Abstract
While the acute phase of coronavirus disease 2019 (COVID-19) is associated with worsening cardiac outcomes, it is unclear whether it affects the outcome of patients with ST-segment elevation myocardial infarction (STEMI) after the acute phase. In addition, while many studies compared the course of STEMI during the COVID-19 pandemic with the years before the outbreak, we evaluated the course of STEMI during the pandemic according to whether or not patients had history of COVID-19. Patients diagnosed with STEMI during the ongoing COVID-19 pandemic were included in the study. The Ministry of Health database was analyzed retrospectively, and patients with (n = 191) and without (n = 127) a history of polymerase chain reaction (PCR) confirmed COVID-19 infection were divided into groups. Clinical and angiographic characteristics were assessed. The rates of in-hospital major adverse cardiac events (MACE) were higher in those who had a history of PCR-verified COVID-19 infection. Angiographic and procedural findings indicating successful reperfusion were better in patients without a history of COVID-19. A history of COVID-19 infection (odds ratio 1.40, 95% confidence interval 1.25–1.60, P < .01) independently predicted MACE. A history of COVID-19 infection is a predictor of worse outcomes following coronary intervention and in-hospital MACE among patients with STEMI.
Keywords: COVID-19, ST-elevation myocardial infarction, primary percutaneous coronary intervention
Introduction
Coronavirus disease 2019 (COVID-19) has significantly impacted healthcare worldwide, and the World Health Organization (WHO) declared it as a pandemic on March 11, 2020.1 During this pandemic, a decrease in the number of hospitalizations, treatment delays, and worsened cardiac outcomes in cases of ST-elevation myocardial infarction (STEMI) have been observed.2-6 A few studies have reported the impact of the COVID-19 pandemic on STEMI patients.7,8 These studies compared the incidence, time course, and outcomes of patients with STEMI before and during the COVID-19 pandemic. Ultimately, the pandemic environment has been shown to affect acute coronary syndrome (ACS) treatment at all stages, from patient presentation to hospital treatment and admission, and reports have documented significant delays from symptom onset to presentation and revascularization.9-13 There are also reports of increased cardiovascular manifestations and thrombotic complications in patients presenting with COVID-19.14,15
Little is known about the impact of having a history of COVID-19 on the outcome of STEMI. Thus, present study evaluated the effect of having a polymerase chain reaction (PCR)-confirmed history of COVID-19 on clinical and angiographic outcomes in STEMI patients after excluding those with positive PCR results during hospitalization and within the last 1 month.
Methods
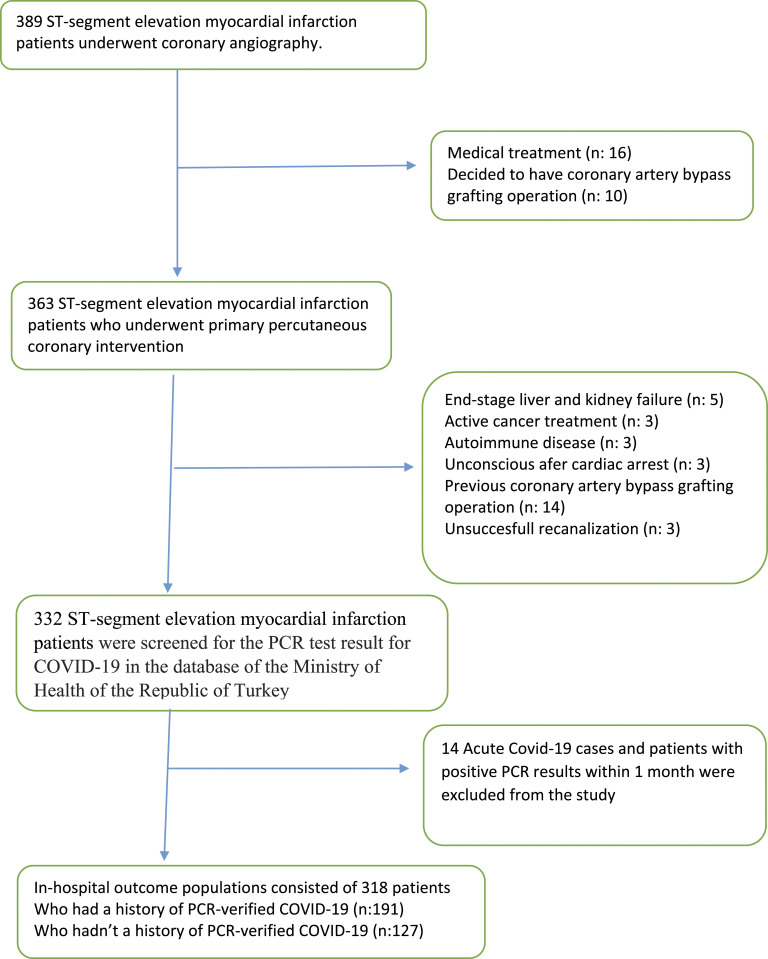
This was a two-center (Atlas University Medical Faculty Medicine Hospital, Istanbul and Acıbadem University Atakent Hospital, Istanbul) retrospective, observational cohort study that enrolled consecutive unselected non-randomized eligible patients who were hospitalized for STEMI and underwent primary percutaneous coronary intervention (pPCI) at our institutions from January 2021 to June 2022. A total of 389 patients were screened for inclusion. Among these patients, 71 patients were excluded (Figure 1). Finally, a total of 318 patients (85 females, 233 males, age range 35–83) were included in the study population.
Figure 1.
Flow diagram demonstrating enrollment and follow-up study patients.
All STEMI patients were adjudicated for inclusion and retrospectively confirmed to meet acute myocardial infarction definition. All STEMI patients were screened for the PCR test result for COVID-19 in the database of the Ministry of Health of the Republic of Turkey. Acute COVID-19 cases and patients with positive PCR results within 1 month were excluded from the study. The remaining 318 patients were divided into 2 groups according to who had a history of PCR-verified COVID-19 infection (group 1) or without (group 2). Clinical events of patients included in the study which developed in-hospital were recorded, and retrospective analysis was performed. Major adverse cardiac events (MACE) that developed in-hospital were evaluated separately. Our local ethics committee approved the study protocol by the Declaration of Helsinki, and all patients provided written informed consent for pPCI.
Demographic, clinical, and laboratory data at admission were collected from our hospital electronic database. Patients who had undergone pPCI before and who had ≥50% stenosis in major coronary arteries and side branches ≥1.5 mm on coronary angiography were defined as coronary artery disease. The diagnosis of STEMI was made according to current guidelines.16
Cardiac enzymes, creatinine levels, and hemogram parameters were studied daily during hospitalization. Peak troponin levels during hospitalization were evaluated in the study. All patients underwent echocardiography within 48–72 h after pPCI. The modified Simpson method was used to calculate the left ventricular ejection fraction (LVEF).
Angiographic Analysis
Primary percutaneous coronary intervention was undertaken according to the European Society of Cardiology Guidelines17 and the operator’s preference. The selection of the specific type of revascularization, procedural devices, stent types, and antiaggregant treatments were based on the decision of the operator. All angiographic end points were evaluated before and after pPCI. The patency of the infarct-related artery (IRA) was classified according to the thrombolysis in myocardial infarction (TIMI) flow grade (TFG) and TIMI myocardial perfusion grade (TMPG), which were assessed using previously described techniques.18,19 Thrombus burden grade was defined according to Gibson et al.20 Only patients with thrombus-containing lesion grades 2, 3, or 4 at diagnostic angiography were included. In brief, in TIMI thrombus grade 0, no cineangiographic characteristics of thrombus are present. In TIMI thrombus grade 1, possible thrombus is present, with angiography characteristics such as reduced contrast density, haziness, irregular lesion contour, or a smooth convex “meniscus” at the site of total occlusion, suggestive, but not diagnostic of thrombus. In TIMI thrombus grade 2, there is definite thrombus, with greatest dimensions 1/2 the vessel diameter. In TIMI thrombus grade 3, there is definite thrombus but with greatest linear dimension >1/2 but <2 vessel diameters. In TIMI thrombus grade 4, there is definite thrombus with the largest dimension 2 vessel diameters; and in TIMI thrombus grade 5, there is total occlusion. Collateral grading was carried out according to Rentrop grading system that ranges from 0 (no collateral filling) to 3 (complete vessel opacification by retrograde flow).21
Electrocardiogram Assessment of ST Resolution
The sum of ST elevation was assessed in three contiguous leads in the infarct zone, 60 msec from the J point. The extent of ST-segment resolution was assessed 90 minutes after pPCI and expressed as the percentage of the ST-segment elevation shown on presentation.22 309 electrocardiograms (97.16% of the 318 total cases, 187 electrocardiograms (ECGs) of patients with group 1 and 122 ECGs of patients with Group 2) were completely interpreted. In the remaining cases, ECGs were not assessed because of missing data, inadequate strips, and presence of idioventricular rhythm, functioning ventricular pacemaker, or new-onset left bundle-branch block.
Clinical Endpoint Definitions
Information about the in-hospital outcome was obtained from an electronic centralized clinical database. In-hospital clinical results were collected by a physician who was unaware of the initial clinical, laboratory, and angiographic results.
In-hospital mortality was defined as all-cause mortality during hospitalization. In-hospital MACEs were defined as the combined endpoint of cardiac death, reinfarction, target vessel revascularization, heart failure, and arrhythmic events (ventricular fibrillation and ventricular tachycardia).
Statistical Methods
All data were presented as mean ± SD or median (interquartile range) and as percentages for categorical variables. Continuous variables were checked for normal distribution assumption using the Kolmogorov–Smirnov test. Differences between MACE(+) patients and MACE(−) patients were evaluated using the Mann–Whitney U test or the Student t test as appropriate. Categorical variables were tested by the Pearson χ2 test or Fisher exact test. Univariate and multivariate binary logistic regression analyses were performed to identify the independent predictors of in-hospital mortality and MACE separately. Forward stepwise multivariable regression models using parameters with P < .10 were included in logistic regression analyses. The appropriateness of the model created was assessed using the Hosmer and Lemeshow test. All P values were 2 sided, and a P < .05 was considered statistically significant. All statistical assessments were carried out using the Statistical package for Social Sciences (SPSS for Windows, version 23.0. IBM Corp. Armonk, NY, USA) software.
Results
Baseline demographic, clinical, and laboratory data were analyzed and compared between the two groups. Comparison results are given in Table 1. Patients with a diagnosis of STEMI with a PCR-confirmed history of COVID-19 (group 1) were significantly older. The two groups were similar with respect to sex distribution, frequencies of diabetes mellitus, hypertension, hypercholesterolemia, the rate of smoking, history of previous stroke, and previous AMI.
Table 1.
Baseline Demographic, Clinical, and Laboratory Characteristics of Patients.
| Parameters | Who had a history of polymerase chain reaction (PCR)-verified coronavirus disease 2019 (COVID-19) (n:191) | Who did not have a history of polymerase chain reaction (PCR)-verified coronavirus disease 2019 (COVID-19) (n:127) | P value |
|---|---|---|---|
| Demographic parameters | |||
| Age | 58.74 ± 9.4 | 50.21 ± 9.5 | <.01 |
| Gender (m/f) | 139/52 | 94\33 | .72 |
| Hypertension, n (%) | 65 (34.0%) | 41 (32.3%) | .81 |
| Diabetes mellitus, n (%) | 42 (21.9%) | 24 (18.8%) | .58 |
| Hypercholesterolemia, n (%) | 57 (29.8%) | 40 (31.5%) | .82 |
| Smoker, n (%) | 116 (60.7%) | 71 (50.4%) | 0,33 |
| Previous myocardial infarction, n(%) | 21 (10.9%) | 11 (8.6%) | .53 |
| Previous stroke, n (%) | 11 (5.7%) | 5 (3.9%) | .48 |
| Killip score >1 | 38 (19.8%) | 13 (10.2%) | .04 |
| ST-segment resolution < 50% | 72 (37.6%) | 26 (20.4%) | <.01 |
| Tirofiban use, n (%) | 48 (25.1%) | 16 (12.5%) | .02 |
| Left ventricular ejection fraction (LVEF) (%) | 54 (45–60) | 60 (48–63) | <.01 |
| Inotropic treatment, n(%) | 36 (18.8%) | 11 (8.6%) | .03 |
| IV diuretic treatment, n (%) | 37 (19.3%) | 13 (10.2%) | .05 |
| Intra-aortic balloon pump (IABP), n (%) | 5 (2.6%) | 1 (.7%) | .24 |
| Length of hospitalization (days) | 5 (4–5) | 4 (4–5) | .31 |
| Mortality, n (%) | 4 (2.0%) | 1 (.7%) | .24 |
| Major adverse cardiac events (MACE), n (%) | 24 (12.5%) | 6 (4.7%) | .03 |
| Laboratory parameters | |||
| Blood glucose at admission (mg/dl) | 145 (116–199) | 137 (112–198) | .194 |
| Creatinine level at admission (mg/dl) | .91 (.8–1.1) | .87 (.7–1.0) | .02 |
| Creatinine level at post-procedural (mg/dl) | .98 (.8–1.2) | .91 (.79–1.1) | <.01 |
| Peak troponin I (ng/L) | 39.7 (15.8–80.9) | 28.9 (11.3–69.7) | <.01 |
| C-reactive protein (mg/dl) | 2.1 (.7–10.1) | 1.4 (.5–4.1) | <.01 |
| Hemoglobin level (g/dl) | 14.7 (13.5–15.7) | 14.1 (13.7–15.3) | .123 |
| White blood cells (×103 μ/L) | 13.3 (11.5–15.3) | 8.7 (7.4–9.9) | <.01 |
| Neutrophil (×103cells/μL) | 9.6 (7.3–11.8) | 5.3 (4.1–7.1) | <.01 |
| Lymphocyte ((×103cells/μL) | 2.5 (1.6–4.1) | 2.2 (1.5–2.9) | <.01 |
| Neutrophil/lymphocyte ratio | 4,1 (3.5–7.2) | 3.0 (1,8–4,0) | <.01 |
| Platelet (×103/μL) | 275 (232–322) | 228 (191–275) | <.01 |
| Mean platelet volume (fL) | 8.8 (8.1–9.9) | 8.0 (7.2–8.8) | <.01 |
Regarding clinical parameters, group 1 had a higher heart rate than that in group 2 (P = .04), and patients with Killip > 1 score (P = .04) in need of intravenous inotropic (P = .03), intravenous diuretic (P = .05), and glycoprotein IIb/IIIa inhibitor therapy (P = .02) were higher than that in group 2. In group 1, LVEF was lower than that in group 2 (P < .01). On admission (P = .02) and post-procedural 48 h (P < .01) creatinine level and on admission C-reactive protein (CRP) values (P < .01) were higher in group 1. In group 1, peak troponin I levels (ng/ml) were higher than those in group 2 (P < .01). Hematological parameters were analyzed and white blood cells (WBC), mean platelet volume (MPV), platelet, neutrophil, lymphocyte levels, and neutrophil-to-lymphocyte ratio were greater in group 1 (P < .01).
Angiographic and procedural characteristics of patients included in the study are presented in Table 2. Thrombus burden score was significantly higher in group 1 (P = .04). The overall rate of impaired epicardial arterial flow (TFG 0/1/2) was 14.9% in the group 2 and 30.3% in group 1. Impaired epicardial arterial flow was significantly higher in group 1 than group 2 (P = .01). TIMI myocardial perfusion grade was worse in group 1 than in group 2 (P < .01). Abnormal myocardial perfusion (TMPG 0/1/2) was 62.3% in group 1 and 33.8% group 2. Good collateral vascular development (Rentrop >1) was significantly worse in group 1 (P = .01). Less than 50% ST-segment resolution on ECGs were 51.9% in group 1 and this result was higher (P = .01) than in group 2 (29.5%).
Table 2.
In-Hospital Angiographic and Procedural Characteristics of Patients.
| Parameters | Who had a history of PCR-verified COVID-19 (n:191) | Who hadn’t a history of PCR-verified COVID-19 (n:127) | P |
|---|---|---|---|
| Multivessel disease (n, %) | 107 (56.0%) | 60 (47.2%) | .36 |
| Infarct-related artery | |||
| LAD, n (%) | 84 (43.9%) | 61 (48.0%) | .66 |
| CX, n (%) | 38 (19.8%) | 25 (19.6%) | .96 |
| RCA, n (%) | 68 (35.6%) | 41 (32.3%) | .66 |
| LMCA | 1 (.5%) | 0 | |
| Stent implantation, (n, %) | 188 (98.4%) | 120 (94.5%) | .80 |
| Thrombus aspiration, (n, %) | 66 (34.5%) | 37 (29.1%) | .12 |
| Impaired Epicardial arterial flow TFG <3 flow | 58 (30.3%) | 19 (14.9%) | .01 |
| Impaired TIMI myocardial perfusion grade TMPG <3 | 119 (62.3%) | 43 (33.8%) | <.01 |
| Thrombus burden score | 4.9 ± 0.4 | 4.4 ± 0.9 | .04 |
| Good collateral flow (Rentrop 2–3) | 36 (18.8%) | 45 (35.4%) | .01 |
IRA, infarct-related artery; LAD, left anterior descending artery; LCx, left circumflex artery, RCA, right coronary artery.
TFG, Thrombolysis In Myocardial Infarction (TIMI) Flow Grade.
TMPG, TIMI Myocardial Perfusion Grade.
The average length of hospital stay (days) was 4.5 ± 1.2 and there was no significant difference between the two groups. A total of five patients died during hospital follow-up and four of them were group 1. All of these patients had been admitted with cardiogenic shock. During the in-hospital period, the group 1 MACE rate was higher than that of group 2 (P = .03). Reintervention because of recurrent infarction was needed for 7 patients (5 in group 1 and 2 in group 2) during hospital follow-up. Decompensated heart failure and ventricular tachycardia/fibrillation developed in 10 patients (8 in group 1 and 2 in group 2) and 7 patients (4 in group 1 and 3 in group 2) respectively. Reintervention because of recurrent infarction and decompensated heart failure rates were higher in group 1 than group 2.
Univariate and multivariate regression analyses were performed to investigate the possible predictors of MACE in the study population. In univariate regression analysis, age, sex, presence of hypertension, presence of diabetes, multivessel disease, post-TIMI 3 flow, Killip Score > 1, troponin I, creatinine, CRP, and history of PCR-verified COVID-19 infection were correlated with MACE. In multivariate regression analysis, using model adjusted for aforementioned parameters, age, sex, multivessel disease, Killip Score > 1, troponin I, CRP and history of PCR-verified. COVID-19 infection (odds ratio (OR) 1.40, 95% confidence interval (CI) 1.25–1.60, P < .01) independently predicted MACE (Table 3).
Table 3.
Independent Predictors Of Major Adverse Cardiac Events According to the Univariate and Multivariate Regression Analyses in the Study Population.
| Variables | Unadjusted, OR (95% CI) | P | Adjusted, OR (95% CI)* | P |
|---|---|---|---|---|
| Age | 1.04 (1.02–1.06) | <.01 | 1.03 (1.01–1.05) | .04 |
| Female sex | .38 (.12–.64) | <.01 | .37 (.10–.73) | .01 |
| Multivessel disease | 1.06 (.93–.98) | <.01 | 1.08 (.93–.99) | .03 |
| Killip score 3 or 4 | 5.62 (2.36–13.38) | <.01 | 3.06 (1.03–9.09) | .04 |
| Troponin I | 1.03 (1.02–1.04) | <.01 | 1.02 (1.01–1.04) | <.01 |
| CRP | 1.02 (1.01–1.04) | <.01 | 1.02 (1.01–1.03) | .04 |
| History of COVID-19 infection | 1.40 (1.25–1.60) | <.01 | 1.32 (1.13–1.54) | <.01 |
Parameters with P < .10 in univariate model were entered to the multivariate regression analysis.
Discussion
In our study, there was a significant relationship between a history of PCR-verified COVID-19 infection and in-hospital MACEs in patients underwent pPCI due to STEMI. Regression analysis results showed that a history of PCR-verified COVID-19 infection can make an additional contribution for risk determination in STEMI patients. Our study revealed that impaired epicardial arterial flow (TFG < 3) and impaired TMPG < 3, thrombus burden score of IRA, and distal embolization were significantly higher whereas ST-segment resolution and collateral vascular development were significantly lower in patients with a history of PCR-verified COVID-19 infection.
Little is known about the impact of public health emergencies like a community outbreak of infectious disease on STEMI systems of care. In COVID-19 patients, the prevalence of cardiovascular disease (CVD) was found to be very high and it was shown that the presence of CVD is also associated with increased mortality.23,24
COVID-19 appears to contribute to the development of cardiovascular events such as myocardial damage, arrhythmias, acute coronary syndrome (ACS), and arterial and venous thromboembolism, as well as being more severe and fatal in those with CVD. This indicates the existence of a bidirectional interaction between COVID-19 and the cardiovascular system, but the underlying mechanisms of this interaction are still not fully elucidated. It has been suggested that the systemic burden of inflammation caused by COVID-19 accelerates the development of subclinical disease or causes new cardiovascular damage. It is thought that direct viral invasion may also be an important mechanism in the pathogenesis of all extrapulmonary manifestations seen during COVID-19, including CVDs, since ACE2, the receptor for SARS-CoV-2 to enter the host cell, is found in many tissues outside the lung. Other mechanisms that may have a place in the pathogenesis are endothelial damage and thromboinflammation, and dysregulation of the renin-angiotensin-aldosterone system in which ACE2 is involved.23,24
Severe systemic inflammation increases the risk of atherosclerotic plaque disruption and AMI.25,26 A 2018 study found that influenza and other select viral illnesses were associated with an increased risk of AMI within the first 7 days of disease diagnosis, with an incidence ratio of 6.1 for influenza and 2.8 for other viruses.25 Another study of patients hospitalized for community-acquired pneumonia found an increased risk of active CVD that remained active for several years after hospitalization.27 Due to extensive inflammation and hypercoagulability, the risk of AMI is likely present in patients with acute COVID-19. Importantly, the infection itself can precipitate cardiovascular complications.25,26 Mononuclear myocardial infiltration, myocardial injury, and myocarditis have been documented with COVID-19.28 Moreover, through overt inflammation, plaque destabilization, coagulation disturbances, and alterations in myocardial supply and demand, COVID-19 infection can result in ischemia and acute myocardial infarction (AMI).29 Nonetheless, results of studies addressing more lasting effects of infections on CVD risk have been conflicting. Likewise, it is not clear whether having COVID-19 in the years after the acute period has an effect on the prognosis of AMI. In the present study, we found that patients having a history of COVID-19 had an increased risk of STEMI outcome that remained active for several years after infection.
The mechanisms by which infections might increase CVD risk in the short term are discussed elsewhere.30 However, the mechanism by which infections could affect long-term risk of CVD are poorly understood. Experimental models of infection in mice prone to atherosclerosis and autopsy studies in humans suggest that infections can induce pro-inflammatory changes in the cellular composition of the atherosclerotic lesions and make them more vulnerable to cause coronary and cerebrovascular events.31 Persistent systemic inflammatory activity is a known risk factor for CVD.32 Although more than 80% of patients hospitalized for systemic infections recover by one week, half of these patients continue exhibiting high circulating inflammatory markers.33 Furthermore, higher interleukin-6 levels at hospital discharge are associated with increased cardiovascular mortality at 1 year after systemic infections.34 Thus, persistent inflammation after systemic infections can contribute to subsequent progression to CVD. Similarly, survivors of systemic infections hospitalization may also have a persistent procoagulant state, and higher levels of coagulation markers at hospital discharge have been associated with increased risk of cardiovascular deaths.34 As we have shown in our study, the mechanisms of the long-term adverse effects of COVID-19 infection on the course of CVD should be examined more closely in future studies.
Our study has several implications. First, previous studies have examined the outcome, clinical, and angiographic features of patients presenting with STEMI during the acute phase of COVID-19 infection. Second, previous studies compared patients who presented with STEMI during a peak period of the COVID-19 pandemic with patients who presented during the same time period a year earlier, prior to the pandemic. Against this, the objective of the present study examines outcome, clinical, and angiographic markers in patients who have had STEMI within a few years of infection, excluding those with acute COVID-19 infection. Moreover, this research is carried out in patients with and without COVID-19 infection under the same conditions during the COVID-19 pandemic. Finally, the higher in-hospital mortality rate among STEMI patients observed in previous studies has been attributed to a higher incidence of late admissions and significantly longer door-to-door as a result of failure to maintain social distancing and avoidance of medical care in healthcare settings for fear of contracting COVID-19.5-10 In our study, STEMI patients were evaluated under the same conditions during the COVID-19 pandemic. Thus, the direct effect of COVID-19 infection on the outcome of STEMI was evaluated.
Several limitations associated with the present study warrant mention. First, this was a retrospective, observational, two-center study conducted in Istanbul. Second, COVID-19 PCR tests were performed markedly less frequently in our country than in Europe or the United States because of lack of resources, transportation, and qualified staff for COVID-19 tests. In this regard, asymptomatic infections might have been missed in patients in the group with no history of PCR-confirmed COVID-19 infection. Additionally, since the criteria for PCR testing were not clear in our database, the vulnerability of our population may have had an impact on our findings. Third, the severity, duration, and amount of organ involvement of COVID-19 infection could not be evaluated in patients in the study group with a history of COVID-19 infection. One of the biggest limitations of our study was the lack of data on the course and severity of the infection in patients with a positive history of COVID-19. Finally, we only evaluated in-hospital mortality, and there was no long-term follow-up.
Conclusion
Our results illustrate that a history of PCR-verified COVID-19 infection was associated with worse outcomes in STEMI patients treated with pPCI and independently predictor for in-hospital MACEs. Further studies are needed to confirm and to reveal clinical implications of our findings.
Footnotes
Author Contribution: All authors contributed to: (1) substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data, (2) drafting the article or revising it critically for important intellectual content, and (3) final approval of the version to be published.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Zeki Doganismail https://orcid.org/0000-0002-5620-7268
İsmail Erden https://orcid.org/0000-0002-0253-6429
Gokhan Bektasoglu https://orcid.org/0000-0002-4571-7908
Ahmet Karabulut https://orcid.org/0000-0002-2001-9142
References
- 1.Mahase E. COVID-19: WHO declares pandemic because of “alarming levels” of spread, severity and inaction. BMJ. 2020;368:1036. [DOI] [PubMed] [Google Scholar]
- 2.De Luca G, Verdoia M, Cercek M, et al. Impact of COVID-19 pandemic on mechanical reperfusion for patients with STEMI. J Am Coll Cardiol. 2020;76:2321-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodriguez-Leor O, Cid-Alvarez B, Ojeda S, et al. Impact of the COVID-19 pandemic on interventional cardiology activity in Spain. REC Interv Cardiol. 2020;2:82-9. [Google Scholar]
- 4.Metzler B, Siostrzonek P, Binder RK, Bauer A, Reinstadler SJ.Decline of acute coronary syndrome admissions in Austria since the outbreak of COVID-19: the pandemic response causes cardiac collateral damage. Eur Heart J. 2020;41:1852-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia S, Albaghdadi MS, Meraj PM, et al. Reduction in ST-segment elevation cardiac catheterization laboratory activations in the United States during COVID-19 pandemic. J Am Coll Cardiol. 2020;75:2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Rosa S, Spaccarotella C, Basso C, et al. Reduction of hospitalizations for myocardial infarction in Italy in the COVID-19 era. Eur Heart J. 2020;41:2083-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yerasi C, Case BC, Forrestal BJ, et al. Treatment of ST-segment elevation myocardial infarction during COVID-19 pandemic. Cardiovasc Revasc Med. 2020;21:1024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stefanini GG, Montorfano M, Trabattoni D, et al. ST-elevation myocardial infarction in patients with COVID-19. Circulation. 2020;141:2113-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mafham MM, Spata E, Goldacre R, et al. COVID-19 pandemic and admission rates for and management of acute coronary syndromes in England. Lancet. 2020;396:381-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solomon MD, McNulty EJ, Rana JS, et al. The COVID-19 pandemic and the incidence of acute myocardial infarction. N Engl J Med. 2020;383:691–3. [DOI] [PubMed] [Google Scholar]
- 11.De Filippo O, D’Ascenzo F, Angelini F, et al. Reduced rate of hospital admissions for ACS during COVID-19 outbreak in Northern Italy. N Engl J Med. 2020;383:88–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toner L, Koshy AN, Hamilton GW, Clark D, Farouque O, Yudi MB.Acute coronary syndromes undergoing percutaneous coronary intervention in the COVID-19 era: comparable case volumes but delayed symptom onset to hospital presentation. Eur Heart J Qual Care Clin Outcomes. 2020;6:225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tam CF, Cheung KS, Lam S, et al. Impact of coronavirus disease 2019 (COVID-19) outbreak on ST-segment-elevation myocardial infarction care in Hong Kong, China. Circ Cardiovasc Qual Outcomes. 2020;13:e006631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thakkar S, Arora S, Kumar A, et al. A systematic review of the cardiovascular manifestations and outcomes in the setting of coronavirus-19 disease. Clin Med Insights Cardiol 2020;14:1179546820977196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bilaloglu S, Aphinyanaphongs Y, Jones S, Iturrate E, Hochman J, Berger JS. Thrombosis in hospitalized patients with COVID-19 in a New York City health system. JAMA 2020;324:799–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119–77. [DOI] [PubMed] [Google Scholar]
- 17.Authors/Task Force Members. Silber S, Albertsson P, Aviles FF, et al. Guidelines for percutaneous coronary interventions: the task force for percutaneous coronary interventions of the European society of cardiology. Eur Heart J. 2005;26:804–47. [DOI] [PubMed] [Google Scholar]
- 18.The TIMI Study Group . The thrombolysis in myocardial infarction (TIMI) trial: phase i findings. N Engl J Med. 1985;312:932-6. [DOI] [PubMed] [Google Scholar]
- 19.Van’t Hof AW, Liem A, Suryapranata H, et al. Angiographic assessment of myocardial reperfusion in patients treated with primary angioplasty for acute myocardial infarction: myocardial blush grade. Myocardial Infarction Study Group. Circulation. 1998;97:2302-6 [DOI] [PubMed] [Google Scholar]
- 20.Gibson CM, Murphy SA, Morrow DA, et al. Angiographic perfusion score: An angiographic variable that integrates both epicardial and tissue level perfusion before and after facilitated percutaneous coronary intervention in acute myocardial infarction. Am Heart J. 2004;148:336-40 [DOI] [PubMed] [Google Scholar]
- 21.Rentrop KP, Cohen M, Blanke H, Phillips RA. Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J Am Coll Cardiol. 1985;5:587-92. [DOI] [PubMed] [Google Scholar]
- 22.Schro¨der R, Dissmann R, Bru¨ggemann T, et al. Extent of early ST-segmental elevation resolution: a simple but strong predictor of outcome in patients with acute myocardial infarction. J Am Coll Cardiol. 1994;24:384-91 [DOI] [PubMed] [Google Scholar]
- 23.Nishiga M, Wang DW, Han Y, Lewis DB., Wu JC. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol 2020;17:543−58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta A, Madhavan MV, Sehgal K, et al. Extrapulmonary manifestations of COVID-19. Nat Med 2020;26:1017−32. [DOI] [PubMed] [Google Scholar]
- 25.Kwong JC, Schwartz KL, Campitelli MA, et al. Acute myocardial infarction after laboratory-confirmed influenza infection. N Engl J Med 2018;378:345–53 [DOI] [PubMed] [Google Scholar]
- 26.Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5:811-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corrales-Medina VF, Alvarez KN, Weissfeld LA, et al. Association between hospitalization for pneumonia and subsequent risk of cardiovascular disease. JAMA. 2015;313:264–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol 2020;17:259-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system. JAMA Cardiol 2020;5:831-40 [DOI] [PubMed] [Google Scholar]
- 30.Corrales-Medina VF, Madjid M, Musher DM. Role of acute infection in triggering acute coronary syndromes. Lancet Infect Dis. 2010;10:83-92 [DOI] [PubMed] [Google Scholar]
- 31.Madjid M, Vela D, Khalili-Tabrizi H, Casscells SW, Litovsky S.Systemic infections cause exaggerated local inflammation in atherosclerotic coronary arteries: clues to the triggering effect of acute infections on acute coronary syndromes. Tex Heart Inst J. 2007;34:11-18 [PMC free article] [PubMed] [Google Scholar]
- 32.Kaptoge S, Seshasai SR, Gao P, et al. Inflammatory cytokines and risk of coronary heart disease: new prospective study and updated meta-analysis. Eur Heart J. 2014;35:578-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kellum JA, Kong L, Fink MP, et al. GenIMS Investigators . Understanding the Inflammatory Cytokine Response in Pneumonia and Sepsis. Arch Intern Med. 2007;167:1655-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yende S, D’Angelo G, Kellum JA, et al. Inflammatory markers at hospital discharge predict subsequent mortality after pneumonia and sepsis. Am J Respir Crit Care Med. 2008;177:1242-7. [DOI] [PMC free article] [PubMed] [Google Scholar]