Abstract
Background:
Approximately 50% of intensive care survivors experience persistent psychological symptoms. Eye-movement desensitisation and reprocessing (EMDR) is a widely recommended trauma-focussed psychological therapy, which has not been investigated systematically in a cohort of intensive care survivors: We therefore conducted a randomised pilot feasibility study of EMDR, using the Recent Traumatic Episode Protocol (R-TEP), to prevent psychological distress in intensive care survivors. Findings will determine whether it would be possible to conduct a fully-powered clinical effectiveness trial and inform trial design.
Method:
We aimed to recruit 26 patients who had been admitted to intensive care for over 24 h with COVID-19 infection. Consenting participants were randomised (1:1) to receive either usual care plus remotely delivered EMDR R-TEP or usual care alone (controls). The primary outcome was feasibility. We also report factors related to safety and symptom changes in post-traumatic stress disorder, (PTSD) anxiety and depression.
Results:
We approached 51 eligible patients, with 26 (51%) providing consent. Intervention adherence (sessions offered/sessions completed) was 83%, and 23/26 participants completed all study procedures. There were no attributable adverse events. Between baseline and 6-month follow-up, mean change in PTSD score was −8 (SD = 10.5) in the intervention group versus +0.75 (SD = 15.2) in controls (p = 0.126). There were no significant changes to anxiety or depression.
Conclusion:
Remotely delivered EMDR R-TEP met pre-determined feasibility and safety objectives. Whilst we achieved group separation in PTSD symptom change, we have identified a number of protocol refinements that would improve the design of a fully powered, multi-centre randomised controlled trial, consistent with currently recommended rehabilitation clinical pathways.
Trial registration:
ClinicalTrials.gov: NCT04455360.
Keywords: Critical care, intensive care, COVID, PTSD, anxiety, depression, psychology, EMDR, early EMDR intervention, R-TEP, feasibility
Introduction
Intensive care survivors frequently experience a range of health sequelae, widely referred to as ‘Post Intensive Care Syndrome’. 1 In addition to physical and cognitive impairment, meta-analyses show that 20%–25% experience symptoms of post-traumatic stress disorder (PTSD), in the year following hospital discharge,2,3 and the prevalence of anxiety and depressive symptoms is 32%–40% 4 and 28%–30%, respectively. 5 These symptoms frequently co-exist 6 and are associated with reduced quality of life,4,5,7 increased healthcare use, 8 delayed or no return to work 9 and unhealthy coping behaviours. 10 The survivorship phase is frequently overlooked by healthcare providers, and psychological services are widely lacking. 11
During the severe acute respiratory syndrome coronavirus two (SARS CoV-2) pandemic, admission illness severity was higher than in previously documented populations. 12 Intensive care services were stretched by unprecedented demand, acute staff shortages and high levels of personal protective equipment. 13 Data from previous infective outbreaks, 14 suggest that clinicians may witness an increased incidence of post-ICU psychopathology, following the pandemic. 15
Research into attenuating strategies, such as patient diaries, 16 follow-up clinics 17 and nurse-led psychological care 18 has provided mixed evidence of benefit. More recently, calls have grown for collaboration with our colleagues in mental health.19,20 Eye movement desensitisation and reprocessing (EMDR) is a trauma-focussed psychotherapy believed to reduce distress by facilitating recall, processing and integration of traumatic memories within a positive emotional and cognitive framework. 21 Meta-analyses report reductions in post-traumatic, anxiety and depressive symptoms following a range of traumatic events, including life-threatening medical events.22,23 International organisations recommend EMDR as an effective and cost-effective treatment for PTSD.24,25 EMDR reduces post-traumatic symptoms in patients with co-morbid psychotic, depressive, anxiety and substance misuse disorders 26 ; an important consideration given the association between pre-existing psychiatric diagnosis and post-intensive care psychopathology. 27 In 2018, Hulme 28 reported reductions in PTSD symptom severity, following EMDR therapy, in a non-randomised pilot study of 10 ICU-survivors. Two recent case studies describe positive treatment effect following ICU admission.29,30
The Recent Traumatic Episode Protocol, (R-TEP) 31 is an EMDR intervention, adapted for early delivery, that allows for processing of fragmented, traumatic memories; frequently reported by ICU survivors and associated with post-ICU PTSD development. 32 EMDR R-TEP has reduced PTSD symptoms following missile attacks,33,34 and life-threatening medical events.35,36 The aforementioned, case study 30 described a positive treatment response to EMDR R-TEP, following ICU admission.
A number of systematic reviews report uncertainty regarding the timing of psychological interventions, to prevent or ameliorate traumatic stress symptoms. An International Society of Traumatic Stress Studies (ISTSS) review, concluded that there is no strong evidence for early, preventative intervention irrespective of symptomology. 37 Reviews focussing on life-threatening medical events 38 and ICU-survivorship specifically,39,40 could not identify optimal timing of preventative interventions. Moreover, none of the reviewed studies investigated a protocolised, trauma-focussed psychological therapy aimed at prevention of downstream post-ICU mental health morbidity.
Given the pervasiveness of post-ICU PTSD, paucity of robust evidence and partial support for preventative interventions, we identified both timing of intervention and pre-screening for symptoms, as key uncertainties in our study programme. We therefore elected to investigate delivery of an early EMDR R-TEP intervention, offered to all survivors, to prevent development of PTSD, symptom entrenchment and to avoid excessive suffering.
This study investigated the feasibility of conducting a randomised controlled trial of online EMDR R-TEP with a cohort of intensive care survivors. Through the inclusion of a control group (CG) who received usual care, we aimed to gather preliminary evidence of possible clinical effectiveness. Findings will inform the development and delivery of a subsequent, fully-powered randomised controlled trial (RCT), in a broader cohort of intensive care survivors, which may inform psychological care pathways for this underserved population.
Method
Trial design
COVEMERALD was an investigator-initiated, single-centre, pilot feasibility study. Registered on ClinicalTrials.gov (NCT04455360), in advance of beginning the trial: London-Fulham Research Ethics Committee granted ethical approval on 24th August 2020 (Reference: 20/HRA/3633). At the time of this study, only COVID-19 related research would be considered by UK Health Research Authority. The full study protocol has been published elsewhere. 41 The study was conducted according to Medical Research Council (MRC) guidance on developing complex interventions 42 and is reported according to Consolidated Standards of Reporting Trials (CONSORT) extension to randomised pilot and feasibility trials. 43 All study activity was undertaken at University Hospital Southampton (UHS) National Health Service Foundation Trust (NHS FT), a large regional centre servicing a population of 1.9 million in central southern United Kingdom.
Patients
Patients were eligible to enrol in the study if they had been admitted to intensive care for at least 24 h following a positive COVID-19 test (polymerase chain reaction), were aged 18 years or over, had capacity to provide informed consent, and had been discharged from hospital for less than 3 months. Patients were excluded if they had cognitive impairment, a pre-existing diagnosis of psychosis, suffered acute brain injury or were not expected to survive beyond hospital discharge. Initial inclusion criteria included 24 h of mechanical ventilation, but this was removed on the advice of our patient and public involvement (PPI) group, following reports of distress associated with non-invasive positive pressure ventilation.
Recruitment occurred between October 2020 and April 2021. Consecutive patients were screened for eligibility, following hospital-discharge. The Chief Investigator telephoned potential participants once eligibility criteria were confirmed. Patient information sheets were posted or e-mailed, and a follow-up phone call arranged. If the patient expressed a desire to participate in the study, research staff documented the conversation and recorded consent in writing. Consenting participants were emailed a link to complete a demographic questionnaire and baseline assessments on an electronic data management system, ALEA Clinical™. All trial procedures were completed remotely due to ongoing COVID-19 restrictions.
Randomisation and treatment
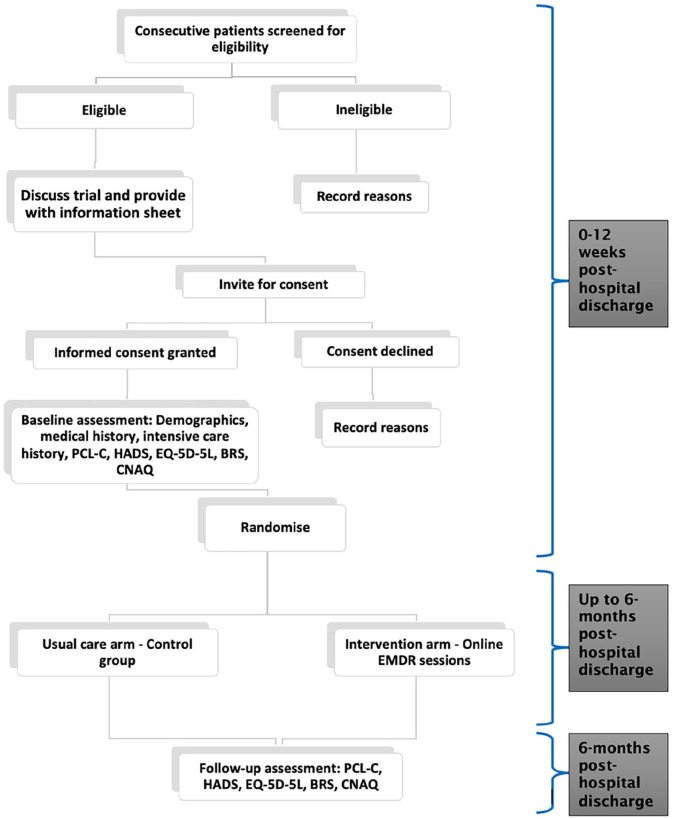
We assigned participants in a 1:1 ratio to receive either usual care (control group CG) or usual care plus online EMDR (Intervention) using computer generated random permutation ( ALEA Clinical™): no stratification factors were applied. A brief description of usual care is provided in Supplemental File: Usual care description. Following consent, the study team provided contact details of participants in the intervention arm to the Intensive Psychological Therapies Service (IPTS) at Dorset Healthcare University NHS FT: all sessions took place via ZoomTN videoconferencing platform. The EMDR R-TEP intervention is described in detail according to the Template for Intervention Description and Replication Checklist 44 (see Supplemental File: TIDieR Checklist). Briefly, the sessions consisted of eight phases: history taking; preparation with attention to safety and containment; assessment of points of disturbance (using 0–10 scale of Subjective Units of Distress [SUD] 0 = no distress, 10 = highest anxiety/distress ever felt); focussed processing and desensitisation with bilateral stimulation; installation of positive cognition with bilateral stimulation; episode body scan; episode closure; re-evaluation of SUD and validity of positive cognition. Each session lasted between 60 and 90 min. Additional sessions were offered if SUD scores were ⩾2 on re-evaluation. Up to eight sessions of EMDR were offered. If no points of disturbance were identified (SUD ⩽ 1), sessions were discontinued. Participant flow through the study is shown in Figure 1: Participant flow diagram.
Figure 1.
Participant flow diagram.
Outcome measures and data collection
Our primary aim was to assess the feasibility of delivering online EMDR to adult survivors of COVID-19 related critical illness. Feasibility objectives were selected from MRC and National Institute for Health and Care Research guidance 45 and pre-published 41 : (i) recruitment rate >30% of patients approached; (ii) intervention session adherence >75%, calculated from sessions completed as a proportion of sessions offered; (iii) protocol adherence >75% of all participants, based upon deviations and violations; (iv) trial completion of >75% of study activities completed; and (v) review of serious events attributable to trial procedures. These were not defined as progression criteria but would inform refinement of study design.
We recorded baseline demographic data, ICU-admission history and medical history; comorbidities, intensive care bed days, length of hospital inpatient stay, total benzodiazepine use, total days of ventilation, (intubated and non-invasive positive pressure ventilation) and illness severity using the Acute Physiology and Chronic Health Evaluation (APACHE) II score. Secondary clinical outcomes were assessed by comparing change in self-reported symptoms from baseline to follow-up (6-month post-hospital discharge), between the control (CG) and intervention groups. The Post-traumatic Stress Disorder Checklist-Civilian version (PCL-C); is a 17 question, patient-reported outcome measure, widely-used and validated in populations including intensive care survivors.6,46,47 Participants report frequency of experiencing PTSD symptoms, giving a total score between 17 and 85. PCL-C has estimated sensitivity and specificity for PTSD caseness, in primary care populations of 28–30, 48 with an estimated minimal clinically important difference (MCID) in the range of 5.7–10.2 (midpoint of 7.9) based upon comparison with clinician assessment. 49
Anxiety and depressive symptoms were measured by the Hospital Anxiety and Depression Scale (HADS) 50 ; HADS was the most frequently used assessment tool in a meta-analysis of post-ICU depressive symptoms 51 and was used in the UK’s largest study of post-ICU mental health outcomes. 6 Scores can be reported separately for anxiety and depression sub-scales, with ⩾8 52 defining caseness for each. HADS MCID, for both subscales, is estimated between 1.7 53 and 2 54 points.
PTSD is associated with a range of sequelae, which will be of interest in the main trial and future research workstreams. The following exploratory outcomes were measured in order to explore uncertainty around follow-up rates, questionnaire response rate and time needed to clean and analyse the data; Quality of life was measured using EuroQol Five Dimension-Five level scale (EQ-5D-5L) 55 ; We used the Brief Resilience Scale (BRS) 56 to assess resilience. Emerging research is exploring whether bolstering resilience, may offer innovative techniques in ameliorating PTSD symptoms. 57 We used the Council of Nutrition Appetite Questionnaire (CNAQ) 58 to measure appetite and predicted weight change, as PTSD is independently associated with both weight gain and loss. 59 We originally intended to assess cognitive function, physical activity, functional disability and report episodes of delirium in ICU: however, lack of researcher time meant we were unable to perform remote cognition testing, our PPI group recommended removal of functional disability assessment due to participant burden, COVID restrictions denied the opportunity to use physical activity monitors, and delirium episodes had been recorded in the ICU notes only rarely, due to necessary adaptation of clinical practices. Full details and definitions of outcome variables are available in Supplemental File: Table S1. Patient reported outcomes were completed online. All other data were collected by research staff and stored securely, using ALEA Clinical™.
Statistical analysis
This was a feasibility trial in which the effectiveness of EMDR was not evaluated, so a formal power calculation is not appropriate. Sample size was based upon recommendations for feasibility studies, 60 and previously-reported ICU recovery feasibility studies of complex interventions. 61 Twenty-six consenting participants ensured a comprehensive evaluation of feasibility, with 13 randomised to CG and 13 to EMDR. The study statistician was blind to group allocation and downloaded data from ALEA™ to IBM SPSS™ to perform statistical analyses of clinical outcomes. Demographics and baseline characteristics were compared using the Pearson Chi-Square test, or the Fisher’s exact test, if nominal, or the Student’s t test, or Mann–Whitney U test, if quantitative. Demographic data are reported as numbers (percentage), mean (standard deviation (SD)) and median (inter-quartile range (IQR)) where appropriate. Clinical outcome data are reported as change from Baseline to Follow-up. These data were assessed for normal distribution using the Shapiro-Wilk test. 62 Normally distributed variables are reported as mean (SD). Non-normally distributed variables are reported as median (IQR). Where appropriate, variables are reported as number (percentage) of the study population.
Results
Feasibility
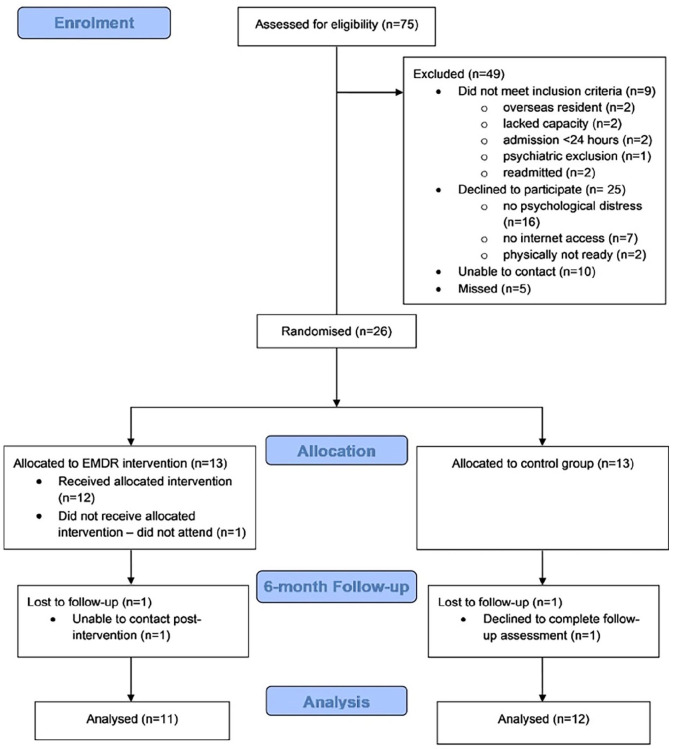
Seventy-five consecutive, discharged patients were screened for inclusion between October 2020 and April 2021. Nine did not meet inclusion criteria. We could not find contact details for 10 patients and five were missed due to lack of research time for the CI. Fifty-one eligible patients were approached, with 26(51%) consenting to participation over the 7-month recruitment period. Thirteen participants were allocated to the CG, and 13 to the intervention group. Recruitment, randomisation, retention and trial completion data are shown in Figure 2: Study flowchart (CONSORT) diagram. Sixteen (62%) males and 10 (38%) females were recruited, matching the proportion of patients admitted with severe COVID-19. Demographic and clinical characteristics are summarised in Table 1. There were no significant differences between groups in age, gender, ethnicity, BMI, admission severity (APACHEII), median ICU and hospital length of stay (LOS). Benzodiazepine use was higher in the EMDR R-TEP group (46%) versus CG (23%), although this was not statistically significant.
Figure 2.
Study flowchart (CONSORT diagram).
Table 1.
Demographic and clinical characteristics at baseline.
| Variables | All (N = 26) | Control (N = 13) | EMDR (N = 13) | p-value |
|---|---|---|---|---|
| Age, mean (SD), years | 58.0 (15.3) | 58.3 (16.5) | 57.7 (14.8) | 0.923 |
| Gender, male n (%) | 16 (61.5) | 8 (61.5) | 8 (61.5) | 1.00 |
| BMI | 32.7 (6.82) | 32.5 (6.70) | 32.9 (7.21) | 0.885 |
| Ethnicity n (%) | 0.593 | |||
| White (British) | 23 (88.5) | 11 (84.6) | 12 (92.3) | |
| White (Other) | 2 (7.7) | 1 (7.7) | 1 (7.7) | |
| Unknown | 1 (3.8) | 1 (7.7) | 0 (0.0) | |
| Medical history n (%) | ||||
| Anxiety | 1 (3.8) | 0 (0.0) | 1 (7.7) | 0.308 |
| Bipolar | 1 (3.8) | 0 (0.0) | 1 (7.7) | 0.308 |
| Cancer | 1 (3.8) | 1 (7.7) | 0 (0.0) | 0.308 |
| Cardiovascular | 4 (15.4) | 4 (30.8) | 0 (0.0) | 0.030 |
| Depression | 1 (3.8) | 1 (7.7) | 0 (0.0) | 0.308 |
| Endocrine | 5 (19.2) | 2 (15.4) | 3 (23.1) | 0.619 |
| Gastrointestinal | 3 (11.5) | 1 (7.7) | 2 (15.4) | 0.539 |
| Musculoskeletal | 3 (11.5) | 2 (15.4) | 1 (7.7) | 0.539 |
| Neurological | 1 (3.8) | 1 (7.7) | 0 (0.0) | 0.308 |
| PTSD | 1 (3.8) | 0 (0.0) | 1 (7.7) | 0.308 |
| Renal | 1 (3.8) | 0 (0.0) | 1 (7.7) | 0.308 |
| Respiratory | 4 (15.4) | 3 (23.1) | 1 (7.7) | 0.277 |
| APACHE II score^ | 11 (7.13) | 11 (8.12) | 11 (7.13) | 0.757 |
| ICU LoS^ | 8 (5.18) | 6 (5.18) | 9 (7.17) | 0.719 |
| Hospital LoS^ | 16 (10.30) | 13(10.30) | 9(7.17) | 0.976 |
| Total ventilation days^ | 6 (4.15) | 6 (4.19) | 5 (3.13) | 0.881 |
| Benzodiazepine use n (%) | 9 (34.6) | 3 (23.1) | 6 (46.2) | 0.216 |
SD: standard deviation; IQR: inter-quartile range; BMI: body mass index; PTSD: post-traumatic stress disorder; APACHE: acute physiology and chronic health evaluation; ICU: intensive care unit; LoS: length of stay. Data are presented as mean (SD), ^median (IQR) or n (%).
One participant allocated to intervention did not undertake any EMDR sessions and did not give a reason: the 12 remaining participants attended 34 of 41 arranged sessions, giving an intervention session adherence of 83%. Five sessions were missed due to physical ill health, one due to denial of psychological disturbance, and one due to confusion over appointment date. Mean session attendance was 3.25 per participant. Five participants needed only one session as their Baseline SUD was 1/10. One patient from each group did not complete the 6-month follow-up assessments. One declined but gave no reason and one could not be contacted. Twenty-three participants (88%) completed all study procedures. There were no protocol deviations and no reported adverse events.
Secondary outcomes
The mean Baseline PCL-C score for the whole intervention group was 29.2 although 48.7 in the seven participants who required more than one session. Clinical outcomes are summarised in Table 2. Mean PCL-C score decreased by eight points (Standard deviation (SD) 10.49) in the intervention group but increased by 0.75 (SD 15.17) in the CG (p = 0.126). There was wide variability in response among participants in the intervention group: 9 reported a reduction in PCL-C scores, (from −3 to −29), one participant reported no change, and one reported an increase of 10 points (a combat veteran with previously reported PTSD diagnosis). In the CG, three of 12 participants reported a reduced PCL-C score (ranging from −5 to −37), three reported no change, six reported increased PCL-C scores (from +3 to +24).
Table 2.
Change from baseline to 6-month in clinical outcomes in intervention and control groups.
| Questionnaire | Control (N = 12) | Intervention (N = 11) | p-value |
|---|---|---|---|
| PCL-C | 0.75 (15.17) | −8.00 (10.49) | 0.126 |
| HADS overall | −0.42 (6.63) | −0.91 (4.21) | 0.835 |
| HADS anxiety | −0.83 (4.02) | −0.45 (2.30) | 0.787 |
| HADS depression* | 1.00 (−1.50, 2.00) | −2.00 (−3.00, 1.00) | 0.263 |
| BRS* | 0.00 (−0.33, 0.17) | −0.17 (−0.33, 0.50) | 0.658 |
| CNAQ | 1.50 (2.54) | 1.6 (3.95) | 0.943 |
| EQ-5D-5L score | −0.02 (0.15) | −0.04 (0.14) | 0.657 |
| EQ-5D-5L VAS | 10.33 (15.33) | 11.2 (13.10) | 0.889 |
PCL-C: post traumatic stress disorder checklist: Civilian; HADS: hospital anxiety and depression scale; BRS: brief resilience scale; CNAQ: council of nutrition and appetite questionnaire; EQ-5D-5L: EuroQol 5 dimensions-5 levels; VAS: visual analogue scale.
Data are presented as mean (Standard Deviation) and p-value reported from t-test, or *median (Inter Quartile Range) and p-value reported from Wilcoxon rank-sum test.
Mean change in overall HADS scores was comparable between groups, with a reduction of 0.91 (SD 4.21) in intervention group and a reduction of 0.42 (SD 6.63) in the CG (p = 0.835). Mean HADS-Anxiety scores decreased by 0.45 (SD 2.30) in the intervention group and 0.83 (SD 4.02) in the CG (p = 0.787); median HADS-Depression scores fell by 2 (Inter Quartile Range (IQR) −3,1) in the intervention but increased by 1 (IQR −1.5,2) in the CG (p = 0.263). Median change in resilience score was −0.17 (IQR −0.03,0.50) in the intervention group, and 0 (IQR −0.33,0.17) in the CG (p = 0.658). Mean change in CNAQ was 1.6 (SD 3.95) in intervention group and 1.5 (SD 2.54) in the CG (p = 0.943). Mean EQ-5D-5L scores declined by 0.04 (SD 0.14) in the intervention group and −0.02 (SD 0.15) in the CG (p = 0.657): mean change in EQ-5D-5L visual analogue score was 11.2 (SD 13.10) in the intervention group and 10.33 (SD 15.33) in the CG (p = 0.889).
Discussion
To our knowledge COVEMERALD is the first investigation of a protocolised EMDR intervention, following an intensive care admission. We exceeded our pre-published feasibility thresholds and safely delivered online EMDR R-TEP to a cohort of intensive care survivors. We report findings that will inform design changes, and improve the chances of delivering a future fully-powered effectiveness RCT. Our clinical findings indicate that such an investigation of EMDR is warranted, in a broader cohort of intensive care survivors.
The primary outcome of this study was feasibility. We met recruitment target in 7 month, with a mean of 3.7 participants per month, during a period of unprecedented clinical pressure. We were able to recruit 51% of eligible patients approached, exceeding our published target of 30%. To achieve our recruitment target (n = 26) we screened 75 patients. Accounting for exclusions, missed patients and trial decliners, 35% of screened patients consented to trial participation. Meaningful comparison of recruitment rates, are difficult due to the novelty of this intervention in this cohort. However, a review of publicly funded trials in the UK noted that the median recruitment rate was 0.98 participants per centre per month, with 50% of RCTs failing to meet recruitment targets. 63
Consecutive patients were approached for COVE-MERALD participation and the demographic characteristics of the study sample were largely representative of the wider patient population: however, the self-declared ethnicity of study participants (96% white) indicates an under-representation of other ethnic groups, based on ICU patient populations. Between September 2020 and April 2021, 28% of patients admitted to UK intensive care units with COVID-19, were of black, Asian, mixed or other ethnicity 12 : 23% of patients admitted to our unit during the recruitment period were black, Asian, mixed or other ethnicity yet in this study >90% of participants were white. Furthermore, 14% of patients who we approached declined participation in our online intervention study, due to lack of digital access. Widely recognised as a social determinant of health 64 and exacerbated by the COVID-19 requirement for social distancing, the digital divide presents an increasing risk of exacerbating health inequality. 65 Recently the UK National Institute for Health and Care Research (NIHR) has published guidance for ensuring inclusivity in research, 66 which will inform the approach to recruitment in future studies.
A key uncertainty of our trial was whether EMDR R-TEP, delivered early (within 3 month of hospital discharge), could work as a protective intervention against development of persistent post-traumatic stress symptoms, irrespective of symptomology at the time of recruitment. Eligible patients most frequently cited lack of psychological distress as the main reason for trial decline. Moreover, of the 12 participants who received the intervention, five patients only had one session, due to no psychological distress. Our cohort was too small to undertake meaningful sub-group analysis, comparing symptom resolution between those above and below clinical cut-offs. We believe our findings assert that future studies should focus on screening for PTSD symptoms before offering EMDR, consistent with international treatment guidance.24,25,67
Screening for psychological symptoms at 3 month is further supported by our experience of intervention session adherence: although 34 of 41 (83%) organised sessions were completed suggesting that participants found the intervention acceptable, five of these seven missed sessions were due to physical illness in the early rehabilitation phase. To promote RCT scalability and clinical implementation, we propose aligning the psychological screening with the 3-month post-hospital discharge follow-up visit, recommended in ICU rehabilitation clinical pathways. 68 A recently published survey reported increasing provision of UK follow-up services, yet highlighted important gaps, most commonly in psychological support. 11 Our work supports the author’s conclusion that improving the evidence base will be key to expanding service delivery and impacting upon patient-centred outcomes.
The known relationship between EMDR intervention fidelity and treatment effect size 69 has important implications for future studies of clinical effectiveness. The COVEMERALD EMDR R-TEP intervention was performed by a Consultant clinical psychologist and two trained, experienced psychological therapists. An EMDR consultant offered clinical supervision: however, we could not formally check intervention fidelity due to time and resource constraints. Future studies should consider using an EMDR fidelity rating scale,70,71 to ensure validity and enable replication, and provide an account of possible relationships between intervention fidelity and treatment effect size, including individual dose-response variability. Moreover, there are fewer EMDR R-TEP practitioners than those trained in standard protocol EMDR. Careful consideration should be given to which EMDR protocol is most useful and scalable in this context.
There were no protocol deviations or safety incidents, consistent with systematic reviews of EMDR, including those studies in survivors of life-threatening medical events. 72 COVEMERALD exceeded the reported mean completion rate (75%) of seven other studies investigating psychological interventions for ICU survivors 39
Clinical outcomes
Our study was not powered to detect efficacy of the intervention compared to usual practice. The reported values do match findings from a systematic review of studies of EMDR in survivors of other life-threatening medical events 72 and show a trend towards symptom reduction in PTSD (−8) and depressive symptoms (−2). These are in the ranges defined as MCID of 5.7–10.249 and −253 respectively, however, clinical relevance should not be ascribed to these results, given the study design limitations. We do, however, believe these results support the case for further investigations of EMDR for symptom reduction in survivors of critical illness.
This trial was conducted during an ongoing global pandemic, with recognised adverse effect on population mental health. To adequately explore interaction between our patient cohort, contextual and cultural factors, we recommend that future researchers adopt a mixed-methods approach, in larger samples. This would enhance understanding of when, how and under which circumstances EMDR is effective and may offer insight into the wide treatment response variability.
Limitations
The study has a number of design limitations which may affect generalisability, many of which have been outlined in the discussion; this was a small, single-centre study, with inadequate representation of under-served populations, failure to address digital exclusion and lack of intervention fidelity checks. Moreover, there is a high risk of bias associated with non-blinded clinical outcome measures. Our follow-up period was limited to 6 month due to lack of funding. Given the uncertain mental health trajectory following ICU discharge, future studies should report clinical outcomes up to a minimum of 12 month post-discharge, preferably longer. Our study was undertaken during a period of unprecedented clinical pressure, using a patient population limited to sufferers of COVID-19. Rapid changes to the UK’s research rules meant that we were limited to undertaking research in this cohort. While this may limit generalisability of our study, emerging evidence suggests that post-discharge challenges faced by COVID patients are comparable to those in wider ICU-survivor cohorts. 73 However, this study does need to be repeated in a more representative cohort of ICU-survivors. Remaining uncertainties require refinement of trial design, before proceeding to a definitive RCT of clinical effectiveness.
Conclusion
This study met feasibility and safety targets. However, fundamental design changes will need to be applied before progression to an adequately powered, multi-centre RCT of clinical effectiveness. A future trial of EMDR for intensive care survivors should consider a larger number of simultaneously recruiting sites, and adopting strategies to ensure representative inclusion of under-served ethnic, socio-economic and digitally-excluded populations. We recommend psychological screening of participants, consistent with recommended ICU clinical rehabilitation pathways. The EMDR intervention should be fidelity-checked, and offered online or face-to-face. To support scalability and rapid translation of findings, the RCT should be embedded within established clinical referral pathways. A mixed-methods approach, should be adopted, in order to capture the complexity of interaction between the intervention, outcome, context, culture and mechanisms of change.
Supplemental Material
Supplemental material, sj-docx-1-inc-10.1177_17511437221136828 for A randomised pilot feasibility study of eye movement desensitisation and reprocessing recent traumatic episode protocol, to improve psychological recovery following intensive care admission for COVID-19 by Andrew Bates, Hannah Golding, Sophie Rushbrook, Elan Shapiro, Natalie Pattison, David S Baldwin, Michael P W Grocott and Rebecca Cusack in Journal of the Intensive Care Society
Acknowledgments
We would like to thank the Intensive care staff and patients at University Hospital Southampton, for their advice, support, and patience during this period of unprecedented pressure. In particular we acknowledge the efforts of Fiona Hall (UHS NHSFT ICU follow-up senior sister) and Dr Kathleen Nolan (UHS NHSFT ICU and follow-up Consultant), Janet Hathaway, project manager at Dorset Healthcare University NHSFT, Lesley Hawkins, senior project manager at UHS NHSFT, Tara Walker and Nicola Coulter for delivering the intervention and participating in invaluable trial team meetings. This trial was possible because of their unfailing enthusiasm and support.
Footnotes
Authors’ contributions: AB conceived and designed the study, acquired and interpreted the data, and led manuscript preparation, under the supervision of NP, DSB, RC and MPWG. HG acquired and interpreted the data, designed and formatted the tables. SR conceived and designed the study and led intervention delivery and supervision. ES developed the EMDR R-TEP intervention, trained the psychological therapists and participated in study design. NP provided intellectual input and critical revision of the manuscript. DSB contributed to study design, intellectual input and critical revision of the manuscript. MPWG designed the study, provided intellectual input and critical revision of the manuscript. RC conceived and designed the study, acquired and interpreted the data, provided intellectual input and critical revision of the manuscript. All authors contributed to, edited and approved the final manuscript.
Availability of data and materials: Datasets used in preparation of this manuscript can be accessed from the corresponding author on reasonable request.
Consent for publication: Individual’s data is not included in this manuscript, therefore consent for publication is not required.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval and consent to participate: London-Fulham Research Ethics Committee, United Kingdom granted ethical approval on 24th August 2020. (Reference: 20/HRA/3633). The full study protocol has been published elsewhere(41). Due to the ongoing requirement to maintain social distancing during the pandemic, verbal consent was obtained and documented during telephone consultation between participants and research staff. Consent forms were posted to all participants.
Funding: Andrew Bates is funded by a National Institute for Health Research (NIHR) (pre-doctoral clinical academic fellowship) for this research project. This article presents independent research funded by the National Institute for Health Research (NIHR). The views expressed are those of the author and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
ORCID iDs: Andrew Bates  https://orcid.org/0000-0002-3614-0270
https://orcid.org/0000-0002-3614-0270
Hannah Golding  https://orcid.org/0000-0001-6147-8858
https://orcid.org/0000-0001-6147-8858
Rebecca Cusack  https://orcid.org/0000-0003-2863-2870
https://orcid.org/0000-0003-2863-2870
Bibliography
- 1. Flaatten H, Waldmann C. The post-ICU syndrome, history and definition. In: Preiser J-C, Herridge M, Azoulay E. (eds) Post-intensive care syndrome. Cham, Switzerland: Springer, 2020, 3–12. [Google Scholar]
- 2. Righy C, Rosa RG, da Silva RTA, et al. Prevalence of post-traumatic stress disorder symptoms in adult critical care survivors: a systematic review and meta-analysis. Crit Care 2019; 23: 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Parker AM, Sricharoenchai T, Raparla S, et al. Posttraumatic stress disorder in critical illness survivors: a meta-analysis. Crit Care Med 2015; 43: 1121–1129. [DOI] [PubMed] [Google Scholar]
- 4. Nikayin S, Rabiee A, Hashem MD, et al. Anxiety symptoms in survivors of critical illness: a systematic review and meta-analysis. Gen Hosp Psychiatry 2016; 43: 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davydow DS, Gifford JM, Desai SV, et al. Posttraumatic stress disorder in general intensive care unit survivors: a systematic review. Gen Hosp Psychiatry 2008; 30: 421–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hatch R, Young D, Barber V, et al. Anxiety, depression and post traumatic stress disorder after critical illness: a UK-wide prospective cohort study. Crit Care 2018; 22: 310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Davydow DS, Gifford JM, Desai SV, et al. Depression in general intensive care unit survivors: a systematic review. Intensive Care Med 2009; 35: 796–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davydow DS, Hough CL, Zatzick D, et al. Psychiatric symptoms and acute care service utilization over the course of the year following medical-surgical ICU admission: a longitudinal investigation. Crit Care Med 2014; 42: 2473–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McPeake J, Mikkelsen ME, Quasim T, et al. Return to employment after critical illness and its association with psychosocial outcomes. a systematic review and meta-analysis. Ann Am Thorac Soc 2019; 16: 1304–1311. [DOI] [PubMed] [Google Scholar]
- 10. Davydow DS, Zatzick D, Hough CL, et al. A longitudinal investigation of alcohol use over the course of the year following medical-surgical intensive care unit admission. Psychosomatics 2013; 54: 307–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Connolly B, Milton-Cole R, Adams C, et al. Recovery, rehabilitation and follow-up services following critical illness: an updated UK national cross-sectional survey and progress report. BMJ Open 2021; 11: e052214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Intensive Care National Audit and Research Centre. ICNARC report on COVID-19 in critical care: England, Wales and Northern Ireland, https://www.icnarc.org/Our-Audit/Audits/Cmp/Reports (2021, accessed 17 December 2021).
- 13. Neville TH, Hays RD, Tseng C-H, et al. Survival after Severe COVID-19: long-term outcomes of patients admitted to an intensive care unit. J Intensive Care Med 2022; 37: 1019–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gardner PJ, Moallef P. Psychological impact on SARS survivors: critical review of the English language literature. Can Psychol/Psychol Can 2015; 56: 123–135. [Google Scholar]
- 15. Barker-Davies RM, O’Sullivan O, Senaratne KPP, et al. The Stanford Hall consensus statement for post-COVID-19 rehabilitation. Br J Sports Med 2020; 54: 949–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McIlroy PA, King RS, Garrouste-Orgeas M, et al. The effect of ICU diaries on psychological outcomes and quality of life of survivors of critical illness and their relatives: a systematic review and meta-analysis. Crit Care Med 2019; 47: 273–279. [DOI] [PubMed] [Google Scholar]
- 17. Cuthbertson BH, Rattray J, Campbell MK, et al. The PRaCTICaL study of nurse led, intensive care follow-up programmes for improving long term outcomes from critical illness: a pragmatic randomised controlled trial. BMJ 2009; 339: b3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wade DM, Mouncey PR, Richards-Belle A, et al. Effect of a Nurse-Led preventive psychological intervention on symptoms of posttraumatic stress disorder among critically ill patients: a randomized clinical trial. JAMA 2019; 321: 665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bieber ED, Philbrick KL, Shapiro JB, et al. Psychiatry’s role in the prevention of post-intensive care mental health impairment: stakeholder survey. BMC Psychiatry 2022; 22: 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fernando SM, Ranzani OT, Herridge MS. Mental health morbidity, self-harm, and suicide in ICU survivors and caregivers. Intensive Care Med 2022; 48: 1084–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Novo Navarro P, Landin-Romero R, Guardiola-Wanden-Berghe R, et al. 25 years of eye movement desensitization and reprocessing (EMDR): the EMDR therapy protocol, hypotheses of its mechanism of action and a systematic review of its efficacy in the treatment of post-traumatic stress disorder. Rev Psiquiatr Salud Ment (Engl Ed) 2018; 11: 101–114. [DOI] [PubMed] [Google Scholar]
- 22. Mavranezouli I, Megnin-Viggars O, Daly C, et al. Psychological treatments for post-traumatic stress disorder in adults: a network meta-analysis. Psychol Med 2020; 50: 542–555. [DOI] [PubMed] [Google Scholar]
- 23. Bisson JI, Roberts NP, Andrew M, et al. Psychological therapies for chronic post-traumatic stress disorder (PTSD) in adults. Cochrane Database Syst Rev 2013; 2013: CD003388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Van Ommeren M. Guidelines for the management of conditions specifically related to stress. World Health Organisation. https://www.who.int/publications/i/item/guidelines-for-the-management-of-conditions-that-are-specifically-related-to-stress (2013, accessed January 2022). [PubMed]
- 25. National Institute for Health and Care Excellence. Post-traumatic stress disorder: NICE guideline, https://www.nice.org.uk/guidance/NG116 (2018, accessed January 2022). [PubMed]
- 26. Valiente-Gómez A, Moreno-Alcázar A, Treen D, et al. EMDR beyond PTSD: a systematic literature review. Front Psychol 2017; 8: 1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Patel MB, Jackson JC, Morandi A, et al. Incidence and risk factors for intensive care unit-related post-traumatic stress disorder in veterans and civilians. Am J Respir Crit Care Med 2016; 193: 1373–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hulme T. Using eye movement therapy to reduce trauma after intensive care. Nursing Times 2018; 114: 18–21. [Google Scholar]
- 29. Wake S, Kitchiner D. Post-traumatic stress disorder after intensive care. BMJ 2013; 346: f3232. [DOI] [PubMed] [Google Scholar]
- 30. Clarke R. The EMDR recent traumatic episode protocol with an intensive care survivor: a case study. J EMDR Pract Res 2022; 16: 50–60. [Google Scholar]
- 31. Shapiro E, Laub B. Early EMDR Intervention (EEI): a summary, a theoretical model, and the recent traumatic episode protocol (R-TEP). J EMDR Pract Res 2008; 2: 79–96. [Google Scholar]
- 32. Jones C, Griffiths RD, Humphris G, et al. Memory, delusions, and the development of acute posttraumatic stress disorder-related symptoms after intensive care. Crit Care Med 2001; 29: 573–580. [DOI] [PubMed] [Google Scholar]
- 33. Shapiro E, Laub B. Early EMDR intervention following a community critical incident: a randomized clinical trial. J EMDR Pract Res 2015; 9: 17–27. [Google Scholar]
- 34. Shapiro E, Laub B, Rosenblat O. Early EMDR intervention following intense rocket attacks on a town: a randomised clinical trial. Clin Neuropsychiatry J Treat Eval 2018; 15: 194–205. [Google Scholar]
- 35. Tofani LR, Wheeler K. The recent-traumatic episode protocol: outcome evaluation and analysis of three case studies. J EMDR Pract Res 2011; 5: 95–110. [Google Scholar]
- 36. Gil-Jardiné C, Evrard G, Al Joboory S, et al. Emergency room intervention to prevent post concussion-like symptoms and post-traumatic stress disorder. A pilot randomized controlled study of a brief eye movement desensitization and reprocessing intervention versus reassurance or usual care. J Psychiatr Res 2018; 103: 229–236. [DOI] [PubMed] [Google Scholar]
- 37. Roberts NP, Kitchiner NJ, Kenardy J, et al. Early psychological intervention following recent trauma: a systematic review and meta-analysis. Eur J Psychotraumatol 2019; 10: 1695486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Birk JL, Sumner JA, Haerizadeh M, et al. Early interventions to prevent posttraumatic stress disorder symptoms in survivors of life-threatening medical events: a systematic review. J Anxiety Disord 2019; 64: 24–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wade DF, Moon Z, Windgassen SS, et al. Non-pharmacological interventions to reduce ICU-related psychological distress: a systematic review. Minerva Anestesiol 2016; 82: 465–478. [PubMed] [Google Scholar]
- 40. Roberts MB, Glaspey LJ, Mazzarelli A, et al. Early interventions for the prevention of posttraumatic stress symptoms in survivors of critical illness: a qualitative systematic review. Crit Care Med 2018; 46: 1328–1333. [DOI] [PubMed] [Google Scholar]
- 41. Bates A, Rushbrook S, Shapiro E, et al. CovEMERALD: assessing the feasibility and preliminary effectiveness of remotely delivered eye movement desensitisation and reprocessing following Covid-19 related critical illness: a structured summary of a study protocol for a randomised controlled trial. Trials 2020; 21: 929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Craig P, Dieppe P, Macintyre S, et al. Developing and evaluating complex interventions: the new medical research council guidance. BMJ 2008; 337: a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Eldridge SM, Chan CL, Campbell MJ, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ 2016; 355: i5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014; 348: g1687. [DOI] [PubMed] [Google Scholar]
- 45. Skivington K, Matthews L, Simpson SA, et al. A new framework for developing and evaluating complex interventions: update of Medical Research Council guidance. BMJ 2021; 374: n2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Karstoft KI, Andersen SB, Bertelsen M, et al. Diagnostic accuracy of the posttraumatic stress disorder checklist–civilian version in a representative military sample. Psychol Assess 2014; 26: 321–325. [DOI] [PubMed] [Google Scholar]
- 47. Blevins CA, Weathers FW, Davis MT, et al. The posttraumatic stress disorder checklist for DSM-5 (PCL-5): development and initial psychometric evaluation. J Trauma Stress 2015; 28: 489–498. [DOI] [PubMed] [Google Scholar]
- 48. Lang AJ, Laffaye C, Satz LE, et al. Sensitivity and specificity of the PTSD checklist in detecting PTSD in female veterans in primary care. J Trauma Stress 2003; 16: 257–264. [DOI] [PubMed] [Google Scholar]
- 49. Stefanovics EA, Rosenheck RA, Jones KM, et al. Minimal clinically important differences (MCID) in assessing outcomes of post-traumatic stress disorder. Psychiatr Q 2018; 89: 141–155. [DOI] [PubMed] [Google Scholar]
- 50. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983; 67: 361–370. [DOI] [PubMed] [Google Scholar]
- 51. Rabiee A, Nikayin S, Hashem MD, et al. Depressive symptoms after critical illness: a systematic review and meta-analysis. Crit Care Med 2016; 44: 1744–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bjelland I, Dahl AA, Haug TT, et al. The validity of the hospital anxiety and depression scale. An updated literature review. J Psychosom Res 2002; 52: 69–77. [DOI] [PubMed] [Google Scholar]
- 53. Lemay KR, Tulloch HE, Pipe AL, et al. Establishing the minimal clinically important difference for the hospital anxiety and depression scale in patients with cardiovascular disease. J Cardiopulm Rehabil Prev 2019; 39: E6–E11. [DOI] [PubMed] [Google Scholar]
- 54. Wynne SC, Patel S, Barker RE, et al. Anxiety and depression in bronchiectasis: response to pulmonary rehabilitation and minimal clinically important difference of the hospital anxiety and depression scale. Chron Respir Dis 2020; 17: 1479973120933292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011; 20: 1727–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Smith BW, Dalen J, Wiggins K, et al. The brief resilience scale: assessing the ability to bounce back. Int J Behav Med 2008; 15: 194–200. [DOI] [PubMed] [Google Scholar]
- 57. Horn SR, Charney DS, Feder A. Understanding resilience: new approaches for preventing and treating PTSD. Exp Neurol 2016; 284: 119–132. [DOI] [PubMed] [Google Scholar]
- 58. Wilson MM, Thomas DR, Rubenstein LZ, et al. Appetite assessment: simple appetite questionnaire predicts weight loss in community-dwelling adults and nursing home residents. Am J Clin Nutr 2005; 82: 1074–1081. [DOI] [PubMed] [Google Scholar]
- 59. LeardMann CA, Woodall KA, Littman AJ, et al. Post-traumatic stress disorder predicts future weight change in the millennium cohort study. Obesity (Silver Spring) 2015; 23: 886–892. [DOI] [PubMed] [Google Scholar]
- 60. Hertzog MA. Considerations in determining sample size for pilot studies. Res Nurs Health 2008; 31: 180–191. [DOI] [PubMed] [Google Scholar]
- 61. Sosnowski K, Mitchell ML, White H, et al. A feasibility study of a randomised controlled trial to examine the impact of the ABCDE bundle on quality of life in ICU survivors. Pilot Feasibility Stud 2018; 4: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mohd Razali N, Bee Wah Y. Power comparisons of Shapiro-Wilk, Kolmogorov-Smirnov, Lilliefors and Anderson-Darling tests. J Stat Model Analytics 2011; 2: 21–33. [Google Scholar]
- 63. Walters SJ, Bonacho Dos Anjos Henriques-Cadby I, Bortolami O, et al. Recruitment and retention of participants in randomised controlled trials: a review of trials funded and published by the United Kingdom Health Technology Assessment Programme. BMJ Open 2017; 7: e015276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ramsetty A, Adams C. Impact of the digital divide in the age of COVID-19. J Am Med Inform Assoc 2020; 27: 1147–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Stone E, Nuckley P, Shapiro R. Digital inclusion in health and care: lessons learned from the NHS widening digital participation programme (2017-2020). London, UK. https://www.goodthingsfoundation.org/insights/digital-participation-lessons-learned/. (2020, accessed 17 July 2022).
- 66. Witham MD, Anderson E, Carroll C, et al. Developing a roadmap to improve trial delivery for under-served groups: results from a UK multi-stakeholder process. Trials 2020; 21: 694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Forbes D, Bisson JI, Monson CM, et al. Effective treatments for PTSD: practice guidelines from the international society for traumatic stress studies. 3rd ed. New York: The Guildford Press, 2020. [Google Scholar]
- 68. National Institute for Health and Care Excellence. Rehabilitation after critical illness in adults. Quality standard [QS158] NICE, https://www.nice.org.uk/guidance/QS158 (2017, accessed January 2022). [PubMed]
- 69. Maxfield L, Hyer L. The relationship between efficacy and methodology in studies investigating EMDR treatment of PTSD. J Clin Psychol 2002; 58: 23–41. [DOI] [PubMed] [Google Scholar]
- 70. Korn D, Maxfield L, Stickgold R, et al. EMDR fidelity rating scale | EMDR foundation [Internet]. https://emdrfoundation.org/research-grants/emdr-fidelity-rating-scale/ (2018, accessed 10 January 2021).
- 71. Shapiro E, Laub B. EMDR recent traumatic episode protocol (EMDR R-TEP) fidelity scale. EMDR Foundation. https://emdrfoundation.org/toolkit/rtep-fidelity-checklist.pdf (2014, accessed January 2022).
- 72. Haerizadeh M, Sumner JA, Birk JL, et al. Interventions for posttraumatic stress disorder symptoms induced by medical events: a systematic review. J Psychosom Res 2020; 129: 109908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hodgson CL, Higgins AM, Bailey MJ, et al. Comparison of 6-month outcomes of survivors of COVID-19 versus non–COVID-19 critical illness. Am J Respir Crit Care Med 2022; 205: 1159–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-inc-10.1177_17511437221136828 for A randomised pilot feasibility study of eye movement desensitisation and reprocessing recent traumatic episode protocol, to improve psychological recovery following intensive care admission for COVID-19 by Andrew Bates, Hannah Golding, Sophie Rushbrook, Elan Shapiro, Natalie Pattison, David S Baldwin, Michael P W Grocott and Rebecca Cusack in Journal of the Intensive Care Society