Abstract
Dry eye is one of the most common chronic diseases in ophthalmology. It affects quality of life and has become a public health problem that cannot be ignored. The current treatment methods mainly include artificial tear replacement therapy, anti-inflammatory therapy, and local immunosuppressive therapy. These treatments are mainly limited to improvement of ocular surface discomfort and other symptoms. In recent years, regenerative medicine has developed rapidly, and ophthalmologists are working on new methods to treat dry eye. Mesenchymal stromal cells (MSCs) have anti-inflammatory, tissue repair, and immune regulatory effects, and have become a promising tool for the treatment of dry eye. These effects can also be produced by MSC-derived exosomes (MSC-Exos). As a cell-free therapy, MSC-Exos are hypoimmunogenic, serve more stable entities, and compared with MSCs, reduce the safety risks associated with the injection of live cells. This article reviews current knowledge about MSCs and MSC-Exos, and highlights the latest progress and future prospects of MSC-based therapy in dry eye treatment.
Keywords: dry eye, mesenchymal stromal cell, exosome, regenerative medicine, cornea
Introduction
Dry eye is a chronic and progressive disease, which can cause various forms of ocular discomfort and/or visual dysfunction. Globally, the incidence of dry eye ranges from 5.5% to 33.7%1. It is estimated that the total cost of dry eye patient management calls in the US health care system is US$54.4 billion per year2. Dry eye has become the most common chronic disease in ophthalmology, so it has become a public health problem that cannot be ignored.
Dry eye is characterized by an unstable tear film or unbalanced ocular surface microenvironment. The main functions of the tear film include lubrication, nutrition, cleansing, maintaining the optical properties of the cornea, and resisting bacterial infections of the cornea and conjunctiva. The human tear film contains secretions from specific tissues on the ocular surface (Fig. 1). Meibomian gland secretions are composed of tear film lipids, the aqueous layer is mainly secreted by the lacrimal gland, and mucin by the conjunctival goblet cells3. Dysfunction of these tissues may cause abnormal secretions, resulting in tear film instability4. At present, it is believed that local inflammation and immune response are the important mechanisms leading to pathological damage in dry eye5. Therefore, the medical treatment of dry eye usually focuses on topical drugs such as cyclosporine and glucocorticoids to reduce inflammation and immune response6. However, dry eye is a chronic progressive disease. These treatments can temporarily relieve the ocular surface symptoms, but they cannot completely resolve them. In recent years, regenerative medicine has developed rapidly, and scientists have explored other dry eye treatment methods such as stem cell–based therapy.
Figure 1.
The composition of the tear secretion unit, the function of each tissue, and the structure of the tear film.
Mesenchymal stromal cells (MSCs) isolated from different tissues have been shown to show distinct differentiation potentials. MSCs can be isolated from bone marrow, placenta, fat, umbilical cord, and other tissues7,8. The anti-inflammatory, immune regulatory, and tissue regeneration potential of MSCs has been widely studied. MSCs can interact directly with target tissues through intercellular contact9,10. In addition to direct effects, they can also produce indirect effects through paracrine functions to modify the microenvironment.
Despite the clear advantages of MSC transplantation, the risks of allograft and cell rejection and small vessel lodging and obstruction remain11. However, exosomes can avoid these potential risks. Exosomes are small membrane vesicles (30~150 nm) formed by intracellular endocytic vesicle membrane compression and are released into the extracellular environment by fusion with the membrane12. MSC-derived exosomes (MSC-Exos) have functions similar to MSCs and are more secure, stable, and easy to store than cells. Therefore, MSC-Exos therapy as a cell-free therapy has been developed as a safer and more advantageous alternative to MSC therapy, and some research results have demonstrated these benefits.
Recently, MSCs and MSC-Exos have been widely used in the treatment of clinical diseases, and stem cell therapies have attracted increasing attention from ophthalmologists. The purpose of this article is to explore the latest research findings, potential applications, and challenges related to MSC-based treatment of dry eye. In view of the evidence that MSCs have roles in inflammation, immune regulation, and tissue regeneration, the treatment of dry eye based on MSCs shows promise, as described in this article.
Dry Eye
Pathology of Dry Eye
The pathogenesis of dry eye is complex and remains unclear. In 2017, the Tear Film and Ocular Surface Dry Eye Workshop (TFOS DEWS) II redefined dry eye as the following:
a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles3.
The current research on the mechanism of dry eye is mainly focused on the following aspects.
Inflammation in Dry Eye
Inflammation has long been considered one of the main pathogenic factors in dry eye13. Measurement of the levels of tear cytokines in patients with dry eye provides evidence of ocular surface inflammation. Previous studies showed that the levels of interleukin (IL)-1, IL-4, IL-6, matrix metalloproteinase-3 (MMP-3), MMP-9, IL-8, IL-10, IL-17A, tumor necrosis factor (TNF)-β, and TNF-α in patients with dry eye were higher than those in controls, and were correlated with the severity of dry eye14–18. Among them, the expression of IL-6 was particularly high in dry eye. After IL-6 binds to the receptor, it can activate phosphorylation signal transduction, induce the transcription of activator-3, make Th17 cells secrete IL-17 and other inflammatory factors, and aggravate the inflammatory response and cell apoptosis19. In addition, IL-17 promotes the release of MMP-3 and MMP-920,21. MMPs involved in wound healing and inflammation. During the development of dry eye, MMPs break down the corneal barrier by disrupting the tight junctions between corneal epithelial cells.
In 2017, Rhee and Mah22 proposed a vicious circle of dry eye: multiple factors in the ocular and external environment cause tear film instability, resulting in tear hyperosmolarity, leading to corneal and conjunctival apoptosis, triggering neurogenic inflammation, and then stimulating the lacrimal glands and meibomian gland, further aggravating tear film instability. Therefore, anti-inflammatory therapy has always been an essential part of dry eye treatment.
Immune Response of Dry Eye
T cells and related factors play a vital role in pathogenesis of dry eye. The ocular surface tissues in dry eye express high levels of C-C motif chemokine ligand (CCL) 3, CCL5, CCL20, and other Th cell chemokines, which can induce the migration of Th cells to the ocular surface. This may be one of the pathways for pathological CD4+T cells to reach and infiltrate the ocular surface. A study by De Paiva et al.23 showed that both Th1 and Th17 cells exist in the goblet cell–rich area of the conjunctiva, accompanied by high expression of interferon-γ (IFN-γ) and IL-17. Strategies that inhibit Th17 cell function or target IL-17 can significantly reduce the severity and progression of dry eye24. In dry eye, Th17 cell homing is mediated by CCL20. IFN-γ can significantly reduce mucin secretion of goblet cells, which is important for maintaining immune tolerance on the ocular surface24,25. Regulatory T cells (Tregs) suppress the immune response by suppressing autoreactive T cells and dry eye.
Other Pathologies of Dry Eye
Dysfunction of any link in the nerve conduction pathway may trigger dry eye disease. The cornea is densely populated with sensory nerves. Changes in the ocular surface microenvironment (such as increased tear osmotic pressure and release of tear inflammatory factors) reach the nerve center through afferent signals, and efferent innervation stimulates ocular surface gland secretion and blink activity. Normal blinking allows the tear film to spread over the entire ocular surface26. However, abnormal corneal nerve function will further aggravate ocular surface injury, leading to the persistence of dry eye–related inflammation27. In addition, studies have demonstrated that conjunctival goblet cell secretion granules are regulated by peripheral sympathetic or parasympathetic nerves. Normal conjunctival nerve function is an important factor in regulating goblet cell secretion and maintaining ocular surface function27. In addition, previous studies have shown that oxidative stress can induce damage to ocular surface tissue in dry eye. Tear film components also reportedly change seasonally with the increase of 8-hydroxy-2′-deoxyguanosine (8-OHdG) and malondialdehyde (MDD)28.
MSCs and MSC-Exos
MSCs have been widely studied due to their easy access and wide range of sources. The physiological functions of MSCs have made MSC therapy a potential treatment for many diseases. The Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy has proposed minimum standards for defining human MSCs for laboratory scientific investigation and preclinical research. First, MSCs must be plastic-adherent under standard culture conditions. Second, MSCs must express CD105, CD73, and CD90, lacking expression of CD45, CD34, CD14, CD11b, CD79alpha, or CD19, and human leukocyte antigen (HLA)-DR surface molecules. Third, MSCs must differentiate in vitro into osteoblasts, adipocytes, and chondrocytes29.
Sources of MSCs
MSCs are pluripotent stromal cells that exist in various tissues throughout the development process. To date, MSCs have been isolated from adipose tissue, umbilical cord, dental tissue, bone marrow, cornea, skeletal muscle, synovium, and periosteum8. Among them, bone marrow (BM)-MSCs, adipose-derived (AD)-MSCs, umbilical cord (UC)-MSCs, and cornea-derived MSCs are widely used in laboratory and clinical research (Fig. 2).
Figure 2.
The derivation of the four types of MSCs and their therapeutic application in dry eye. MSC: mesenchymal stromal cell.
The Biological Characteristics of MSCs and MSC-Exos
Immunogenicity of MSCs
MSCs have low immunogenicity; they are able to escape the immune system recognition mechanisms and evade the host’s immune defense30. According to the results of previous studies, MSCs have low expression of major histocompatibility complex (MHC) I and MHC II, and do not express CD40, CD40L, B71, B72, or other costimulators. These characteristics enable MSCs to escape the surveillance of the immune system and enter the body without being rejected by the immune system, an advantage known as immune privilege. Therefore, the infusion of MSCs can reduce the autoimmune side effects caused by engraftment. However, recent studies have demonstrated generation of antibodies against and immune rejection of allogenic MSCs, suggesting that MSCs do not have absolute immune privilege30.
Immunoregulatory and anti-inflammatory effects of MSCs
In the resting state, MSCs tend to be immunosuppressed and display characteristics of immune homeostasis. When MSCs are exposed to various pro-inflammatory factors, such as TNF-α and IL-1α, their immunosuppressive properties are significantly enhanced, leading to their differentiation into an immunosuppressive phenotype. MSCs can regulate a variety of immune cells and exert immune regulatory and anti-inflammatory effects.
MSCs can inhibit T cell proliferation, activation and differentiation, and induce T cell apoptosis and Treg recruitment31,32. Previous studies have shown that MSCs can increase the proportion of CD8(+)CD28(−) T cells and enhance their ability to inhibit the proliferation and activation of primary CD4(+) T cells. MSCs reduce the production of IFN-γ by activating CD4(+) T cells and induce apoptosis of activated CD4(+) T cells33. Present studies have shown that the effect of MSCs on immune balance is related to IFN-γ-induced enzyme indoleamine 2,3-dioxygenase (IDO) and other molecules34–36. Many studies have found abnormal regulation of IDO in patients with autoimmune diseases such systemic lupus erythematosus and Sjögren’s syndrome (SS)35,37. IDO represses effector T cells by finely modulating innate and adaptive immune responses by degrading the tryptophan to kynurenine and other downstream metabolites38. The kynurenine metabolic pathway can be stimulated by IFN-γ and other cytokines to activate IDO in SS, thereby interfering with serotonergic and glutamatergic neurotransmission, thus affecting the ocular surface34,39.
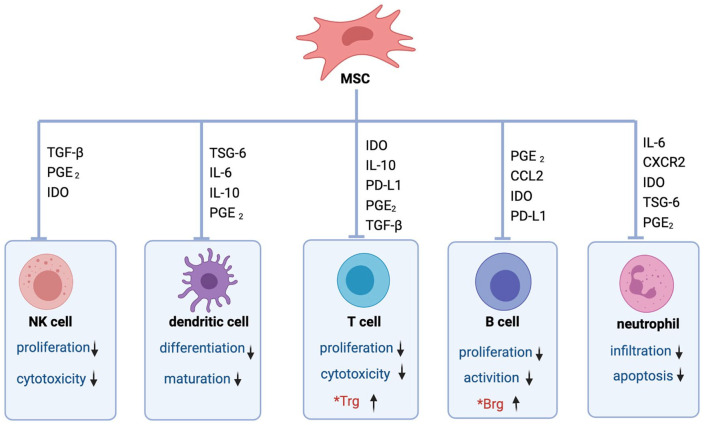
MSCs can also induce cell cycle arrest by downregulating CuclinD2 and upregulating p27Kip1 in T cells, resulting in the inability of activated T cells to divide. In addition, MSCs inhibit the proliferation and cytotoxicity of natural killer (NK) cells, suppress differentiation and maturation of dendritic cells (DCs), and the infiltration and apoptosis of neutrophils (Fig. 3). This is mainly achieved through the secretion of cytokines, including IL-10, human leukocyte antigen-G5 (HLAG), transforming growth factor-β (TGF-β), prostaglandin E2, IDO, and inducible nitric oxide synthase (iNOS)30,40. Therefore, MSCs have been usedto treat several autoimmune diseases based on theirimmunoregulatory and anti-inflammatory properties41,42.
Figure 3.
MSCs regulate the immune response through soluble factors and direct cell-to-cell contact. MSCs inhibit the proliferation and cytotoxicity of NK cells, suppress dendritic cells’ differentiation and maturation, inhibit the proliferation and cytotoxicity of T cells, inhibit the proliferation and activation of B cells, inhibit the infiltration and apoptosis of neutrophil, and also induce differentiation of Tregs and Bregs. Bregs: regulatory B cells; CCL: C-C motif chemokine ligand; IDO: indoleamine 2,3-dioxygenase; PD-L1: programmed cell death ligand; IL: interleukin; MSCs: mesenchymal stromal cells; NK: natural killer; PGE2: prostaglandin E2; TGF-β: transforming growth factor-β; Tregs: regulatory T cells; TSG-6: tumor necrosis factor-α stimulated gene/protein 6.
Tissue regeneration by MSCs
MSCs also have potential for tissue repair, with important possibilities in the treatment of several diseases. To date, regenerative medicine has been widely used to treat many organs in clinical practice, including transplantation of tissue-derived MSCs and treatment by stem cell–derived cytokines, using the potent tissue repair potential of MSCs. When tissues are damaged, stressed or necrotic cells release pro-inflammatory cytokines and chemokines. MSCs can secrete numerous chemokine receptors, and after binding to chemokines, MSCs migrate to damaged tissues. This characteristic is known as homing ability43,44, but the specific mechanism of this ability in MSCs has not been fully described. When MSCs migrate to injured tissue, they differentiate into osteoblasts, fibroblasts, or other cells needed for tissue repair under the regulation of the local environment45. Concurrently, MSCs secrete many cytokines related to tissue repair, including vascular endothelial growth factor (VEGF), fibroblast growth factor 2 (FGF-2), insulin-like growth factor 1 (IGF-1), and hepatocyte growth factor (HGF)9,46. These characteristics of MSCs make collaborative contributions to tissue repair.
MSC-Exos
The exosomes are composed of nucleic acids, proteins, and lipids. Fundamentally, an exosome is an antigen-presented vesicle with a long circulating half-life. Studies have shown that MSC-Exos themselves can act as therapeutic entities in tissue repair, immunomodulation, and in anti-inflammatory function47. MSC-Exo carries anti-inflammatory cytokines and miRNAs and exerts anti-inflammatory effects. Previous studies have shown that exosomes can suppress the TLR4-MyD88-NF-κB pathway, reduce the levels of pro-inflammatory factors (IL-1β, IL-6, TNF-α, and IL-12), and increase the levels of anti-inflammatory factors (IL-10 and TGF-β)48,49. MSC-Exos can also regulate T cells, B cells, NK cells, DCs, and other immune cells50–52.
Furthermore, exosomes have the advantages of high permeability, stability, and low tumorigenicity, which compensate for the limitations of MSC treatment53. Exosomes can freely pass through various biological barriers without blocking microvascular circulation, and they have stability, making them ideal drug carriers54. In addition, MSC-Exo is considered nonimmunogenic so that it can be used in clinical applications on a large scale. However, there are few toxicological studies on exosomes, and their application in vivo remains to be fully evaluated (Fig. 4).
Figure 4.
The functions of MSC-derived exosomes, their advantages, and challenges in clinical application. MHC: major histocompatibility complex; MSCs: mesenchymal stem cells.
Application of MSCs in Dry Eye Treatment
According to the dry eye consensus of the Asia Dry Eye Society, tear film instability is the core mechanism of dry eye. Tear film stability is maintained by various ocular tissues such as those of the lacrimal gland, conjunctiva, cornea, and eyelid55, dysfunction of which will affect tear film homeostasis and may lead to dry eye syndrome. As explained earlier, this may in turn damage the abovementioned tissues and exacerbate the syndrome in a vicious circle.
Many studies have been conducted on MSCs in the dry eye treatment. The goal of MSCs in dry eye treatment is to regenerate new tissue to repair the corneal, conjunctival, and other damage caused by inflammation, and to restore tear film stability. Recently, several preclinical in vivo studies have shown that MSCs have significant potential in the treatment of dry eye (Table 1).
Table 1.
A Summary of Methods Used in In Vivo Studies on MSCs in the Treatment of Dry Eye Syndrome.
| Type of treatment | Cells | Model | Animal (number) | Year | References |
|---|---|---|---|---|---|
| Lacrimal gland injection | Allogeneic AD-MSCs | Keratoconjunctivitis sicca | Dog (n = 12) | 2015 | Villatoro et al.101 |
| Intraperitoneal injection | Allogeneic BM-MSCs | Sjögren’s syndrome dry eye | Mouse (n = 20) | 2017 | Aluri et al.56 |
| Periorbital injection | Human MSCs | Dry eye | Mouse (N/A) | 2015 | Lee et al.57 |
| Topical drop | Allogeneic BM-MSCs | Dry eye | Rat (n = 16) | 2014 | Beyazyildiz et al.66 |
| Intravenous injection | UC-MSCs | Sjögren’s syndrome dry eye | Rabbit (n = 36) | 2020 | Lu et al.69 |
| Topical drop | Allogeneic AD-MSCs | Dry eye | Mouse (n = 18) | 2021 | Wang et al.58 |
| Lacrimal gland injection | Allogeneic AD-MSCs | Keratoconjunctivitis sicca | Dog (n = 15) | 2016 | Bittencourt et al.59 |
| Intravenous injection | Olfactory ectomesenchymal stem cells | Sjögren’s syndrome | Mouse (n = 24) | 2021 | Rui et al.60 |
AD-MSCs: adipose-derived mesenchymal stem cells; BM-MSCs: bone marrow–derived mesenchymal stem cells; MSCs: mesenchymal stem cells; N/A: not applicable; UC-MSCs: umbilical cord–derived mesenchymal stem cells.
Some studies have also applied MSCs in clinical practice to explore their therapeutic effect on human dry eye61,62. Michael et al. injected allogeneic AD-MSCs into the lacrimal glands of five patients with aqueous-deficient dry eye. They found that the tear secretion increased and the tear film osmolarity decreased after treatment63. Weng et al.64 used intravenous MSCs to treat dry eye and found that MSCs were a safe and potentially effective method for the treatment of cGVHD53 secondary dry eye. As of February 2022, there were three clinical trials on the ClinicalTrials.gov website (Table 2). In previous studies, MSC treatment of ocular surface diseases mainly included tissue transplantation65, periorbital injection57,63, topical drops66, and intravenous injection67 (Fig. 2).
Table 2.
Ongoing Clinical Trials Using Mesenchymal Stem Cells in Dry Eye Treatment, Sourced From the Clinical Trials Database ClinicalTrials.gov (February 2022).
| No. | Study title | Condition | Drug | Interventions | Participants’ number | Locations | Last update | References |
|---|---|---|---|---|---|---|---|---|
| 1. | Treatment with Allogeneic Adipose-derived Mesenchymal Stem Cells in Patients with Aqueous Deficient Dry Eye Disease (MESADDE) | Dry eye Keratoconjunctivitis sicca Aqueous tear deficiency |
AD-MSCs | Lacrimal gland injection | 7 | Rigshospitalet, Copenhagen, Denmark | October 8, 2020 | NCT03878628 |
| 2. | Effect of UMSCs Derived Exosomes on Dry Eye in Patients With cGVHD | Dry eye | UMSC-Exo | Transconjunctival injection | 27 | Zhongshan Ophthalmic Center, Guangzhou, Guangdong, China | February 21, 2020 | NCT04213248 |
| 3. | Mesenchymal Stem Cell Therapy of Dry Eye Disease in Patients with Sjögren’s Syndrome (AMASS) | Keratoconjunctivitis sicca Sjögren’s syndrome |
AD-MSCs | Transconjunctival injection | 40 | Rigshospitalet, Copenhagen, Denmark | November 12, 2020 | NCT04615455 |
AD-MSCs: adipose-derived mesenchymal stem cells; cGVHD: chronic Graft-versus-host disease; UMSC-Exo: umbilical cord mesenchymal stem cells-Exsomes.
The Role of MSCs in Corneal Repair
Corneal inflammation, nerve damage, and limbal stem cell deficiency (LSCD) occur with dry eye, seriously affecting patients’ vision and quality of life. Since MSCs have anti-inflammatory, regenerative, and neuroprotective properties, scientists have explored their applications in corneal repair63,68–71. In addition, MSCs have immunoregulatory capacity and they can induce immunosuppression in vivo and in vitro, making them a promising option for ocular surface reconstruction and dry eye72–74.
Application of AD-MSCs in corneal tissue
Adipose tissue has been the main source of MSCs for research. Previous studies have shown that AD-MSCs have a significant impact on wound healing and regenerative medicine. Galindo et al.75 used amniotic membrane containing human AD-MSCs for corneal transplantation to treat rabbit LSCD, and found that the ocular surface has good tolerance to human AD-MSCs and that human AD-MSCs have anti-inflammatory and anti-angiogenic effects. Other studies have used AD-MSCs or the acellular products of AD-MSCs after in vitro culture for corneal transplantation, including allograft, xenograft, and autologous AD-MSC corneal transplantation, demonstrating their potential for this purpose76–78. Conversely, a previous in vivo study found that local or intravenous administration of AD-MSCs during corneal transplantation did not improve graft-versus-host response and instead increased inflammation and neovascularization79. The discrepancy may be due to the use of different injection sites and different amounts of MSCs.
AD-MSCs may also act indirectly through paracrine signaling. It is known that AD-MSCs can produce paracrine factors, such as VEGF, IGF, and TGF-β, with the potential to improve tissue wound healing80,81. In the treatment of corneal injury, paracrine factors of AD-MSCs can promote corneal repair by reducing immune infiltration82. A study which cocultured AD-MSCs with human corneal epithelial cells found that AD-MSC secretions inhibited TGF-β-induced corneal epithelial–mesenchymal transition83.
Application of BM-MSCs in corneal lesions
MSCs were originally isolated from bone marrow, and current research focuses on BM-MSCs. Many studies to date have focused on corneal treatment with BM-MSCs, including subconjunctival injection, intravenous injection, and corneal transplantation. Among them, a clinical trial of corneal transplantation using allogenic MSCs conducted by Calonge et al.68 in 2018 showed that BM-MSCs could promote the proliferation of corneal epithelium, which verified the effectiveness of BM-MSCs in the treatment of LSCD. A number of studies have shown that in the repair of corneal injury, BM-MSCs exert anti-inflammatory, anti-fibrosis, and proregeneration effects through the secretion of TSG-6 and HGF67,70,84,85. Sharad K. M et al. treated corneal injury in mice with intravenous BM-MSCs and found that MSCs have the capacity to inhibit the generation of myofibroblasts, and the corneal transparency is also restored due to high levels of HGF secretion. HGF was significantly upregulated on stimulation with recombinant human IL-1β86. In addition, BM-MSCs can reduce corneal oxidative stress87.
Application of UC-MSCs in corneal lesions
UC-MSCs are derived from umbilical cord blood, which is easier than other MSC sources to collect and more abundant in supply, alleviating ethical disputes.
Azmi et al.88 cocultured human UC-MSCs with corneal epithelial cells and found that the MSCs promoted epithelial cell growth and function by downregulating the expression of HLA class I and diphenylamine IFN-γ stimulated human telomerase perpetuators. Park et al.71 found less ocular damage and decreased cell apoptosis in mice intravenously injected with UC-MSCs. UC-MSCs tend to aggregate and attach to the damaged tissue area, and differentiate into cell types according to their specific microenvironment. Compared with BM-MSCs, the expression profile of UC-MSCs is closer to that of embryonic stem cells, which can differentiate into corneal epithelial, stromal, and endothelial cells. UC-MSCs also have low immunogenicity and low incidence of rejection and graft-versus-host disease. Therefore, many studies have used UC-MSCs for corneal transplantation89.
However, the current methods for isolating and culturing stem cells from the umbilical cord are cumbersome, resulting in low yields of primary cells, and limiting the application of UC-MSCs in corneal therapy90.
Application of cornea-derived MSCs in treatment of corneal lesions
Cornea-derived MSCs are mainly distributed in the anterior part of the corneal stroma near the limbal stem cells. They are MSCs with multidirectional differentiation potential. Research has shown that they can induce differentiation into corneal cells in vitro91, but there is some controversy about this. In 2005, a team from the University of Pittsburgh isolated and identified corneal stromal mesenchymal stem cells and found them to have a greater potential to differentiate into corneal cells in vitro than BM-MSCs, AD-MSCs, or UC-MSCs92. Multiple studies have also shown that coculture of cornea-derived MSCs with limbal epithelial cells is superior to culture with limbal epithelial cells alone93.
The therapeutic effect of cornea-derived MSCs is not yet clear. Chronic corneal inflammation in patients with dry eye often leads to corneal interstitial damage and fibrosis, scarring, and reduced transparency. Stem cells derived from the limbal stroma may avoid the formation of corneal scars94, but the mechanism by which MSCs act against fibrosis has not yet been elucidated. With the progression of dry eye, further damage to the cornea may lead to the generation of corneal neovascularization. Eslani et al.95 used human corneal stem cells to treat mouse cornea and found that cornea-derived MSCs can induce macrophage apoptosis by secreting factors such as pigment epithelial–derived factor, thereby inhibiting corneal neovascularization.
The Role of MSCs in Conjunctival Repair
Goblet cells in the conjunctival epithelium secrete mucosal protein, which is the main component of the tear film mucoprotein layer. The loss of goblet cells will destroy the stability of the tear film, while the activation of inflammatory factors and the reduction of tear nutrient factors will change the differentiation of the conjunctiva and reduce goblet cells23,96. The loss of goblet cells in the conjunctival epithelium is a widely recognized feature of dry eye97–99. Severe dry eye, such as in Stevens–Johnson syndrome, may feature a complete loss of conjunctival goblet cells. Previous studies have shown that the number of conjunctival goblet cells increases after treatment with MSCs in animal dry eye models57,66. Lee et al.100 used periorbital injections of BM-MSCs to treat a mouse model of dry eye induced by an intraorbital injection of concanavalin A, and found increased tear secretion and goblet cell numbers and decreased levels of inflammatory factors on the ocular surface after treatment.
The Role of MSCs in Lacrimal Gland Repair
Lacrimal glands play an important role in the physiological and pathological processes of the ocular surface. Abnormalities such as lacrimal gland inflammation and apoptosis can lead to decreased tear secretion. Some studies have shown that MSCs can promote lacrimal gland regeneration and increase tear secretion101. Villatoro et al.102 injected AD-MSCs into the lacrimal glands of dogs with dry eye and found improvement in dry eye signs and good tolerance. Michael et al. conducted a clinical trial in which allograft AD-MSCs were injected into the lacrimal glands of patients with severe lacrimal defects. The results similarly showed an improvement in signs and symptoms with good tolerance63.
The Role of MSCs in Meibomian Gland Repair
The meibomian gland secretes meibum, the main component of the tear lipid layer which acts as a barrier to the evaporation of tear fluid. In meibomian gland dysfunction (MGD), the quality and quantity of the meibum will change, thereby negatively affecting the tear film stability, leading to reduced tears and dry eyes. Beyazyıldız et al. used MSCs to treat a benzalkonium chloride–induced rat model of dry eye and found that the ocular surface condition of the rats was improved after treatment. MSC infiltration into cells could be seen in the eyelid gland, but their therapeutic effect on the gland has not yet been demonstrated66.
Application of MSC-Exos in Dry Eye Treatment
Over the last decade, research on MSC-Exos has made great progress in the field of regenerative medicine. Various regulatory functions of MSC-Exos have been reported, including differentiation, inflammation, and immunosuppression73,103. In addition, exosomes secreted from stromal stem cells have been shown to have anti-fibrosis, anti-inflammatory, and regenerative effects on injured corneas104,105. Shen et al.106 found greater proliferation and less apoptosis in rabbit corneal stromal cells cultured with rabbit adipose MSC-Exos than in those without MSC-Exos. Wang et al.58 treated dry eye in mice using mouse AD-MSC-derived exosomes (mADSC-Exos) and found that mADSC-Exos could decrease the cytokines, promote corneal epithelial repair, and increase tear secretion by inhibiting the NLRP3-IL-1β signaling pathway.
Previous laboratory studies have shown that cornea-derived MSCs can prevent scar formation after corneal injury in mice, stimulate the regeneration of hyaline interstitial tissue, and this function depends on exosomes to deliver miRNAs105. In addition, the human cornea-derived MSCs can be absorbed by corneal epithelial cells and accelerate the healing of corneal injury104. Zhou et al. used MSC-Exos to treat chronic Graft versus host disease-associated dry eye in mice and refractory dry eye in patients.The study demonstrated that MSC-Exos could relieve dry eye symptoms and found that Mir-204 contained in MSC-Exos played a role in the treatment107. Previous studies have shown that microRNAs including mioRNA-466, miRNA-205, miRNA-122, and others can promote the healing of injured corneas108–110. However, no further clinical or laboratory studies have been conducted using these miRNAs to treat dry eye.
At present, exosome therapy has many limitations, which hinder its application in dry eye treatment. Specific problems are as follows: (1) Due to technical limitations, it is difficult to obtain MSC-Exos with high purity and high yield. Therefore, technology needs to be further developed to improve separation efficiency and productivity. (2) The direct relationship between the specific content of MSC-Exos (lipid, protein, and nucleic acid) and their positive therapeutic effects remains unclear. The therapeutic effect, safety, and effective dose of various loaded substances need to be further studied. (3) MSC-Exos as a drug delivery carrier is a current focus of research, and drug delivery efficiency is another challenge that needs to be overcome.
Conclusion
In recent decades, MSCs have demonstrated great potential and availability in human and animal studies on the treatment of ocular surface diseases, and some laboratory studies have shown that MSCs can effectively treat dry eye. However, there is a lack of clinical studies such as randomized controlled trials to confirm their efficacy in the treatment of dry eye and to clarify their mechanisms which may include tissue regeneration, anti-inflammatory, and immunomodulatory synergistic effects. Although MSC therapy is promising in the treatment of dry eye, limited survival time and the implantation of bioactive agents are bottlenecks in disease treatment. Sustained maintenance and improvement of the survival and secretion of MSCs remain challenging in this field and require further study.
Since their biological characteristics are very similar to those of their source cells, MSC-Exos are considered an effective cell-free treatment strategy. The exosomes derived from MSCs are easy to store and can be directly transported to the diseased site through targeted modification or local injection, circumventing many limitations of cell therapy. However, due to technical limitations, clinical transformation associated with MSC-Exo therapy remains a major challenge. Future research will require the development of efficient technical methods that can deliver sufficient doses of drugs into MSC-Exos while maintaining its physical integrity and biological activity. Although further research is needed, MSC-based therapy of dry eye is feasible and its scope for treatment of damaged tissue is exciting.
Acknowledgments
The authors thank Quanzhou Medical Research Center for continuous support of this work. Illustration created with BioRender.com
Footnotes
Ethical Approval: This study was approved by our institutional review board.
Statement of Human and Animal Rights: This article does not contain any studies with human or animal subjects.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Science and Technology Bureau of Quanzhou (2020CT003), and the Medical Innovation project of Fujian Province (2021CXA027).
ORCID iD: Yingying Gao  https://orcid.org/0000-0002-6869-3013
https://orcid.org/0000-0002-6869-3013
References
- 1. Rabina G, Boguslavsky II, Mimouni M, Kaiserman I. The association between preoperative dry eye symptoms and postoperative discomfort in patients underwent photorefractive keratectomy. J Ophthalmol. 2019;2019:7029858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yu J, Asche CV, Fairchild CJ. The economic burden of dry eye disease in the United States: a decision tree analysis. Cornea. 2011;30(4):379–87. [DOI] [PubMed] [Google Scholar]
- 3. Craig JP, Nichols KK, Akpek EK, Caffery B, Dua H, Liu ZS, Joo C, Nelson JD, Nichols JJ, Tsubota K, Stapleton F. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276–83. [DOI] [PubMed] [Google Scholar]
- 4. You IC, Li Y, Jin R, Ahn M, Choi W, Yoon KC. Comparison of 0.1%, 0.18%, and 0.3% hyaluronic acid eye drops in the treatment of experimental dry eye. J Ocul Pharmacol Ther. 2018;34(8):557–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Niu L, Zhang S, Wu J, Chen L, Wang Y. Upregulation of NLRP3 inflammasome in the tears and ocular surface of dry eye patients. PLoS ONE. 2015;10(5):e0126277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stonecipher KG, Chia J, Onyenwenyi A, Villanueva L, Hollander DA. Health claims database study of cyclosporine ophthalmic emulsion treatment patterns in dry eye patients. Ther Clin Risk Manag. 2013;9:409–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wu X, Zhao X, Puertollano R, Bonifacino JS, Eisenberg E, Greene LE. Adaptor and clathrin exchange at the plasma membrane and trans-Golgi network. Mol Biol Cell. 2003;14(2):516–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brady K, Dickinson SC, Guillot PV, Polak J, Blom AW, Kafienah W, Hollander AP. Human fetal and adult bone marrow-derived mesenchymal stem cells use different signaling pathways for the initiation of chondrogenesis. Stem Cells Dev. 2014;23(5):541–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. da Silva Meirelles L, Fontes AM, Covas DT, Caplan AI. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009;20(5–6):419–27. [DOI] [PubMed] [Google Scholar]
- 10. Mahiddine FY, Kim JW, Qamar AY, Ra JC, Kim SH, Jung EJ, Kim MJ. Conditioned medium from canine amniotic membrane-derived mesenchymal stem cells improved dog sperm post-thaw quality-related parameters. Animals (Basel). 2020;10(10):1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marote A, Teixeira FG, Mendes-Pinheiro B, Salgado AJ. MSCs-derived exosomes: cell-secreted nanovesicles with regenerative potential. Front Pharmacol. 2016;7:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2(8):569–79. [DOI] [PubMed] [Google Scholar]
- 13. Bron AJ, de Paiva CS, Chauhan SK, Bonini S, Gabison EE, Jain S, Knop E, Markoulli M, Ogawa Y, Perez V, Uchino Y, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15(3):438–510. [DOI] [PubMed] [Google Scholar]
- 14. Sugaya S, Sakimoto T, Shoji J, Sawa M. Regulation of soluble interleukin-6 (IL-6) receptor release from corneal epithelial cells and its role in the ocular surface. Jpn J Ophthalmol. 2011;55(3):277–82. [DOI] [PubMed] [Google Scholar]
- 15. Choi M, Han SJ, Ji YW, Choi YJ, Jun I, Alotaibi MH, Ko BY, Kim EK, Kim T-I, Nam SM, Seo KY. Meibum expressibility improvement as a therapeutic target of intense pulsed light treatment in meibomian gland dysfunction and its association with tear inflammatory cytokines. Sci Rep. 2019;9(1):7648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu X, Chen X, Ma Y, Lin X, Yu X, He S, Luo C, Xu W. Analysis of tear inflammatory molecules and clinical correlations in evaporative dry eye disease caused by meibomian gland dysfunction. Int Ophthalmol. 2020;40(11):3049–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pinto-Fraga J, Enríquez-de-Salamanca A, Calonge M, González-García MJ, López-Miguel A, López-de la, Rosa A, García-Vázquez C, Calder V, Stern ME, Fernández I. Severity, therapeutic, and activity tear biomarkers in dry eye disease: an analysis from a phase III clinical trial. Ocul Surf. 2018;16(3):368–76. [DOI] [PubMed] [Google Scholar]
- 18. Boehm N, Riechardt AI, Wiegand M, Pfeiffer N, Grus FH. Proinflammatory cytokine profiling of tears from dry eye patients by means of antibody microarrays. Investig Ophthalmol Vis Sci. 2011;52(10):7725–30. [DOI] [PubMed] [Google Scholar]
- 19. Fujimura T, Fujimoto T, Itaya-Hironaka A, Miyaoka T, Yoshimoto K, Sakuramoto-Tsuchida S, Yamauchi A, Takeda M, Tsujinaka H, Tanaka Y, Takasawa S. Significance of Interleukin-6/STAT pathway for the gene expression of REG Iα, a new autoantigen in Sjögren’s syndrome patients, in salivary duct epithelial cells. Clin Rev Allergy Immunol. 2017;52(3):351–63. [DOI] [PubMed] [Google Scholar]
- 20. Tan X, Sun S, Liu Y, Zhu T, Wang K, Ren T, Wu Z, Xu H, Zhu L. Analysis of Th17-associated cytokines in tears of patients with dry eye syndrome. Eye (Lond). 2014;28(5):608–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. De Paiva CS, Chotikavanich S, Pangelinan SB, Pitcher JD, 3rd, Fang B, Zheng X, Ma P, Farley WJ, Siemasko KF, Niederkorn JY, Stern ME, et al. IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunol. 2009;2(3):243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rhee MK, Mah FS. Inflammation in dry eye disease: how do we break the cycle? Ophthalmology. 2017;124(Suppl 11):S14–19. [DOI] [PubMed] [Google Scholar]
- 23. De Paiva CS, Villarreal AL, Corrales RM, Rahman HT, Chang VY, Farley WJ, Stern ME, Niederkorn JY, Li DQ, Pflugfelder SC. Dry eye-induced conjunctival epithelial squamous metaplasia is modulated by interferon-γ. Invest Ophthalmol Vis Sci. 2007;48(6):2553–60. [DOI] [PubMed] [Google Scholar]
- 24. Chauhan SK, El Annan J, Ecoiffier T, Goyal S, Zhang Q, Saban DR, Dana R. Autoimmunity in dry eye is due to resistance of Th17 to Treg suppression. J Immunol. 2009;182(3):1247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Albertsmeyer AC, Kakkassery V, Spurr-Michaud S, Beeks O, Gipson IK. Effect of pro-inflammatory mediators on membrane-associated mucins expressed by human ocular surface epithelial cells. Exp Eye Res. 2010;90(3):444–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Labetoulle M, Baudouin C, Calonge M, Merayo-Lloves J, Boboridis KG, Akova YA, Aragona P, Geerling G, Messmer EM, Benítez-Del-Castillo J. Role of corneal nerves in ocular surface homeostasis and disease. Acta Ophthalmol. 2019;97(2):137–45. [DOI] [PubMed] [Google Scholar]
- 27. Dartt DA, McCarthy DM, Mercer HJ, Kessler TL, Chung EH, Zieske JD. Localization of nerves adjacent to goblet cells in rat conjunctiva. Curr Eye Res. 1995;14(11):993–1000. [DOI] [PubMed] [Google Scholar]
- 28. Li Y, Liu H, Zeng W, Wei J. Edaravone protects against hyperosmolarity-induced oxidative stress and apoptosis in primary human corneal epithelial cells. PLoS ONE. 2017;12(3):e0174437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC, Krause DS, Deans RJ, Keating A, Prockop DJ, Horwitz EM. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–17. [DOI] [PubMed] [Google Scholar]
- 30. Krampera M, Galipeau J, Shi Y, Tarte K, Sensebe L; MSC Committee of the International Society for Cellular Therapy (ISCT). Immunological characterization of multipotent mesenchymal stromal cells—The International Society for Cellular Therapy (ISCT) working proposal. Cytotherapy. 2013;15(9):1054–61. [DOI] [PubMed] [Google Scholar]
- 31. Glennie S, Soeiro I, Dyson PJ, Lam EWF, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105(7):2821–27. [DOI] [PubMed] [Google Scholar]
- 32. Almeida CR, Caires HR, Vasconcelos DP, Barbosa MA. NAP-2 secreted by human NK cells can stimulate mesenchymal stem/stromal cell recruitment. Stem Cell Reports. 2016;6(4):466–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu Q, Zheng H, Chen X, Peng Y, Huang W, Li X, Li G, Xia W, Sun Q, Xiang AP. Human mesenchymal stromal cells enhance the immunomodulatory function of CD8(+)CD28(–) regulatory T cells. Cell Mol Immunol. 2015;12(6):708–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. de Oliveira FR, Fantucci MZ, Adriano L, Valim V, Cunha TM, Louzada-Junior P, Rocha EM. Neurological and inflammatory manifestations in Sjögren’s syndrome: the role of the kynurenine metabolic pathway. Int J Mol Sci. 2018;19(12):60–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bengtsson AA, Trygg J, Wuttge DM, Sturfelt G, Theander E, Donten M, Moritz T, Sennbro CJ, Torell F, Lood C, Surowiec I, et al. Metabolic profiling of systemic lupus erythematosus and comparison with primary Sjögren’s syndrome and systemic sclerosis. PLoS ONE. 2016;11(7):e0159384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang G, Cao K, Liu K, Xue Y, Roberts AI, Li F, Han Y, Rabson AB, Wang Y, Shi Y. Kynurenic acid, an IDO metabolite, controls TSG-6-mediated immunosuppression of human mesenchymal stem cells. Cell Death Differ. 2018;25(7):1209–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang D, Huang S, Yuan X, Liang J, Xu R, Yao G, Feng X, Sun L. The regulation of the Treg/Th17 balance by mesenchymal stem cells in human systemic lupus erythematosus. Cell Mol Immunol. 2017;14(5):423–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Selvan SR, Dowling JP, Kelly WK, Lin J. Indoleamine 2,3-dioxygenase (IDO): biology and target in cancer immunotherapies. Curr Cancer Drug Targets. 2016;16(9):755–64. [DOI] [PubMed] [Google Scholar]
- 39. Filippini P, Del Papa N, Sambataro D, Del Bufalo A, Locatelli F, Rutella S. Emerging concepts on inhibitors of indoleamine 2,3-dioxygenase in rheumatic diseases. Curr Med Chem. 2012;19(31):5381–93. [DOI] [PubMed] [Google Scholar]
- 40. Soleymaninejadian E, Pramanik K, Samadian E. Immunomodulatory properties of mesenchymal stem cells: cytokines and factors. Am J Reprod Immunol. 2012;67(1):1–8. [DOI] [PubMed] [Google Scholar]
- 41. Gowhari Shabgah A, Shariati-Sarabi Z, Tavakkol-Afshari J, Ghasemi A, Ghoryani M, Mohammadi M. A significant decrease of BAFF, APRIL, and BAFF receptors following mesenchymal stem cell transplantation in patients with refractory rheumatoid arthritis. Gene. 2020;732:144336. [DOI] [PubMed] [Google Scholar]
- 42. Shi B, Qi J, Yao G, Feng R, Zhang Z, Wang D, Chen C, Tang X, Lu L, Chen W, Sun L. Mesenchymal stem cell transplantation ameliorates Sjögren’s syndrome via suppressing IL-12 production by dendritic cells. Stem Cell Res Ther. 2018;9(1):308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kang SK, Shin IS, Ko MS, Jo JY, Ra JC. Journey of mesenchymal stem cells for homing: strategies to enhance efficacy and safety of stem cell therapy. Stem Cells Int. 2012;2012:342968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cofano F, Boido M, Monticelli M, Zenga F, Ducati A, Vercelli A, Garbossa D. Mesenchymal stem cells for spinal cord injury: current options, limitations, and future of cell therapy. Int J Mol Sci. 2019;20(11):2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shohara R, Yamamoto A, Takikawa S, Iwase A, Hibi H, Kikkawa F, Ueda M. Mesenchymal stromal cells of human umbilical cord Wharton’s jelly accelerate wound healing by paracrine mechanisms. Cytotherapy. 2012;14(10):1171–81. [DOI] [PubMed] [Google Scholar]
- 46. Kang JW, Kang KS, Koo HC, Park JR, Choi EW, Park YH. Soluble factors-mediated immunomodulatory effects of canine adipose tissue-derived mesenchymal stem cells. Stem Cells Dev. 2008;17(4):681–93. [DOI] [PubMed] [Google Scholar]
- 47. Lo Sicco C, Reverberi D, Balbi C, Ulivi V, Principi E, Pascucci L, Becherini P, Bosco MC, Varesio L, Franzin C, Pozzobon M, et al. Mesenchymal stem cell-derived extracellular vesicles as mediators of anti-inflammatory effects: endorsement of macrophage polarization. Stem Cells Transl Med. 2017;6(3):1018–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhang B, Yin Y, Lai RC, Tan SS, Choo ABH, Lim SK. Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev. 2014;23(11):1233–44. [DOI] [PubMed] [Google Scholar]
- 49. Lee B-C, Kang I, Yu K-R. Therapeutic features and updated clinical trials of mesenchymal stem cell (MSC)-derived exosomes. J Clin Med. 2021;10(4):711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Abumaree MH, Bahattab E, Alsadoun A, Al Dosaimani A, Abomaray FM, Khatlani T, Kalionis B, El-Muzaini MF, Alawad AO, Alaskar AS. Characterization of the interaction between human decidua parietalis mesenchymal stem/stromal cells and natural killer cells. Stem Cell Res Ther. 2018;9(1):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Martínez HR, Molina-Lopez JF, González-Garza MT, Moreno-Cuevas JE, Caro-Osorio E, Gil-Valadez A, Gutierrez-Jimenez E, Zazueta-Fierro OE, Meza JA, Couret-Alcaraz P, Hernandez-Torre M. Stem cell transplantation in amyotrophic lateral sclerosis patients: methodological approach, safety, and feasibility. Cell Transplant. 2012;21(9):1899–907. [DOI] [PubMed] [Google Scholar]
- 52. Cosenza S, Toupet K, Maumus M, Luz-Crawford P, Blanc-Brude O, Jorgensen C, Noël D. Mesenchymal stem cells-derived exosomes are more immunosuppressive than microparticles in inflammatory arthritis. Theranostics. 2018;8(5):1399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. He L, He T, Xing J, Zhou Q, Fan L, Liu C, Chen Y, Wu D, Tian Z, Liu B, Rong L. Bone marrow mesenchymal stem cell-derived exosomes protect cartilage damage and relieve knee osteoarthritis pain in a rat model of osteoarthritis. Stem Cell Res Ther. 2020;11(1):276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Xunian Z, Kalluri R. Biology and therapeutic potential of mesenchymal stem cell-derived exosomes. Cancer Sci. 2020;111(9):3100–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tsubota K, Yokoi N, Shimazaki J, Watanabe H, Dogru M, Yamada M, Kinoshita S, Kim H, Tchah H, Hyon JY, Yoon K, et al. New perspectives on dry eye definition and diagnosis: a consensus report by the Asia Dry Eye Society. Ocul Surf. 2017;15(1):65–76. [DOI] [PubMed] [Google Scholar]
- 56. Aluri HS, Samizadeh M, Edman MC, Hawley DR, Armaos HL, Janga SR, Meng Z, Sendra VG, Hamrah P, Kublin CL, Hamm-Alvarez SF, et al. Delivery of bone marrow-derived mesenchymal stem cells improves tear production in a mouse model of sjögren’s syndrome. Stem Cells Int. 2017;2017:3134543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lee MJ, Ko AY, Ko JH, Lee HJ, Kim MK, Wee WR, Khwarg SI, Oh JY. Mesenchymal stem / stromal cells protect the ocular surface by suppressing inflammation in an experimental dry eye. Mol Ther. 2015;23(1):139–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wang G, Li H, Long H, Gong X, Hu S, Gong C. Exosomes derived from mouse adipose-derived mesenchymal stem cells alleviate benzalkonium chloride-induced mouse dry eye model via inhibiting NLRP3 inflammasome. Ophthalmic Res. 2022;65(1):40–51. [DOI] [PubMed] [Google Scholar]
- 59. Bittencourt MKW, Barros MA, Martins JFP, Vasconcellos JPC, Morais BP, Pompeia C, Bittencourt MD, Evangelho KDS, Kerkis I, Wenceslau CV. Allogeneic mesenchymal stem cell transplantation in dogs with keratoconjunctivitis sicca. Cell Med. 2016;8(3):63–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rui K, Hong Y, Zhu Q, Shi X, Xiao F, Fu H, Yin Q, Xing Y, Wu X, Kong X, Xu H, et al. Olfactory ecto-mesenchymal stem cell-derived exosomes ameliorate murine Sjögren’s syndrome by modulating the function of myeloid-derived suppressor cells. Cell Mol Immunol. 2021;18(2):440–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Oh JY, Lee RH. Mesenchymal stromal cells for the treatment of ocular autoimmune diseases. Prog Retin Eye Res. 2021;85:100967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Xu J, Wang D, Liu D, Fan Z, Zhang H, Liu O, Ding G, Gao R, Zhang C, Ding Y, Bromberg JS, et al. Allogeneic mesenchymal stem cell treatment alleviates experimental and clinical Sjögren syndrome. Blood. 2012;120(15):3142–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Møller-Hansen M, Larsen AC, Toft PB, Lynggaard CD, Schwartz C, Bruunsgaard H, Haack-Sørensen M, Ekblond A, Kastrup J, Heegaard S. Safety and feasibility of mesenchymal stem cell therapy in patients with aqueous deficient dry eye disease. Ocul Surf. 2021;19:43–52. [DOI] [PubMed] [Google Scholar]
- 64. Weng J, He C, Lai P, Luo C, Guo R, Wu S, Geng S, Xiangpeng A, Liu X, Du X. Mesenchymal stromal cells treatment attenuates dry eye in patients with chronic graft-versus-host disease. Mol Ther. 2012;20(12):2347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yan D, Yan C, Yu F, Zhang S, Chen L, Wu N, Shao C, Yao Q, Sun H, Fu Y. Exploitation of human mesenchymal stromal cell derived matrix towards the structural and functional restoration of the ocular surface. Biomater Sci. 2020;8(17):4712–27. [DOI] [PubMed] [Google Scholar]
- 66. Beyazyıldız E, Pınarlı FA, Beyazyıldız O, Hekimoğlu ER, Acar U, Demir MN, Albayrak A, Kaymaz F, Sobacı G, Delibaşı T. Efficacy of topical mesenchymal stem cell therapy in the treatment of experimental dry eye syndrome model. Stem Cells Int. 2014;2014:250230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yun YIN, Park SEY, Lee HJU, Ko JHWA, Kim MEEKUM, Wee WONR, Reger RL, Gregory CA, Choi H, Fulcher SF, Prockop DJ, et al. Comparison of the anti—inflammatory effects of induced pluripotent stem cell—derived and bone marrow—derived mesenchymal stromal cells in a murine model of corneal injury. Cytotherapy. 2017;19(1):28–35. [DOI] [PubMed] [Google Scholar]
- 68. Calonge M, Pérez I, Galindo S, Nieto-Miguel T, López-Paniagua M, Fernández I, Alberca M, García-Sancho J, Sánchez A, Herreras JM. A proof-of-concept clinical trial using mesenchymal stem cells for the treatment of corneal epithelial stem cell deficiency. Transl Res. 2019;206:18–40. [DOI] [PubMed] [Google Scholar]
- 69. Lu X, Li N, Zhao L, Guo D, Yi H, Yang L, Liu X, Sun D, Nian H, Wei R. Human umbilical cord mesenchymal stem cells alleviate ongoing autoimmune dacryoadenitis in rabbits via polarizing macrophages into an anti-inflammatory phenotype. Exp Eye Res. 2020;191:107905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhang N, Luo X, Zhang S, Liu R, Liang L, Su W, Liang D. Subconjunctival injection of tumor necrosis factor-α pre-stimulated bone marrow-derived mesenchymal stem cells enhances anti-inflammation and anti-fibrosis in ocular alkali burns. Graefes Arch Clin Exp Ophthalmol. 2021;259(4):929–40. [DOI] [PubMed] [Google Scholar]
- 71. Park SY, Oh IY, Kim JH, Kim HJ, Seo B, Kwon OY, Song WJ, Kwon HS, Cho YS, Moon HB, Kim TB. Therapeutic effects of mesenchymal stem cells on a Stevens-Johnson syndrome/toxic epidermal necrolysis model. J Korean Med Sci. 2020;35(15):2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Li F, Zhao SZ. Control of cross talk between angiogenesis and inflammation by mesenchymal stem cells for the treatment of ocular surface diseases. Stem Cells Int. 2016;2016:7961816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Burrello J, Monticone S, Gai C, Gomez Y, Kholia S, Camussi G. Stem cell-derived extracellular vesicles and immune-modulation. Front Cell Dev Biol. 2016;4:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110(10):3499–506. [DOI] [PubMed] [Google Scholar]
- 75. Galindo S, Herreras JM, López-Paniagua M, Rey E, de la Mata A, Plata-Cordero M, Calonge M, Nieto-Miguel T. Therapeutic effect of human adipose tissue-derived mesenchymal stem cells in experimental corneal failure due to limbal stem cell niche damage. Stem Cells. 2017;35(10):2160–74. [DOI] [PubMed] [Google Scholar]
- 76. Venugopal B, Shenoy SJ, Mohan S, Anil Kumar PR, Kumary TV. Bioengineered corneal epithelial cell sheet from mesenchymal stem cells—A functional alternative to limbal stem cells for ocular surface reconstruction. J Biomed Mater Res B Appl Biomater. 2020;108(3):1033–45. [DOI] [PubMed] [Google Scholar]
- 77. Holan V, Trosan P, Cejka C, Javorkova E, Zajicova A, Hermankova B, Chudickova M, Cejkova J. A comparative study of the therapeutic potential of mesenchymal stem cells and limbal epithelial stem cells for ocular surface reconstruction. Stem Cells Transl Med. 2015;4(9):1052–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Nieto-Miguel T, Galindo S, Reinoso R, Corell A, Martino M, Pérez-Simón JA, Calonge M. In vitro simulation of corneal epithelium microenvironment induces a corneal epithelial-like cell phenotype from human adipose tissue mesenchymal stem cells. Curr Eye Res. 2013;38(9):933–44. [DOI] [PubMed] [Google Scholar]
- 79. Fuentes-Julián S, Arnalich-montiel F, Jaumandreu L. Adipose-derived mesenchymal stem cell administration does not improve corneal graft survival outcome. PLoS One. 2015;10(3):e0117945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Yao Y, Huang J, Geng Y, Qian H, Wang F, Liu X, Shang M, Nie S, Liu N, Du X, Dong J, et al. Paracrine action of mesenchymal stem cells revealed by single cell gene profiling in infarcted murine hearts. PLoS ONE. 2015;10(6):e0129164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Yang JA, Chung HM, Won CH, Sung JH. Potential application of adipose-derived stem cells and their secretory factors to skin: discussion from both clinical and industrial viewpoints. Expert Opin Biol Ther. 2010;10(4):495–503. [DOI] [PubMed] [Google Scholar]
- 82. Al-Jaibaji O, Swioklo S, Shortt A, Figueiredo FC, Connon CJ. Hypothermically stored adipose-derived mesenchymal stromal cell alginate bandages facilitate use of paracrine molecules for corneal wound healing. Int J Mol Sci. 2020;21(16):5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Shibata S, Hayashi R, Okubo T, Kudo Y, Baba K, Honma Y, Nishida K. The secretome of adipose-derived mesenchymal stem cells attenuates epithelial–mesenchymal transition in human corneal epithelium. Regen Ther. 2019;11:114–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Di G, Du X, Qi X, Zhao X, Duan H, Li S, Xie L, Zhou Q. Mesenchymal stem cells promote diabetic corneal epithelial wound healing through TSG—6—dependent stem cell activation and macrophage switch. Invest Ophthalmol Vis Sci. 2017;58(10):4344–54. [DOI] [PubMed] [Google Scholar]
- 85. Roddy GW, Oh JY, Lee RH, Bartosh TJ, Ylostalo J, Coble K, Rosa RH, Jr, Prockop DJ. Action at a distance: systemically administered adult stem/progenitor cells (MSCs) reduce inflammatory damage to the cornea without engraftment and primarily by secretion of TNF-α stimulated gene/protein 6. Stem Cells. 2011;29(10):1572–79. [DOI] [PubMed] [Google Scholar]
- 86. Mittal SK, Omoto M, Amouzegar A, Sahu A, Rezazadeh A, Katikireddy KR, Shah DI, Sahu SK, Chauhan SK. Restoration of corneal transparency by mesenchymal stem cells. Stem Cell Reports. 2016;7(4):583–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Cejkova J, Trosan P, Cejka C, Lencova A, Zajicova A, Javorkova E, Kubinova S, Sykova E, Holan V. Suppression of alkali-induced oxidative injury in the cornea by mesenchymal stem cells growing on nanofiber scaffolds and transferred onto the damaged corneal surface. Exp Eye Res. 2013;116:312–23. [DOI] [PubMed] [Google Scholar]
- 88. Azmi SM, Salih M, Abdelrazeg S, Roslan FF, Mohamed R, Tan JJ, Shaharuddin B. Human umbilical cord-mesenchymal stem cells: a promising strategy for corneal epithelial regeneration. Regen Med. 2020;15(3):1381–97. [DOI] [PubMed] [Google Scholar]
- 89. Coulson-Thomas VJ, Caterson B, Kao WW. Transplantation of human umbilical mesenchymal stem cells cures the corneal defects of mucopolysaccharidosis VII mice. Stem Cells. 2013;31(10):2116–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ziaei M, Zhang J, Patel DV, McGhee CNJ. Umbilical cord stem cells in the treatment of corneal disease. Surv Ophthalmol. 2017;62(6):803–15. [DOI] [PubMed] [Google Scholar]
- 91. Jabbehdari S, Yazdanpanah G, Kanu LN, Anwar KN, Shen X, Rabiee B, Putra I, Eslani M, Rosenblatt MI, Hematti P, Djalilian AR. Reproducible derivation and expansion of corneal mesenchymal stromal cells for therapeutic applications. Transl Vis Sci Technol. 2020;9(3):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Du Y, Funderburgh ML, Mann MM, SundarRaj N, Funderburgh JL. Multipotent stem cells in human corneal stroma. Stem Cells. 2005;23(9):1266–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zhang J, Huang C, Feng Y, Li Y, Wang W. Comparison of beneficial factors for corneal wound-healing of rat mesenchymal stem cells and corneal limbal stem cells on the xenogeneic acellular corneal matrix in vitro. Mol Vis. 2012;18:161–73. [PMC free article] [PubMed] [Google Scholar]
- 94. Basu S, Hertsenberg AJ, Funderburgh ML, Burrow MK, Mann MM, Du Y, Lathrop KL, Syed-Picard FN, Adams SM, Birk DE, Funderburgh JL. Human limbal biopsy-derived stromal stem cells prevent corneal scarring. Sci Transl Med. 2014;6(266):266ra172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Eslani M, Putra I, Shen X, Hamouie J, Afsharkhamseh N, Besharat S, Rosenblatt MI, Dana R, Hematti P, Djalilian AR. Corneal mesenchymal stromal cells are directly antiangiogenic via PEDF and sFLT-1. Investig Ophthalmol Vis Sci. 2017;58(12):5507–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Murube J, Rivas L. Biopsy of the conjunctiva in dry eye patients establishes a correlation between squamous metaplasia and dry eye clinical severity. Eur J Ophthalmol. 2003;13(3):246–56. [DOI] [PubMed] [Google Scholar]
- 97. Tseng SC, Hirst LW, Maumenee AE, Kenyon KR, Sun TT, Green WR. Possible mechanisms for the loss of goblet cells in mucin-deficient disorders. Ophthalmology. 1984;91(6):545–52. [DOI] [PubMed] [Google Scholar]
- 98. Volpe EA, Henriksson JT, Wang C, Barbosa FL, Zaheer M, Zhang X, Pflugfelder SC, de Paiva CS. Interferon-gamma deficiency protects against aging-related goblet cell loss. Oncotarget. 2016;7(40):64605–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Pflugfelder SC, De Paiva CS, Moore QL, Volpe EA, Li DQ, Gumus K, Zaheer ML, Corrales RM. Aqueous tear deficiency increases conjunctival interferon-γ (IFN-γ) expression and goblet cell loss. Invest Ophthalmol Vis Sci. 2015;56(12):7545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lee MJ, Ko AY, Ko JH, Lee HJ, Kim MK, Wee WR, Khwarg SI, Oh JY. Mesenchymal stem/stromal cells protect the ocular surface by suppressing inflammation in an experimental dry eye. Mol Ther. 2015;23(1):139–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Dietrich J, Ott L, Roth M, Witt J, Geerling G, Mertsch S, Schrader S. MSC transplantation improves lacrimal gland regeneration after surgically induced dry eye disease in mice. Sci Rep. 2019;9(1):18299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Villatoro AJ, Fernández V, Claros S, Rico-Llanos GA, Becerra J, Andrades JA. Use of adipose-derived mesenchymal stem cells in keratoconjunctivitis sicca in a canine model. Biomed Res Int. 2015;2015:527926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Xian P, Hei Y, Wang R, Wang T, Yang J, Li J, Di Z, Liu Z, Baskys A, Liu W, Wu S, et al. Mesenchymal stem cell-derived exosomes as a nanotherapeutic agent for amelioration of inflammation-induced astrocyte alterations in mice. Theranostics. 2019;9(20):5956–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Samaeekia R, Rabiee B, Putra I, Shen X, Park YJ, Hematti P, Eslani M, Djalilian AR. Effect of human corneal mesenchymal stromal cell-derived exosomes on corneal epithelial wound healing. Investig Ophthalmol Vis Sci. 2018;59(12):5194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Shojaati G, Khandaker I, Funderburgh ML, Mann MM, Basu R, Stolz DB, Geary ML, Dos Santos A, Deng SX, Funderburgh JL. Mesenchymal stem cells reduce corneal fibrosis and inflammation via extracellular vesicle-mediated delivery of miRNA. Stem Cells Transl Med. 2019;8(11):1192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Shen T, Zheng QQ, Shen J, Li QS, Song XH, Luo HB, Hong CY, Yao K. Effects of adipose-derived mesenchymal stem cell exosomes on corneal stromal fibroblast viability and extracellular matrix synthesis. Chin Med J (Engl). 2018;131(6):704–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Zhou T, He C, Lai P, Yang Z, Liu Y, Xu H, Lin X, Ni B, Ju R, Yi W, Liang L, et al. miR-204-containing exosomes ameliorate GVHD-associated dry eye disease. Sci Adv. 2022;8(2):eabj9617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Wang T, Li F, Geng W, Ruan Q, Shi W. MicroRNA-122 ameliorates corneal allograft rejection through the downregulation of its target CPEB1. Cell Death Discov. 2017;3(1):17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Seo M, Choi JS, Rho CR, Joo CK, Lee SK. MicroRNA miR-466 inhibits lymphangiogenesis by targeting prospero-related homeobox 1 in the alkali burn corneal injury model. J Biomed Sci. 2015;22(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Lin D, Halilovic A, Yue P, Bellner L, Wang K, Wang L, Zhang C. Inhibition of miR-205 impairs the wound-healing process in human corneal epithelial cells by targeting KIR4.1 (KCNJ10). Investig Ophthalmol Vis Sci. 2013;54(9):6167–78. [DOI] [PubMed] [Google Scholar]