Abstract
This review presents information from several studies that have demonstrated the antiviral activity of extracts (Andrographis paniculata, Artemisia annua, Artemisia afra, Cannabis sativa, Curcuma longa, Echinacea purpurea, Olea europaea, Piper nigrum, and Punica granatum) and phytocompounds derived from medicinal plants (artemisinins, glycyrrhizin, and phenolic compounds) against SARS-CoV-2. A brief background of the plant products studied, the methodology used to evaluate the antiviral activity, the main findings from the research, and the possible mechanisms of action are presented. These plant products have been shown to impede the adsorption of SARS-CoV-2 to the host cell, and prevent multiplication of the virus post its entry into the host cell. In addition to antiviral activity, the plant products have also been demonstrated to exert an immunomodulatory effect by controlling the excessive release of cytokines, which is commonly associated with SARS-CoV-2 infections.
Keywords: Antiviral activity, COVID-19, medicinal plant, SARS-CoV-2
Impact Statement
We currently live in the midst of a pandemic caused by a viral pathogen that has undergone conformational changes due to constant mutations, and has spread worldwide. While every tool against the virus – including vaccines, masks, social distancing, and antivirals – is vital, the development of efficient antivirals is still challenging. Several studies have demonstrated the promising in vitro effects of medicinal plants against SARS-CoV-2, and plant-derived compounds should therefore be considered for development as therapeutic agents. Here, we compiled the results for different plant extracts and compounds that exhibit promising antiviral activity. Further investigations involving animal models can help realize the potential of these molecules and help control SARS-CoV-2 infection.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the etiologic agent of the coronavirus disease 2019 (COVID-19), and was identified by the World Health Organization (WHO) as the causative agent of the outbreak of pneumonia of unknown cause that started in Wuhan City, Hubei Province, China, in December 2019. 1 SARS-CoV-2 is a positive-sense single-stranded enveloped RNA virus belonging to the Coronaviridae family. 2 While its initial records were from Wuhan, China, at the end of 2019, it was soon noticed on a global scale; thus, it was decreed by the WHO as a new coronavirus that caused the COVID-19. 3
This virus can reach different types of cells by interacting with the angiotensin-converting enzyme 2 (ACE2) receptor, 4 which is abundantly present in the lung and intestinal epithelia, as well as in the smooth muscle and endothelial cells of all organs, thus providing possible pathways for viral entry. 5 Consequently, COVID-19 is associated with multiple organ failure, which is one of the main causes of death due to SARS-CoV-2 infection. 6 Therefore, investigating molecules that can interfere with the interaction of SARS-CoV-2 with the host ACE2 receptors can offer new therapeutic options for COVID-19.
We now have anti-SARS-CoV-2 vaccines that have been shown to be effective against the virus, preventing, in most cases, the evolution to a more serious disease. In addition to vaccines, research efforts are focused on developing drugs that can act against the virus, with the aim of having one more weapon against the pathogen. The therapeutic effectiveness of remdesivir and corticosteroids has been demonstrated in some clinical trials.7,8 However, significant results have not been reported for the other drugs tested, including chloroquine, hydroxychloroquine, and lopinavir.9,10 Regardless, the scientific community has continued the search for more efficient therapeutic molecules.
Given the current pandemic scenario and the limited availability of effective prophylactic and curative drug therapies against SARS-CoV-2 infections, there is an urgent need to find effective therapeutics that are inexpensive and biocompatible. Perhaps, it is in the nature the active principle that will have the ability to interfere with the infectivity of SARS-CoV-2. In this article, we gathered recent in vitro studies that have demonstrated the antiviral effect of products derived from medicinal plants against SARS-CoV-2. Indeed, these plant candidates contain several bioactive molecules, such as polyphenols, which have been already reported to exhibit potent antiviral activity against several viruses. 11
Therefore, this review aims to compile and present promising results from recent studies that address the use of medicinal plant extracts and phytocompounds, to interfere with the actions of SARS-CoV-2 in host cells. Here, we have added a brief background on the plant products studied by the researchers, followed by the methodology used to evaluate their antiviral activity, and the main results of the research, as well as probable mechanism of action of the compounds (Table 1). The discovery of complementary products, such as those of plant origin, could serve as an additional therapeutic intervention against the new coronavirus that has spread worldwide, causing high mortality. Possible therapeutic approaches could be preventive or curative, using different pharmaceutical forms, such as syrups, elixirs, capsules, and tablets, which could take products from just one plant species or a mixture of them, given the possibilities of individual interactions of each product with the different sites in SARS-CoV-2 and also in the host cell. Therefore, the purpose of this work is to demonstrate the potential of using natural products as a strategy to control SARS-CoV-2. Although the studies presented here demonstrate in vitro activity, their findings could facilitate efficient screening plant compounds with potential for development as drugs against SARS-CoV-2 and other viruses. Another limitation of this study would be the reasonable amount of plant species or biocomponents researched. Therefore, we hope that this work is the first in a sequence of other studies produced by our group on the ability of plant products to control or eliminate emergency pathogens.
Table 1.
Summary of the main findings in the analyzed articles on the interaction of plant products with SARS-CoV-2.
| Plant product | Main findings | Site of action | Reference |
|---|---|---|---|
| Andrographis paniculata | 1. Inhibition of the virion reproduction. | - | Sa-Ngiamsuntorn et al. 17 |
| 2. More potent in the late stages of the viral life cycle than in the early stages of viral genome replication and protein synthesis. | Figure 1(A) and (B) | ||
| 3. Interference in the entry of SARS-CoV-2 into the host cell. | Figure 1(C) | ||
| Artemisia annua | 1. Action after entry of the SARS-CoV-2 into the host cell. | Figure 1(A) | Nair et al. 22 |
| 2. Regulation of the expression of pro-inflammatory cytokines by the infected cell. | – | ||
| Artemisia annua and Artemisia afra | 1. Synthesis of a transition complex through the iron atoms of the heme group and O1. | – | Nie et al. 31 |
| Artemisinins | 1. Inhibition of the SARS-CoV-2 nucleoprotein. | Figure 1(D) | Cao et al. 35 |
| 2. Increase of the endosomal pH. | Figure 1(E) | ||
| Cannabis sativa | 1. Regulation of ACE2 gene expression. | Figure 1(F) | Wang et al. 37 |
| 2. Decreased levels of TMPRSS2. | Figure 1(G) | ||
| Curcuma longa L. | Impaired binding of the spike glycoprotein of SARS-CoV-2 to the receptor ACE2. | Figure 1(H) | Bormann et al. 50 |
| Curcuma longa L. and Piper nigrum | 1. Impaired viral adsorption. | Figure 1(H) | Roshdy et al. 57 |
| 2. Deformations in surface proteins of the coronavirus. | – | ||
| 3. Regulation of the levels of cytokines Ikβα, TNF-α, and IL-6. | – | ||
| 4. Blocking of NF-κβ pathway. | – | ||
| Echinacea purpurea | 1. Impaired binding to receptors in the cell membrane. | Figure 1(F) to (H) | Signer et al. 59 |
| Glycyrrhiza glabra L. and glycyrrhizin | 1. Action on the SARS-CoV-2 protease Mpro, which mediates the proteolytic cleavage of viral polyproteins. | Figure 1(I) | van de Sand et al. 65 |
| 2. Impaired viral entry into the cell. | Figure 1(C) | ||
| Olea europaea L. | 1. Changes in the spike protein. | Figure 1(K) | Takeda et al. 75 |
| 2. Damage to the viral genome, compromising the protein translation by the coronavirus. | Figure 1(L) | ||
| Phenolic compounds | 1. High binding capability to the SARS-CoV-2 RBD-spike protein, impairing its adsorption to the host cell. | Figure 1(H) and (K) | Goc et al. 83 |
| Punica granatum L. | 1. Inhibition of the binding of the viral spike protein to the ACE2 receptor. | Figure 1(H) | Tito et al. 94 |
| 2. Inhibition of 5α-reductase and protease Mpro, which are closely related to SARS-CoV-2 replication. | – |
ACE2: angiotensin-converting enzyme 2; TMPRSS2: transmembrane protease, serine 2; Ikβα: inhibitor kappa beta alpha protein; TNF-α: tumor necrosis factor-α; IL-6: interleukin-6; NF-κβ: nuclear factor kappa β; RBD: receptor-binding domain.
Andrographis paniculata
Background
A. paniculata has been reported to exhibit several pharmacological activities, 12 including resistance against common cold, diarrhea, and fever caused by infections, in addition to being used as a health tonic. 13
Andrographolide is one of the main bioactive components of this vegetable, 14 which has been reported to exhibit broad-spectrum antiviral activity. 15 It was found to inhibit the action of the main protease of SARS-CoV-2. 16 In addition, Sa-Ngiamsuntorn et al. 17 evaluated the efficacy of the A. paniculata extract and andrographolide in an in vitro model of SARS-CoV-2 infection induced in lung epithelial cells (Calu-3).
Methodology
Sa-Ngiamsuntorn et al. 17 used kidney epithelial cells extracted from the African green monkey (Cercopithecus aethiops) (Vero), Vero cell derivative (Vero E6), human liver cancer cell line (HepG2), human colon cancer cell line (Caco-2), human airway epithelial cell line (Calu-3), human neuroblastoma cell line (SH-SY5Y), human normal kidney (HK-2), and an immortalized hepatocyte-like cell line (imHC). SARS-CoV-2 was isolated from a patient with COVID-19 via a nasopharyngeal swab. The effects of A. paniculata crude extract and the phytocompound andrographolide were evaluated on the virus, normal cells, and infected cells. For evaluation of antiviral activity, Calu-3 cells infected with SARS-CoV-2 were treated for 48 h with different dilutions of A. paniculata extract or andrographolide, and extracellular viral RNA levels were measured using quantitative reverse transcription–polymerase chain reaction (qRT-PCR). In addition, the cytotoxicity of the plant products was investigated in the different cell types mentioned above using the MTT assay.
Main findings
Both the A. paniculata extract and androgradophile were shown to inhibit SARS-CoV-2 replication in a dose-dependent manner, as demonstrated by the reduction in viral plaque in Calu-3 cultures. The plant products also reduced extracellular levels of viral RNA, suppressing the infectivity of the coronavirus. No cytotoxicity was observed for all the cell types studied, with CC50 > 100 μg/mL.
Mechanisms of action
A. paniculata products were able to inhibit virion reproduction. In addition, they may be more potent in the late stages of the viral life cycle in Calu-3 cells (Figure 1(A)) than in the early stages of viral genome replication and protein synthesis (Figure 1(B)). These products can also interfere with the entry of SARS-CoV-2 into the host cell (Figure 1(C)), as demonstrated by a reduction in the amount of viral RNA in the cell cultures.
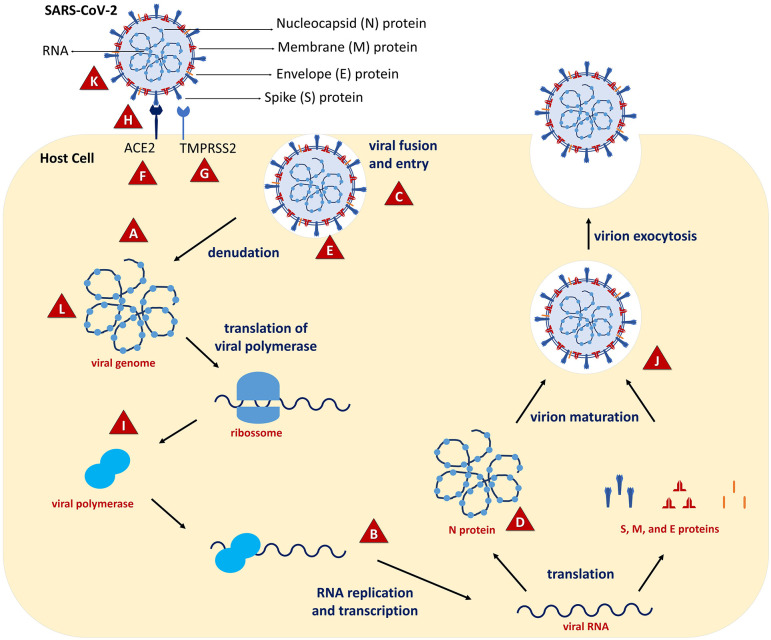
Figure 1.
Interaction sites of the plant products within the SARS-CoV-2 replication cycle. Some plant products are able to impair the entry of the virus onto host cell (C). They can bind the surface proteins of the virus, such as the spike glycoprotein, impairing its binding to the ACE2 receptor in the host cell (H). In addition, the plant products can reduce the activity of the ACE2 and other receptors, such as TMPRSS2 (F and G). They also cause deformations in the spike protein (K), which compromises its interaction with ACE2 and viral envelope molecules. The plant products can also interfere with some phases of virus multiplication after viral penetration into the host cells. Studies demonstrated that they can inhibit some viral enzymes responsible for SARS-CoV-2 replication (J), such as protease Mpro and 5α-reductase (A, L, I, and B). Also, an inhibitory effect on the SARS-CoV-2 nucleoprotein was observed (D), and damage to the viral genome has also been reported (L). Endosomal pH changes were also observed, leading to destruction of viral particles inside the host cell (E). Plants products and interaction sites: A. paniculata (A to C); Artemisinins (D and E); C. sativa (F and G); C. longa L. and P. nigrum (H); E. purpurea (F and H); G. glabra L. and glycyrrhizin (C, I, and J); O. europaea L. (K and L); phenolic compounds (H and K); P. granatum L. (H). (A color version of this figure is available in the online journal.)
Artemisia annua
Background
Native to Eastern Europe and Asia, A. annua is distributed worldwide. 18 As a medicinal plant, it has been used for centuries in Asia for the treatment of recurrent fever. Over the years, its use has also been indicated in herbal therapy against malaria. 19 A major component of A. annua is the flavonoid artemisinin, which is a known antimalarial compound, and also exhibits antineoplastic, antimicrobial and anti-inflammatory activities. 20 The antiviral capacity of A. annua has been investigated against the coronavirus strain that caused the first SARS epidemic in 2002, with encouraging results obtained regarding the in vitro inhibition of SARS-CoV-1 in a dose-dependent manner. 21 Thus, in the recent study by Nair et al., 22 the antiviral efficacy of the A. annua extract was verified against SARS-CoV-2 and its two variants in an in vitro infection model to help develop the plant as an economical and safe therapeutic alternative.
Methodology
The study used seven cultures of A. annua, the leaves of which were obtained in different years and from countries on four different continents. Infusions were prepared by boiling dried leaves in hot water, followed by sieving, cooling, and sterilization of the extract by filtration. Dry leaf extracts were also prepared using dichloromethane as solvent. The concentrations of artemisinin and flavonoids in the hot water extracts were then determined. Vero E6 and Calu-3 cell lines were inoculated in vitro with a SARS-CoV-2 isolate and the UK (B1.1.7) and South African (B1.351) variants. The infected cell cultures were subsequently treated with A. annua extract or the phytocompounds amodiaquine, artesunate, artemether, artemisinin, deoxy artemisinin, and dihydroartemisinin to compare their inhibitory effects on coronaviruses. In addition, control Vero E6 cells were treated with the extract to determine any potential cytotoxic effects of the plant products.
Main findings
The A. annua hot water extracts were found to contain 20.1–149.4 µg/mL artemisinin and 7.3–37.2 µg/mL flavonoids. Extracts from all seven A. annua cultures tested exhibited antiviral activity against all three SARS-CoV-2 variants, with maximum inhibitory concentrations between 13.5 and 57.4 µg/mL of dry weight. In addition, the hot water extracts did not cause any significant decrease in post-treatment cell viability, unlike the cytotoxic effects of the dichloromethane extracts. Among the constituent phytocompounds, amodiaquine, artemisinin, and artemether also exhibited antiviral activity, although the last two substances were also identified as cytotoxic. No protective antiviral activity was observed for artesunate or dihydroartemisinin. Surprisingly, higher maximum inhibitory concentrations were observed with increased concentrations of artemisinin and flavonoids contrary to expectations, suggesting that another phytocompound derived from A. annua was responsible for the suppression of SARS-CoV-2.
Mechanism of action
The mechanism behind the control of SARS-CoV-2 infection by A. annua is not yet fully understood. Considering the findings described in this study, it is believed that the viral inhibition promoted by A. annua extract is related to a stage of virus multiplication after entry into the host cell (Figure 1(A)). Recent studies have demonstrated that some bioactive compounds from A. annua may bind to the active site and inhibit the viral protease Mpro, which is essential for the replication process of the coronavirus. 23
A. annua and its constituent compounds have also been suggested to act as modulators of the human immune system. The plant extract can regulate the expression of pro-inflammatory cytokines by inhibiting toll-like receptors and other proteins that participate in the signaling cascade, such as tyrosine kinase and phospholipase, providing relief from the cytokine storm observed in SARS-CoV-2 infections. 24
Artemisia annua and Artemisia afra
Background
Artesunate and artemisinin are phytocompounds of A. annua that are indicated by the UN to act against malaria, as they may have few side effects. 25 Artemisinin and its synthetic derivatives have been demonstrated to act against parasitic diseases, 26 some types of cancer, 27 and viruses, 28 both in vitro and in human clinical trials.
A. annua extract has been demonstrated to exhibit in vitro antiviral activity against SARS-CoV-1 and SARS-CoV-2. 21 In a recent study, artesunate was shown to exhibit better in vitro activity against SARS-CoV-2 than A. annua extract. 29
At the beginning of the pandemic, traditional medicines made from plant extracts were used in several countries to prevent or treat COVID-19. The use of A. afra tea has been reported in South Africa, although there is no clear scientific evidence. 30
Methodology
In a recent study, extracts of A. annua and A. afra, as well as “Covid-Organics” were investigated for their anti-SARS-CoV-2 effects in vitro 31 using Vero E6 and Crandell-Rees Feline Kidney (CRFK) cell lines. The anti-SARS-CoV-2 activity of the plant products was investigated using the SARS-CoV-2 BavPat 1 isolate (SARS-CoV-2/human/Germany/BavPat 1/2020) and a feline coronavirus (FCoV). The plant products prepared at 10 different concentrations were mixed with the coronaviruses. These suspensions were then added to the cultures of Vero E6 and CRFK, and the inhibitory effect on plaque formation was determined. The cytotoxic effect of the plant products was evaluated using a Cell Counting Kit-8.
Main findings
The plant products impaired viral replication in a dose-dependent manner, with significant reduction in their antiviral activity at concentrations below 1 mg/mL. The drink “Covid-Organics” demonstrated a moderate inhibitory activity against FCoV. While treatment with both plant extracts caused a reduction in SARS-CoV-2 plaque formation, A. annua was observed to be more effective against FCoV than A. afra. Cytotoxicity tests revealed that the concentrations of extracts employed to inhibit SARS-CoV-2 infection were nontoxic for the cells.
Mechanism of action
The mechanism of action of artemisinin has been suggested to involve the construction of a transition complex through the iron atoms of the heme group and O1. The position of artemisinin in relation to heme is due to stereoelectronic interactions between them, which affect the rearrangement of the complex until the breakage of the Fe–O bond. Further studies are required to understand the relationship between the molecular structures of heme and artemisinin to understand their interaction with their respective receptors. 32
Artemisinins
Background
Artemisinin, extracted from A. annua, was first cited in Chemical Abstracts. Interestingly, this vegetable is named after its bitter taste. Considering the potential of chloroquine and its derivative hydroxychloroquine to inhibit SARS-CoV-2 replication, it is possible that artemisinins, which are known to exhibit antimalarial and immunomodulatory action, could also be effective.33,34
Studies have demonstrated the broad-spectrum antiviral potential of artemisinin-derived molecules such as artesunate, which has demonstrated efficacy against cytomegalovirus and human herpes simplex virus (HSV), among a range of DNA and RNA viruses. 28
Methodology
Cao et al. 35 investigated the anti-SARS-CoV-2 effect of some artemisinins in vitro, using Vero E6 cells and the nCoV-2019BetaCoV/Wuhan/WIV04/2019 strain of SARS-CoV-2. The plant compounds arteannuin B, arteether, artemether, artemisinic acid, artemisinin, artemisone, artesunate, dihydroartemisinin, and lumefantrine were analyzed. Cytotoxicity was determined using a cell counting kit-8 assay. For evaluation of antiviral activity, Vero E6 cell cultures were first exposed to the plant compounds and then infected with the virus. Viral RNA was extracted from these samples, and quantified using qRT-PCR to determine the viral load. SARS-CoV-2 nucleoprotein was detected using immunofluorescence assays and western blotting. Pharmacokinetic modeling and simulations were performed using the Simcyp simulator (version 18), and all simulations were based on virtual clinical trials composed of groups of prevalidated built-in “Healthy Volunteer” populations.
Main findings
Half-maximal effective concentrations (EC50) against SARS-CoV-2 were determined to be >100 μM (artemisic acid), 73.80 ± 26.91 μM (artemether), 64.45 ± 2.58 μM (artemisinin), 49.64 ± 1.85 μM (artemisone), 31.86 ± 4.72 μM (arteether), 23.17 ± 3.22 μM (lumefantrine), 13.31 ± 1.24 μM (dihydroartemisinin), 12.98 ± 5.30 μM (artesunate), and 10.28 ± 1.12 μM (arteannuin B). Immunofluorescence assays revealed inhibition of SARS-CoV-2 nucleoprotein in a dose-dependent manner with several artemisinins. Complete inhibition of nucleoprotein expression was observed with arteannuin B (25 μM), and partial inhibition with artesunate (25 μM), dihydroartemisinin (25 μM), and lumefantrine (100 μM). In particular, arteannuin B (25 μM) and lumefantrine (100 μM) were found to be effective only after the virus entered the cell, exerting an inhibitory effect on viral RNA and its proteins. Thus, it is suggested that such phytocompounds could be useful for blocking intracellular events such as viral biosynthesis. The pharmacokinetic assay revealed that lumefantrine had low hepatic clearance and low renal excretion, with a prolonged half-life of up to six days, as observed in healthy volunteers. After six oral doses for three days, lumefantrine (EC50 = 480 mg) was detected in the plasma and lungs. These findings demonstrate the anti-SARS-CoV-2 potential of lumefantrine.
Mechanism of action
The probable mechanism of action of artemisinins, specifically arteannuin B and lumefantrine, is related to their inhibitory effect on the SARS-CoV-2 nucleoprotein (Figure 1(D)), as demonstrated by Cao et al. 35 In addition, artemisinins have also been suggested to increase endosomal pH, and thus cause shedding of the virus already present within the cell 36 (Figure 1(E)).
Cannabis sativa
Background
Wang et al. 37 investigated the potential of C. sativa, especially one of its cannabinoids, cannabidiol (CBD), to modulate the expression of ACE2, which is responsible for the entry of SARS-CoV-2 into the host cell.
CBD has been reported to exert analgesic, anti-inflammatory, anxiolytic, antioxidant, anticonvulsant, and cytotoxic effects. In addition, C. sativa can affect gene expression and control inflammation. 38 Other studies have also assessed the therapeutic potential of C. sativa in combating cancer and various inflammatory diseases.38,39 Therefore, patients and physicians are increasingly interested in CBD (inhaled, oral, or topical) as a potential adjuvant or alternative therapeutic agent against some diseases.38,40,41
Methodology
In the study by Wang et al., 37 the therapeutic effect of CBD extracts was evaluated on inflammation induced by pro-inflammatory cytokines (10 ng/mL tumor necrosis factor-α [TNF-α]/interferon gamma [IFN-γ]) in three-dimensional artificial human tissue models of the oral cavity, respiratory tract, and small intestinal epithelium. Gene expression of ACE2 and the serine protease TMPRSS2, which also plays an important role in SARS-CoV-2 infection, was analyzed at the mRNA level.
Main findings
As a result, downregulation of ACE2 gene expression was observed on treatment with several extracts of C. sativa. The extracts modulated ACE2 expression in inflammation-stimulated artificial tissues, whereas CBD or cannabidiol alone did not interfere with ACE2 levels. Notably, despite similar amounts and molar proportions of the main cannabinoids, tetrahydrocannabinol and CBD in the analyzed extracts, not all extracts were equally effective, with some extracts exerting unwanted molecular effects on ACE2 and TMPRSS2 gene levels. This finding emphasizes that different medicinal cannabis crops can have different effects, and that the potency of each cannabis crop must be analyzed in detail. The extracts also differed in specificity; the same extract positively or negatively regulated the expression of ACE2 depending on the type of tissue in which it was used. Importantly, the results of the study cannot be extrapolated to the effects of marijuana smoking. A recent study has shown that smoking increases ACE2 levels and exacerbates the clinical outcomes of COVID-19. 42
Mechanism of action
The mechanism of antiviral action is related to the regulation of ACE2 gene expression (Figure 1(F)) triggered by C. sativa extracts. 37 However, the mechanism by which the plant compounds induce the downregulation of gene expression is still not clear. Furthermore, some C. sativa extracts decreased the levels of TMPRSS2 (Figure 1(G)), another critical protein in the entry of SARS-CoV-2 into the host organism, in contrast to upregulation by CBD alone. 37 Thus, further studies are required to facilitate better understanding of the mechanisms involved in antiviral action.
Curcuma longa L.
Background
C. longa L. contains a wide variety of bioactive phytochemicals, 43 among which curcumin has been extensively reported to exhibit antioxidant, anti-inflammatory, antibacterial, antifungal, antiviral, antitumor, and hepatoprotective activities.44 –46
Curcumin has been reported to be effective against several viruses such as dengue virus, human immunodeficiency virus, Kaposi sarcoma–associated herpesvirus, enterovirus, Zika virus, chikungunya virus, vesicular stomatitis virus, human respiratory syncytial virus, viral hemorrhagic septicemia virus, influenza A virus, herpes simplex type 2, norovirus, and hepatitis C virus.47,48 Chen et al. 49 demonstrated, for example, a 90% reduction in influenza virus yield on curcumin treatment, which was also reflected in the inhibition of hemagglutination.
Methodology
Based on these findings, the possibility that curcumin can also act effectively against SARS-CoV-2 has been explored. Bormann et al. 50 investigated the ability of C. longa root extract, a curcumin-containing nutritional supplement, and pure curcumin to neutralize this coronavirus in vitro.
In this study, the aqueous extract of C. longa L. root, a nutritional supplement capsule containing curcumin (640 mg of C. longa L. powder, 105 mg of C. longa L. extract with 99.9 mg of curcumin, and 5 mg of black pepper with 4.7 mg of piperine) and curcumin were tested. To verify their ability to neutralize SARS-CoV-2, the plant products were added at different concentrations to the cultures of an SARS-CoV-2 isolate obtained from the nasopharyngeal swab of a hospitalized patient with COVID-19, and the virus was then used to infect Vero 6 and Calu-3 cells. The ability to neutralize SARS-CoV-2 was evaluated in the Vero E6 cells, and using an in-cell ELISA (icELISA)-based neutralization test in the Calu-3 cells. The Orangu colorimetric test was used for cell viability analysis. SARS-CoV-2 RNA levels after treatment with the plant products were quantified using qRT-PCR.
Main findings
Complete neutralization of SARS-CoV-2 was achieved using the aqueous extract at 1:32 dilution. Furthermore, this dilution did not exert a cytotoxic effect on Vero E6 and Calu-3 cells. The curcumin supplement capsules and pure curcumin promoted similar neutralization of the coronavirus at 14.6 and 15.6 µg/mL, respectively, without exerting any cytotoxicity. In addition, curcumin reduced SARS-CoV-2 RNA levels in both cell supernatants at a concentration of 14 µg/mL, indicating a legitimate antiviral effect. Curcumin probably acts by triggering the inhibition of viral entry into cells.
Mechanism of action
These results suggest that curcumin and C. longa L. extract may interfere with the binding of the spike glycoprotein of SARS-CoV-2 to its host cell receptor ACE250,51 (Figure 1(H)). Furthermore, adding piperine has been proven to increase the bioavailability of curcumin by 2000% in rats and humans, without adverse events, indicating that piperine could be a good adjunct to curcumin supplementation 52 .
Curcuma longa L. and Piper nigrum
Background
C. longa L. belongs to the Zingiberaceae family, and has been used in cooking as a food colorant and additive, as well as being widely investigated as a phytotherapeutic agent. Its main phytocompound, curcumin, is responsible for numerous biological effects, including suppression of tumor cell growth, 53 anti-inflammatory effects in chronic diseases 54 and antiviral action against Zika and chikungunya viruses. 55 However, the low bioavailability of curcumin has necessitated studies on other agents that can be added to improve this limiting pharmacokinetic factor. Piperine, the main ingredient of P. nigrum, is one such compound that can promote up to 2000% higher bioavailability of curcumin when they are administered together orally. 56
Consequently, the herbal extract EGYVIR was created as a combination of the root extract of C. longa L. with P. nigrum extract, exerting immunomodulatory and antiviral activity against SARS-CoV-2. 57
Methodology
According Roshdy et al., 57 the EGYVIR extract was prepared using 40 mg of C. longa L. root and 100 mL of P. nigrum extract. The chemical composition of EGYVIR was analyzed using gas chromatography–mass spectrometry. Vero E6 cells were infected with SARS-CoV-2 (HCoV-19/Egypt/NRC-03/2020). Antiviral activity of EGYVIR was verified using a plate reduction assay after 1 h exposure of the virus to different EGYVIR concentrations. The viral RNA levels in the supernatants were analyzed using qRT-PCR. To understand the mechanism of action of the plant product, the viral replication, viral adsorption, and virucidal mechanisms were studied. TNF-α, interleukin-6 (IL-6), nuclear factor kappa β (NF-κB p50), and inhibitor kappa beta alpha protein (Ikβα) levels were also quantified in supernatants of human hepatocellular carcinoma (Huh7) cells infected with SARS-CoV-2 and treated with EGYVIR, using ELISA and qRT-PCR. In addition, the cytotoxicity of EGYVIR was evaluated using MTT assay.
Main findings
Fifty-three compounds were detected in EGYVIR: with pentatriacontane (41.04%), amyrin (9.49%), lupeol (8.86%), turmerone (8.13%), sitosterol (7.61%), bisdemethoxycurcumin (6.8%), piperine (4.6%), vitamin D3 (1.76%), and curcumin (1.3%) being the most abundant. EGYVIR was verified to be noncytotoxic to Vero E6 at the concentrations used, while exerting significant control of in vitro infection by SARS-CoV-2, with up to 92% plaque reduction. The levels of viral RNA detected by qRT-PCR also decreased significantly with EGYVIR treatment, to 78% that of the untreated control. The transcriptional levels Ikβα and TNF-α decreased two- to four-fold, and that of IL-6 reduced in the Huh7 cell supernatant on EGYVIR administration in a dose- and time-dependent manner compared to the untreated control.
Mechanism of action
Roshdy et al. 57 verified that EGYVIR impaired the binding of SARS-CoV-2 to its receptor in the host cell (prevented viral adsorption) (Figure 1(H)), and caused deformations in surface proteins of the virus (virucidal activity), in addition to inhibiting its replication. It was shown to act directly on the surface proteins of the coronavirus, such as receptor-binding domain (RBD), thus preventing its binding to ACE2 on the host cell. In addition to its antiviral mechanism, EGYVIR regulated the levels of cytokines Ikβα, TNF-α, and IL-6. In addition, EGYVIR may act by blocking the NF-κβ pathway and inhibiting the nuclear translocation of the p50 subunit, resulting in a reduction in the synthesis of IL-6 and TNF-α.
Echinacea purpurea
Background
E. purpurea is one of the main plants used in the treatment of respiratory symptoms resulting from upper airway infections. It is found mostly in eastern North America and is used extensively in the United States and Europe. 58
The beneficial effects of Echinaforce®, a commercial product containing E. purpurea extract in a 65% alcoholic solution, have been demonstrated to exhibit, with significant antiviral activity, facilitating its application as a therapeutic option for cases of influenza infection. 58
Subsequently, the effectiveness of Echinaforce® in controlling other viruses that cause respiratory infections, such as HCoV-229E, MERS-CoV, SARS-CoV-1, and SARS-CoV-2, was analyzed. In addition, the protective capacity of this herbal medicine against human airway epithelium-like cells has also been studied. 59
Methodology
For this study, Echinaforce® was obtained from a hydroethanolic extraction, with a final concentration of 65% ethanol and 16 mg/mL of E. purpurea dry mass. Several cell lines, including human liver cells (Huh-7), Vero cells, Vero E6 cells, and areolar adipose tissue cells (A9) were used in the study. In addition to respiratory viruses, other viruses such as mouse parvovirus, yellow fever virus (YFV), and vaccinia virus (VACV) were also chosen. In vitro reconstituted human airway epithelia were also used as a testing model, in addition to cells obtained from three healthy donors. Human cells were exposed in vitro to a series of dilutions of Echinaforce® and then incubated for measurement of cell viability using MTT or Alamar Blue assays. HCoV-229E was incubated with different concentrations of Echinaforce® as pretreatment, and the solutions were then washed and filtered to estimate residual infectivity. In addition, human cells were pretreated with Echinaforce®, followed by removal of the extract and introduction of HCoV-229. The infected cell culture was then washed and again subject to postinfection treatment with Echinaforce®. Similarly, respiratory cells from the reconstituted human nasal epithelium were pretreated with Echinaforce® on the apical surface. After mucus removal, cells were infected with HCoV-229E. The culture was then apically washed, and viruses were determined using a limiting dilution assay. This procedure was replicated for other coronaviruses (MERS-CoV, SARS-CoV-1, and SARS-CoV-2), YFV, VACV, and mouse minute virus (MVM). For viral quantification, the samples were serially diluted, incubated, and stained with crystal violet
Main findings
Treatment with Echinaforce® caused a dose-dependent reduction in the infectivity of HCoV-229E, with a half-maximal inhibitory concentration (IC50) of 3.2 µg/mL and complete inactivation of the virus using 50 µg/mL extract. In addition, cell viability in samples treated with Echinaforce® was not impaired by the application of the evaluated concentrations. The findings from this study indicate that pretreatment of virions with Echinaforce® has greater therapeutic potential than treatment of HCoV-229E-infected cells. Furthermore, prophylaxis in uninfected cells was ineffective at all concentrations of the extract. In the samples of reconstituted respiratory epithelium, pretreatment of the cells was efficient in inhibiting the replication of the virus after infection, with a lower viral titer observed with 50 µg/mL extract than that with 10 µg/mL extract. Thus, the alcoholic extract of E. purpurea was found to exhibit antiviral activity against infections caused by HCoV-229E, in addition to a protective effect against MERS-CoV, SARS-CoV-1, SARS-CoV-2, and YFV. Total inactivation of these viruses was observed using Echinaforce® at 50 µg/mL. In contrast, VACV and MVM infections are resistant to the use of this herbal medicine.
Mechanism of action
Although the mechanism of antiviral action of Echinaforce® is not fully understood, it is known to act on extracellular viruses, preventing their binding to receptors present in the cell membrane (Figure 1(F) to (H)). Signer et al. 59 observed inhibition of the binding of HCoV and other enveloped RNA viruses to cells. This effect caused the permanent inactivation of the virions and reduction of their infectivity by altering the conformation of structural proteins and molecules of the viral envelope.
In addition, Echinaforce® was found to reduce the signs and symptoms of upper airway infections such as sneezing, coughing, and nasal discharge. A reversal of the discharge of cytokines, especially IL-6 and IL-8, was observed on treatment with Echinaforce®, thus elucidating its anti-inflammatory effect. 60
Glycyrrhiza glabra L. and glycyrrhizin
Background
G. glabra L., popularly called licorice, belongs to the Fabaceae family, is native to Eurasia, and is currently used in some countries for commercial purposes. Its dried root is consumed as a sweet, and its extract is used as a flavoring agent, sweetener in cooking, and an herbal medicine. 61 One of its main phytocompounds is glycyrrhizin, which has recognized pharmacological potential, 62 including an inhibitory effect on HSV, HIV, and coronaviruses.63,64
Methodology
In a recent study, van de Sand et al. 65 verified the ability of G. glabra L. extract and glycyrrhizin to inhibit SARS-CoV-2 replication. G. glabra L. root extract and pure glycyrrhizin were analyzed for their activity on Vero E6 cells infected with a clinical isolate of SARS-CoV-2 obtained from the nasopharyngeal swab of a patient with COVID-19. SARS-CoV-2 was pretreated with the plant extract for 1 h, and then inoculated into the Vero E6 cell culture. Viral plaques were stained with crystal violet dye and counted to determine the neutralization effect of the plant extract. The antiviral potential of glycyrrhizin was analyzed in cells already infected with coronaviruses. Viral RNA levels, as well as the inhibitory effect on the main protease (Mpro) of SARS-CoV-2, were quantified by qRT-PCR. In addition, the cytotoxic effect of the plant products on Vero E6 cells was also verified using the Orangu cell counting solution test, after exposure periods of 5 min, 4, 12, and 24 h.
Main findings
The G. glabra L. extract showed a significant antiviral effect at a subtoxic concentration of 2 mg/mL, as determined using Vero E6 cells. Likewise, the phytocompound glycyrrhizin also blocked SARS-CoV-2 replication at 0.5 mg/mL with pre- and post-viral-entry treatment, and at 1 mg/mL with only postentry treatment. Glycyrrhizin also significantly reduced viral RNA levels in cell supernatants, at a concentration of 1 mg/mL, and inhibited Mpro activity at 1.6 mg/mL, which confirms its antiviral effect.
Mechanism of action
The antiviral effect of G. glabra L. can be attributed to the action exerted by glycyrrhizin on the SARS-CoV-2 protease Mpro, also called 3CL protease, which mediates the proteolytic cleavage of polyproteins necessary for viral replication and transcription (Figure 1(I)). 66 The phytocompound impaired viral entry into the cell (Figure 1(C)) as well as viral replication (Figure 1(J)), as demonstrated by the significant decrease in viral RNA levels.
Olea europaea L.
Background
Both O. europaea extract and olive oil are rich in the polyphenol hydroxytyrosol (HT). 67 Several biological activities have been attributed to olive polyphenols, such as antioxidant, anti-inflammatory, anticancer, antimicrobial, and antiviral effects, mainly against the influenza A virus and human immunodeficiency virus (HIV).68 –70
The commercially available product HIDROX® contains a hydroxytyrosol-rich aqueous olive pulp extract whose biocompatibility has been demonstrated in vitro and in vivo. 71 In addition, it has been shown to confer several beneficial effects, such as anti-inflammatory action in mice 72 and improvement of routine activities in individuals with rheumatoid arthritis. 73 However, studies indicate that the biological effects are more pronounced when hydroxytyrosol-rich extract is used rather than the isolated phytocompound. 74
The anti-SARS-CoV-2 activity of isolated HT and 12% HIDROX® has been evaluated in the form of solutions and creams for topical dermatological use. 75
Methodology
This study used SARS-CoV-2 (JPN/TY/WK-521) with Vero E6 cells expressing serine transmembrane protease 2 (Vero E6/TMPRSS2). After cell growth, SARS-CoV-2 was inoculated in the culture medium. For the analysis of antiviral activity, a suspension of SARS-CoV-2 was added to a solution containing HIDROX® or HT, in a proportion of 1:9. These solutions were inoculated into cells to assess the cytopathic effects and viral titers. Western blotting was used to evaluate the effect of the products on the structures of viral proteins, such as spike protein S1 subunit, RBD, S2 subunit, and nucleocapsid protein. The interaction of the plant products with the carbohydrate chains in the spike protein was evaluated using PNGase F PRIME Glycosidase. The antiviral effect of the HIDROX® cream was tested by adding 20 mg of the product to a polyethylene terephthalate film (2.25 cm2) that came into contact with a viral suspension.
Main findings
SARS-CoV-2 was inactivated on treatment with HIDROX® at different concentrations and exposure times, such as 4.5 mg/mL/0.5 h (99.68%), 0.9 mg/mL/1 h (98.53%), 0.45 mg/mL/1 h (90%), 5.63 mg/mL/5 min (86.67%), and 11.25 mg/mL/5 min (95.78%). In addition, the extent of SARS-CoV-2 inactivation on treatment with the isolated HT did not differ significantly from that on treatment with the HIDROX® extract. Western blotting revealed that both HIDROX® and HT affected the molecular weight of SARS-CoV-2 structural proteins, especially the spike protein expressed on the viral surface. However, the extract was again more potent than the isolated phytocompound. In addition, neither plant product interfered with the carbohydrate chain of the spike protein, suggesting that this may not be a target for HIDROX® or HT. Real-time RT-PCR verified that the evaluated products also significantly impacted the viral genome. Inactivation of SARS-CoV-2 was also observed on treatment with the HIDROX® cream at different concentrations and exposure times, such as 10%/10 min (94.38%), 5%/10 min (79.47%), and 2%/30 min (94.38%). Therefore, cream containing HIDROX® could be an alternative product for hand hygiene, for example, to eliminate SARS-CoV-2 from the surface.
Mechanism of action
HIDROX® probably acts by causing structural changes in the spike protein present on the viral surface (Figure 1(K)), which would result in the aggregation of the polyphenol–protein complex. 76 Another potential mechanism of action is related to damage to the viral genome (Figure 1(L)), which could compromise the effectiveness of viral protein translation. A study on green tea catechins found that HT was also able to generate reactive oxygen, and thus damage nucleotides. 77
Phenolic compounds
Background
Polyphenols constitute an important and the largest group of phytochemicals, presenting a wide spectrum of properties and acting in different physiological and biochemical processes.78 –80 They can be classified as hydroxybenzoic acids, hydroxycinnamic acids, flavonoids, stilbenes, and lignans, many of which have been proven to be therapeutically effective. 81 The antiviral potential of these phytocompounds has been recognized, as well as their safety and efficacy in integrative and complementary therapeutic approaches. 82
Methodology
Goc et al. 83 evaluated the potential of phenolic compounds to inhibit the binding and entry of SARS-CoV-2 into the host cell. In this study, a human alveolar epithelial cell line (A549) stably overexpressing the hACE2 receptor (hACE2/A549) and eGFP-luciferase-SARS-CoV-2 spike glycoprotein pseudotyped particles was used. Several phytocompounds – including phenolic acids, flavonoids, stilbenes, alkaloids, and terpenes – were evaluated. The binding reaction of SARS-CoV-2 to the hACE2 RBD was performed using the SARS-CoV-2 surrogate virus neutralization test kit, while that of SARS-CoV-2 pseudovirus to hACE2 was performed using a protocol developed by GenScript. In addition, cell–cell fusion, TMPRSS2 activity, cathepsin L activity, ACE2 activity, endosomal and polysomal pH, and cell viability assays were performed.
Main findings
Of the 56 phytocompounds evaluated, only three showed significant ability to inhibit the binding of horseradish peroxidase (HRP)-conjugated RBD-SARS-CoV-2 spike protein to the immobilized hACE2 receptor. Brazilin, theaflavin-3,3’-digallate (TF-3), and curcumin showed the highest inhibitory effect. Furthermore, these phytocompounds were able to solubilize the hACE2 receptor after 1 h exposure. No significant reduction in cell viability was observed up to 3 h exposure, although the cell viability decreased after 48 h. These phytocompounds inhibited the binding of virions pseudotyped with SARS-CoV-2 spike protein to hACE2/A549 in a dose-dependent manner. In addition, inhibition of TMPRSS2 activity was observed; however, ACE2 and TMPRSS2 levels were not affected by treatment with the phytocompounds. Inhibition of cathepsin L activity and an increase in endosomal and lysosomal pH were also observed.
Mechanism of action
Brazilin, TF-3, and curcumin exhibited the highest binding to the SARS-CoV-2 RBD-spike protein (Figure 1(K)), with all three compounds inhibiting viral binding to the cell surface (Figure 1(H)) regardless of the exposure time or incubation pattern. The ability of SARS-CoV-2 to bind the ACE2 receptor decreased (Figure 1(H)) on preincubation of the virus with these compounds or interaction after infection was induced. In addition, these phytocompounds reduced the fusion of the spike protein to its receptor in the host cell (ACE2), indicating that the three compounds have inhibitory properties especially directed toward RBD-SARS-CoV-2, as well as on cellular proteases involved in the steps of viral infection. Although the mechanism behind inhibition of TMPRSS2 activity is not clear, it is certain that this action impedes viral binding to the cell surface. 83
Punica granatum L.
Background
P. granatum L. contains several polyphenols in its fruit, including ellagitannins, gallic acid, ellagic acid (EA), and anthocyanins, 84 which are responsible for its numerous biological activities, such as anticancer and antidiabetic properties, and effectiveness against atherosclerosis and cardiovascular disease. In addition, it is effective against bacteria and viruses.85 –87
The antiviral action of some polyphenols has been reported against the Epstein–Barr virus, 88 enterovirus, 89 HSV, 90 and influenza virus. 91 Several studies on the action of polyphenols against SARS-CoV-2 have also been conducted using curcumin, kaempferol, catechin, naringenin, quercetin, hesperidin, rutin, and diosmin.92,93
Methodology
Tito et al. 94 verified the potential of pomegranate peel extract (PPE) inhibit in vitro the interaction of the SARS-CoV-2 spike protein with its ACE2 receptor, and consequently, the entry of the coronavirus in the host cell. In addition, its ability to impair the action of 3CL viral protease was verified. The extract from the seeds of P. granatum L. was analyzed using high-resolution mass spectrometry to determine its composition, and its antioxidant activity was also determined. The ability of PPE to inhibit spike–ACE interaction was analyzed using the SARS-CoV-2 inhibitor screening assay kit, in human kidney cells (HK-2) infected with lentivirus pseudotyped with SARS-CoV-2 spike protein. Using microscale thermophoresis, the ability of the plant extract to bind ACE2 and spike protein was evaluated. The levels of ACE2 and TMPRSS2 in HK-2 cells were verified using RT-PCR. In addition, the activities of 5α-reductase and 3CL protease were evaluated.
Main findings
P. granatum L. extract contained several phytocompounds, the most abundant being ellagitannins, gallic acid, and EA. The most frequently detected polyphenols were pedunculagin and punicalin. The plant extract inhibited the binding between spike/ACE2 by approximately 74%. In addition, binding of PPE components to the spike protein was 10-fold stronger than to the ACE2 receptor. The expression levels of both ACE2 and TMPRSS2 in HK-2 cells were significantly reduced by 30% and 70%, respectively. Similarly, the P. granatum extract reduced the activity of 5α-reductase and 3CL protease proteins.
Mechanism of action
Based on these results, it was possible to verify that the polyphenols in the P. granatum extract were able to inhibit the binding of the viral spike protein to its ACE2 receptor (Figure 1(H)). In addition, the extract can inhibit the activities of 5α-reductase and 3CL protease, which are closely related to SARS-CoV-2 replication. The most effective polyphenols in the PPE were PC and EA, probably because of a chemical interaction of the hydroxyl and galloyl groups. Lentivirus infection was almost entirely eliminated in the extract containing polyphenols.94,95 Finally, PPE was shown to inhibit 3CL protease activity by up to 80%, demonstrating its biological importance in decreasing the chance of the virus to be internalized in cells. 94
Final considerations and conclusions
We have presented in this review several in vitro studies that have demonstrated the antiviral activity of medicinal plant extracts and phytocompounds against SARS-CoV-2. The evaluated plant products have been shown to interfere with the viral pathogenesis in the following stages:
Adsorption of the virus onto the host cell: some plant products are able to bind to the surface proteins of the virus, such as the spike glycoprotein, impairing its binding to the ACE2 receptor in the host cell. In addition, the plant products reduce the activity of the ACE2 and other receptors, such as TMPRSS2. They also cause deformations in the spike protein, which compromises its interaction with ACE2 and viral envelope molecules.
During viral multiplication within the cell, including the inhibition of viral enzymes: the plant products could also significantly interfere with some phases of virus multiplication after viral penetration into the host cells. Studies demonstrated that the phytocompounds inhibit some viral enzymes responsible for SARS-CoV-2 replication, such as protease Mpro and 5α-reductase. In addition, an inhibitory effect on the SARS-CoV-2 nucleoprotein was observed, and damage to the viral genome has also been reported. Endosomal pH changes were also observed, leading to destruction of viral particles inside the host cell.
Release of chemical mediators such as cytokines by host cells stimulated by SARS-CoV-2: besides their antiviral activity, the plant products also promoted an immunomodulatory effect against viral infection. Treatment with the plant products reduced the cellular production of excess cytokines that is commonly associated with SARS-CoV-2 infections. This effect can be explained by the ability of these products to inhibit toll-like receptors and other proteins that act in the signaling cascade, such as tyrosine kinase and phospholipase.
Based on the findings demonstrated in the studies addressed in this article, we suggest that products derived from medicinal plants have the potential to control SARS-CoV-2 infection and could be another effective tool against COVID-19. From the proven effective and noncytotoxic concentrations determined in vitro, animal studies and clinical trials must be conducted to further ascertain the antiviral activity and biocompatibility and potential for their therapeutic use in human patients.
Acknowledgments
GOdN and JCdSK thank to Anhembi Morumbi University for Scientific Initiation Scholarships (Edital PIPCD – 09/2021).
Footnotes
Authors’ Contributions: BSA, GOdN, JCdSK, JVBO, KGdSS, and MAdTC wrote the manuscript. JRdO and CRO reviewed the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Jonatas Rafael de Oliveira  https://orcid.org/0000-0003-2398-6506
https://orcid.org/0000-0003-2398-6506
Jaqueline Cadorini de Souza Kawall  https://orcid.org/0000-0001-9932-6761
https://orcid.org/0000-0001-9932-6761
References
- 1. Helmy YA, Fawzy M, Elaswad A, Sobieh A, Kenney SP, Shehata AA. The COVID-19 pandemic: a comprehensive review of taxonomy, genetics, epidemiology, diagnosis, treatment, and control. J Clin Med 2020;9:1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chan JF, Lau SK, To KK, Cheng VC, Woo PC, Yuen KY. Middle East respiratory syndrome coronavirus: another zoonotic betacoronavirus causing SARS-like disease. Clin Microbiol Rev 2015;28:465–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang JT, Ran RX, Lv ZH, Feng LN, Ran CY, Tong YQ, Li D, Su HW, Zhu CL, Qiu SL, Yang J, Xiao MY, Liu MJ, Yang YT, Liu SM, Li Y. Chronological changes of viral shedding in adult inpatients with COVID-19 in Wuhan, China. Clin Infect Dis 2020;71:2158–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol 2020;5:536–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. J Pathol 2004;203:631–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS;, China Medical Treatment Expert Group for Covid-19 . Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez de, Castilla D, Finberg RW, Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA, Short WR, Touloumi G, Lye DC, Ohmagari N, Oh MD, Ruiz-Palacios GM, Benfield T, Fätkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M, Makowski M, Osinusi A, Nayak S, Lane HC;, ACTT-1 Study Group Members. Remdesivir for the treatment of COVID-19: final report. N Engl J Med 2020;383:1813–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tomazini BM, Maia IS, Cavalcanti AB, Berwanger O, Rosa RG, Veiga VC, Avezum A, Lopes RD, Bueno FR, Silva MVAO, Baldassare FP, Costa ELV, Moura RAB, Honorato MO, Costa AN, Damiani LP, Lisboa T, Kawano-Dourado L, Zampieri FG, Olivato GB, Righy C, Amendola CP, Roepke RML, Freitas DHM, Forte DN, Freitas FGR, Fernandes CCF, Melro LMG, Junior GFS, Morais DC, Zung S, Machado FR, Azevedo LCP; COALITION COVID-19 Brazil III, Investigators. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA 2020;324:1307–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dequin PF, Heming N, Meziani F, Plantefève G, Voiriot G, Badié J, François B, Aubron C, Ricard JD, Ehrmann S, Jouan Y, Guillon A, Leclerc M, Coffre C, Bourgoin H, Lengellé C, Caille-Fénérol C, Tavernier E, Zohar S, Giraudeau B, Annane D, Le Gouge A, CAPE COVID Trial Group the CRICS-TriGGERSep Network . Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients with COVID-19: a randomized clinical trial. JAMA 2020;324:1298–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 2020;30:269–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kamboj A, Saluja AK, Kumar M, Atri P. Antiviral activity of plant polyphenols. J Pharm Res 2012;5:2402–12 [Google Scholar]
- 12. Pholphana N, Rangkadilok N, Saehun J, Ritruechai S, Satayavivad J. Changes in the contents of four active diterpenoids at different growth stages in Andrographis paniculata (Burm.f.) Nees (Chuanxinlian). Chin Med 2013;8:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hossain MS, Urbi Z, Sule A, Hafizur Rahman KM. Andrographis paniculata (Burm.f.) Wall. ex Nees: a review of ethnobotany, phytochemistry, and pharmacology. Sci World J 2014;2014:274905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koteswara Rao Y, Vimalamma G, Rao CV, Tzeng YM. Flavonoids and andrographolides from Andrographis paniculata. Phytochemistry 2004;65:2317–21 [DOI] [PubMed] [Google Scholar]
- 15. Gupta S, Mishra KP, Ganju L. Broad-spectrum antiviral properties of andrographolide. Arch Virol 2017;162:611–23 [DOI] [PubMed] [Google Scholar]
- 16. Shi TH, Huang YL, Chen CC, Pi WC, Hsu YL, Lo LC, Chen WY, Fu SL, Lin CH. Andrographolide and its fluorescent derivative inhibit the main proteases of 2019-nCoV and SARS-CoV through covalent linkage. Biochem Biophys Res Commun 2020;533:467–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sa-Ngiamsuntorn K, Suksatu A, Pewkliang Y, Thongsri P, Kanjanasirirat P, Manopwisedjaroen S, Charoensutthivarakul S, Wongtrakoongate P, Pitiporn S, Chaopreecha J, Kongsomros S, Jearawuttanakul K, Wannalo W, Khemawoot P, Chutipongtanate S, Borwornpinyo S, Thitithanyanont A, Hongeng S. Anti-SARS-CoV-2 activity of Andrographis paniculata extract and its major component andrographolide in human lung epithelial cells and cytotoxicity evaluation in major organ cell representatives. J Nat Prod 2021;84:1261–70 [DOI] [PubMed] [Google Scholar]
- 18. Simon JE, Charles D, Cebert E, Grant L, Janick J, Whipkey A. Artemisia annua L.: a promising aromatic and medicinal. In: Janick J, Simon JE. Advances in new crops. Portland, OR: Timber Press, 1990, pp.522–6. [Google Scholar]
- 19. Brown GD. The biosynthesis of artemisinin (Qinghaosu) and the phytochemistry of Artemisia annua L. (Qinghao). Molecules 2010;15:7603–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Garcia LC. A Review of Artemisia annua L.: its genetics, biochemical characteristics, and anti-malarial efficacy. Int J Sci Tech 2015;5:38–46 [Google Scholar]
- 21. Li SY, Chen C, Zhang HQ, Guo HY, Wang H, Wang L, Zhang X, Hua SN, Yu J, Xiao PG, Li RS, Tan X. Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antiviral Res 2005;67:18–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nair MS, Huang Y, Fidock DA, Polyak SJ, Wagoner J, Towler MJ, Weathers PJ. Artemisia annua L. extracts inhibit the in vitro replication of SARS-CoV-2 and two of its variants. J Ethnopharmacol 2021;274:114016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Johnson TO, Adegboyega AE, Ojo AO, Yusuf AJ, Iwaloye O, Ugwah-Oguejiofor CJ, Asomadu RO, Chukwuma IF, Ejembi SA, Ugwuja EI. Identification of possible inhibitors of SARS-CoV-2 main protease from some bioactive compounds of Artemisia annua: an in silico approach. Research Square 2021, https://assets.researchsquare.com/files/rs-612899/v2/d211f386-6f3b-4c80-80a0-2dd80698f000.pdf?c=1637245461
- 24. Fuzimoto AD. An overview of the anti-SARS-CoV-2 properties of Artemisia annua, its antiviral action, protein-associated mechanisms, and repurposing for COVID-19 treatment. J Integr Med 2021;19:375–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Klayman DL. Qinghaosu (artemisinin): an antimalarial drug from China. Science 1985;228:1049–55 [DOI] [PubMed] [Google Scholar]
- 26. Adam I, Elhardello OA, Elhadi MO, Abdalla E, Elmardi KA, Jansen FH. The antischistosomal efficacies of artesunate-sulfamethoxypyrazine-pyrimethamine and artemether-lumefantrine administered as treatment for uncomplicated, Plasmodium falciparum malaria. Ann Trop Med Parasitol 2008;102:39–44 [DOI] [PubMed] [Google Scholar]
- 27. Efferth T. From ancient herb to modern drug: Artemisia annua and artemisinin for cancer therapy. Semin Cancer Biol 2017;46:65–83 [DOI] [PubMed] [Google Scholar]
- 28. Efferth T, Romero MR, Wolf DG, Stamminger T, Marin JJ, Marschall M. The antiviral activities of artemisinin and artesunate. Clin Infect Dis 2008;47:804–11 [DOI] [PubMed] [Google Scholar]
- 29. Zhou Y, Gilmore K, Ramirez S, Settels E, Gammeltoft KA, Pham LV, Fahnøe U, Feng S, Offersgaard A, Trimpert J, Bukh J, Osterrieder K, Gottwein JM, Seeberger PH. In vitro efficacy of artemisinin-based treatments against SARS-CoV-2. Sci Rep 2021;11:14571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dandara C, Dzobo K, Chirikure S. COVID-19 Pandemic and Africa: from the situation in Zimbabwe to a case for precision herbal medicine. OMICS 2021;25:209–12 [DOI] [PubMed] [Google Scholar]
- 31. Nie C, Trimpert J, Moon S, Haag R, Gilmore K, Kaufer BB, Seeberger PH. In vitro efficacy of artemisia extracts against SARS-CoV-2. Virol J 2021;18:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Costa MS, Kiralj R, Ferreia MMC. Theoretical study of the interaction between artemisinin and heme. Quim Nova 2007;30:25–31 [Google Scholar]
- 33. Ho WE, Peh HY, Chan TK, Wong WS. Artemisinins: pharmacological actions beyond anti-malarial. Pharmacol Ther 2014;142:126–39 [DOI] [PubMed] [Google Scholar]
- 34. Cheong DHJ, Tan DWS, Wong FWS, Tran T. Anti-malarial drug, artemisinin and its derivatives for the treatment of respiratory diseases. Pharmacol Res 2020;158:104901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cao R, Hu H, Li Y, Wang X, Xu M, Liu J, Zhang H, Yan Y, Zhao L, Li W, Zhang T, Xiao D, Guo X, Li Y, Yang J, Hu Z, Wang M, Zhong W. Anti-SARS-CoV-2 potential of artemisinins in vitro. ACS Infect Dis 2020;6:2524–31 [DOI] [PubMed] [Google Scholar]
- 36. Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, Fu S, Gao L, Cheng Z, Lu Q, Hu Y, Luo G, Wang K, Lu Y, Li H, Wang S, Ruan S, Yang C, Mei C, Wang Y, Ding D, Wu F, Tang X, Ye X, Ye Y, Liu B, Yang J, Yin W, Wang A, Fan G, Zhou F, Liu Z, Gu X, Xu J, Shang L, Zhang Y, Cao L, Guo T, Wan Y, Qin H, Jiang Y, Jaki T, Hayden FG, Horby PW, Cao B, Wang C. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial (Erratum in Lancet 2020;395:1694). Lancet 2020;395:1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang B, Kovalchuk A, Li D, Rodriguez-Juarez R, Ilnytskyy Y, Kovalchuk I, Kovalchuk O. In search of preventive strategies: novel high-CBD Cannabis sativa extracts modulate ACE2 expression in COVID-19 gateway tissues. Aging 2020;12:22425–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fitzcharles MA, Clauw DJ, Hauser W. A cautious hope for cannabidiol (CBD) in rheumatology care. Arthritis Care Res. Epub ahead of print 7 March 2020. DOI: 10.1002/acr.24176. [DOI] [PubMed] [Google Scholar]
- 39. Kovalchuk O, Kovalchuk I. Cannabinoids as anticancer therapeutic agents. Cell Cycle 2020;19:961–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Häuser W, Finn DP, Kalso E, Krcevski-Skvarc N, Kress HG, Morlion B, Perrot S, Schäfer M, Wells C, Brill S. European Pain Federation (EFIC) position paper on appropriate use of cannabis-based medicines and medical cannabis for chronic pain management. Eur J Pain 2018;22: 1547–64 [DOI] [PubMed] [Google Scholar]
- 41. Fitzcharles MA, Niaki OZ, Hauser W, Hazlewood G, Canadian Rheumatology Association. Position statement: a pragmatic approach for medical cannabis and patients with rheumatic diseases. J Rheumatol 2019;46:532–8 [DOI] [PubMed] [Google Scholar]
- 42. Brake SJ, Barnsley K, Lu W, McAlinden KD, Eapen MS, Sohal SS. Smoking upregulates angiotensin-converting enzyme-2 receptor: a potential adhesion site for novel coronavirus SARS-CoV-2 (COVID-19). J Clin Med 2020;9:841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stanić Z. Curcumin, a compound from natural sources, a true scientific challenge: a review. Plant Foods Hum Nutr 2017;72:1–12 [DOI] [PubMed] [Google Scholar]
- 44. Moghadamtousi SZ, Kadir HA, Hassandarvish P, Tajik H, Abubakar S, Zandi K. A review on antibacterial, antiviral, and antifungal activity of curcumin. Biomed Res Int 2014;2014:186864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Perrone D, Ardito F, Giannatempo G, Dioguardi M, Troiano G, Lo Russo L, DE Lillo A, Laino L, Lo Muzio L. Biological and therapeutic activities, and anticancer properties of curcumin. Exp Ther Med 2015;10: 1615–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kunnumakkara AB, Bordoloi D, Padmavathi G, Monisha J, Roy NK, Prasad S, Aggarwal BB. Curcumin, the golden nutraceutical: multitargeting for multiple chronic diseases. Br J Pharmacol 2017;174:1325–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Anggakusuma Colpitts CC, Schang LM, Rachmawati H, Frentzen A, Pfaender S, Behrendt P, Brown RJ, Bankwitz D, Steinmann J, Ott M, Meuleman P, Rice CM, Ploss A, Pietschmann T, Steinmann E. Turmeric curcumin inhibits entry of all hepatitis C virus genotypes into human liver cells. Gut 2014;63:1137–49 [DOI] [PubMed] [Google Scholar]
- 48. Jennings MR, Parks RJ. Curcumin as ana Agent. Viruses 2020;12:1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chen D, Shien J, Tiley L, Chiou S, Wang S, Chang T, Lee Y, WeiChan K, Hsu W. Curcumin inhibits influenza virus infection and haemagglutination activity. Food Chem 2010;119:1346–51 [Google Scholar]
- 50. Bormann M, Alt M, Schipper L, van de Sand L, Le-Trilling VTK, Rink L, Heinen N, Madel RJ, Otte M, Wuensch K, Heilingloh CS, Mueller T, Dittmer U, Elsner C, Pfaender S, Trilling M, Witzke O, Krawczyk A. Turmeric root and its bioactive ingredient curcumin effectively neutralize SARS-CoV-2 in vitro. Viruses 2021;13:1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jena AB, Kanungo N, Nayak V, Chainy GBN, Dandapat J. Catechin and curcumin interact with S protein of SARS-CoV2 and ACE2 of human cell membrane: insights from computational studies. Sci Rep 2021;11:2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med 1998;64:353–6. [DOI] [PubMed] [Google Scholar]
- 53. Larasati YA, Yoneda-Kato N, Nakamae I, Yokoyama T, Meiyanto E, Kato JY. Curcumin targets multiple enzymes involved in the ROS metabolic pathway to suppress tumor cell growth. Sci Rep 2018;8:2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shehzad A, Rehman G, Lee YS. Curcumin in inflammatory diseases. Biofactors 2013;39:69–77 [DOI] [PubMed] [Google Scholar]
- 55. Mounce BC, Cesaro T, Carrau L, Vallet T, Vignuzzi M. Curcumin inhibits Zika and chikungunya virus infection by inhibiting cell binding. Antiviral Res 2017;142:148–57 [DOI] [PubMed] [Google Scholar]
- 56. Prasad S, Tyagi AK, Aggarwal BB. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: the golden pigment from golden spice. Cancer Res Treat 2014;46:2–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Roshdy WH, Rashed HA, Kandeil A, Mostafa A, Moatasim Y, Kutkat O, Abo Shama NM, Gomaa MR, El-Sayed IH, El Guindy NM, Naguib A, Kayali G, Ali MA. EGYVIR: An immunomodulatory herbal extract with potent antiviral activity against SARS-CoV-2. PLoS ONE 2020;15:e0241739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pleschka S, Stein M, Schoop R, Hudson JB. Anti-viral properties and mode of action of standardized Echinacea purpurea extract against highly pathogenic avian influenza virus (H5N1, H7N7) and swine-origin H1N1 (S-OIV). Virol J 2009;6:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Signer J, Jonsdottir HR, Albrich WC, Strasser M, Züst R, Ryter S, Ackermann-Gäumann R, Lenz N, Siegrist D, Suter A, Schoop R, Engler OB. In vitro virucidal activity of Echinaforce®, an Echinacea purpurea preparation, against coronaviruses, including common cold coronavirus 229E and SARS-CoV-2 (Erratum in: Virol J 2020;17:172). Virol J 2020;17:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schapowal A. The triple action of the herbal medicine Echinaforce in the treatment of cold and flu-like infections. Swiss J Integr Med 2011; 23:15 [Google Scholar]
- 61. Lim TK. Glycyrrhiza glabra. In: Lim TK. (ed.) Edible medicinal and non-medicinal plants. Dordrecht: Springer, 2016, pp.354–57 [Google Scholar]
- 62. van Gelderen CE, Bijlsma JA, van Dokkum W, Savelkoul TJ. Glycyrrhizic acid: the assessment of a no effect level. Hum Exp Toxicol 2000;19:434–9 [DOI] [PubMed] [Google Scholar]
- 63. Cinatl J, Morgenstern B, Bauer G, Chandra P, Rabenau H, Doerr HW. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet 2003;361:2045–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Huang W, Chen X, Li Q, Li P, Zhao G, Xu M, Xie P. Inhibition of intercellular adhesion in herpes simplex virus infection by glycyrrhizin. Cell Biochem Biophys 2012;62:137–40 [DOI] [PubMed] [Google Scholar]
- 65. van de Sand L, Bormann M, Alt M, Schipper L, Heilingloh CS, Steinmann E, Todt D, Dittmer U, Elsner C, Witzke O, Krawczyk A. Glycyrrhizin effectively inhibits SARS-CoV-2 replication by inhibiting the viral main protease. Viruses 2021;13:609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jin Z, Du X, Xu Y, Deng Y, Liu M, Zhao Y, Zhang B, Li X, Zhang L, Peng C, Duan Y, Yu J, Wang L, Yang K, Liu F, Jiang R, Yang X, You T, Liu X, Yang X, Bai F, Liu H, Liu X, Guddat LW, Xu W, Xiao G, Qin C, Shi Z, Jiang H, Rao Z, Yang H. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature 2020;582:289–93 [DOI] [PubMed] [Google Scholar]
- 67. Soni MG, Burdock GA, Christian MS, Bitler CM, Crea R. Safety assessment of aqueous olive pulp extract as an antioxidant or antimicrobial agent in foods. Food Chem Toxicol 2006;44:903–15 [DOI] [PubMed] [Google Scholar]
- 68. Karković Marković A, Torić J, Barbarić M, Jakobušić Brala C. Hydroxytyrosol, tyrosol and derivatives and their potential effects on human health. Molecules 2019;24:2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yamada K, Ogawa H, Hara A, Yoshida Y, Yonezawa Y, Karibe K, Nghia VB, Yoshimura H, Yamamoto Y, Yamada M, Nakamura K, Imai K. Mechanism of the antiviral effect of hydroxytyrosol on influenza virus appears to involve morphological change of the virus. Antiviral Res 2009;83:35–44 [DOI] [PubMed] [Google Scholar]
- 70. Bedoya LM, Beltrán M, Obregón-Calderón P, García-Pérez J, de la Torre HE, González N, Pérez-Olmeda M, Auñón D, Capa L, Gómez-Acebo E, Alcamí J. Hydroxytyrosol: a new class of microbicide displaying broad anti-HIV-1 activity. AIDS 2016;30:2767–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Christian MS, Sharper VA, Hoberman AM, Seng JE, Fu L, Covell D, Diener RM, Bitler CM, Crea R. The toxicity profile of hydrolyzed aqueous olive pulp extract. Drug Chem Toxicol 2004;27:309–30 [DOI] [PubMed] [Google Scholar]
- 72. Bitler CM, Viale TM, Damaj B, Crea R. Hydrolyzed olive vegetation water in mice has anti-inflammatory activity. J Nutr 2005;135:1475–9 [DOI] [PubMed] [Google Scholar]
- 73. Bitler CM, Matt K, Irving M, Hook G, Yusen J, Eagar F, Kirschner K, Walker B, Crea R. Olive extract supplement decreases pain and improves daily activities in adults with osteoarthritis and decreases plasma homocysteine in those with rheumatoid arthritis. Nutr Res 2007;27:470–7 [Google Scholar]
- 74. Di Rosa G, Brunetti G, Scuto M, Trovato Salinaro A, Calabrese EJ, Crea R, Schmitz-Linneweber C, Calabrese V, Saul N. Healthspan enhancement by olive polyphenols in C. Elegans Wild Type and Parkinson’s Models. Int J Mol Sci 2020;21:3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Takeda Y, Jamsransuren D, Matsuda S, Crea R, Ogawa H. The SARS-CoV-2-inactivating activity of hydroxytyrosol-rich aqueous olive pulp extract (HIDROX®) and its use as a virucidal cream for topical application. Viruses 2021;13:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Brudzynski K, Alvarez LM. Polyphenol–protein complexes and their consequences for the redox activity, structure and function of honey: a current view and new hypothesis—a review. Pol J Food Nutr Sci 2015;65:71–80 [Google Scholar]
- 77. Furukawa A, Oikawa S, Murata M, Hiraku Y, Kawanishi S. (−)-Epigallocatechin gallate causes oxidative damage to isolated and cellular DNA. Biochem Pharmacol 2003;66:1769–78 [DOI] [PubMed] [Google Scholar]
- 78. Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev 2009;2:270–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Patil UK, Saraogi R. Natural products as potential drug permeation enhancer in transdermal drug delivery system. Arch Dermatol Res 2014; 306:419–26 [DOI] [PubMed] [Google Scholar]
- 80. Upadhyay S, Dixit M. Role of polyphenols and other phytochemicals on molecular signaling. Oxid Med Cell Longev 2015;2015:504253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Cory H, Passarelli S, Szeto J, Tamez M, Mattei J. The role of polyphenols in human health and food systems: a mini-review. Front Nutr 2018;5:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Paraiso IL, Revel JS, Stevens JF. Potential use of polyphenols in the battle against COVID-19. Curr Opin Food Sci 2020;32:149–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Goc A, Sumera W, Rath M, Niedzwiecki A. Phenolic compounds disrupt spike-mediated receptor-binding and entry of SARS-CoV-2 pseudo-virions. PLoS ONE 2021;16:e0253489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Reddy MK, Gupta SK, Jacob MR, Khan SI, Ferreira D. Antioxidant, antimalarial and antimicrobial activities of tannin-rich fractions, ellagitannins and phenolic acids from Punica granatum L. Planta Med 2007;73:461–7 [DOI] [PubMed] [Google Scholar]
- 85. Viuda-Martos M, Fernández-López J, Pérez-Álvarez J. Pomegranate and its many functional components as related to human health: a review. Compr Rev Food Sci Food Saf 2010;9:635–54 [DOI] [PubMed] [Google Scholar]
- 86. Landete J. Ellagitannins, ellagic acid and their derived metabolites: a review about source, metabolism, functions and health. Food Res Int 2011;44:1150–60 [Google Scholar]
- 87. Howell AB, D’Souza DH. The pomegranate: effects on bacteria and viruses that influence human health. Evid Based Complement Alternat Med 2013;2013:606212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Yiu CY, Chen SY, Chang LK, Chiu YF, Lin TP. Inhibitory effects of resveratrol on the Epstein–Barr virus lytic cycle. Molecules 2010;15:7115–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zhang L, Li Y, Gu Z, Wang Y, Shi M, Ji Y, Sun J, Xu X, Zhang L, Jiang J, Shi W. Resveratrol inhibits enterovirus 71 replication and pro-inflammatory cytokine secretion in rhabdosarcoma cells through blocking IKKs/NF-κB signaling pathway. PLoS ONE 2015;10:e0116879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Annunziata G, Maisto M, Schisano C, Ciampaglia R, Narciso V, Tenore GC, Novellino E. Resveratrol as a novel anti-herpes simplex virus nutraceutical agent: an overview. Viruses 2018;10:473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lin CJ, Lin HJ, Chen TH, Hsu YA, Liu CS, Hwang GY, Wan L. Correction: Polygonum cuspidatum and its active components inhibit replication of the influenza virus through toll-like receptor 9-induced interferon beta expression. PLoS ONE 2015;10:e0125288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Khaerunnisa S, Kurniawan H, Awaluddin R, Suhartati S, Soetjipto S. Potential inhibitor of COVID-19 main protease (Mpro) from several medicinal plant compounds by molecular docking study. Preprints 2020:2020030226, https://www.preprints.org/manuscript/202003.0226/v1 [Google Scholar]
- 93. Adem S, Eyupoglu V, Sarfraz I, Rasul A, Ali M. Identification of potent COVID-19 main protease (Mpro) inhibitors from natural polyphenols: an in silico strategy unveils a hope against CORONA. Preprints 2020:2020030333, https://www.preprints.org/manuscript/202003.0333/v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Tito A, Colantuono A, Pirone L, Pedone E, Intartaglia D, Giamundo G, Conte I, Vitaglione P, Apone F. Pomegranate peel extract as an inhibitor of SARS-CoV-2 spike binding to human ACE2 receptor (in vitro): a promising source of novel antiviral drugs. Front Chem 2021;9:638187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Suručić R, Tubić B, Stojiljković MP, Djuric DM, Travar M, Grabež M, Šavikin K, Škrbić R. Computational study of pomegranate peel extract polyphenols as potential inhibitors of SARS-CoV-2 virus internalization. Mol Cell Biochem 2021;476:1179–93 [DOI] [PMC free article] [PubMed] [Google Scholar]