). As per our study design, we could not evaluate whether MCHr dropped during the first postnatal days or the predictive value of neonatal MCHr for the later development of anemia or impaired neurodevelopment. Further regular monitoring of the erythrocyte indices would be warranted within the first months of life, to examine whether IDMs develop iron-deficient anemia at a higher rate compared to controls.
In conclusion, neonatal MCHr was significantly lower in IDM compared to infants from uncomplicated pregnancies and neonatal MCHr was significantly associated with maternal diabetes and preeclampsia after adjusting for perinatal factors. Neonatal MCHr during the first postnatal day presented a significant discordance with neonatal ferritin. Further studies are warranted to evaluate the predictive value of MCHr in the later development of anemia and impaired neurodevelopment in high-risk infants.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Aristotle University ethics committee (29285/20-06-2018). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
EB and VS conceptualized and developed the study design. Data collection was performed by EB, TM, and KD, DR performed the statistical analysis and wrote the initial draft. EB, HC, GM, TM, KD, CT, AK, DG, and VS reviewed and revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.1011897/full#supplementary-material
References
- 1. Lozoff B, Smith JB, Kaciroti N, Clark KM, Guevara S, Jimenez E. Functional significance of early-life iron deficiency: Outcomes at 25 years. J Pediatr (2013) 163(5):1260–6. doi: 10.1016/j.jpeds.2013.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bastian TW, Rao R, Tran PV, Georgieff MK. The effects of early-life iron deficiency on brain energy metabolism. Neurosci Insights (2020) 15:2633105520935104. doi: 10.1177/2633105520935104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bahr TM, Baer VL, Ohls RK, Christensen TR, Ward DM, Bennett ST, et al. Reconciling markedly discordant values of serum ferritin versus reticulocyte hemoglobin content. J Perinatol (2021) 41(3):619–26. doi: 10.1038/s41372-020-00845-2 [DOI] [PubMed] [Google Scholar]
- 4. Riggins T, Miller NC, Bauer PJ, Georgieff MK, Nelson CA. Consequences of low neonatal iron status due to maternal diabetes mellitus on explicit memory performance in childhood. Dev Neuropsychol (2009) 34(6):762–79. doi: 10.1080/87565640903265145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Soubasi V, Petridou S, Sarafidis K, Tsantali C, Diamanti E, Buonocore G, et al. Association of increased maternal ferritin levels with gestational diabetes and intra-uterine growth retardation. Diabetes Metab (2010) 36(1):58–63. doi: 10.1016/j.diabet.2009.06.010 [DOI] [PubMed] [Google Scholar]
- 6. Georgieff MK, Landon MB, Mills MM, Hedlund BE, Faassen AE, Schmidt RL, et al. Abnormal iron distribution in infants of diabetic mothers: Spectrum and maternal antecedents. J Pediatr (1990) 117(3):455–61. doi: 10.1016/s0022-3476(05)81097-2 [DOI] [PubMed] [Google Scholar]
- 7. Halterman JS, Kaczorowski JM, Aligne CA, Auinger P, Szilagyi PG. Iron deficiency and cognitive achievement among school-aged children and adolescents in the united states. Pediatrics (2001) 107(6):1381–6. doi: 10.1542/peds.107.6.1381 [DOI] [PubMed] [Google Scholar]
- 8. Shafir T, Angulo-Barroso R, Jing Y, Angelilli ML, Jacobson SW, Lozoff B. Iron deficiency and infant motor development. Early Hum Dev (2008) 84(7):479–85. doi: 10.1016/j.earlhumdev.2007.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brugnara C, Zurakowski D, DiCanzio J, Boyd T, Platt O. Reticulocyte hemoglobin content to diagnose iron deficiency in children. JAMA (1999) 281(23):2225–30. doi: 10.1001/jama.281.23.2225 [DOI] [PubMed] [Google Scholar]
- 10. Torsvik IK, Markestad T, Ueland PM, Nilsen RM, Midttun O, Bjorke Monsen AL. Evaluating iron status and the risk of anemia in young infants using erythrocyte parameters. Pediatr Res (2013) 73(2):214–20. doi: 10.1038/pr.2012.162 [DOI] [PubMed] [Google Scholar]
- 11. Christensen RD, Henry E, Bennett ST, Yaish HM. Reference intervals for reticulocyte parameters of infants during their first 90 days after birth. J Perinatol (2016) 36(1):61–6. doi: 10.1038/jp.2015.140 [DOI] [PubMed] [Google Scholar]
- 12. American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes Care (2014) 37 Suppl 1:S81–90. doi: 10.2337/dc14-S081 [DOI] [PubMed] [Google Scholar]
- 13. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults–the evidence report. National institutes of health. Obes Res (1998) 6 Suppl 2:51S–209S. doi: 10.1002/j.1550-8528.1998.tb00690.x [DOI] [PubMed] [Google Scholar]
- 14. Geng F, Mai X, Zhan J, Xu L, Georgieff M, Shao J, et al. Timing of iron deficiency and recognition memory in infancy. Nutr Neurosci (2022) 25(1):1–10. doi: 10.1080/1028415X.2019.1704991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. MacQueen BC, Christensen RD, Ward DM, Bennett ST, O’Brien EA, Sheffield MJ, et al. The iron status at birth of neonates with risk factors for developing iron deficiency: A pilot study. J Perinatol (2017) 37(4):436–40. doi: 10.1038/jp.2016.234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dao MC, Sen S, Iyer C, Klebenov D, Meydani SN. Obesity during pregnancy and fetal iron status: Is hepcidin the link? J Perinatol (2013) 33(3):177–81. doi: 10.1038/jp.2012.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Georgieff MK, Schmidt RL, Mills MM, Radmer WJ, Widness JA. Fetal iron and cytochrome c status after intrauterine hypoxemia and erythropoietin administration. Am J Physiol (1992) 262(3 Pt 2):R485–91. doi: 10.1152/ajpregu.1992.262.3.R485 [DOI] [PubMed] [Google Scholar]
- 18. Kling PJ, Winzerling JJ. Iron status and the treatment of the anemia of prematurity. Clin Perinatol (2002) 29(2):283–94. doi: 10.1016/s0095-5108(02)00002-7 [DOI] [PubMed] [Google Scholar]
- 19. Verner AM, Manderson J, Lappin TR, McCance DR, Halliday HL, Sweet DG. Influence of maternal diabetes mellitus on fetal iron status. Arch Dis Child Fetal Neonatal Ed (2007) 92(5):F399–401. doi: 10.1136/adc.2006.097279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bard H, Prosmanne J. Relative rates of fetal hemoglobin and adult hemoglobin synthesis in cord blood of infants of insulin-dependent diabetic mothers. Pediatrics (1985) 75(6):1143–7. doi: 10.1542/peds.75.6.1143 [DOI] [PubMed] [Google Scholar]
- 21. Perrine SP, Greene MF, Faller DV. Delay in the fetal globin switch in infants of diabetic mothers. N Engl J Med (1985) 312(6):334–8. doi: 10.1056/NEJM198502073120602 [DOI] [PubMed] [Google Scholar]
- 22. Chouliaras GL, Stamoulakatou A, Tsiftis G, Perissaki G, Premetis E, Lycopoulou L. Age, beta thalassaemia trait, and iron-deficient anaemia significantly affect reticulocyte indices in pre-school children. Eur J Pediatr (2010) 169(9):1097–104. doi: 10.1007/s00431-010-1186-7 [DOI] [PubMed] [Google Scholar]
- 23. Vicinanza P, Vicinanza M, Cosimato V, Terracciano D, Cancellario S, Massari A, et al. Mean reticolocyte hemoglobin content index plays a key role to identify children who are carriers of beta-thalassemia. Transl Med UniSa (2017) 17:31–6. [PMC free article] [PubMed] [Google Scholar]
- 24. Brown MS. Effect of transfusion and phlebotomy on serum ferritin levels in low birth weight infants. J Perinatol (1996) 16(1):39–42. [PubMed] [Google Scholar]
- 25. Christensen RD, Yaish HM, Henry E, Bennett ST. Red blood cell distribution width: Reference intervals for neonates. J Matern Fetal Neonatal Med (2015) 28(8):883–8. doi: 10.3109/14767058.2014.938044 [DOI] [PubMed] [Google Scholar]
- 26. German K, Vu PT, Irvine JD, Juul SE. Trends in reticulocyte hemoglobin equivalent values in critically ill neonates, stratified by gestational age. J Perinatol (2019) 39(9):1268–74. doi: 10.1038/s41372-019-0434-6 [DOI] [PubMed] [Google Scholar]
- 27. Al-Ghananim RT, Nalbant D, Schmidt RL, Cress GA, Zimmerman MB, Widness JA. Reticulocyte hemoglobin content during the first month of life in critically ill very low birth weight neonates differs from term infants, children, and adults. J Clin Lab Anal (2016) 30(4):326–34. doi: 10.1002/jcla.21859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ennis KM, Dahl LV, Rao RB, Georgieff MK. Reticulocyte hemoglobin content as an early predictive biomarker of brain iron deficiency. Pediatr Res (2018) 84(5):765–69. doi: 10.1038/s41390-018-0178-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lorenz L, Peter A, Arand J, Springer F, Poets CF, Franz AR. Reticulocyte haemoglobin content declines more markedly in preterm than in term infants in the first days after birth. Neonatology (2017) 112(3):246–50. doi: 10.1159/000477124 [DOI] [PubMed] [Google Scholar]
- 30. Mateos ME, De-la-Cruz J, Lopez-Laso E, Valdes MD, Nogales A. Reticulocyte hemoglobin content for the diagnosis of iron deficiency. J Pediatr Hematol Oncol (2008) 30(7):539–42. doi: 10.1097/MPH.0b013e31817580ca [DOI] [PubMed] [Google Scholar]
- 31. Ullrich C, Wu A, Armsby C, Rieber S, Wingerter S, Brugnara C, et al. Screening healthy infants for iron deficiency using reticulocyte hemoglobin content. JAMA (2005) 294(8):924–30. doi: 10.1001/jama.294.8.924 [DOI] [PubMed] [Google Scholar]
- 32. Lofving A, Domellof M, Hellstrom-Westas L, Andersson O. Reference intervals for reticulocyte hemoglobin content in healthy infants. Pediatr Res (2018) 84(5):657–61. doi: 10.1038/s41390-018-0046-4 [DOI] [PubMed] [Google Scholar]
- 33. Geng F, Mai X, Zhan J, Xu L, Zhao Z, Georgieff M, et al. Impact of fetal-neonatal iron deficiency on recognition memory at 2 months of age. J Pediatr (2015) 167(6):1226–32. doi: 10.1016/j.jpeds.2015.08.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Georgieff MK. Iron assessment to protect the developing brain. Am J Clin Nutr (2017) 106(Suppl 6):1588S–93S. doi: 10.3945/ajcn.117.155846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Breymann C. Iron deficiency anemia in pregnancy. Semin Hematol (2015) 52(4):339–47. doi: 10.1053/j.seminhematol.2015.07.003 [DOI] [PubMed] [Google Scholar]
- 36. Siddappa AM, Rao R, Long JD, Widness JA, Georgieff MK. The assessment of newborn iron stores at birth: A review of the literature and standards for ferritin concentrations. Neonatology (2007) 92(2):73–82. doi: 10.1159/000100805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cullis JO, Fitzsimons EJ, Griffiths WJ, Tsochatzis E, Thomas DW, British Society for H. Investigation and management of a raised serum ferritin. Br J Haematol (2018) 181(3):331–40. doi: 10.1111/bjh.15166 [DOI] [PubMed] [Google Scholar]
- 38. Ogawa C, Tsuchiya K, Maeda K. Reticulocyte hemoglobin content. Clin Chim Acta (2020) 504:138–45. doi: 10.1016/j.cca.2020.01.032 [DOI] [PubMed] [Google Scholar]
- 39. Piva E, Brugnara C, Spolaore F, Plebani M. Clinical utility of reticulocyte parameters. Clin Lab Med (2015) 35(1):133–63. doi: 10.1016/j.cll.2014.10.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.