Abstract
In the last decades, astrocytes have emerged as important regulatory cells actively involved in brain function by exchanging signaling with neurons. The endocannabinoid (eCB) signaling is widely present in many brain areas, being crucially involved in multiple brain functions and animal behaviors. The present review presents and discusses current evidence demonstrating that astrocytes sense eCBs released during neuronal activity and subsequently release gliotransmitters that regulate synaptic transmission and plasticity. The eCB signaling to astrocytes and the synaptic regulation mediated by astrocytes activated by eCBs are complex phenomena that exhibit exquisite spatial and temporal properties, a wide variety of downstream signaling mechanisms, and a large diversity of functional synaptic outcomes. Studies investigating this topic have revealed novel regulatory processes of synaptic function, like the lateral regulation of synaptic transmission and the active involvement of astrocytes in the spike‐timing dependent plasticity, originally thought to be exclusively mediated by the coincident activity of pre‐ and postsynaptic neurons, following Hebbian rules for associative learning. Finally, the critical influence of astrocyte‐mediated eCB signaling on animal behavior is also discussed.
Keywords: 2‐AG, anandamide, astrocytes, CB1receptor, endocannabinoids, synaptic plasticity
Main Points
Astrocytes sense eCB signaling via CB1R activation and respond to eCB released during neuronal activity regulating synaptic plasticity.
The eCB signaling is widely expressed in many brain areas being crucial in many brain functions and animal behaviors.
1. INTRODUCTION
The endocannabinoid (eCB) system is a relevant intercellular signaling system that regulates multiple physiological functions in multiple organ systems (Ahn et al., 2008; Alger & Kim, 2011; Castillo et al., 2012; di Marzo, 2009; Ohno‐Shosaku & Kano, 2014; Piomelli, 2003). eCB signaling has been identified to play important roles in the peripheral and central nervous systems, including the cortex, basal ganglia, spinal cord, cerebellum, hippocampus and olfactory areas, thus being involved in a plethora of brain functions such as pain perception, food intake, learning and memory, anxiety or cognition (Navarrete et al., 2014).
The endocannabinoid system comprises mainly of two types of G‐protein coupled receptors (CB1Rs and CB2Rs), two major types of endogenous neurotransmitters or endocannabinoids, anandamide (AEA) and 2‐arachidonoylglycerol (2‐AG), enzymes for the specific synthesis and degradation of these eCBs, and transporters (for reviews, see Zou & Kumar, 2018). The eCBs are lipids derived from the arachidonic acid that are released on‐demand from the membrane of the postsynaptic neurons in a calcium‐dependent manner regulated by neuronal activity (Castillo et al., 2012; Katona & Freund, 2012; Ohno‐Shosaku & Kano, 2014; Piomelli, 2003).
The involvement of the eCB signaling in multiple brain functions is thought to be mainly mediated through the regulation of synaptic transmission and plasticity in different brain areas. The canonical cellular mechanisms underlying the eCB‐induced synaptic regulation are the activity‐dependent release of eCBs by the postsynaptic neuron, their action as retrograde messengers to activate presynaptic CB1Rs, and the activation of intracellular pathways that lead to the short‐ or long‐term depression of presynaptic neurotransmitter release (Araque et al., 2017).
In addition to this canonical process, additional cellular mechanisms have been proposed to exist. Indeed, the presence of functional CB1Rs that can be activated by endogenous and exogenous ligands has been reported in different types of glial cells (for detailed references, see other articles in the present special issue). Specifically, astrocytes, which are emerging as key neuromodulatory cells that regulate synaptic function through the so‐called tripartite synapses, have been found to respond to neuronal release of eCBs and to regulate synaptic transmission.
In the present review, we will first introduce the eCB‐induced synaptic regulation processes mediated by neuronal mechanisms. While this exciting topic will be succinctly introduced, the reader may find more exhaustive details in excellent recent reviews (Zou & Kumar, 2018). We will then focus on discussing the current evidence of the eCB‐induced synaptic regulation by astrocytes.
2. ECB‐INDUCED SYNAPTIC REGULATION BY EXCLUSIVE NEURONAL MECHANISMS
At the end of the twentieth century, eCBs were found to regulate synaptic transmission in a form of short‐term synaptic plasticity. Initial studies in the hippocampus and cerebellum described that the depolarization of postsynaptic neurons led to a transient decrease in the inhibitory synaptic efficacy, a phenomenon called depolarization‐induced suppression of inhibition (DSI), and that was found to depend on eCB signaling (Llano et al., 1991; Pitler & Alger, 1992; Wilson & Nicoll, 2002). A few years later, a similar phenomenon was found to occur in excitatory synaptic terminals in the cerebellum, being named depolarization‐induced suppression of excitation (DSE; Kreitzer & Regehr, 2001). Both DSE and DSI have been proposed to share similar mechanisms. First, calcium increase in the postsynaptic neuron is the initial step for the release of eCBs. Calcium enters through voltage‐gated calcium channels (VGCC) with NMDA receptors also as a possible source of intracellular calcium elevations (Castillo et al., 2012). Additional mechanisms involving the neurotransmitters glutamate and acetylcholine released from presynaptic terminals have also been implicated in eCB release. The activation of group I metabotropic glutamate receptors (mGluRs) or muscarinic acetylcholine receptors (mAChRs) by their endogenous agonists induce the activation of the enzyme phospholipase C (PLC) that leads to the calcium mobilization from the internal stores (Agulhon et al., 2008; Kofuji & Araque, 2020). Then, the activation of the calcium‐sensitive enzymes diacylglycerol lipase (DGL) and N‐acetylphosphatidylethanolamine‐hydrolysing phospholipase D (NAPE‐PLD) produce 2‐AG and AEA, which, after being released, act retrogradely activating CB1Rs located presynaptically (Castillo et al., 2012). Different mechanisms, not mutually exclusive, have been proposed to decrease the synaptic transmitter release, including inhibition of Ca2+ influx through presynaptic VGCCs and activation of presynaptic K+ channels (Castillo et al., 2012). Finally, eCB signaling ends by the reuptake of the eCBs and the enzymatic degradation of 2‐AG by monoacylglycerol lipase (MGL) (Dinh et al., 2002) and AEA by fatty acid amide hydrolase (FAAH) (Cravatt et al., 1996; Hillard et al., 1995; McKinney & Cravatt, 2005).
Besides mediating DSE and DSI as short‐term forms of plasticity, eCBs participate in diverse forms of long‐term synaptic plasticity. For example, Chevaleyre and Castillo reported a long‐term depression of inhibition (iLTD) induced by eCBs release in the hippocampus. They postulated that this iLTD depends on the amount of eCBs being released and the duration of the CB1R activation. Notably, this iLTD showed spatial selectivity because it was selectively observed in the dendritic inhibitory inputs, unlike DSI that was generally manifested in dendritic and somatic inhibitory synapses. In addition, the iLTD required mGluR5 activation in postsynaptic pyramidal neurons and CB1R activation in presynaptic GABAergic terminals. By revealing that eCB‐induced synaptic regulation was controlled by the magnitude and spatial–temporal properties of the eCB signaling, this study reveals the exquisiteness of eCB signaling in regulating synaptic function (Chevaleyre & Castillo, 2003).
The ECB signaling has also been shown to mediate some forms of the long‐term potentiation (LTP) of synaptic transmission through network interaction. In the somatosensory cortex, the DSI induced in a subpopulation of neurons enhanced the postsynaptic Ca2+ spikes, which leads to the induction of the LTP, whereas subpopulation of neurons that underwent DSE instead of DSI, Ca2+ spikes were unaffected and LTP was absent (Maglio et al., 2018), further illustrating the spatial‐ and cell‐specific selectivity of eCB signaling described above. Moreover, this form of LTP required the activation of the tyrosine receptor kinase B (TrkB) receptor by BDNF, in agreement with other studies showing eCB and BDNF signaling cross‐talk to induce long‐term synaptic plasticity in different brain areas (Gangarossa et al., 2020; Huang et al., 2008; Zhao et al., 2015). These studies also exemplify the functional relevance of the interaction between eCBs and other neurotransmitter systems, as described above for glutamatergic and cholinergic systems.
Besides the canonical mechanism of action of eCBs as retrograde signals, autocrine effects have also been reported. Activation of postsynaptic CB1Rs by eCBs released by the stimulated neuron induced a self‐inhibition by the activation of inward rectifying K+ channels that hyperpolarized the neuron and reduced the generation of action potential (Bacci et al., 2004; Marinelli et al., 2008).
3. ASTROCYTES SENSE ECB SIGNALING VIA CB1R ACTIVATION
Since the early 20th century, the classical view of astrocytes as passive, supportive cells has been expanded by a new concept in which astrocytes actively participate in synaptic information processing and regulation. The development of organic and genetically encoded calcium indicators (GECIs) led to the realization that astrocytes display a form of excitability reflected as calcium changes in their processes and soma. These increases in the astrocytic calcium levels can be induced by various neurotransmitters including eCBs through activation of CB1Rs. Calcium elevations induced by eCBs in astrocytes is not only restricted to a single astrocyte but can travel to surrounding astrocytes through gap‐junctions, modulating the calcium excitability in neighboring astrocytes (Figure 1) (Navarrete & Araque, 2010). The presence and functionality of CB1Rs in astrocytes has been a topic of controversy (Metna‐Laurent & Marsicano, 2015; Stella, 2010). Nevertheless, in the past few years, many studies have supported the key role of astroglial CB1R in the modulation of synaptic transmission and its essential function in learning and memory processes (Han et al., 2012; Martín et al., 2015; Martin‐Fernandez et al., 2017; Navarrete & Araque, 2008, 2010; Robin et al., 2018).
FIGURE 1.
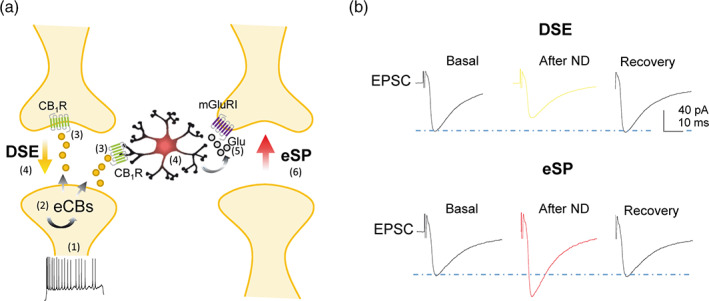
Endocannabinoids induce DSE and eSP signaling to neurons and astrocytes. (a) (1) The increase of the activity in the presynaptic terminal (2) induces the release of eCBs. (3) Binding of eCBs to the presynaptic CB1R produces the decrease in the glutamate release inducing depolarization‐induced suppression of excitation (DSE)(4). (3) Likewise, the interaction of eCBs to the astrocytic CB1R (4) increase the mobilization of calcium from the internal stores and subsequently the exocytosis of glitransmitter glutamate and, in turn, (5) the interaction with presynaptic mGluRI in the heteroneuronal synapse generating excitatory short potentiation (eSP)(6). (b) Representative EPSC traces before, after neuronal depolarization (ND) and during recovery, showing DSE (upper traces) and eSP (bottom traces)
In neuronal cells, activation of CB1Rs usually mediate an inhibitory effect in cellular excitability. CB1Rs via Gi/o proteins suppress neurotransmitter release in presynaptic terminals. In astrocytes, CB1Rs are coupled to Gq/11 class of G‐proteins (Navarrete & Araque, 2008). Gq/11 activates phospholipase C that generates the synthesis of inositol triphosphate which leads, ultimately, to release of calcium from internal stores through inositol triphosphate receptor type 2 (IP3R2) (Navarrete & Araque, 2008). The astrocytic calcium elevations induced by neuronal released eCBs have been shown to stimulate the release of neuroactive substances, called gliotransmitters, like glutamate, ATP or D‐serine that can potentially influence synaptic transmission, as discussed below (Andrade‐Talavera et al., 2016; Covelo & Araque, 2018; Navarrete & Araque, 2010).
The calcium signal in astrocytes, therefore, plays a key role in the release of gliotransmitters and, hence, in the regulation of synaptic function and neuronal network activity. While important progress was initially made to define the properties, mechanisms and consequences of the astrocyte calcium signal in brain slices, which provide an accessible experimental approach to study cellular activity (Perea & Araque, 2005; Porter & McCarthy, 1996; Rusakov, 2015; Volterra et al., 2014), but which present limitations in assessing normal astrocyte function in vivo. Astrocyte calcium activity in vivo has been monitored using confocal or two‐photon microcopy in anesthetized or head‐restrained animals (Hirase et al., 2004; Lines et al., 2020; Navarrete et al., 2012; Rusakov, 2015; Sekiguchi et al., 2016; Volterra et al., 2014), but its use is largely restricted to outer brain structures. Additional techniques, like grin lenses and fiber photometry systems, that allow monitoring calcium signal in deep brain structures, in vivo and in freely‐moving behaving animals (Cobar et al., 2022; Corkrum et al., 2020; Tsunematsu et al., 2021), are expected to provide further details of the calcium dynamics in vivo while animals perform behavioral tasks. The combination of this calcium imaging techniques with the recently developed endocannabinoid fluorescent sensor (Dong et al., 2022) is expected to provide valuable information on how endocannabinoids control astrocyte calcium signaling in vivo.
4. ECBS REGULATE SYNAPTIC TRANSMISSION THROUGH ACTIVATION OF ASTROCYTES
The participation of astrocytes in the regulation of different forms of synaptic transmission and plasticity has been demonstrated by a large number of studies in different brain areas, including the hippocampus, cortex, dorsal and ventral striatum, amygdala and cerebellum (see Kofuji & Araque, 2021). The astrocyte responsiveness with calcium elevations to different synaptically‐released neurotransmitters stimulates the release of gliotransmitters that activate neuronal receptors, thus regulating neural function and synaptic activity (Araque et al., 2001; Covelo & Araque, 2016; Haydon & Carmignoto, 2006; Navarrete et al., 2014; Nedergaard et al., 2003; Parpura & Zorec, 2010; Perea et al., 2009; Theodosis et al., 2008; Volterra & Meldolesi, 2005).
The experimental test of the idea that astrocytic calcium increases induce the release of gliotransmitters that regulate synaptic transmission and that the eCBs induced these calcium elevations, led to the description of new forms of synaptic regulatory phenomena mediated by the eCB signaling to astrocytes, such as the so‐called Lateral regulation of synaptic transmission (Navarrete & Araque, 2010). Studies using the minimal stimulation technique that allows monitoring synaptic transmission at single CA1 hippocampal synapses showed that eCBs induced DSE in excitatory CA3‐CA1 hippocampal synapses through the canonical mechanism of direct activation of presynaptic CB1Rs by eCBs released by the stimulated neuron. In addition, these eCBs led to the synaptic potentiation of relatively distant synapses in a neighboring neuron through the activation of astrocytic CB1Rs, which elevate the astrocyte calcium levels and stimulate the release of astrocytic glutamate that, acting on presynaptic mGluR1receptors, potentiate synaptic transmitter release (Covelo & Araque, 2018; Navarrete & Araque, 2010). This eCB‐induced astrocyte‐mediated lateral regulation of synaptic transmission is not restricted to the hippocampus; rather it seems to be a general phenomenon, as it has also been reported in the dorsal striatum (Martín et al., 2015) and somatosensory cortex (Baraibar et al., 2022).
Therefore, the eCB signaling, which depresses synaptic transmission when it exclusively engages neuronal processes can be transformed into a synaptic potentiating signaling when it involves astrocytes, which adds further complexity to the functional synaptic outcomes of the eCB signaling. The contrasting depressing and potentiating effects of eCBs through direct activation of neuronal processes in active synapses or indirect activation of astrocytic processes in inactive synapses, respectively, may serve as a homeostatic mechanism to maintain homogeneous synaptic function in ensembles of synapses within a circuit. Furthermore, the fact that the depressing and potentiating synaptic regulation by eCBs occurs in nearby or relatively distant synapses, respectively, further indicates the subtility of the spatial signaling by eCBs.
In addition to glutamate, the synaptic regulation by eCBs signaling to astrocytes may also involve other gliotransmitters. In the centromedial amygdala, astrocytic CB1R activation led to the release of ATP/adenosine and the consequent differential regulation of synaptic inputs. While ATP/adenosine depressed excitatory synapses from basolateral amygdala via A1 adenosine receptor activation, these gliotransmitters potentiated inhibitory synapses from the centrolateral amygdala through A2A receptor activation (Martin‐Fernandez et al., 2017). Likewise, in the suprachiasmatic nucleus, which regulates the circadian rhythm, eCBs induced a decrease in the inhibitory transmission through activation of astrocytes and presynaptic A1 receptors (Hablitz et al., 2020).
Besides directly responding to eCBs, astrocytes have also been reported to be involved in the eCB‐induced synaptic regulation by controlling extracellular eCB diffusion. In the hypothalamic supraoptic and paraventricular nucleus, where extensive neuronal‐glial remodeling occurs in response to physiological conditions such as chronic dehydration and lactation (Hatton, 1997; Oliet et al., 2001; Theodosis & Poulain, 1999), eCB‐mediated DSI was absent in control conditions but present under conditions of glial retraction or impaired astrocytic function (Di et al., 2013), suggesting that astrocytes control eCB spillover and eCB‐induced synaptic regulation.
Finally, whether astrocytes are capable of releasing eCBs to regulate synaptic transmission remains largely unexplored. Initial studies reported the ability of astrocytes in culture to produce eCBs (Walter et al., 2002; Walter & Stella, 2003) upon different stimuli, like ionomycin, endothelin‐1 or ATP (Walter et al., 2002, 2004; Walter & Stella, 2003). However, since these studies were performed in cultured astrocytes, which undergo strong phenotypic changes in culture, it is questionable whether such phenomenon occurs in more intact conditions. Nevertheless, a recent study in hippocampal slices has proposed that astrocytes activated by group II metabotropic glutamate receptors release eCBs that induce a transient heterosynaptic depression (tHSD) (Smith et al., 2020), although alternative astrocytic signaling independent of astrocytic eCB release may underly the observed tHSD. Further studies are required to conclusively determined whether astrocytes in situ are capable of releasing eCBs.
5. ECBS REGULATE LONG‐TERM SYNAPTIC PLASTICITY THROUGH ACTIVATION OF ASTROCYTES
Synaptic plasticity, defined as experience‐dependent changes in the structure and function of the synapses is a fundamental process in brain function (Glazewski et al., 1996; Glazewski & Fox, 1996; Kuhlman et al., 2014). Long‐term synaptic plasticity, which is considered the cellular mechanism underlying learning and memory (Bliss & Collingridge, 1993), can be manifested as long‐term potentiation (LTP) and long‐term depression (LTD) of the synaptic strength. Numerous pieces of evidence indicate that both phenomena can be regulated by astrocytes (see e.g., Adamsky et al., 2018; Cavaccini et al., 2020; Durkee et al., 2021; Henneberger et al., 2010; Navarrete et al., 2012, 2019; Noriega‐Prieto et al., 2021; Pascual et al., 2005; Perea & Araque, 2007; Savtchouk et al., 2019; Suzuki et al., 2011), and specifically by astrocytes activated by eCBs.
In the hippocampus, direct activation of CB1Rs in astrocytes by exogenous cannabinoids was found to be sufficient to trigger LTD in CA3‐CA1 synapses, which was associated with working memory impairments (see below; Han et al., 2012). Marsicano's group (Robin et al., 2018) also showed that high frequency stimulation of those synaptic connections induced an NMDAR‐dependent LTP that required astroglial CB1Rs. Activation of these receptors elevated astrocyte calcium and supplied the necessary gliotransmitter D‐serine, co‐agonist of hippocampal synaptic NMDARs, to induce the LTP (Robin et al., 2018; Savtchouk et al., 2019). On the other hand, the eCB‐induced astrocyte‐mediated lateral synaptic regulation described above may trigger LTP in CA3‐CA1 synapses when is temporally coincident with nitric oxide signaling (Gómez‐Gonzalo et al., 2015). Additionally, in this brain area, the spike timing‐dependent plasticity, a synaptic plasticity paradigm based on the precise temporal coincidence of pre‐ and postsynaptic activity that is thought to be the cellular basis of associative learning, has been found to be controlled by eCB signaling to astrocytes. Rodriguez‐Moreno's group found that the spike timing‐dependent long‐term depression (t‐LTD) in hippocampal CA3‐CA1 synapses required activation of astrocytic CB1Rs, astrocyte calcium elevations and the release of the gliotransmitter d‐serine (Andrade‐Talavera et al., 2016). Furthermore, this group has more recently shown that the STDP protocol that triggered the eCB‐mediated t‐LTD in immature (<21 days old) mice elicited a t‐LTP in older (> 21 days old) mice. This t‐LTP still requires astrocytic signaling (involving the release of the gliotransmitters glutamate and ATP/adenosine) but is independent of eCB signaling (Falcón‐Moya et al., 2020), indicating that a developmentally regulated switch of synaptic plasticity from t‐LTD to t‐LTP that requires functional modification of the eCB signaling to astrocytes.
In the somatosensory barrel cortex, the t‐LTD was also found to involve eCB signaling to astrocytes (Min & Nevian, 2012). In this case, the spike timing‐dependent protocol induced CB1R‐mediated calcium elevations in astrocytes that stimulated the release of the gliotransmitter glutamate, which activating presynaptic NMDARs induced the long‐term depression of layer 2/3 synapses (Min & Nevian, 2012).
Altogether, these studies demonstrated the crucial involvement of eCB signaling to astrocytes in the induction of certain forms of synaptic plasticity. Such involvement in the spike timing‐dependent plasticity has important implications. This synaptic plasticity paradigm was originally thought to be exclusively mediated by the coincident activity of the pre‐ and postsynaptic neuron, following Hebbian rules for associative learning (Markram et al., 2011). This idea is, however, challenged by the fact that eCB and astrocytic signaling are crucially involved in spike timing‐dependent plasticity phenomena, suggesting that astrocyte activity triggered by eCB signaling is involved in associative learning and brain storage information.
6. ECBS INFLUENCE ANIMAL BEHAVIOR THROUGH ACTIVATION OF ASTROCYTES
In addition to regulatory mechanisms of synaptic transmission and plasticity by astrocytic eCB signaling, several studies have shown that astrocytic responsiveness to endocannabinoids, as well as exogenous cannabinoids, may influence animal behavior. The use of transgenic mice in which the CB1Rs can be selectively deleted in astrocytes, e.g., crossing mice carrying the “floxed” CB1R gene (CB1f/f) (Marsicano et al., 2003) with mice expressing the Cre recombinase under an astrocytic promoter, like GFAP (e.g., GFAP‐CreERT2 mice) (Hirrlinger et al., 2006), has been instrumental in assessing the impact of astrocytic eCB signaling on behavior. Using mice lacking CB1Rs selectively in astrocytes (obtained by crossing CB1f/f mice with GFAP‐CreERT2 mice), Han et al. showed that Δ9‐tetrahydrocannabinol (THC), the major psychoactive ingredient of marijuana, induced the long‐term depression of hippocampal CA3‐CA1 synapses LTD that was dependent on astrocyte CB1Rs. Authors further showed that associated with these synaptic plasticity changes, the acute exposure to the exogenous cannabinoid impaired the spatial working memory in control mice, an effect that was absent in mice lacking CB1Rs in astrocytes, suggesting that the impairment of working memory by marijuana and cannabinoids was due to the activation of astroglial CB1Rs (Han et al., 2012).
Using these transgenic mice, Marsicano's group also demonstrated a key role of CB1Rs in recognition memory (Robin et al., 2018). These authors showed that mice lacking CB1Rs in astrocytes displayed reduced LTP at CA3‐CA1 hippocampal synapses and impaired object recognition memory. These effects could be reverted by elevation d‐serine levels, suggesting that eCB signaling stimulates the release of the gliotransmitter d‐serine, which, acting as co‐agonist of NMDARs, is necessary for hippocampal LTP induction (Robin et al., 2018).
The behavior output of the amygdala function has also been shown to be influenced by astrocyte activity. As described above, we found that CB1R activation of astrocytes in the centromedial amygdala, the main output nucleus of the amygdala that mediates the fear behavior, depressed excitatory transmission and potentiated inhibitory transmission in that nucleus. These effects could be mimicked by direct chemogenetic activation of astrocytes and resulted in relatively silencing the neuronal firing. Consistent with this, the fear behavior exhibited by mice with stimulated astrocytes was reduced compared to control animals, suggesting that the selective and differential regulation of excitatory and inhibitory synapses exerted by eCB‐stimulated astrocytes determines animal fear responses (Martin‐Fernandez et al., 2017).
7. CONCLUDING REMARKS
For the last 20 years, astrocytes have emerged as important regulatory cells actively involved in brain function by exchanging signaling with neurons. Astrocytes can sense synaptic transmission by expressing neurotransmitter receptors that, upon activation by synaptically released neurotransmitters, elevates their intracellular calcium levels, In turn, they release gliotransmitters that, acting on neuronal receptors, regulate synaptic transmission and plasticity, which results in influencing network function and animal behavior. These astrocyte‐neuron interactions, embodied in the tripartite synapse concept, have led to a paradigm shift in modern neuroscience that postulates that brain function and animal behavior result not only from neuronal activity but from the coordinated activity of astrocytes and neurons.
The demonstration of eCB‐mediated neuron‐astrocyte signaling has been instrumental in advancing this novel concept. As discussed in this article, evidence showing that astrocytes respond to eCBs released during neuronal activity and that they subsequently release gliotransmitters that control synaptic transmission and plasticity has not only supported this idea, but has also revealed novel synaptic regulatory phenomena with exquisite spatial and temporal properties.
The eCB signaling is widely expressed in many brain areas, being crucially involved in many brain functions and animal behaviors. While current available evidence shows that the eCB‐mediated astrocytic regulation of synaptic transmission occurs in the studied paradigmatic brain regions, such as the cortex, hippocampus and amygdala, it is feasible that it is a general phenomenon present throughout the brain. Further studies are required to test this likely hypothesis. Furthermore, studies discussed above revealed a wide variety of downstream signaling mechanisms and functional outcomes of the synaptic regulation mediated by astrocytes activated by eCBs. Additional studies in other brain areas involved in different animal behaviors may reveal novel properties and functions of this phenomenon.
AUTHOR CONTRIBUTIONS
Noriega‐Prieto J, Kofuji P and Araque A designed the scope and drafted the manuscript. All authors approved the final version.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
This work was supported by grants from National Institutes of Health (NIH‐MH R01MH119355; NIH‐NINDS R01NS097312; and NIH‐NIDA R01DA048822) and Department of Defense (W911NF2110328) to Alfonso Araque.
Noriega‐Prieto, J. A. , Kofuji, P. , & Araque, A. (2023). Endocannabinoid signaling in synaptic function. Glia, 71(1), 36–43. 10.1002/glia.24256
Funding information National Institutes of Health, Grant/Award Numbers: NIH‐MH R01MH119355, NIH‐NIDA R01DA048822, NIH‐NINDS R01NS097312; U.S. Department of Defense, Grant/Award Number: W911NF2110328
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- Adamsky, A. , Kol, A. , Kreisel, T. , Doron, A. , Ozeri‐Engelhard, N. , Melcer, T. , Refaeli, R. , Horn, H. , Regev, L. , Groysman, M. , London, M. , & Goshen, I. (2018). Astrocytic activation generates De novo neuronal potentiation and memory enhancement. Cell, 174, 59–71. [DOI] [PubMed] [Google Scholar]
- Agulhon, C. , Petravicz, J. , McMullen, A. B. , Sweger, E. J. , Minton, S. K. , Taves, S. R. , Casper, K. B. , Fiacco, T. A. , & McCarthy, K. D. (2008). What is the role of astrocyte calcium in neurophysiology? Neuron, 59, 932–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn, K. , McKinney, M. K. , & Cravatt, B. F. (2008). Enzymatic pathways that regulate endocannabinoid signaling in the nervous system. Chemical Reviews, 108, 1687–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alger, B. E. , & Kim, J. (2011). Supply and demand for endocannabinoids. Trends in Neurosciences, 34, 304–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade‐Talavera, Y. , Duque‐Feria, P. , Paulsen, O. , & Rodríguez‐Moreno, A. (2016). Presynaptic spike timing‐dependent long‐term depression in the mouse hippocampus. Cerebral Cortex, 26, 3637–3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque, A. , Carmignoto, G. , & Haydon, P. G. (2001). Dynamic signaling between astrocytes and neurons. Annual Review of Physiology, 63, 795–813. [DOI] [PubMed] [Google Scholar]
- Araque, A. , Castillo, P. E. , Manzoni, O. J. , & Tonini, R. (2017). Synaptic functions of endocannabinoid signaling in health and disease. Neuropharmacology, 124, 13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacci, A. , Huguenard, J. R. , & Prince, D. A. (2004). Long‐lasting self‐inhibition of neocortical interneurons mediated by endocannabinoids. Nature, 431, 312–316. [DOI] [PubMed] [Google Scholar]
- Baraibar, A. M. , Belisle, L. , Marsicano, G. , Matute, C. , Mato, S. , Araque, A. , & Kofuji, P. (2022). Spatial organization of neuron‐astrocyte interactions in the somatosensory cortex. bioRxiv, 2022.02.03.479046. 10.1101/2022.02.03.479046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss, T. V. P. , & Collingridge, G. L. (1993). A synaptic model of memory: Long‐term potentiation in the hippocampus. Nature, 361(6407), 31–39. [DOI] [PubMed] [Google Scholar]
- Castillo, P. E. , Younts, T. J. , Chávez, A. E. , & Hashimotodani, Y. (2012). Endocannabinoid signaling and synaptic function. Neuron, 76(1), 70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaccini, A. , Durkee, C. , Kofuji, P. , Tonini, R. , & Araque, A. (2020). Astrocyte signaling gates long‐term depression at Corticostriatal synapses of the direct pathway. Journal of Neuroscience, 40, 5757–5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre, V. , & Castillo, P. E. (2003). Heterosynaptic LTD of hippocampal GABAergic synapses: A novel role of endocannabinoids in regulating excitability. Neuron, 38, 461–472. [DOI] [PubMed] [Google Scholar]
- Cobar, L. F. , Kashef, A. , Bose, K. , & Tashiro, A. (2022). Opto‐electrical bimodal recording of neural activity in awake head‐restrained mice. Scientific Reports, 12, 736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corkrum, M. , Covelo, A. , Lines, J. , Bellocchio, L. , Pisansky, M. , Loke, K. , Quintana, R. , Rothwell, P. E. , Lujan, R. , Marsicano, G. , Martin, E. D. , Thomas, M. J. , Kofuji, P. , & Araque, A. (2020). Dopamine‐evoked synaptic regulation in the nucleus Accumbens requires astrocyte activity. Neuron, 105, 1036–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covelo, A. , & Araque, A. (2016). Lateral regulation of synaptic transmission by astrocytes. Neuroscience, 323, 62–66. [DOI] [PubMed] [Google Scholar]
- Covelo, A. , & Araque, A. (2018). Neuronal activity determines distinct gliotransmitter release from a single astrocyte. eLife, 7, e32237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt, B. F. , Giang, D. K. , Mayfield, S. P. , Boger, D. L. , Lerner, R. A. , & Gilula, N. B. (1996). Molecular characterization of an enzyme that degrades neuromodulatory fatty‐acid amides. Nature, 384, 83–87. [DOI] [PubMed] [Google Scholar]
- di Marzo, V. (2009). The endocannabinoid system: Its general strategy of action, tools for its pharmacological manipulation and potential therapeutic exploitation. Pharmacological Research, 60, 77–84. [DOI] [PubMed] [Google Scholar]
- Di, S. , Popescu, I. R. , & Tasker, J. G. (2013). Glial control of endocannabinoid Heterosynaptic modulation in hypothalamic magnocellular neuroendocrine cells. The Journal of Neuroscience, 33, 18331–18342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh, T. P. , Freund, T. F. , & Piomelli, D. (2002). A role for monoglyceride lipase in 2‐arachidonoylglycerol inactivation. Chemistry and Physics of Lipids, 121, 149–158. [DOI] [PubMed] [Google Scholar]
- Dong, A. , He, K. , Dudok, B. , Farrell, J. S. , Guan, W. , Liput, D. J. , Puhl, H. L. , Cai, R. , Wang, H. , Duan, J. , Albarran, E. , Ding, J. , Lovinger, D. M. , Li, B. , Soltesz, I. , & Li, Y. (2022). A fluorescent sensor for spatiotemporally resolved imaging of endocannabinoid dynamics in vivo. Nature Biotechnology, 40, 787–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durkee, C. , Kofuji, P. , Navarrete, M. , & Araque, A. (2021). Astrocyte and neuron cooperation in long‐term depression. Trends in Neurosciences, 44, 837–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcón‐Moya, R. , Pérez‐Rodríguez, M. , Prius‐Mengual, J. , Andrade‐Talavera, Y. , Arroyo‐García, L. E. , Pérez‐Artés, R. , Mateos‐Aparicio, P. , Guerra‐Gomes, S. , Oliveira, J. F. , Flores, G. , & Rodríguez‐Moreno, A. (2020). Astrocyte‐mediated switch in spike timing‐dependent plasticity during hippocampal development. Nature Communications, 11, 4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangarossa, G. , Perez, S. , Dembitskaya, Y. , Prokin, I. , Berry, H. , & Venance, L. (2020). BDNF controls bidirectional endocannabinoid plasticity at corticostriatal synapses. Cerebral Cortex, 30, 197–214. [DOI] [PubMed] [Google Scholar]
- Glazewski, S. , Chen, C.‐M. , Silva, A. , & Fox, K. (1996). Requirement for alpha ‐CaMKII in experience‐dependent plasticity of the barrel cortex. Science, 272, 421–423. [DOI] [PubMed] [Google Scholar]
- Glazewski, S. , & Fox, K. (1996). Time course of experience‐dependent synaptic potentiation and depression in barrel cortex of adolescent rats. Journal of Neurophysiology, 75, 1714–1729. [DOI] [PubMed] [Google Scholar]
- Gómez‐Gonzalo, M. , Navarrete, M. , Perea, G. , Covelo, A. , Martín‐Fernández, M. , Shigemoto, R. , Luján, R. , & Araque, A. (2015). Endocannabinoids induce lateral long‐term potentiation of transmitter release by stimulation of gliotransmission. Cerebral Cortex, 25, 3699–3712. [DOI] [PubMed] [Google Scholar]
- Hablitz, L. M. , Plá, V. , Giannetto, M. , Vinitsky, H. S. , Stæger, F. F. , Metcalfe, T. , Nguyen, R. , Benrais, A. , & Nedergaard, M. (2020). Circadian control of brain glymphatic and lymphatic fluid flow. Nature Communications, 11, 4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, J. , Kesner, P. , Metna‐Laurent, M. , Duan, T. , Xu, L. , Georges, F. , Koehl, M. , Abrous, D. N. , Mendizabal‐Zubiaga, J. , Grandes, P. , Liu, Q. , Bai, G. , Wang, W. , Xiong, L. , Ren, W. , Marsicano, G. , & Zhang, X. (2012). Acute cannabinoids impair working memory through Astroglial CB1 receptor modulation of hippocampal LTD. Cell, 148, 1039–1050. [DOI] [PubMed] [Google Scholar]
- Hatton, G. I. (1997). Function‐related plasticity in hypothalamus. Annual Review of Neuroscience, 20, 375–397. [DOI] [PubMed] [Google Scholar]
- Haydon, P. G. , & Carmignoto, G. (2006). Astrocyte control of synaptic transmission and neurovascular coupling. Physiological Reviews, 86, 1009–1031. [DOI] [PubMed] [Google Scholar]
- Henneberger, C. , Papouin, T. , Oliet, S. H. R. , & Rusakov, D. A. (2010). Long‐term potentiation depends on release of d‐serine from astrocytes. Nature, 463, 232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard, C. J. , Wilkison, D. M. , Edgemond, W. S. , & Campbell, W. B. (1995). Characterization of the kinetics and distribution of N‐arachidonylethanolamine (anandamide) hydrolysis by rat brain. Biochimica et Biophysica Acta, 1257, 249–256. [DOI] [PubMed] [Google Scholar]
- Hirase, H. , Qian, L. , Barthó, P. , & Buzsáki, G. (2004). Calcium dynamics of cortical astrocytic networks in vivo. PLoS Biology, 2, E96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirrlinger, P. G. , Scheller, A. , Braun, C. , Hirrlinger, J. , & Kirchhoff, F. (2006). Temporal control of gene recombination in astrocytes by transgenic expression of the tamoxifen‐inducible DNA recombinase variant CreERT2. Glia, 54, 11–20. [DOI] [PubMed] [Google Scholar]
- Huang, Y. , Yasuda, H. , Sarihi, A. , & Tsumoto, T. (2008). Roles of endocannabinoids in heterosynaptic long‐term depression of excitatory synaptic transmission in visual cortex of young mice. The Journal of Neuroscience, 28, 7074–7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona, I. , & Freund, T. F. (2012). Multiple functions of endocannabinoid signaling in the brain. Annual Review of Neuroscience, 35, 529–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofuji, P. , & Araque, A. (2020). G‐protein‐coupled receptors in astrocyte–neuron communication. Neuroscience, 456, 71–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofuji, P. , & Araque, A. (2021). Astrocytes and behavior. Annual Review of Neuroscience, 44, 49–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer, A. C. , & Regehr, W. G. (2001). Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron, 29, 717–727. [DOI] [PubMed] [Google Scholar]
- Kuhlman, S. J. , O'Connor, D. H. , Fox, K. , & Svoboda, K. (2014). Structural plasticity within the barrel cortex during initial phases of whisker‐dependent learning. Journal of Neuroscience, 34, 6078–6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lines, J. , Martin, E. D. , Kofuji, P. , Aguilar, J. , & Araque, A. (2020). Astrocytes modulate sensory‐evoked neuronal network activity. Nature Communications, 11, 3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano, I. , Leresche, N. , & Marty, A. (1991). Calcium entry increases the sensitivity of cerebellar Purkinje cells to applied GABA and decreases inhibitory synaptic currents. Neuron, 6(4), 565–574. [DOI] [PubMed] [Google Scholar]
- Maglio, L. E. , Noriega‐Prieto, J. A. , Maraver, M. J. , & Fernández de Sevilla, D. (2018). Endocannabinoid‐dependent long‐term potentiation of synaptic transmission at rat barrel cortex. Cerebral Cortex, 28, 1568–1581. [DOI] [PubMed] [Google Scholar]
- Marinelli, S. , Pacioni, S. , Bisogno, T. , di Marzo, V. , Prince, D. A. , Huguenard, J. R. , & Bacci, A. (2008). The endocannabinoid 2‐arachidonoylglycerol is responsible for the slow self‐inhibition in neocortical interneurons. The Journal of Neuroscience, 28, 13532–13541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram, H. , Gerstner, W. , & Sjöström, P. J. (2011). A history of spike‐timing‐dependent plasticity. Frontiers in Synaptic Neuroscience, 3, 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano, G. , Goodenough, S. , Monory, K. , Hermann, H. , Eder, M. , Cannich, A. , Azad, S. C. , Cascio, M. G. , Ortega‐Gutiérrez, S. , van der Stelt, M. , López‐Rodríguez, M. L. , Casanova, E. , Schütz, G. , Zieglgänsberger, W. , di Marzo, V. , Behl, C. , & Lutz, B. (2003). CB1 cannabinoid receptors and on‐demand defense against excitotoxicity. Science, 302, 84–88. [DOI] [PubMed] [Google Scholar]
- Martín, R. , Bajo‐Grañeras, R. , Moratalla, R. , Perea, G. , & Araque, A. (2015). Circuit‐specific signaling in astrocyte‐neuron networks in basal ganglia pathways. Science (New York, N.Y.), 349, 730–734. [DOI] [PubMed] [Google Scholar]
- Martin‐Fernandez, M. , Jamison, S. , Robin, L. M. , Zhao, Z. , Martin, E. D. , Aguilar, J. , Benneyworth, M. A. , Marsicano, G. , & Araque, A. (2017). Synapse‐specific astrocyte gating of amygdala‐related behavior. Nature Neuroscience, 20, 1540–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney, M. K. , & Cravatt, B. E. (2005). Structure and function of fatty acid amide hydrolase. Annual Review of Biochemistry, 74, 411–432. [DOI] [PubMed] [Google Scholar]
- Metna‐Laurent, M. , & Marsicano, G. (2015). Rising stars: Modulation of brain functions by astroglial type‐1 cannabinoid receptors. Glia, 63, 353–364. [DOI] [PubMed] [Google Scholar]
- Min, R. , & Nevian, T. (2012). Astrocyte signaling controls spike timing‐dependent depression at neocortical synapses. Nature Neuroscience, 15, 746–753. [DOI] [PubMed] [Google Scholar]
- Navarrete, M. , & Araque, A. (2008). Endocannabinoids mediate neuron‐astrocyte communication. Neuron, 57, 883–893. [DOI] [PubMed] [Google Scholar]
- Navarrete, M. , & Araque, A. (2010). Endocannabinoids potentiate synaptic transmission through stimulation of astrocytes. Neuron, 68, 113–126. [DOI] [PubMed] [Google Scholar]
- Navarrete, M. , Cuartero, M. I. , Palenzuela, R. , Draffin, J. E. , Konomi, A. , Serra, I. , Colié, S. , Castaño‐Castaño, S. , Hasan, M. T. , Nebreda, Á. R. , & Esteban, J. A. (2019). Astrocytic p38α MAPK drives NMDA receptor‐dependent long‐term depression and modulates long‐term memory. Nature Communications, 10, 2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete, M. , Díez, A. , & Araque, A. (2014). Astrocytes in endocannabinoid signalling. Philosophical Transactions of the Royal Society B: Biological Sciences, 369, 20130599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete, M. , Perea, G. , de Sevilla, D. F. , Gómez‐Gonzalo, M. , Núñez, A. , Martín, E. D. , & Araque, A. (2012). Astrocytes mediate in vivo cholinergic‐induced synaptic plasticity. PLoS Biology, 10, e1001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard, M. , Ransom, B. , & Goldman, S. A. (2003). New roles for astrocytes: Redefining the functional architecture of the brain. Trends in Neurosciences, 26, 523–530. [DOI] [PubMed] [Google Scholar]
- Noriega‐Prieto, J. A. , Maglio, L. E. , Zegarra‐Valdivia, J. A. , Pignatelli, J. , Fernandez, A. M. , Martinez‐Rachadell, L. , Fernandes, J. , Núñez, Á. , Araque, A. , Torres‐Alemán, I. , & Fernández de Sevilla, D. (2021). Astrocytic IGF‐IRs induce adenosine‐mediated inhibitory downregulation and improve sensory discrimination. The Journal of Neuroscience, 41, 4768–4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno‐Shosaku, T. , & Kano, M. (2014). Endocannabinoid‐mediated retrograde modulation of synaptic transmission. Current Opinion in Neurobiology, 29, 1–8. [DOI] [PubMed] [Google Scholar]
- Oliet, S. H. R. , Piet, R. , & Poulain, D. A. (2001). Control of glutamate clearance and synaptic efficacy by glial coverage of neurons. Science, 292, 923–926. [DOI] [PubMed] [Google Scholar]
- Parpura, V. , & Zorec, R. (2010). Gliotransmission: Exocytotic release from astrocytes. Brain Research Reviews, 63, 83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual, O. , Casper, K. , Kubera, C. , Zhang, J. , Revilla‐Sanchez, R. , Sul, J. , Takano, H. , Moss, S. , McCarthy, K. , & Haydon, P. (2005). Astrocytic purinergic signaling coordinates synaptic networks. Science, 310, 113–116. [DOI] [PubMed] [Google Scholar]
- Perea, G. , & Araque, A. (2005). Glial calcium signaling and neuron‐glia communication. Cell Calcium, 38, 375–382. [DOI] [PubMed] [Google Scholar]
- Perea, G. , & Araque, A. (2007). Astrocytes potentiate transmitter release at single hippocampal synapses. Science, 317, 1083–1086. [DOI] [PubMed] [Google Scholar]
- Perea, G. , Navarrete, M. , & Araque, A. (2009). Tripartite synapses: Astrocytes process and control synaptic information. Trends in Neurosciences, 32, 421–431. [DOI] [PubMed] [Google Scholar]
- Piomelli, D. (2003). The molecular logic of endocannabinoid signalling. Nature Reviews Neuroscience, 4, 873–884. [DOI] [PubMed] [Google Scholar]
- Pitler, T. A. , & Alger, B. E. (1992). Postsynaptic spike firing reduces synaptic GABA(A) responses in hippocampal pyramidal cells. Journal of Neuroscience, 12, 4122–4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter, J. T. , & McCarthy, K. D. (1996). Hippocampal astrocytes in situ respond to glutamate released from synaptic terminals. Journal of Neuroscience, 16, 5073–5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin, L. M. , Oliveira da Cruz, J. F. , Langlais, V. C. , Martin‐Fernandez, M. , Metna‐Laurent, M. , Busquets‐Garcia, A. , Bellocchio, L. , Soria‐Gomez, E. , Papouin, T. , Varilh, M. , Sherwood, M. W. , Belluomo, I. , Balcells, G. , Matias, I. , Bosier, B. , Drago, F. , van Eeckhaut, A. , Smolders, I. , Georges, F. , … Marsicano, G. (2018). Astroglial CB1 receptors determine synaptic D‐serine availability to enable recognition memory. Neuron, 98, 935–944. [DOI] [PubMed] [Google Scholar]
- Rusakov, D. A. (2015). Disentangling calcium‐driven astrocyte physiology. Nature Reviews Neuroscience, 16, 226–233. [DOI] [PubMed] [Google Scholar]
- Savtchouk, I. , di Castro, M. A. , Ali, R. , Stubbe, H. , Luján, R. , & Volterra, A. (2019). Circuit‐specific control of the medial entorhinal inputs to the dentate gyrus by atypical presynaptic NMDARs activated by astrocytes. Proceedings of the National Academy of Sciences of the United States of America, 116, 13602–13610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi, K. J. , Shekhtmeyster, P. , Merten, K. , Arena, A. , Cook, D. , Hoffman, E. , Ngo, A. , & Nimmerjahn, A. (2016). Imaging large‐scale cellular activity in spinal cord of freely behaving mice. Nature Communications, 7, 11450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, N. A. , Bekar, L. K. , & Nedergaard, M. (2020). Astrocytic endocannabinoids mediate hippocampal transient Heterosynaptic depression. Neurochemical Research, 45, 100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella, N. (2010). Cannabinoid and cannabinoid‐like receptors in microglia, astrocytes, and astrocytomas. Glia, 58, 1017–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, A. , Stern, S. A. , Bozdagi, O. , Huntley, G. W. , Walker, R. H. , Magistretti, P. J. , & Alberini, C. M. (2011). Astrocyte‐neuron lactate transport is required for long‐term memory formation. Cell, 144, 810–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodosis, D. T. , & Poulain, D. A. (1999). Contribution of astrocytes to activity‐dependent structural plasticity in the adult brain. Advances in Experimental Medicine and Biology, 468, 175–182. [DOI] [PubMed] [Google Scholar]
- Theodosis, D. T. , Poulain, D. A. , & Oliet, S. H. R. (2008). Activity‐dependent structural and functional plasticity of astrocyte‐neuron interactions. Physiological Reviews, 88, 983–1008. [DOI] [PubMed] [Google Scholar]
- Tsunematsu, T. , Sakata, S. , Sanagi, T. , Tanaka, K. F. , & Matsui, K. (2021). Region‐specific and state‐dependent astrocyte ca 2+ dynamics during the sleep‐wake cycle in mice. The Journal of Neuroscience, 41, 5440–5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volterra, A. , Liaudet, N. , & Savtchouk, I. (2014). Astrocyte Ca2+ signalling: An unexpected complexity. Nature Reviews. Neuroscience, 15, 327–335. [DOI] [PubMed] [Google Scholar]
- Volterra, A. , & Meldolesi, J. (2005). Astrocytes, from brain glue to communication elements: The revolution continues. Nature Reviews Neuroscience, 6, 626–640. [DOI] [PubMed] [Google Scholar]
- Walter, L. , Dinh, T. , & Stella, N. (2004). ATP induces a rapid and pronounced increase in 2‐arachidonoylglycerol production by astrocytes, a response limited by monoacylglycerol lipase. The Journal of Neuroscience, 24, 8068–8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter, L. , Franklin, A. , Witting, A. , Möller, T. , & Stella, N. (2002). Astrocytes in culture produce anandamide and other acylethanolamides. Journal of Biological Chemistry, 277, 20869–20876. [DOI] [PubMed] [Google Scholar]
- Walter, L. , & Stella, N. (2003). Endothelin‐1 increases 2‐arachidonoyl glycerol (2‐AG) production in astrocytes. Glia, 44, 85–90. [DOI] [PubMed] [Google Scholar]
- Wilson, R. I. , & Nicoll, R. A. (2002). Endocannabinoid signaling in the brain. Science, 296, 678–682. [DOI] [PubMed] [Google Scholar]
- Zhao, L. , Yeh, M. L. W. , & Levine, E. S. (2015). Role for endogenous BDNF in endocannabinoid‐mediated long‐term depression at neocortical inhibitory synapses. eNeuro, 2, ENEURO0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou, S. , & Kumar, U. (2018). Cannabinoid receptors and the endocannabinoid system: Signaling and function in the central nervous system. International Journal of Molecular Sciences, 19, 833. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


