Abstract
Microsphere-based flow cytometry is a highly sensitive emerging technology for specific detection and clinical analysis of antigens, antibodies, and nucleic acids of interest. In this review, studies that focused on the application of flow cytometry as a viable alternative for the investigation of infectious diseases were analyzed. Many of the studies involve research aimed at epidemiological surveillance, vaccine candidates and early diagnosis, non-infectious diseases, specifically cancer, and emphasize the simultaneous detection of biomarkers for early diagnosis, with accurate results in a non-invasive approach. The possibility of carrying out multiplexed assays affords this technique high versatility and performance, which is evidenced in a series of clinical studies that have verified the ability to detect several molecules in low concentrations and with minimal sample volume. As such, we demonstrate that microsphere-based flow cytometry presents itself as a promising technique that can be adopted as a fundamental element in the development of new diagnostic methods for a number of diseases.
Keywords: Flow cytometry, diagnosis, infectious diseases, cancer, microspheres, immunology
Impact Statement
The development of early, sensitive, and specific diagnostic tests is of paramount importance for the control and eradication of various diseases. In recent decades, bead-based flow cytometry immunoassays have been applied for the detection of antigens, antibodies, and nucleic acids of various pathologies and as such have become a widely used method for clinical diagnosis and basic research. In addition to the ability to perform cell characterization, cytometers can also detect microspheres according to their size, shape, stability, and ability to absorb and retain fluorescent dyes, thus allowing the development of different types of flow cytometric assays, in this case, either simple or multiplex. We hope that this article contributes to the diffusion of knowledge regarding the use of this highly sensitive and specific method and that it promotes the development of new applications on several diagnostic fronts.
Introduction
Flow cytometry is a widely used, consolidated technique for clinical diagnosis that is also used in hemovigilance studies, environmental analyses, vaccine development, and standardization of clinical tests, among other applications. Initially, it was developed for cell characterization; however, the capability of this method for detecting microspheres according to their properties has allowed the expansion of its application to other areas.1 –4
The development of flow cytometry assays using microspheres has gained notoriety due to their valuable characteristics, such as high sensitivity, specificity, precision, productivity, and high yield, as well as for allowing simultaneous screening of multiple analytes in a single sample. The tests can be performed with a low sample volume and use reagents that are easily produced in the laboratory. In addition, the analyte panel can be easily altered, thus giving the test greater flexibility.3,5
Microspheres have several properties, such as a variety of sizes and shapes, stability, and the ability to absorb and retain fluorescent dyes, which are useful characteristics in various types of flow cytometry analyses, that is, simple or multiplexed. Another important feature of the diagnosis is the presence of functional groups (amino or carboxyl) in the microspheres, which allow non-covalent and covalent bonds on their surfaces through molecular interactions, and enable chemical coupling or adsorption of proteins, oligonucleotides, polysaccharides, lipids, or peptides.2,4,5
Over the past 25 years, several types of microspheres have been developed for use in diagnostic methods using flow cytometry. They can be easily customized by the researchers according to the research objective, or previously developed commercially available microspheres may be used. 5
In this review, we aim to present the current panorama of the application of microspheres in flow cytometry for the detection of diseases, such as infectious diseases (bacterial, viral, and parasitic) and non-infectious diseases, and emphasize the different methodologies used and flow cytometry’s effectiveness in sensitive and specific diagnoses.
Principle and mechanism of cytometry bead-based assays
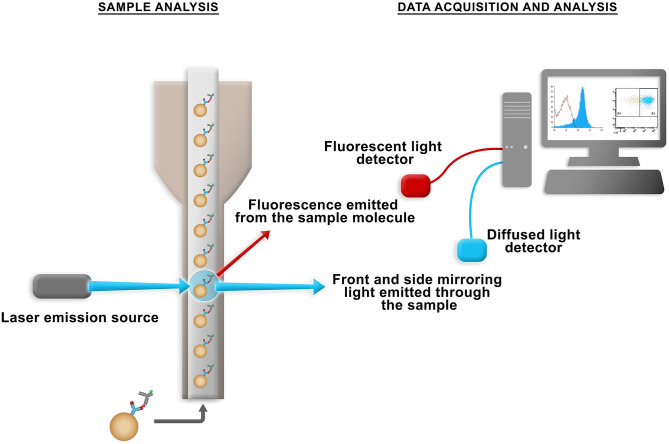
The flow cytometer can simultaneously analyze several parameters of biological and non-biological particles in suspension. In its operation, the flow system aligns the particles so that they are intercepted one by one by lasers. Then, the emission of light and fluorescence from these particles is detected by the optical system that is formed by filters and mirrors. The data obtained are interpreted by the electronic system so that they can be analyzed graphically (Figure 1).1 –3
Figure 1.
Flow chamber where the suspension is aspirated for a mirror and photosensor system and directed to a light capture system, where the computer system later converts it into digital data for analysis. (A color version of this figure is available in the online journal.)
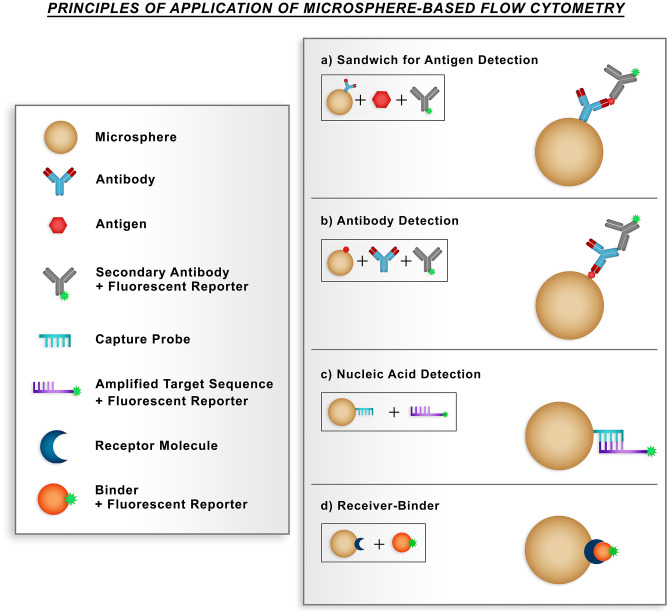
Most applications of beads in flow cytometry occur in the form of immunoassays for protein determination, mainly sandwich and competitive assays, in which the capture antibody coupled to the microspheres allows the binding of the antigen and this enables the binding of the detection antibody labeled with a fluorescent reporter (Figure 2(a)). Antibody detection is also possible using microspheres with a specific antigen attached and also allows the binding of detection antibodies that are labeled with a fluorescent reporter (Figure 2(b)).6,7
Figure 2.
Main applications of flow cytometry immunoassays based on microspheres. In each type of assay, flow cytometry analysis identifies the population of the microspheres and the signal of the fluorescent molecule. (a) Detection of antigen by the sandwich method, in which the capture antibody coupled to microsphere allows the binding of the antigen, thus allowing the binding of the detection antibody marked with a fluorescent reporter. (b) Detection of antibodies using microspheres with a coupled specific antigen, thus allowing the binding of detection antibodies labeled with a fluorescent reporter. (c) In the detection of nucleic acids, a capture probe immobilized on the surface of microspheres binds to an amplified product that has sequences complementary to the probe. (d) The microspheres coupled to the receptor molecule allow interaction with binding molecules associated with the fluorescent reporter. (A color version of this figure is available in the online journal.)
The beads are also used in receptor-ligand assays, which is a method that has already been consolidated, and the microspheres coupled to the receptor molecule allow interactions with ligand molecules associated with the fluorescent reporter. Combined with the high-throughput flow cytometry technique, the application of microspheres for the detection of nucleic acids has gained prominence in recent years due to their ability to identify different types of microorganisms at the molecular level. For nucleic acid detection, a capture probe immobilized on the surface of a population of microspheres binds to an amplified product that has sequences complementary to the probe (Figure 2(c) and (d)).6,7
Diagnostic applications
Infectious diseases
Bacterial, viral, and parasitic diseases are serious public health problems and demand constant actions for their prevention, diagnosis, and treatment. The detection of microorganisms and their biomarkers in a quick, accurate, and low-cost way is still a challenge for research centers and epidemiological surveillance initiatives. 1 The application of bead-based flow cytometry for the detection and identification of infectious diseases has been used in immunoassays, with the simultaneous analysis of diverse pathogens being the object of the development of new diagnostic methods. 5 Several examples are shown in Table 1.
Table 1.
Multiplexed assays for infectious agents.
| Microorganisms detected simultaneously | Test principle | References |
|---|---|---|
| Streptococcus pneumoniae, Moraxella catarrhalis, Staphylococcus aureus, Streptococcus pyogenes, Haemophilus influenza, Mycoplasma pneumoniae, Legionella spp., Pseudomonas aeruginosa, and Klebsiella pneumoniae | PCR-Luminex Multiplex method | Jiang et al. 8 |
| Mycobacterium tuberculosis, Cryptococcus neoformans, Streptococcus pneumoniae, and herpes simplex virus types 1 and 2 | PCR-Luminex Multiplex method | Zhou et al. 9 |
| Adenovirus, rotavirus, norovirus, Salmonella spp., Campylobacter spp., Shigella spp., Clostridium difficile, Escherichia coli, Yersinia enterocolitica, Vibrio cholerae, Giardia lamblia, Entamoeba histolytica, and Cryptosporidium spp. | PCR-Luminex Multiplex method | Claas et al. 10 |
| Shigella spp., Staphylococcus aureus, Vibrio cholerae, Legionella pneumophila, Clostridium botulinum | PCR-Luminex Multiplex method | Zhao et al. 11 |
| Human papillomavirus, Helicobacter pylori, hepatitis C virus, and Poliomavirus JC | Luminex immunoassay | Waterboer et al. 12 |
| Norovirus GI and GII, rotavirus, astrovirus, sapovirus, and adenovirus | PCR-Luminex Multiplex method | Liu et al. 13 |
| Cryptosporidium spp., Giardia intestinalis, Entamoeba histolytica, Ancylostoma duodenale, Ascaris lumbricoides, Necator americanus, and Strongyloides stercoralis | PCR-Luminex Multiplex method | Taniuchi et al. 14 |
| Toxoplasma gondii, rubella, and cytomegalovirus | Luminex immunoassay | Binnicker et al. 15 |
Bacterial infections
The development of precise, sensitive, and reliable methods for the identification of bacteria, antigens, and related antibodies is one of the most advantageous strategies in the areas of research, diagnosis, and epidemiological surveillance of bacterial infections.
Among the modern methods in common use today, flow cytometry stands out as a reliable technique for making the analysis of bacterial cells possible under individual and populational conditions. The evolution of the processes and devices used has allowed the improvement of viable and non-viable bacterial cell counts, with the provision of multiparametric cellular information that would be impossible to obtain with the traditional plate counting method.16 –18
Different studies have demonstrated the possibility of applying flow cytometry in bacterial tests, and the use of spheres and the multiplexing of tests are ways of expanding the spectrum of use of this technique. In addition to antigen–antibody interactions and bacterial detection, there are variations in the technique that are aimed at studying receptor–ligand interactions, enzyme–substrate interactions, as well as the detection of nucleic acids with special hybridization probes.6,7 As an example of this approach, the study by Zeng et al. 19 combined a flow cytometry system with spheres and oligonucleotide probes directed to bacterial RNA.
In a study by Ou et al., 18 a sphere-based flow cytometry method was evaluated and optimized to quantify variable proportions of live and dead Escherichia coli in basic flow cytometers. Plate counting methods were performed simultaneously with flow cytometry measurements in order to obtain the concentration of live bacteria in the final samples. The results obtained were significant and evidenced a linear relationship between the two methods of counting live and dead bacteria, with results ranging from 108 to c. 104 bacteria per milliliter. In addition, the study demonstrated that flow cytometry can be applied in the analysis of other bacterial species.
The detection of bacterial cells can also be performed using spherical flow cytometry combined with molecular biology techniques. An example that can be cited is the detection of Neisseria meningitidis in cerebrospinal fluid using a multiplex PCR system and Luminex detection technology. 20 Another example is an innovative method for detecting Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa, which was developed using a combination of oligonucleotide probes targeting 16S rRNA and a flow cytometry system using spheres. The detection limit reached by the test was 180 CFU/mL and showed great potential for the simultaneous analysis of more than one bacterial species. 19
A significant portion of the studies on bacterial infections has focused efforts on the identification and quantification of staphylococcal antigens and IgG, IgM, and IgA antibodies against these antigens in order to develop new diagnostic and seroepidemiological methods.21,22
Verkaik et al. 21 characterized the heterogeneity of the humoral immune response by S. aureus and, in order to detect an antigen with the potential to act in vaccines, developed a multiplexed cytometry assay based on spheres with 19 staphylococcal antigens to simultaneously detect the IgG, IgA, and IgM levels. The results showed that each patient had a unique immune response and, therefore, vaccines with multiple components are preferable. Den Reijer et al. 22 used the same methodology as in the previous study and characterized the serum levels of IgG and IgA antibodies against 56 staphylococcal antigens. Their results showed heterogeneity in the immune response to the tested staphylococcal antigens.
For the identification of staphylococcal antigens, Simonova et al. 23 developed a multiplex assay with microspheres to detect three staphylococcal toxins, namely enterotoxins A and B and toxic shock syndrome toxin, which were obtained from supernatants from cultures of different strains of S. aureus. The detection limits were 10, 1000, and 5 pg/mL, respectively. The high level of effectiveness of the test showed that it can be used for clinical diagnosis and environmental and food analyses. Sharma et al. 24 presented a two-color multiplex flow cytometry assay based on spheres, which used unique protein domains of the beta chain of T-cell receptors with a high affinity for three staphylococcal and two streptococcal toxins. The level of sensitivity achieved by the tests was between 4 and 80 times greater when compared to the enzyme-linked immunosorbent assay (ELISA) and had the advantage of using fewer reagents and a smaller sample volume.
Experimental studies involving pneumococcal antigens have also been the object of analysis by several research groups, mainly to determine antigens that could be vaccine candidates against pneumococci and to monitor their effectiveness. Some studies have applied multiplex flow cytometry immunoassays based on spheres against pneumococcal antigens.25 –27 The same multiplex technology for the detection of antibodies against the proteins Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis was used to monitor humoral responses in patients up to 13 years old, 27 as well as children with pneumonia acquired in the community. 25 An automated assay with a combination of multiplex tests based on monoclonal antibodies and PCR was developed using Luminex’s sphere-based flow cytometry technology to perform serotyping of pneumococcal isolates. 28
Viral infections
Every year, viral infections affect millions of people worldwide. New sensitive and specific diagnostic methods are indispensable tools for reducing the number of cases. In the last few decades, bead-based flow cytometry assays have emerged as a viable alternative for the diagnosis of several viral diseases.1,29
Recently, there has been a significant increase in the number of studies related to virometry, a promising and highly sensitive technology that is capable of detecting and classifying viral particles with the aid of specific fluorescent antibodies coupled to spheres. This complex is analyzed using flow cytometers and allows the quantification and phenotypic characterization of unique viral particles in supernatants, body fluids, or tissues.30 –32 The detection of viruses using flow cytometry is possible due to the optimization of commercial flow cytometers, which have advanced lasers and an improved digital and optical focus system, and provide the classification based on the arrangements between the light scattering parameters and the fluorescent channels.33 –35
For the diagnosis of HIV, several approaches using flow cytometry are being developed. 32 A new diagnostic method with the potential to simplify HIV monitoring has been developed by Yufenyuy and Parekh 36 from a multiplex assay with beads coupled to an HIV-1 p24-gp41 fusion protein and HIV-2 peptide from the immunodominant region of the gp36 glycoprotein to detect HIV and HIV-2 serotyping, respectively. The results have shown high reproducibility and the ability to diagnose HIV infection, perform serotyping, and detect and distinguish between recent and long-term infections in a single test. 36 Tests have also been developed for the detection of the p24 protein in various subtypes of HIV-1.37,38 The results have shown that the method is 91% more sensitive than the standard ELISA test and also has a lower cost. 37
Fonseca et al. 39 developed a new multiplex, bead-based assay for the detection of hepatitis C virus (HCV), using the NS3, NS4, and NS5 recombinant antigens from HCV, individually in the simplex assay and in a combination of them in the multiplex assay. In this study, 100% sensitivity and specificity were observed, which shows that the method has the potential to become a possible alternative for the diagnosis of HCV, while having a cost that is two to four times lower than the tests usually employed. Neves et al. 40 also performed a flow cytometry test using magnetic beads capable of detecting human IgG against HCV Core and NS5a proteins. The sensitivity of this immunoassay was 93.3% when using beads with the recombinant proteins of Core and NS5a, and it showed a specificity of 100% and 96.67% for the detection of anti-rCore and anti-rctNS5a, respectively. Another alternative use of flow cytometry for HCV diagnosis was developed by Yang et al., 41 which involved a multiplex genotyping assay to determine the six types of HCV genotypes. A matrix of suspended beads was analyzed using flow cytometry and demonstrated the reliability, speed, and efficiency of the analysis. The main advantage shown was the low sample volume required for its execution.
Based on the advantages of multiplexed analysis, a new test for the subtyping of the avian influenza virus (AIV) was developed. This new method can be explored for the rapid determination of immune responses to AIV in a variety of complex systems. The sensitivity of the test allows the detection of nucleic acids from the AIV strains H5N1 and H9N2 at a threshold of 74 and 1 pg, respectively. 42
Parasitic infections
Despite important advances in the control of parasitic infections, many cases still occur worldwide. Given this scenario, several research fronts are looking for new diagnostic methods with high sensitivity and specificity to diagnose semi-immune patients and with low parasitemia. 43 Flow cytometry has been widely used for studies related to malaria, 44 but only in the last 10 years have studies focused on the etiological diagnosis gained strength and, as a result, multiplexed assays combined with spheres have emerged as a reliable alternative for malaria serology. 45
The standardization and validation of a cytometric bead array (CBA) of antibodies against Plasmodium falciparum antigens has proved to be a test with the potential to diagnose malaria and provides specific advantages when compared to ELISA. These include sample conservation, the ability to detect various analytes in a single test, the decrease in sample processing time due to the test kinetics, and the decrease in costs. 46
In a study carried out in endemic regions of Brazil and Papua New Guinea, which aimed to assess naturally acquired humoral responses, the multiplex assay was validated for quantifying IgG antibodies against Plasmodium vivax merozoite surface protein 1. 47 For the detection of antibodies of five candidates for the vaccine for P. falciparum, a comparative CBA was performed with magnetic and non-magnetic spheres and showed that the test with magnetic spheres is a viable alternative in the serology of antibodies against malaria. 45
A new immunoassay capable of detecting histidine-rich protein 2 (HRP2), an important marker of infections by P. falciparum at sub-picogram levels, was developed using a sphere-based flow cytometry system. The test presented is highly specific and economical and allows the processing and screening of a significant number of samples. 48 The system was used to evaluate the results of rapid diagnostic tests for HRP2 under different transmission conditions of P. falciparum and generated estimates for true performance in the field. 48 This system has also been adopted to assess the concentration of HRP2 in patients after treatment, 49 in asymptomatic individuals with malaria, 50 in order to be able to attribute fever to malaria, 51 for PfHRP2 and PfHRP3 gene deletion research 52 and in serosurveillance studies. 53
An innovative test was proposed by Lloyd et al., 54 in which a simple high-throughput flow cytometric assay of THP-1 cells and fluorescent spheres covalently coupled to the VAR2CSA malaria antigen provides the total percentage of antibody-mediated phagocytosis and semi-quantifies the number of internalized antigen-coupled spheres. It has great advantages due to the stability of the spheres for long periods, the need for a small amount of coupled antigen, as well as the fact that it does not require parasite cultures.
Other applications of CBA for other parasitic infections have also been developed, such as the multiplex assay for the identification of IgM and IgG antibodies against Toxaplasma gondii. The test proved to be faster and have a high-throughput for the analysis; however, more studies still need to be carried out to validate the results. 15 The performance of this immunoassay was evaluated in serodiagnosis of critical cases of toxoplasmosis by Guigue et al. 55 and demonstrated that the method is sufficiently sensitive (97.8%) and specific (91.3%) for the detection of IgG and is highly specific (97.4%) for IgM antibodies against T. gondii.
Intestinal parasites are also the subject of much research, mainly in relation to new diagnostic approaches. It is common for intestinal parasites to be caused by more than one species of parasite; therefore, multiplex assays that detect more than one pathogen in a single test are important for epidemiological and serosurveillance studies. 14 A study using the combination of multiplex PCR and probe-based detection with Luminex beads was developed to identify seven intestinal parasites, namely, Cryptosporidium spp., Giardia intestinalis, Entamoeba histolytica, Ancylostoma duodenale, Ascaris lumbricoides, Necator americanus, and Strongyloides stercoralis. The results showed high sensitivity and specificity, and indicated that the test performs well in the detection of several intestinal parasites. 14
Cancer
In this topic, we will address the use of sphere-based flow cytometry for the detection and identification of non-infectious diseases. This technology has been widely applied for the diagnosis of serious diseases, such as cancer, immune and cerebrovascular diseases, and permits detection in the early stages of development, in addition to being more economically attractive when compared to traditional diagnostic methods. Moreover, the processes involved are less invasive and have the advantage that they do not require a large amount of sample in order to be carried out.
Cancer is a disease that has high rates of morbidity and mortality worldwide. The analysis of diagnostic or prognostic biomarkers is the subject of several research fronts that seek to establish effective methods for the identification of the disease in its early stages, thus increasing the likelihood of successful treatment. Sphere-based multiplexed assays are considered a valuable tool for the quick and simultaneous identification of different types of tumor biomarkers such as colorectal, breast, ovary, lung, pancreas, and melanoma biomarkers.3,56
Breast cancer
As in all forms of cancer, success in the treatment of breast cancer is related to the early diagnosis of the disease since it allows surgical resection to occur at an early stage when the patients’ chances of survival are higher. Breast cancer has a heterogeneous behavior and the use of more than one biomarker is appropriate for its identification. 3
The diagnostic performance of a multiplex cancer panel test was evaluated by Hermann et al. 57 Twenty-four tumor-associated parameters were analyzed in sera of breast cancer patients and healthy controls. The authors reported a sensitivity of 33.8% at 95% specificity for discrimination between benign and malignant breast cancer tumors. Cancer antigen 15-3 was the most relevant for differential diagnosis.
Opstal-van Winden et al. 58 developed a bead-based multiplex immunoassay to simultaneously evaluate 10 serum breast cancer biomarkers in the early stages of development. The results of this study showed that the panel of biomarkers used was of no value for early diagnosis; however, it was recommended that combinations of other proteins could present more satisfactory results.
Markou et al. 59 developed a multiplexed PCR-coupled liquid bead array, which was carried out using CTC-mRNA. Biotinylated PCR products were hybridized against fluorescent microspheres modified with gene-specific capture probes and developed with streptavidin-phycoerythrin, which were analyzed using Luminex flow cytometry. This molecular assay proved to be specific for each gene and provided the simultaneous molecular detection of six CTC genes in a limited sample volume, which decreased the cost and time taken for its execution when compared to RT-qPCR. The test can be optimized for the simultaneous detection of up to 100 genes.
Pancreatic cancer
Tests with high sensitivity and precision have been proposed for the diagnosis of pancreatic cancer, which is one of the most aggressive forms of cancer and has high mortality rates and which has no accepted tests for early detection based on the analysis of blood samples. 60 One of the studies carried out for the initial diagnosis of pancreatic cancer was executed by Li et al., 61 who performed a sandwich multiplex assay to determine the patterns of glycosylation of lecithins in the serum of patients with this type of tumor. The authors identified a biomarker that is capable of distinguishing patients with chronic pancreatitis from those with pancreatic cancer.
Using a different approach, Lux et al. 60 sought to identify exosomes released by pancreatic carcinomas that express c-MET and PD-L1 in their membranes. The exosomes were isolated from cultures, coupled to spheres, stained with specific antibodies, and analyzed using flow cytometry. It was shown in this study that PD-L1 has no diagnostic value for pancreatic tumors, but c-MET obtained a sensitivity of 70% and specificity of 85%. c-MET was suggested for a future study with the aim of improving specificity, and the possible combination of the test with an established specific antigen, as well as performing the research with a larger number of patients.
Lung cancer
In 2018, lung cancer was responsible for 1.8 million deaths worldwide. 62 Considered the most common form of cancer, its early diagnosis is crucial; however, most patients are diagnosed late. Aiming at the early detection of the most common type of lung cancer, that of non-small cells, a new cytometric immunoassay based on multiplexed beads was developed by Lee et al. 63 for the characterization of serum biomarkers related to the disease. From the analysis of a multiplexed matrix of 30 serum markers and with the use of a classification algorithm, it was established that the detection of 5 biomarkers (A1AT, CYFRA 21-1, IGF-1, RANTES, AFP) permitted a high-precision diagnosis, which allowed for the distinction between healthy patients and patients with lung cancer.
Another study, carried out by Goebel et al., 64 applied the same methodology for the diagnosis of asymptomatic cancer patients; however, the analysis was performed with 82 biomarkers and, of these, 33 showed diagnostic value for the initial stage of the disease. The method proved to be 90% precise, with 80% sensitivity and 95% specificity. As a continuation of this study, in order to reduce the number of biomarkers used and still maintain the high sensitivity, precision, and specificity of the test, 21 biomarkers were validated with a statistical model (Lung Cancer Detector Test 1), and presented 95.6% precision, 89.1% sensitivity, and 97.7% specificity. In addition, the authors also achieved a decrease in the cost of the procedure. 65
Inflammatory syndrome
Flow cytometry bead-based assays have been applied to the diagnosis of many other diseases. Von Bahr Greenwood et al. 66 applied this technique for the detection of CD25 in serum samples of patients and associated high levels of the molecule and ferritin with secondary (acquired) hemophagocytic lymphohistiocytosis (sHLH).
In a similar way, using CBA, Maruoka et al. 67 identified interferon-inducible protein 10 (IP-10)/CXCL10 and monokine induced by interferon gamma (MIG)/CXCL9 as useful markers for lymphoma-associated hemophagocytic syndrome (LAHS) diagnosis. The authors also suggested the use of this method for therapeutic, LAHS severity, and distinguishing of sepsis.
With cytometric bead assay techniques, Chen et al. 68 analyzed whether the Th1/Th2 cytokine profile could be used to distinguish primary and secondary HLH. The authors reported that lower interleukin (IL)-4 and interferon (IFN)-γ levels in HLH patients are associated with primary HLH, thus demonstrating that CBA can be used as additional tool for differential HLH diagnosis.
Vascular endothelial growth factor (VEGF) measurement using CBA was performed by Maier et al. 69 in diabetic and non-diabetic patients, with reports of an elevation of this marker in vitro. This technique could be applied for polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, and skin abnormalities (POEMS) syndrome, which is a rare multisystem paraneoplastic disease with plasma cell dyscrasia, 70 since the rise in circulating VEGF levels have been reported in patients with POEMS.71 –73 It could also probably be useful in the follow-up of patients with POEMS syndrome as a biomarker of response to treatment.73 –75
Measuring of the IL-10 and IL-10:IL-6 ratio using CBA has also been useful for the diagnostic and prognostic values of the cerebrospinal fluid (CSF) in patients with diffuse large B-cell type (DLBCL) primary central nervous system lymphoma (PCNSL). 76 In the same way, this technique has been used for monitoring of the variation curve of multiple cytokines in cytokine release syndrome (CRS) patients’ peripheral blood samples post chimeric antigen receptor-T cell (CAR-T) therapy. Nguyen-Them et al. 77 reported that IL-10 level detection is a useful tool for the diagnosis of PCNSL.
Allergic reactions
Allergies represent the most prevalent non-infectious diseases worldwide, especially food allergies, which have been increasing in recent decades. Thus, the development of tools for monitoring food allergens is necessary to ensure consumer safety. Several studies have verified the efficiency of the use of beads coupled with antigens to verify the presence of the allergen or for the detection of IgE antibodies. Since the availability of allergenic molecules and high-throughput microtechnologies allow the collection of a large number of IgE results, it can thus be performed and applied in the diagnostic routine.78,79 This type of approach is possible thanks to the use of natural or recombinant components, either using a single reagent for each specimen (singleplex) or through a more complex panel of molecules to be tested in the same test, and can be revealed by flow cytometry. 80
The ability to detect multiple food allergens in different types of food has been evaluated over time and has resulted in the implementation of more sensitive, faster, and lower-cost methods such as the use of beads and cytometry. Gomaa and Boye 81 compared ELISA, liquid chromatography coupled mass spectrometry, and multiplex flow cytometry methods for the detection of multiple allergens incurred simultaneously in a model food system where flow cytometry was performed similarly to ELISA tests. In addition, Pomponi et al. 82 reported characteristics for the implementation of immunoassays based on beads and cytometry, and it was possible to observe good reproducibility of the sensitivity in an assay for IgE detection in a multiplex system using allergenic molecules and fluorescent beads that were analyzed using flow cytometry. 82
The simultaneous detection in different food sources was shown to be possible by Otto et al. 83 who reported the detection of five allergens with median inhibitory concentrations (IC50) that ranged from 2.5 to 15 mg/kg according to the allergen to be detected. Meimaridou et al. 84 developed a flow cytometry-based immunoassay using color-coded microsphere technology to detect benzo[a]pyrene and other polycyclic aromatic hydrocarbons in buffers and food extracts, while Zhou et al. 85 observed IgE-mediated hypersensitivity to polyethylene glycol and the risk of anaphylaxis from the consumption of this product. These results reinforce the importance of screening and identification systems for known or even unknown emerging food allergens.
Clinical hypersensitivity reactions against l-asparaginase have been reported during the treatment of acute lymphoblastic leukemia in up to 70% of patients. Using CBA, Rathod et al. 86 reported the detection of anti-asparaginase IgG1 and IgE antibodies in mice immunized with asparaginase, which presented severe hypersensitivity reactions after being challenged with asparaginase. The authors highlighted the importance of monitoring these antibodies in patients, and that bead-based assays are a viable alternative for this.
Conclusions
In recent years, flow cytometry has been enhanced with the application of encoded microspheres and with the improvement of the equipment used, thus making it possible to carry out multiplexed immunoassays aimed at detecting bacterial, viral, and parasitic infections, in addition to non-infectious diseases. Given the extremely positive results highlighted by this review, which has demonstrated the high sensitivity and specificity of the technique, microsphere-based flow cytometry has the potential to figure as a central axis of new approaches for research and diagnosis. This technology presents itself as a viable and promising alternative that, although it has not yet been applied on a large scale, has the necessary characteristics for this task, mainly in research and diagnostic centers that already work with cytometers.
Acknowledgments
We thank the Instituto Leonidas e Maria Deane – ILMD/Fiocruz Amazonia, the Postgraduate Program in Biotechnology – PPGBIOTEC at the Universidade Federal do Amazonas – UFAM, and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES for funding the scholarship.
Footnotes
Authors’ Contributions: All authors participated in the design and review of the manuscript. AMdF, LAMM, JCG, YOC, and WLLN performed the literature review, and AMdF wrote the manuscript and provided the images.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research received funding from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES and Fundação Oswaldo Cruz – FIOCRUZ.
ORCID iDs: Adélia Marques de Figueiredo  https://orcid.org/0000-0002-9313-3966
https://orcid.org/0000-0002-9313-3966
Juliane Corrêa Glória  https://orcid.org/0000-0002-9122-1145
https://orcid.org/0000-0002-9122-1145
Luis André Morais Mariúba  https://orcid.org/0000-0003-4210-6367
https://orcid.org/0000-0003-4210-6367
References
- 1. Engin ED. The use of multiplexing technology in the immunodiagnosis of infectious agents. J Immunoassay Immunochem 2019;40:109–22 [DOI] [PubMed] [Google Scholar]
- 2. Kellar KL, Iannone MA. Multiplexed microsphere-based flow cytometric assays. Exp Hematol 2002;30:1227–37 [DOI] [PubMed] [Google Scholar]
- 3. Parsa SF, Vafajoo A, Rostami A, Salarian R, Rabiee M, Rabiee N, Rabiee G, Tahriri M, Yadegari A, Vashaee D, Tayebi L, Hamblin MR. Early diagnosis of disease using microbead array technology: a review. Anal Chim Acta 2018;1032:1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Morgan E, Varro R, Sepulveda H, Ember JA, Apgar J, Wilson J, Lowe L, Chen R, Shivraj L, Agadir A, Campos R, Ernst D, Gaur A. Cytometric bead array: a multiplexed assay platform with applications in various areas of biology. Clin Immunol 2004;110:252–66 [DOI] [PubMed] [Google Scholar]
- 5. Graham H, Chandler DJ, Dunbar SA. The genesis and evolution of bead-based multiplexing. Methods 2019;158:2–11 [DOI] [PubMed] [Google Scholar]
- 6. Antal-Szalmás P, Nagy B, Jr, Debreceni IB, Kappelmayer J. Measurement of soluble biomarkers by flow cytometry. EJIFCC 2013;23:135–42 [PMC free article] [PubMed] [Google Scholar]
- 7. Ou F, McGoverin C, White J, Swift S, Vanholsbeeck F. Bead-based flow-cytometric cell counting of live and dead bacteria. Methods Mol Biol 2019;1968:123–34 [DOI] [PubMed] [Google Scholar]
- 8. Jiang L, Ren H, Zhou H, Qin T, Chen Y. Simultaneous detection of nine key bacterial respiratory pathogens using Luminex xTAG® technology. Int J Environ Res Public Health 2017;14:223–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou L, Wu R, Shi X, Feng D, Feng G, Yang Y, Dai W, Bian T, Liu T, He Y, Shi M, Zhao G. Simultaneous detection of five pathogens from cerebrospinal fluid specimens using luminex technology. Int J Environ Res Public Health 2016;13:193–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Claas EC, Burnham CA, Mazzulli T, Templeton K, Topin F. Performance of the xTAG® gastrointestinal pathogen panel, a multiplex molecular assay for simultaneous detection of bacterial, viral, and parasitic causes of infectious gastroenteritis. J Microbiol Biotechnol 2013;23:1041–5 [DOI] [PubMed] [Google Scholar]
- 11. Zhao J, Kang L, Hu R, Gao S, Xin W, Chen W, Wang J. Rapid oligonucleotide suspension array-based multiplex detection of bacterial pathogens. Foodborne Pathog Dis 2013;10:896–903 [DOI] [PubMed] [Google Scholar]
- 12. Waterboer T, Dondog B, Michael KM, Michel A, Schmitt M, Vaccarella S, Franceschi S, Clifford G, Pawlita M. Dried blood spot samples for seroepidemiology of infections with human papillomaviruses, Helicobacter pylori, Hepatitis C Virus, and JC Virus. Cancer Epidemiol Biomarkers Prev 2012;21:287–93 [DOI] [PubMed] [Google Scholar]
- 13. Liu J, Kibiki G, Maro V, Maro A, Kumburu H, Swai N, Taniuchi M, Gratz J, Toney D, Kang G, Houpt E. Multiplex reverse transcription PCR Luminex assay for detection and quantitation of viral agents of gastroenteritis. J Clin Virol 2011;50:308–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Taniuchi M, Verweij JJ, Noor Z, Sobuz SU, Lieshout Lv, Petri WA, Jr, Haque R, Houpt ER. High throughput multiplex PCR and probe-based detection with Luminex beads for seven intestinal parasites. Am J Trop Med Hyg 2011;84:332–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Binnicker MJ, Jespersen DJ, Harring JA. Multiplex detection of IgM and IgG class antibodies to Toxoplasma gondii, rubella virus, and cytomegalovirus using a novel multiplex flow immunoassay. Clin Vaccine Immunol 2010;17:1734–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Buzatu DA, Moskal TJ, Williams AJ, Cooper WM, Mattes WB, Wilkes JG. An integrated flow cytometry-based system for real-time, high sensitivity bacterial detection and identification. PLoS ONE 2014;9:e94254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hildebrandt P, Surmann K, Salazar MG, Normann N, Völker U, Schmidt F. Alternative fluorescent labeling strategies for characterizing gram-positive pathogenic bacteria: flow cytometry supported counting, sorting, and proteome analysis of Staphylococcus aureus retrieved from infected host cells. Cytometry A 2016;89:932–40 [DOI] [PubMed] [Google Scholar]
- 18. Ou F, McGoverin C, Swift S, Vanholsbeeck F. Absolute bacterial cell enumeration using flow cytometry. J Appl Microbiol 2017;123:464–77 [DOI] [PubMed] [Google Scholar]
- 19. Zeng Y, Zhang D, Qi P. Combination of a flow cytometric bead system with 16S rRNA-targeted oligonucleotide probes for bacteria detection. Anal Bioanal Chem 2019;411:2161–8 [DOI] [PubMed] [Google Scholar]
- 20. Miller F, Lécuyer H, Join-Lambert O, Bourdoulous S, Marullo S, Nassif X, Coureuil M. Neisseria meningitidis colonization of the brain endothelium and cerebrospinal fluid invasion. Cell Microbiol 2013;15:512–9 [DOI] [PubMed] [Google Scholar]
- 21. Verkaik NJ, Boelens HA, de Vogel CP, Tavakol M, Bode LG, Verbrugh HA, van Belkum A, van Wamel WJ. Heterogeneity of the humoral immune response following Staphylococcus aureus bacteremia. Eur J Clin Microbiol Infect Dis 2010;29:509–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Den Reijer PM, Lemmens-den Toom N, Kant S, Snijders SV, Boelens H, Tavakol M, Verkaik NJ, van Belkum A, Verbrugh HA, van Wamel WJ. Characterization of the humoral immune response during Staphylococcus aureus bacteremia and global gene expression by Staphylococcus aureus in human blood. PLoS ONE 2013;8:e53391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Simonova MA, Petrova EE, Dmitrenko OA, Komaleva RL, Shoshina NS, Samokhvalova LV, Valyakina TI, Grishin EV. xMAP-based analysis of three most prevalent staphylococcal toxins in Staphylococcus aureus cultures. Anal Bioanal Chem 2014;406:6447–52 [DOI] [PubMed] [Google Scholar]
- 24. Sharma P, Wang N, Chervin AS, Quinn CL, Stone JD, Kranz DM. A Multiplex assay for detection of staphylococcal and streptococcal exotoxins. PLoS ONE 2015;10:e0135986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Borges IC, Andrade DC, Vilas-Boas AL, Fontoura MS, Laitinen H, Ekström N, Adrian PV, Meinke A, Cardoso MR, Barral A, Ruuskanen O, Käyhty H, Nascimento-Carvalho CM. Detection of antibody responses against Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis proteins in children with community-acquired pneumonia: effects of combining pneumococcal antigens, pre-existing antibody levels, sampling interval, age, and duration of illness. Eur J Clin Microbiol Infect Dis 2015;34:1551–7 [DOI] [PubMed] [Google Scholar]
- 26. Andrade DC, Borges IC, Laitinen H, Ekström N, Adrian PV, Meinke A, Barral A, Nascimento-Carvalho CM, Käyhty H. A fluorescent multiplexed bead-based immunoassay (FMIA) for quantitation of IgG against Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis protein antigens. J Immunol Methods 2014;405:130–43 [DOI] [PubMed] [Google Scholar]
- 27. Borges IC, Andrade DC, Cardoso MR, Toppari J, Vähä-Mäkilä M, Ilonen J, Knip M, Hyöty H, Veijola R, Simell O, Jartti T, Käyhty H, Ruuskanen O, Nascimento-Carvalho CM. Natural development of antibodies against Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis protein antigens during the first 13 years of life. Clin Vaccine Immunol 2016;23:878–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yu J, Lin J, Kim KH, Benjamin WH, Jr, Nahm MH. Development of an automated and multiplexed serotyping assay for Streptococcus pneumoniae. Clin Vaccine Immunol 2011;18:1900–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Filomena A, Pessler F, Akmatov M, Krause G, Duffy D, Gärtner B, Gerhard M, Albert ML, Joos TO, Schneiderhan-Marra N. Development of a bead-based multiplex assay for the analysis of the serological response against the six pathogens HAV, HBV, HCV, CMV, T. gondii, and H. pylori. High-Throughput 2017;6:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lippé R. Flow virometry: a powerful tool to functionally characterize viruses. J Virol 2017;92:e01765–10117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Musich T, Jones JC, Keele BF, Jenkins LMM, Demberg T, Uldrick TS, Yarchoan R, Robert-Guroff M. Flow virometric sorting and analysis of HIV quasispecies from plasma. JCI Insight 2017;2:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Niessl J, Baxter AE, Kaufmann DE. Tools for visualizing HIV in cure research. Curr HIV/AIDS Rep 2018;15:39–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gaudin R, Barteneva NS. Sorting of small infectious virus particles by flow virometry reveals distinct infectivity profiles. Nat Commun 2015; 6:6022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zamora JLR, Aguilar HC. Flow virometry as a tool to study viruses. Methods 2018;134–5:87–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Arakelyan A, Fitzgerald W, Margolis L, Grivel JC. Nanoparticle-based flow virometry for the analysis of individual virions. J Clin Invest 2013; 123:3716–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yufenyuy EL, Parekh BS. Development of a multiplex assay for concurrent diagnoses and detection of HIV-1, HIV-2, and recent HIV-1 infection in a single test. AIDS Res Hum Retroviruses 2018;34:1017–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Merbah M, Onkar S, Grivel JC, Vanpouille C, Biancotto A, Bonar L, Sanders-Buell E, Kijak G, Michael N, Robb M, Kim JH, Tovanabutra S, Chenine AL. Standardization of a cytometric p24-capture bead-assay for the detection of main HIV-1 subtypes. J Virol Methods 2016;230:45–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Biancotto A, Brichacek B, Chen SS, Fitzgerald W, Lisco A, Vanpouille C, Margolis L, Grivel JC. A highly sensitive and dynamic immunofluorescent cytometric bead assay for the detection of HIV-1 p24. J Virol Methods 2009;157:98–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fonseca BP, Marques CF, Nascimento LD, Mello MB, Silva LB, Rubim NM, Foti L, Silva ED, Ferreira AG, Krieger MA. Development of a multiplex bead-based assay for detection of hepatitis C virus. Clin Vaccine Immunol 2011;18:802–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Neves WLL, Mariuba LAM, Alves KCS, Coelho KF, Tarragô AM, Costa AG, Chaves YO, Victoria FS, Victoria MB, Malheiro A. Development of an immunoassay for the detection of human IgG against hepatitis C virus proteins using magnetic beads and flow cytometry. Biotechnol Biotechnol Equip 2021;35:103–10 [Google Scholar]
- 41. Yang CM, Yoon JC, Park JH, Lee JM. Hepatitis C virus impairs natural killer cell activity via viral serine protease NS3. PLoS ONE 2017;12:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang F, Zou M, Li J, Xue Q. Cytometric microsphere array for subtyping avian influenza virus. Viral Immunol 2011;24:403–7. [DOI] [PubMed] [Google Scholar]
- 43. WHO Global. World malaria report 2021. Brazzaville, Congo: WHO Regional Office for Africa, 2021 [Google Scholar]
- 44. Shapiro HM, Ulrich H. Cytometry in malaria: from research tool to practical diagnostic approach. Cytometry A 2010;77:500–1 [DOI] [PubMed] [Google Scholar]
- 45. Ondigo BN, Park GS, Ayieko C, Nyangahu DD, Wasswa R, John CC. Comparison of non-magnetic and magnetic beads multiplex assay for assessment of Plasmodium falciparum antibodies. PeerJ 2019;7:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ondigo BN, Park GS, Gose SO, Ho BM, Ochola LA, Ayodo GO, Ofulla AV, John CC. Standardization and validation of a cytometric bead assay to assess antibodies to multiple Plasmodium falciparum recombinant antigens. Malar J 2012;11:427–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fernandez-Becerra C, Sanz S, Brucet M, Stanisic DI, Alves FP, Camargo EP, Alonso PL, Mueller I, del Portillo HA. Naturally-acquired humoral immune responses against the N- and C-termini of the Plasmodium vivax MSP1 protein in endemic regions of Brazil and Papua New Guinea using a multiplex assay. Malar J 2010;9:29–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rogier E, Plucinski M, Lucchi N, Mace K, Chang M, Lemoine JF, Candrinho B, Colborn J, Dimbu R, Fortes F, Udhayakumar V, Barnwell J. Bead-based immunoassay allows sub-picogram detection of histidine-rich protein 2 from Plasmodium falciparum and estimates reliability of malaria rapid diagnostic tests. PLoS ONE 2017;12:e0172139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Plucinski MM, Dimbu PR, Fortes F, Abdulla S, Ahmed S, Gutman J, Kachur SP, Badiane A, Ndiaye D, Talundzic E, Lucchi N, Aidoo M, Udhayakumar V, Halsey E, Rogier E. Posttreatment HRP2 clearance in patients with uncomplicated Plasmodium falciparum malaria. J Infect Dis 2018;217:685–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Plucinski MM, Herman C, Jones S, Dimbu R, Fortes F, Ljolje D, Lucchi N, Murphy SC, Smith NT, Cruz KR, Seilie AM, Halsey ES, Udhayakumar V, Aidoo M, Rogier E. Screening for Pfhrp2/3-deleted Plasmodium falciparum, non-falciparum, and low-density malaria infections by a multiplex antigen assay. J Infect Dis 2019;219:437–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Plucinski MM, Rogier E, Dimbu PR, Fortes F, Halsey ES, Aidoo M, Smith T. Performance of antigen concentration thresholds for attributing fever to malaria among outpatients in Angola. J Clin Microbiol 2019; 57:e01901–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Herman C, Huber CS, Jones S, Steinhardt L, Plucinski MM, Lemoine JF, Chang M, Barnwell JW, Udhayakumar V, Rogier E. Multiplex malaria antigen detection by bead-based assay and molecular confirmation by PCR shows no evidence of Pfhrp2 and Pfhrp3 deletion in Haiti. Malar J 2019;18:380–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rogier E, van den Hoogen L, Herman C, Gurrala K, Joseph V, Stresman G, Presume J, Romilus I, Mondelus G, Elisme T, Ashton R, Chang M, Lemoine JF, Druetz T, Eisele TP, Existe A, Boncy J, Drakeley C, Udhayakumar V. High-throughput malaria serosurveillance using a one-step multiplex bead assay. Malar J 2019;18:402–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lloyd YM, Ngati EP, Salanti A, Leke RGF, Taylor DW. A versatile, high through-put, bead-based phagocytosis assay for Plasmodium falciparum. Sci Rep 2017;7:14705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Guigue N, Menotti J, Hamane S, Derouin F, Garin YJ. Performance of the BioPlex 2200 flow immunoassay in critical cases of serodiagnosis of toxoplasmosis. Clin Vaccine Immunol 2014;21:496–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Vostrý M. Multiplex immunoassays: chips and beads. EJIFCC 2010;20: 162–5 [PMC free article] [PubMed] [Google Scholar]
- 57. Hermann N, Dressen K, Schroeder L, Debald M, Schildberg FA, Walgenbach-Bruenagel G, Hettwer K, Uhlig S, Kuhn W, Hartmann G, Holdenrieder S. Diagnostic relevance of a novel multiplex immunoassay panel in breast cancer. Tumour Biol 2017;39:1–11 [DOI] [PubMed] [Google Scholar]
- 58. Opstal-van Winden AW, Rodenburg W, Pennings JL, van Oostrom CT, Beijnen JH, Peeters PH, van Gils CH, de Vries A. A bead-based multiplexed immunoassay to evaluate breast cancer biomarkers for early detection in pre-diagnostic serum. Int J Mol Sci 2012;13:13587–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Markou A, Strati A, Malamos N, Georgoulias V, Lianidou ES. Molecular characterization of circulating tumor cells in breast cancer by a liquid bead array hybridization assay. Clin Chem 2011;57:421–30 [DOI] [PubMed] [Google Scholar]
- 60. Lux A, Kahlert C, Grützmann R, Pilarsky C. C-Met and PD-L1 on circulating exosomes as diagnostic and prognostic markers for pancreatic cancer. Int J Mol Sci 2019;20:3305–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Li C, Zolotarevsky E, Thompson I, Anderson MA, Simeone DM, Casper JM, Mullenix MC, Lubman DM. A multiplexed bead assay for profiling glycosylation patterns on serum protein biomarkers of pancreatic cancer. Electrophoresis 2011;32:2028–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. World Health Organization (WHO). Lung cancer. In: The cancer atlas, 2020, https://canceratlas.cancer.org/the-burden/lung-cancer/
- 63. Lee HJ, Kim YT, Park PJ, Shin YS, Kang KN, Kim Y, Kim CW. A novel detection method of non-small cell lung cancer using multiplexed bead-based serum biomarker profiling. J Thorac Cardiovasc Surg 2012;143:421–7 [DOI] [PubMed] [Google Scholar]
- 64. Goebel C, Louden CL, McKenna R, Jr, Onugha O, Wachtel A, Long T. Diagnosis of non-small cell lung cancer for early stage asymptomatic patients. Cancer Genomics Proteomics 2019;16:229–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Goebel C, Louden CL, McKenna R, Onugha O, Wachtel A, Long T. Blood test shows high accuracy in detecting stage I non-small cell lung cancer. BMC Cancer 2020;20:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Von Bahr Greenwood T, Palmkvist-Kaijser K, Chiang SC, Tesi B, Bryceson YT, Hjelmqvist H, Henter JI. Elevated ferritin and soluble CD25 in critically ill patients are associated with parameters of (hyper) inflammation and lymphocyte cytotoxicity. Minerva Anestesiol 2019;85:1289–98 [DOI] [PubMed] [Google Scholar]
- 67. Maruoka H, Inoue D, Takiuchi Y, Nagano S, Arima H, Tabata S, Matsushita A, Ishikawa T, Oita T, Takahashi T. IP-10/CXCL10 and MIG/CXCL9 as novel markers for the diagnosis of lymphoma-associated hemophagocytic syndrome. Ann Hematol 2014;93:393–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chen Y, Wang Z, Luo Z, Zhao N, Yang S, Tang Y. Comparison of Th1/Th2 cytokine profiles between primary and secondary haemophagocytic lymphohistiocytosis. Ital J Pediatr 2016;42:50–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Maier R, Weger M, Haller-Schober EM, El-Shabrawi Y, Theisl A, Barth A, Aigner R, Haas A. Application of multiplex cytometric bead array technology for the measurement of angiogenic factors in the vitreous. Mol Vis 2006;12:1143–7 [PubMed] [Google Scholar]
- 70. Dispenzieri A. POEMS Syndrome: 2019 Update on diagnosis, risk-stratification, and management. Am J Hematol 2019;94:812–27 [DOI] [PubMed] [Google Scholar]
- 71. Watanabe O, Arimura K, Kitajima I, Osame M, Maruyama I. Greatly raised vascular endothelial growth factor (VEGF) in POEMS syndrome. Lancet 1996;347:702. [DOI] [PubMed] [Google Scholar]
- 72. Watanabe O, Maruyama I, Arimura K, Kitajima I, Arimura H, Hanatani M, Matsuo K, Arisato T, Osame M. Overproduction of vascular endothelial growth factor/vascular permeability factor is causative in Crow-Fukase (POEMS) syndrome. Muscle Nerve 1998;21:1390–7 [DOI] [PubMed] [Google Scholar]
- 73. D’Souza A, Hayman SR, Buadi F, Mauermann M, Lacy MQ, Gertz MA, Kyle RA, Kumar S, Greipp PR, Lust JA, Russell SJ, Zeldenrust S, Dingli D, Witzig TE, Rajkumar SV, Dispenzieri A. The utility of plasma vascular endothelial growth factor levels in the diagnosis and follow-up of patients with POEMS syndrome. Blood 2011;118:4663–5 [DOI] [PubMed] [Google Scholar]
- 74. Dispenzieri A. POEMS syndrome. Blood Rev 2007;21:285–99 [DOI] [PubMed] [Google Scholar]
- 75. D’Souza A, Lacy M, Gertz M, Kumar S, Buadi F, Hayman S, Dingli D, Zeldenrust S, Kyle R, Ansell S, Inwards D, Johnston P, Micallef I, Porrata L, Litzow M, Gastineau D, Hogan W, Dispenzieri A. Long-term outcomes after autologous stem cell transplantation for patients with POEMS syndrome (osteosclerotic myeloma): a single-center experience. Blood 2012;120:56–62 [DOI] [PubMed] [Google Scholar]
- 76. Nguyen L, Costopoulos M, Tanguy ML, Houillier C, Choquet S, Bennani H, Elias-Shamieh R, Armand M, Faivre G, Glaisner S, Malak S, Vargaftig J, Hoang-Xuan K, Ahle G, Touitou V, Cassoux N, Davi F, Merle-Béral H, Le arff-Tavernier GM, Soussain C. IL10 and IL10: IL6 ratio in CSF is useful at diagnosis but also in the assessment of therapeutic response in patients with primary central nervous system lymphoma (PCNSL). Blood 2014;124:1619 [Google Scholar]
- 77. Nguyen-Them L, Costopoulos M, Tanguy ML, Houillier C, Choquet S, Benanni H, Elias-Shamieh R, Armand M, Faivre G, Glaisner S, Malak S, Vargaftig J, Hoang-Xuan K, Ahle G, Touitou V, Cassoux N, Davi F, Merle-Béral H, Le Garff-Tavernier M, Soussain C, French LOC Network for CNS Lymphoma. The CSF IL-10 concentration is an effective diagnostic marker in immunocompetent primary CNS lymphoma and a potential prognostic biomarker in treatment-responsive patients. Eur J Cancer 2016;61:69–76 [DOI] [PubMed] [Google Scholar]
- 78. Scala E, Alessandri C, Bernardi ML, Ferrara R, Palazzo P, Pomponi D, Quaratino D, Rasi C, Zaffiro A, Zennaro D, Mari A. Cross-sectional survey on immunoglobulin E reactivity in 23,077 subjects using an allergenic molecule-based microarray detection system. Clin Exp Allergy 2010;40:911–21 [DOI] [PubMed] [Google Scholar]
- 79. Scala E, Pomponi D, Giani M. Allergen microbead arrays: the future of allergy diagnostics? Expert Rev Clin Immunol 2013;9:13. [DOI] [PubMed] [Google Scholar]
- 80. Hiller R, Laffer S, Harwanegg C, Huber M, Schmidt WM, Twardosz A, Barletta B, Becker WM, Blaser K, Breiteneder H, Chapman M, Crameri R, Duchêne M, Ferreira F, Fiebig H, Hoffmann-Sommergruber K, King TP, Kleber-Janke T, Kurup VP, Lehrer SB, Lidholm J, Müller U, Pini C, Reese G, Scheiner O, Scheynius A, Shen HD, Spitzauer S, Suck R, Swoboda I, Thomas W, Tinghino R, Van Hage-Hamsten M, Virtanen T, Kraft D, Müller MW, Valenta R. Microarrayed allergen molecules: diagnostic gatekeepers for allergy treatment. FASEB J 2002;16:414–6 [DOI] [PubMed] [Google Scholar]
- 81. Gomaa A, Boye J. Simultaneous detection of multi-allergens in an incurred food matrix using ELISA, multiplex flow cytometry and liquid chromatography mass spectrometry (LC-MS). Food Chem 2015;175: 585–92 [DOI] [PubMed] [Google Scholar]
- 82. Pomponi D, Bernardi ML, Liso M, Palazzo P, Tuppo L, Rafaiani C, Santoro M, Labrada A, Ciardiello MA, Mari A, Scala E. Allergen micro-bead array for IgE detection: a feasibility study using allergenic molecules tested on a flexible multiplex flow cytometric immunoassay. PLoS ONE 2012;7:e35697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Otto G, Lamote A, Deckers E, Dumont V, Delahaut P, Scippo ML, Pleck J, Hillairet C, Gillard N. A flow-cytometry-based method for detecting simultaneously five allergens in a complex food matrix. J Food Sci Technol 2016;53:4179–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Meimaridou A, Haasnoot W, Noteboom L, Mintzas D, Pulkrabova J, Hajslová J, Nielen MW. Color encoded microbeads-based flow cytometric immunoassay for polycyclic aromatic hydrocarbons in food. Anal Chim Acta 2010;672:9–14 [DOI] [PubMed] [Google Scholar]
- 85. Zhou ZH, Stone CA, Jr, Jakubovic B, Phillips EJ, Sussman G, Park J, Hoang U, Kirshner SL, Levin R, Kozlowski S. Anti-PEG IgE in anaphylaxis associated with polyethylene glycol. J Allergy Clin Immunol Pract 2021;9:1731–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Rathod S, Ramsey M, Relling MV, Finkelman FD, Fernandez CA. Hypersensitivity reactions to asparaginase in mice are mediated by anti-asparaginase IgE and IgG and the immunoglobulin receptors FcεRI and FcγRIII. Haematologica 2019;104:319–29 [DOI] [PMC free article] [PubMed] [Google Scholar]