Abstract
In recent years, with the increase of air pollution, smoking, aging, and respiratory infection, the incidence rate and mortality of lung diseases are increasing annually, which has become a major hazard to human health. N6-methyladenosine (m6A) RNA methylation is the most abundant modifications in eukaryotes, and such modified RNA can be specifically recognized and combined by m6A recognition proteins and then mediate RNA splicing, maturation, enucleation, degradation, and translation. More and more studies have revealed that the m6A modification is involved in the pathogenesis and development of some diseases; however, the mechanisms of m6A in lung diseases are poorly understood. In this review, we summarize the latest progress in the biological function of m6A modifications in lung diseases and discuss the potential therapeutic and prognostic strategies. The dysregulation of global m6A levels and m6A regulators may affect the occurrence and development of asthma, chronic obstructive pulmonary disease, lung cancer, and other lung diseases through inflammation and immune function. In lung cancer, this modification has an important impact on malignant cell proliferation, migration, invasion, and drug resistance. In addition, abnormally changed m6A-modified proteins in lung cancer tissue samples and circulating tumor cells (CTCs) may be used as diagnostic and prognostic markers of lung cancer. Models composed of multiple m6A regulators can be used to evaluate the risk prediction or prognosis of asthma and pulmonary fibrosis. In general, the in-depth study of m6A modifications is a frontier direction in disease research. It provides novel insights for understanding of the molecular mechanisms underlying disease occurrence, development, and drug resistance, as well as for the development of effective novel therapeutics.
Keywords: N6-methyladenosine, asthma, chronic obstructive pulmonary disease, idiopathic pulmonary fibrosis, lung cancer
Impact Statement
The N6-methyladenosine (m6A) methylation is the most abundant internal modification on mRNA. It is confirmed to be closely related to the occurrence, development, targeted therapy, diagnosis, and prognosis of multiple lung diseases. In this review, we conclude that, the m6A methylation affects the occurrence and development of asthma, chronic obstructive pulmonary disease, pulmonary interstitial disease, and lung cancer through inflammation and immune function. Meanwhile, their dysregulations are associated with drug resistance and poor prognosis in lung cancer patients. The m6A regulators can be viable therapeutic targets for drug resistance and new drug targets for targeted therapy. The increased level of m6A modification in circulating tumor cells (CTCs) is indicated to be a non-invasive diagnostic method for lung cancer. More importantly, models composed of multiple m6A regulators are also used to evaluate the diagnosis, risk or prognosis of lung diseases. Therefore, we believe that m6A methylation plays an extremely important role in lung diseases.
Introduction
Environmental exposure and inflammation can cause most categories of lung diseases. 1 Lung diseases include asthma, chronic obstructive pulmonary disease (COPD), idiopathic pulmonary fibrosis (IPF), pulmonary hypertension (PH), and lung cancer. 2 COPD is a common chronic disease, which is the fourth leading cause of death in the world, with the characteristics of airflow obstruction. IPF is a kind of chronic progressive fibrosis interstitial pneumonia, which is characterized by dyspnea and gradual deterioration of lung function. 3 High mortality of lung cancer is associated with common diagnosis at an advanced stage that hampers curative therapy and leads to a poor 5-year survival rate. 4
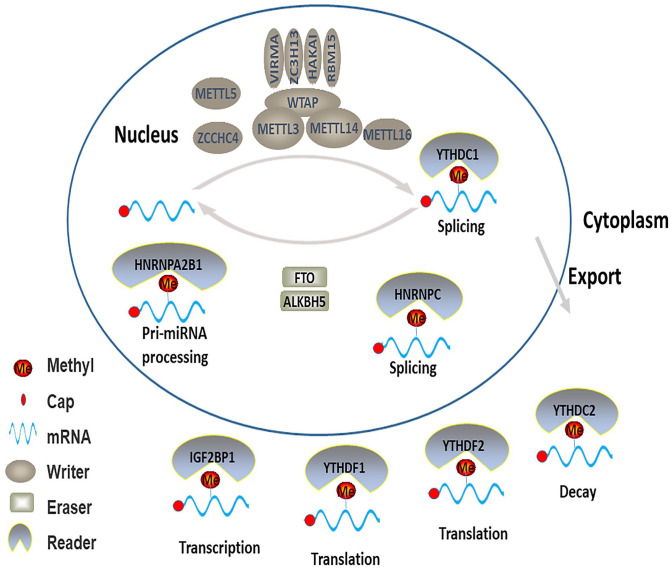
N6-methyladenosine (m6A) refers to the methylation modification of adenine nucleoside N6, which is enriched near the stop codon and 3′-untranslated terminal region (UTR).5,6 There are three types of regulators for RNA m6A methylation modification. The methyltransferases (“writers”) are responsible for catalysis while the demethylases (“erasers”) are responsible for removing methylation. Binding proteins (“readers”) recruit downstream functional complexes to perform their functions (Figure 1). The m6A modification functionally regulates the eukaryotic transcriptome and affects the splicing, nuclear export, translation, and stability of mRNA.5,7,8 Nevertheless, the underlying mechanisms of how m6A RNA methylation plays the biological function are poorly understood. Recently, emerging evidences have reported that m6A modifications play critical roles in a series of cancers, metabolic diseases, infertility, and neural development.9 –15 These modifications appear to play a carcinogenic role in some cancers while have anti-tumor effects in others. Therefore, studying the biological functions of genes regulated by m6A in different diseases and identifying the key m6A target genes are of great significance to understand the pathogenesis of multiple diseases. In this comprehensive review, we describe the roles played by m6A modifications of mRNA in the development of lung diseases.
Figure 1.
The dynamic and reversible processes and consequences of m6A methylation. (A color version of this figure is available in the online journal.)
RNA modification-related proteins
RNA m6A methyltransferases consist of the METTL3 (methyltransferase-like 3) complex, METTL16, METTL5, ZCCHC4 (zinc finger CCHC-type containing 4).16,17 METTL3 complex functions with METTL3 acting as the catalytic core and METTL14 as the RNA-binding platform. 18 While several new components, including WTAP (Wilms’ tumor 1-associated protein), RBM15 (RNA-binding motif protein 15), KIAA1429 (also known as VIRMA), ZC3H13 (zinc finger CCCH domain-containing protein 13), and HAKAI (also known as CBLL1) function as regulatory subunits to regulate the activity of METTL3 in cells.19 –22 Studies have indicated that METTL3 promotes the translation of a large subset of oncogenic mRNAs by interacting with eukaryotic translation initiation factors. 23 New studies suggested that METTL16, METTL5, and ZCCHC4 are also identified as m6A methyltransferases, which can function independently and catalyze m6A modification on some structural RNA.24 –26 As the homologous protein of METTL3, METTL16 regulates the m6A modification of U6 snRNA. 24 METTL5 catalyzes m6A on 18S rRNA, while ZCCHC4 mainly methylates human 28S rRNA.25,26 METTL5 can enhance the metabolic stability in cells by forming a heterodimer complex with a special structure with methyltransferase activator TRMT112.17,26 At the same time, m6A modification of ZCCHC4 mainly affects translation and cell proliferation.
FTO (fat mass and obesity-associated protein) and ALKBH5 (alkB homolog 5) are common demethylases that reverse m6A modification which considered m6A erasers.27,28 FTO is the first discovered RNA demethylase and mediates the demethylation of internal m6A. 27 In a study of lung squamous cell carcinoma, the high expression of FTO was significantly correlated with the poor prognosis of patients. 29 FTO also functioned as an oncogene by regulating the expression of MZF1 which can affect proliferation and invasion, and inhibit apoptosis. 29 As the second m6A demethylase identified, ALKBH5 has been proved to affect biological processes, such as proliferation, invasion, and metastasis.28,30 –32 Interestingly enough, it is found that ALKBH5 plays a carcinogenic or antitumor role in cancers.32,33 Therefore, the potential mechanism of ALKBH5 in cancer is not only unclear, but also controversial. 34
The common RNA m6A modification readers include YTH family proteins (YTHDF1–3 and YTHDC1-2), HNRNP proteins (HNRNPA2B1, HNRNPC, and HNRNPG) and IGF2BPs (insulin-like growth factor 2 mRNA-binding proteins).16,35 These readers can mediate different pathways of function. For instance, YTHDF2/3 has been shown to accelerate mRNA decay,36,37 while YTHDF1 can promote the translation of methylated RNA. 38 YTHDC1 promotes exon inclusion of mRNA. 21 The RNA-binding proteins HNRNPA2B1, HNRNPC, and HNRNPG mediate alternative splicing effects.39 –42 Moreover, HNRNPA2B1 can recruit DGCR8 to RNA to promote primary microRNA (miRNA) processing. 40
Role of m6A RNA methylation in lung diseases
Role of m6A RNA methylation in lung diseases related to environmental poisons
Researchers have conducted in-depth study on the molecular mechanism of lung diseases caused by smoking, whereas the regulatory effect of m6A after exposure to environmental pollutants is unclear. One study showed that the global m6A RNA methylation level was decreased in A549 cells after treating with particulate matter and sodium arsenite. 43 After the analysis of the population study, it was observed that the expression of several m6A regulators in the high PM2.5 exposure group was significantly higher than that in the low exposure control group. 43 Cheng’s group 44 found that the exposure of human bronchial epithelial (HBE) cells to cigarette smoke extract elevated METTL3 expression, which increased the m6A modification of the transcriptional repressor ZBTB4. The downregulation of ZBTB4 was associated with elevated EZH2, which can reduce the level of E-cadherin and induce epithelial–mesenchymal transition (EMT). HBE cells chronically treated with arsenite sodium were identified to have a malignant phenotype and increased levels of cellular proliferation. 45 Further analysis showed that after treating cells with arsenite, the RNA m6A modification which synergistically regulated by METTL3, METTL14, WTAP, and FTO was significantly increased. 45 These studies suggest that m6A modification affects the malignant proliferation and EMT process of HBE cells exposure to environmental toxicants, such as arsenite or particulate matter.
Role of m6A RNA methylation in asthma
Asthma is an immune-related disease. Its airway hyper-responsiveness induces bronchoconstriction and chronic inflammation. 2 In one study, researchers identified 11 significant m6A regulators associated with asthma from the GSE40888 dataset. 46 Subsequently, five candidate m6A regulators were screened according to the nomogram model to predict the risk of childhood asthma. Therefore, we believe that the m6A regulators have assignable influence upon the occurrence and development of childhood asthma. In primary human airway epithelium, the loss of FTO led to destabilization of the mRNA of the master ciliary transcription factor FOXJ1 and a severe loss of ciliated cells. 47 After Fto gene knockout, mice showed a strong asthma like phenotype after allergen stimulation due to the loss of ciliated cells. Therefore, FTO demethylation can stabilize FOXJ1 mRNA which facilitate the formation of motor cilia, and then inhibit the occurrence and development of asthma.
Role of m6A RNA methylation in COPD
COPD is a progressive inflammatory disease of the airways, alveoli, and microvasculature. The key functional characteristic of COPD is the irreversible limitation of airflow. 48 Previous reports showed that METTL3, FTO, IGF2BP3, and YTHDC2 genes were significantly elevated in COPD tissues at mRNA level compared with control samples. 49 According to the analysis of signaling pathways and biological processes, these genes are closely related to promote the development of COPD. This reveals that these m6A regulators may play roles in COPD occurrence. Emphysema refers to parenchymal destruction that causes loss of alveolar units and the characteristic gas trapping and hyperinflation that is found in patients with COPD. 50 Cigarette smoke induced METTL3 overexpression and produced excessive mature miR-93 in bronchial epithelial cells. The miR-93 activated the c-Jun N-terminal kinase (JNK) pathway and elevated the level of matrix metalloproteinases (MMP9 and MMP12) that induced emphysema. 51 Based on the above literatures, METTL3 plays a vital role in pulmonary inflammation.
Role of m6A RNA methylation in IPF
IPF is a kind of progressive fibrosis interstitial pneumonia. It is the end-stage of diffuse pulmonary parenchymal disease and a risk factor for lung cancer.52,53 Han’s group found that both m6A modifications of pri-miR-126 and its binding with DGCR8 were decreased after carbon black treatment, which resulted in the reduction of mature miR-126 and the activation of PI3K/AKT/mTOR pathway, finally driving fibrogenesis in the lungs. 54 A new study showed that the expression of ALKBH5 was both increased in lung tissue of mice inhaled with silica and fibroblasts stimulated by transforming growth factor β1. 55 Their study of the molecular mechanism of these phenomena showed that after demethylation by ALKBH5, the maturation process of pri-miR-320a-3p was blocked and miR-320a-3p decreased. More in-depth study demonstrated that miR-320a-3p regulates fibrosis through binding to the 3’-UTR of FOXM1 mRNA. 55 Therefore, we believe that m6A regulatory protein can promote pulmonary fibrosis by reducing the formation of mature miRNA.
There are some similarities between the current global pandemic coronavirus disease and IPF. As reported, genes related to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are broadly regulated by m6A RNA modification. Li’s group researched the networks of m6A-SARS-CoV-2-related genes in bronchoalveolar lavage cells of IPF patients from the GEO database. 56 Through a variety of regression analysis combined with survival information, eight m6A-related-CoV genes were obtained, and a prognostic risk prediction model was established.
Role of m6A RNA methylation in PH
PH frequently complicates the course of treatment for patients with various chronic lung diseases. 57 However, the incidence and development of PH in lung cancer patients are largely neglected. 58 Post-transcriptional modifications are implicated in the vascular remodeling of PH. Hu’s group demonstrated elevated m6A levels of total RNA in mouse and rat models of PH as well as in patients with idiopathic pulmonary arterial hypertension. 59 Targeting YTHDF1 via gene silencing in human pulmonary artery smooth muscle cells (HPASMCs) and in a novel YTHDF1 knockout mouse attenuates cell proliferation and vascular remodeling in PH, at least in part, by decreased translation of MAGED1. METTL3 mRNA and protein were upregulated in pulmonary artery smooth muscle cells (PASMCs) and hypoxic rat models with PH. 60 The downregulation of METTL3 was accompanied by the weakening of PASMC proliferation and migration. YTHDF2, which can recognize m6A-modified PTEN and promote the degradation, was significantly increased in PASMCs under hypoxia. The decreased expression of PTEN can activate the PI3K/Akt signaling pathway and contribute to the overproliferation of PASMCs. These studies show that METTL3, YTHDF1, and YTHDF2 play significant regulatory roles in the proliferation, migration and vascular remodeling of PASMCs.
Multiple functions of m6A RNA methylation in lung cancer
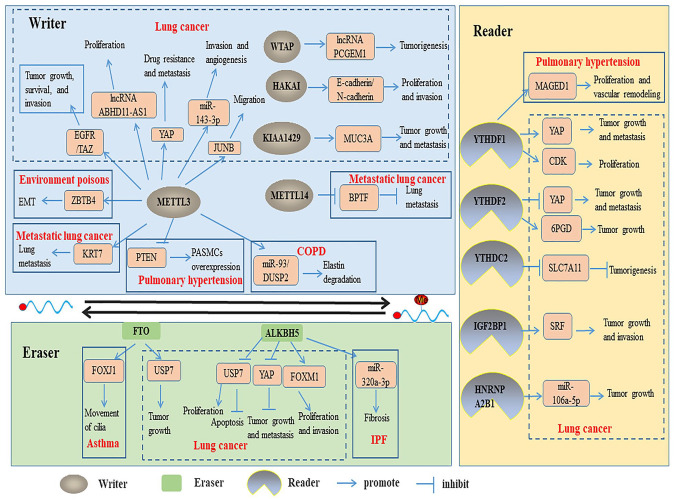
At present, the role and mechanism of m6A modification in tumorigenesis and development are hot topics in tumor biology. In the development of lung cancer, the abnormal changes of m6A gene level are closely related to proliferation, migration, invasion, metastasis, and drug resistance. Therefore, the study of biological function of m6A modification and identify the key factors to clarify the pathogenesis of lung cancer can provide the basis for the diagnosis and treatment of lung cancer. More and more evidence shows that m6A plays a dual role in cancers. It affects the occurrence and development of cancer by enhancing or inhibiting the expression of oncogenes and tumor suppressor genes. We summarize the potential mechanisms of m6A regulators in lung cancer in Table 1. Furthermore, the Figure 2 presents the functions and targets of m6A writers, erasers, and readers in lung diseases.
Table 1.
Multiple functions exerted by m6A RNA methylation in lung cancer.
| Protein | Role in cancer | Biological function | Mechanism | Refs | |
|---|---|---|---|---|---|
| 1 | METTL3 | Oncogene | Promote growth, survival, and invasion | Promote translation of EGFR and TAZ | Lin et al. 61 |
| Promote drug resistance and metastasis | Promote translation of YAP by regulating the lncRNA MALAT1-miR-1914-3p-YAP axis | Jin et al. 62 | |||
| Promote the proliferation | Strengthen the stability of the lncRNA ABHD11-AS1 | Song et al. 63 | |||
| Promote cell migration | Increase mRNA stability of JUNB | Wanna-Udom et al. 64 | |||
| Promote invasion and angiogenesis | Increase the splicing of precursor miR-143-3p and promote miR-143-3p processing | Wang et al. 65 | |||
| 2 | WTAP | Oncogene | Promote tumorigenesis | lncRNA PCGEM1 sponge miR-433-3p | Weng et al. 80 |
| 3 | HAKAI | Oncogene | Promote cell proliferation and invasion | Promote G1/S cell cycle transition, decrease E-cadherin expression and increase expression of MMP2 and MMP9 | Hui et al. 67 |
| Oncogene | Promote cell migration and invasion/ Cisplatin resistance | Decrease E-cadherin and increase N-cadherin/increase AKT activity | Liu et al. 68 | ||
| Oncogene | Promote cell migration/ gefitinib resistance | Decrease E-cadherin | Weng et al. 69 | ||
| 4 | KIAA1429 | Oncogene | Promote tumor growth and metastasis | Promote MUC3A expression | Zhao and Xie 70 |
| 5 | FTO | Oncogene | Promote tumor growth | Increase mRNA stabilize of USP7 | Li et al. 71 |
| Oncogene | Promote cell proliferation, migration, and invasion | R96Q (an FTO missense mutant) inhibit proliferation and invasion | Ding et al. 72 | ||
| 6 | ALKBH5 | Oncogene | Promote proliferation and reduce apoptosis | Decrease mRNA stability of TIMP3 | Zhu et al. 73 |
| Promote proliferation and invasion | Promote translation of FOXM1 | Chao et al. 30 | |||
| Suppressor | inhibit tumor growth and metastasis | Decrease YAP activity by regulating miR-107/LATS2 axis | Jin et al. 31 | ||
| 7 | YTHDF1 | Oncogene | Promote tumor growth and metastasis | Promote translation of YAP | Jin et al. 31 |
| Promote proliferation/ Reduce cisplatin resistance | Promote translation of CDK2, CDK4, and cyclin D1/silent Keap1-Nrf2-AKR1C1 | Shi et al. 74 | |||
| 8 | YTHDF2 | Oncogene | Promote tumor growth and metastasis | Promote YAP mRNA decay via the AGO2 system | Jin et al. 31 |
| Oncogene | Promote tumor growth | Promote translation of 6PGD | Sheng et al. 75 | ||
| 9 | YTHDC2 | Suppressor | Decrease tumorigenesis | Decrease mRNA stability of SLC7A11 | Ma et al. 10 |
| 10 | HNRNPA2B1 | Oncogene | Promote cell growth | Promote the processing of primary miR-106b-5p by LINC01234 | Chen et al. 76 |
| 11 | IGF2BP1 | Oncogene | Promote tumor cell growth and invasion | Enhance SRF-dependent transcriptional activity | Muller et al. 77 |
Figure 2.
Multiple functions of m6A RNA methylation in lung diseases. (A color version of this figure is available in the online journal.)
Role of m6A RNA methylation in lung cancer cell proliferation
Lung cancer is formed by the malignant proliferation of lung epithelial cells, which is related to excessive cell division, a disordered cell cycle and abnormal apoptosis regulation. Existing evidence suggests that m6A RNA methylation is associated with cancer cell proliferation in a variety of cancers. METTL3 expression was increased in both lung adenocarcinoma patients from TCGA (The Cancer Genome Atlas) cohort and lung adenocarcinoma cell lines, and correlated with tumor stage.23,61 METTL3 interacts with translation initiation factors and specifically promotes the translation of initiation factor-dependent reporter mRNAs.14,61,62 As a result, METTL3 increased the expression of epidermal growth factor receptor (EGFR), transcriptional coactivator with PDZ-binding motif (TAZ), and Yes-associated protein (YAP) and promoted cell growth, survival and invasion.61,62 SUMOylation of METTL3 repressed its methyltransferase activity and promoted tumor growth in non–small cell lung cancer (NSCLC). 66 Meanwhile, miRNA-related studies also revealed that miR-33a and miR-600 can inhibit the expression of METTL3 by targeting the mRNA, thereby inhibiting the proliferation NSCLC.78,79 The overexpression of METTL3 enhanced the stability of lncRNA ABHD11-AS1 transcripts and promoted the proliferation of NSCLC. 63 These studies pointed out that METTL3 plays a cancer promoting role in lung cancer. Besides, highly expressed lncRNA PCGEM1 upregulated WTAP and accelerated the progression of NSCLC by sponging miR-433-3p. 80 On the contrary, the inhibition of HAKAI can promote the G1/S cell cycle transition thereby promoting cell proliferation in NSCLC. 67
Some studies indicated that the mRNA and protein levels of FTO were excessive in human NSCLC cell lines and tissues. 71 FTO knockdown with short hairpin RNAs (shRNAs) inhibited the proliferation rate and colony formation ability of lung cancer cells in vitro and in vivo. 71 Mechanistically, the decrease of m6A level caused by FTO contributes to the increase of ubiquitin-specific protease 7 (USP7) mRNA stability, and further promotes the growth of NSCLC cells. 71 In the mice model of intermittent hypoxia, ALKBH5 was increased in lung adenocarcinoma cells, tissues and subcutaneous tumors.30,73 Functionally, ALKBH5 enhanced the proliferation of NSCLC cells and reduced the apoptosis by repressing the stability of TIMP3 mRNA. 73 In lung adenocarcinoma cells, the level of m6A in FOXM1 mRNA was increased with knockdown of ALKBH5, which decreased the translation efficiency and led to the downregulation of FOXM1 protein. 30 These changes significantly inhibited the growth and invasion of lung adenocarcinoma cells. However, a recent study showed that ALKBH5 was significantly underexpressed in NSCLC. ALKBH5 decreased the activity of YAP by regulating the miR-107/LATS2 axis and inhibited proliferation and metastasis, 31 which was not consistent with other studies. Similarly, the m6A methylation modification was removed by FTO and ALKBH5, which increased the stability of USP7 mRNA but decreased the stability of TIMP3 mRNA. Moreover, the results obtained by removing the m6A modification of different genes by ALKBH5 were completely different. We speculated that the m6A modification of different genes was changed and selectively recognized by different m6A reader proteins, resulting in differences in post-transcriptional regulation. The specific molecular mechanism underlying the selective recognition ability of m6A recognition proteins still needs to be further investigated.
YTHDF family and YAP have complex dual functions. YTHDF2 facilitated YAP mRNA decay via the AGO2 system, whereas YTHDF1/3 promoted YAP mRNA translation by interacting with eIF3a/eIF3b.31,62 In addition, one group revealed that YTHDF1 deficiency can affect the translation efficiency of cyclins, thereby inhibiting proliferation, colony formation and xenograft tumor formation. 74 YTHDF2 promoted 6-phosphogluconate dehydrogenase (6PGD) mRNA translation in lung cancer cells by binding to the m6A modification site of 6PGD, 75 to accelerate glucose metabolism through the pentose phosphate pathway and provide raw materials for the malignant proliferation of NSCLC. The increased LINC01234 which mediated by c-Myc interacted with HNRNPA2B1 and promoted the processing of primary miR-106b-5p. While the inhibition of cryptochrome 2 (CRY2) and upregulation of c-Myc caused by miR-106b-5p can enhance NSCLC cell growth. This formed a positive-feedback loop involving the m6A reader, c-Myc, and miR-106b-5p, which can promote the growth of NSCLC cell. 76
Role of m6A RNA methylation in lung cancer cell migration and metastasis
Researches on the effect of m6A RNA methylation on cancer cell migration and metastasis has become increasingly mature. The expression of METTL3 was excessive in EMT which induced by transforming growth factor beta (TGF-β) in lung cancer cells. 64 The knockdown of METTL3 reduced the mRNA stability of JUNB, thereby antagonizing EMT phenotype. Rescue experiments confirmed that JUNB overexpression partially restored EMT inhibited by METTL3 knockdown. 64 METTL3 was down regulated in lung cancer with Simvastatin treatment, which changed the m6A modification of EZH2 mRNA and inhibited the EMT progression. 81 MiR-143-3p is known to enhance the invasiveness and angiogenesis of lung cancer by targeting angiostatin-1. Wang et al. found that METTL3 can promote the expression of miR-143-3p by increasing the splicing of miRNA precursor, and finally promoting the metastasis of lung cancer cells. 65 METTL3 can also enhance metastasis and drug resistance of NSCLC by increasing the stability of YAP mRNA which regulated with lncRNA MALAT1 and miR-1914-3p. 62 In addition to METTL3, KIAA1429 was significantly overexpressed in lung adenocarcinoma (LUAD) with larger tumor diameter, more prone to metastasis, and lower overall survival. KIAA1429 downregulation significantly inhibited the migration, proliferation, and invasion of LUAD cells by inhibiting MUC3A expression and arresting cell cycle in the G1 phase. 70 Moreover, HAKAI regulates the migration and invasion of NSCLC cells by changing the EMT markers E-cadherin and N-cadherin.67,68
Overexpression of FTO can enhance the proliferation, migration, and invasion of lung cancer cells. Nevertheless, the FTO missense mutant R96Q, lacking demethylase activity, blunted the effects. Interestingly, RNA sequencing of FTO or R96Q overexpressed cells indicated that most genes regulated by m6A mRNA demethylation were associated with lung cancer. 72
Role of m6A RNA methylation in drug resistance
Drug resistance refers to the resistance of cancer cells to chemotherapy, radiotherapy, and targeted drugs, which is one of the major causes for cancer recurrence. 82 Recent studies indicated that m6A regulators are dysregulated in a variety of cancers and play not to be neglected roles in drug resistance and cancer recurrence. m6A RNA modification can be considered as a feasible therapeutic target for overcoming drug resistance.
The elevated expression of YAP1 caused by METTL3 enhancing the stability of YAP1 m6A methylation mediated cisplatin resistance in NSCLC. 62 One study suggested that both cancer specimens with acquired gefitinib resistance and gefitinib-resistant NSCLC cells showed increased expression of HAKAI, accompanied by decreased expression of E-cadherin. 69 At the same time, the suppression of HAKAI significantly enhanced chemosensitivity to cisplatin. 68 Notably, the depletion of YTHDF1 renders cisplatin resistant in cancerous cells through the KEAP1/NRF2/AKR1C1 axis. Meanwhile, the high expression of YTHDF1 in patients with NSCLC is often accompanied by better clinical results. 74
In a study of the relationship between m6A modifications and afatinib resistance in NSCLC cells, researchers found that the scores of m6A enrichment in afatinib-resistant lines (H1299) was higher than in afatinib-sensitive lines (A549). 83 Further gene function enrichment analysis revealed that the differentially expressed genes in the two groups were related to the cell cycle. Researchers speculated that m6A-modified genes may cause afatinib resistance in NSCLC by interfering with normal cell cycle.
Role of m6A RNA methylation in lung cancer diagnosis, treatment and prognosis
The pivotal role of m6A RNA methylation in the occurrence and development of cancer provides more opportunities for early diagnosis and treatment. Huang et al. found that m6A modification was upregulated in circulating tumor cells (CTCs) of lung cancer patients, and further analyzed the m6A levels of RNA in individual cells for validation. 84 The results and methods provided a potential basis for evaluating whether lung cancer patients have metastasis and good prognosis. A systematic analysis of m6A regulatory factors in LUAD and normal samples revealed that 12 m6A methylation-related genes displayed aberrant expression. 85 Furthermore, the team finally established a diagnostic score model with 11 genes and a risk score model with 10 genes. It is worth exciting that the diagnostic score model discriminated each stage of lung adenocarcinoma in the training and validation cohorts, even stage IA.
As an important oncogene of many tumors, FTO is considered to be a promising therapeutic target. However, the identified FTO inhibitors have low sensitivity and specificity, and their clinical effects are limited. FTO inhibitor R-2HG enhanced the sensitivity of acute myeloid leukemia (AML) cells to therapeutic agents, while another inhibitor meclofenamic acid inhibited the growth and survival of glioblastoma (GBM) cells.86 –88 According to the characteristics of genetic variation, Li’s group found that mutations in FTO and YTHDF3 were related to poor overall survival. 89 The m6Sig scoring tool they set up had a positive correlation with PD-L1 expression, which could reflect the tumor microenvironment and prognosis of LUAD patients. The therapeutic advantage of the high m6Sig group was also confirmed in the anti PD-L1 immunotherapy cohort. 89 Clinically, most cases of acinar LUAD subtype exhibited simultaneous downregulation of YTHDC2 and elevation of SLC7A11, accompanied by adverse clinical results. 10 The METTL3-eIF3h interaction enhances translation and carcinogenicity. Some studies have confirmed that the depletion of METTL3 can inhibit tumorigenicity and improve the sensitivity of lung cancer cells to BRD4 inhibition. 23 Thus, METTL3-eIF3h can be considered as a possible therapeutic target for lung cancer. The classification of chemotherapy benefit prediction based on m6A regulator constructed by least absolute shrinkage and selection operator (LASSO) Cox model has been proved to be more powerful than other parameters. 90 Further cytological experiments revealed that three m6A regulators-ZCCHC4, G3BP1, and RBMX from the model were considered to be novel targets to overcome chemotherapy resistance of small cell lung cancers (SCLCs).
The information obtained from TCGA databases showed that compared with normal patients, some m6A-related genes were upregulated in LUAD and differentially expressed in different races, ages, and tumor, node, metastasis (TNM) stages. 91 Patients with high expression of METTL3, YTHDF1, and YTHDF2 had better survival rate and were promising biomarkers for the prognosis of LUAD. 91 Wu’s group developed a prognostic model of m6A regulatory gene composed by IGF2BP1, IGF2BP2, HNRNPA2B1, METTL3, and HNRNPC, which can accurately assess the risk level of LUAD patients. 92 IGF2BP1 promoted growth and invasion of lung cancer cells by promoting serum response factor (SRF)-dependent transcriptional activity. Meanwhile, SRF/IGF2BP1-dependent genes indicated a poor overall survival probability in lung cancer. 77 Importantly, nomogram based on prognostic characteristics can accurately predict the survival probability of LUAD patients and provide beneficial guidance for clinical treatment.
Role of m6A RNA methylation in metastatic lung cancer
Metastatic growth is the last but most fatal stage in the malignant progression of tumors. 93 Metastatic lung cancer refers to any part of the malignant tumor that has spread through a variety of ways of metastasis to form a lung tumor, which can be caused by hematogeneous dissemination, lymphatic metastasis, or direct invasion of adjacent organs. 94 The incidence of lung metastasis is different in different parts of the tumor, among which thyroid cancer, breast cancer, renal cancer, choriocarcinoma, and osteosarcoma have the highest incidence. Researchers found that METTL3 mRNA was increased, while FTO was decreased in established breast cancer lung metastasis cells. 95 Specifically, increased METTL3 methylated KRT7-AS to increase the stability of a KRT7-AS/KRT7 mRNA duplex. Furthermore, YTHDF1/eEF-1 was involved in translational elongation of FTO regulated KRT7 mRNA. In general, m6A methylation of KRT7 mRNA promoted breast cancer lung metastasis. Utilizing TCGA and researchers’ own datasets, they found that METTL14 was low expressed in metastatic renal cell carcinoma samples with poor prognosis. 96 From the mechanism analysis, m6A modification mediated by METTL14 negatively regulated the mRNA stability of BPTF and drove lung metastasis.
Discussion
M6A modification is the most common post-transcriptional modification in mRNA. Increasing evidence shows that m6A plays an assignable role in tumorigenesis, metastasis, and angiogenesis. Within recent years, extensive experimental data have shown that global m6A levels and regulators are abnormally expressed in multiple lung diseases. They affect the occurrence and development of asthma, COPD, IPF, and lung cancer through inflammation and immune function. Meanwhile, their dysregulation will also affect the drug resistance and poor prognosis of cancer patients. Studies in human lung cancer cell lines have indicated that m6A regulators, such as METTL3, YTHDF1, and HAKAI can be viable therapeutic targets for drug resistance.62,68,69,74 The increased level of m6A modification in CTCs was indicated to be a non-invasive diagnostic method for lung cancer. 84 Models composed of multiple m6A regulators have also been used to evaluate the diagnosis, risk or prognosis of lung diseases, such as asthma, SARS-CoV-2, and lung cancer.46,56,85,91 In recent years, targeted therapy focused on m6A modification has become a research hotspot for new drug targets, and it mainly includes FTO inhibitors, METTL3-14/WTAP activators, and combination therapy.86,97,98
With the discovery of m6A-related proteins and the study of potential mechanisms, unknown problems have been gradually exposed. First, many m6A methyltransferases have been identified; however, research on lung diseases focuses on METTL3, with few studies focused on other methyltransferases. Thus, whether other methyltransferases are involved in the occurrence and development of lung diseases has not been clarified. Second, the affect of m6A modification in lung cancer is also controversial. Some m6A-related proteins have dual effects and simultaneously promote and inhibit cancer. For example, ALKBH5 can promote the proliferation and invasion of NSCLC and reduce apoptosis.30,73 whereas another study indicated that it can inhibit the growth and metastasis of NSCLC. 31 YTHDF1 deficiency can inhibit the proliferation of NSCLC, although its high expression is associated with good prognosis. 74 How these m6A-modified proteins play different roles and how different m6A recognition proteins selectively recognize and bind to target RNA will be the focus of future research. Third, the development of inhibitors or activators of m6A-related proteins is still in its infancy and is mainly used in preclinical experimental research; thus, its therapeutic effect on cancer patients has not been reported.
With the in-depth research of m6A-modified protein, the detection methods have also been developed. Traditional methods for the analysis of m6A, such as MeRIP-seq and miCLIP-seq, cannot accurately locate and quantify m6A, and these methods rely on the specific antibody of m6A. The specificity and sensitivity of antibodies are limited due to changes in affinity and batch effects as well as the cross reaction between the antibody and other similar modifications (such as m6Am). Therefore, it has become the most popular research hotspot in the field to detect the level of RNA modification with new technology. Based on the sensitivity of the newly discovered RNA endonuclease MazF to m6A, a new high-throughput m6A identification method can quantify the methylation level with single base resolution, accurately detect the whole transcriptome range of m6A, and does not rely on antibodies, which overcomes the limitations of traditional m6A identification methods. Nanopore sequencing calculates the modifications carried by nucleotides by detecting the electrical signals of nucleotides passing through the nanopore. Although these two high-throughput sequencing technologies have good application prospects, but the current calculation methods and technologies still have high false-positive rates, which need to be further improved. However, the realization of these technologies will be the key to clarifying the dynamic regulatory mechanism and molecular function of m6A modification.
In addition to m6A, new chemical modifications of RNA, such as N1 methyladenosine modification (m1A), methylcytosine modification (m5C) and m6Am, have also been hot spots in recent research. At present, research on m5C and m1A is still in its infancy. Whether they play a regulatory role in multiple biological processes, like as m6A modification, needs to be further studied in the future. The development of more accurate detection methods, such as nanopore sequencing and single base resolution technology, is very important for the establishment of high reliability RNA modification profiles. The function of m6Am is still controversial. First, m6Am was identified to be related to RNA stability. Later studies found that m6Am only affected a small part of the half-life of mRNA, and m6Am could also affect the translation efficiency of mRNA. Therefore, further studies are needed on the role of m6Am in mRNA fate determination.
Overall, the study of m6A modification in disease is a new frontier. It not only provides new insights into the molecular mechanisms of disease occurrence, immune response, and drug resistance, but also contributes to the development of new therapies. Targeting dysregulated m6A regulators by inhibitors alone or combined with other therapeutic drugs may have potential efficacy.
Footnotes
Authors’ Contributions: XLM had the idea for the article, reviewed the full text articles, and wrote the initial draft of the manuscript. ZLY conducted the literature search, reviewed full text articles, and contributed to the extraction of informations. YCX extracted information from articles and plotted the figure. XLM wrote the final version in collaboration with LLQ. All authors read and approved the final manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the Natural Science Foundation of Zhejiang Province (Grant No. LQ19H160001) and Public Technology Applied Research Program of Huzhou City (Grant No. 2018GY02).
ORCID iD: Limin Xu  https://orcid.org/0000-0003-1491-7058
https://orcid.org/0000-0003-1491-7058
References
- 1. Perlman DM, Maier LA. Occupational lung disease. Med Clin North Am 2019;103:535–48 [DOI] [PubMed] [Google Scholar]
- 2. Khateeb J, Fuchs E, Khamaisi M. Diabetes and lung disease: a neglected relationship. Rev Diabet Stud 2019;15:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigris JJ, Wells AU, Ancochea J, Bouros D, Carvalho C, Costabel U, Ebina M, Hansell DM, Johkoh T, Kim DS, King TE, Jr, Kondoh Y, Myers J, Muller NL, Nicholson AG, Richeldi L, Selman M, Dudden RF, Griss BS, Protzko SL, Schunemann HJ; Fibrosis AEJACoIP. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30 [DOI] [PubMed] [Google Scholar]
- 5. Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR. Comprehensive analysis of mRNA methylation reveals enrichment in 3’ UTRs and near stop codons. Cell 2012;149:1635–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ke S, Alemu EA, Mertens C, Gantman EC, Fak JJ, Mele A, Haripal B, Zucker-Scharff I, Moore MJ, Park CY, Vagbo CB, Kussnierczyk A, Klungland A, Darnell JE, Jr, Darnell RB. A majority of M6a residues are in the last exons, allowing the potential for 3’ UTR regulation. Genes Dev 2015;29:2037–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roignant JY, Soller M. m(6)A in mRNA: an ancient mechanism for fine-tuning gene expression. Trends Genet 2017;33:380–90 [DOI] [PubMed] [Google Scholar]
- 8. Berulava T, Rahmann S, Rademacher K, Klein-Hitpass L, Horsthemke B. N6-adenosine methylation in MiRNAs. PLoS ONE 2015;10:e0118438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li H, Zhang Y, Guo Y, Liu R, Yu Q, Gong L, Liu Z, Xie W, Wang C. ALKBH1 promotes lung cancer by regulating m6A RNA demethylation. Biochem Pharmacol 2020;189:114284. [DOI] [PubMed] [Google Scholar]
- 10. Ma L, Chen T, Zhang X, Miao Y, Tian X, Yu K, Xu X, Niu Y, Guo S, Zhang C, Qiu S, Qiao Y, Fang W, Du L, Yu Y, Wang J. The m(6)A reader YTHDC2 inhibits lung adenocarcinoma tumorigenesis by suppressing SLC7A11-dependent antioxidant function. Redox Biol 2021;38:101801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Peng W, Li J, Chen R, Gu Q, Yang P, Qian W, Ji D, Wang Q, Zhang Z, Tang J, Sun Y. Upregulated METTL3 promotes metastasis of colorectal Cancer via miR-1246/SPRED2/MAPK signaling pathway. J Exp Clin Cancer Res 2019;38:393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang S, Zhao BS, Zhou A, Lin K, Zheng S, Lu Z, Chen Y, Sulman EP, Xie K, Bogler O, Majumder S, He C, Huang S. m(6)A demethylase ALKBH5 maintains tumorigenicity of glioblastoma stem-like cells by sustaining FOXM1 expression and cell proliferation program. Cancer Cell 2017;31:591–606.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang Y, Su R, Sheng Y, Dong L, Dong Z, Xu H, Ni T, Zhang ZS, Zhang T, Li C, Han L, Zhu Z, Lian F, Wei J, Deng Q, Wang Y, Wunderlich M, Gao Z, Pan G, Zhong D, Zhou H, Zhang N, Gan J, Jiang H, Mulloy JC, Qian Z, Chen J, Yang CG. Small-molecule targeting of oncogenic FTO demethylase in acute myeloid leukemia. Cancer Cell 2019;35:677–91.e10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dai D, Wang H, Zhu L, Jin H, Wang X. N6-methyladenosine links RNA metabolism to cancer progression. Cell Death Dis 2018;9:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ding C, Zou Q, Ding J, Ling M, Wang W, Li H, Huang B. Increased N6-methyladenosine causes infertility is associated with FTO expression. J Cell Physiol 2018;233:7055–66 [DOI] [PubMed] [Google Scholar]
- 16. Gu C, Shi X, Dai C, Shen F, Rocco G, Chen J, Huang Z, Chen C, He C, Huang T, Chen C. RNA m(6)A modification in cancers: molecular mechanisms and potential clinical applications. Innovation 2020;1: 100066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ruszkowska A. METTL16, methyltransferase-like protein 16: current insights into structure and function. Int J Mol Sci 2021;22:2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang L, Jia G, Yu M, Lu Z, Deng X, Dai Q, Chen W, He C. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol 2014;10:93–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ping XL, Sun BF, Wang L, Xiao W, Yang X, Wang WJ, Adhikari S, Shi Y, Lv Y, Chen YS, Zhao X, Li A, Yang Y, Dahal U, Lou XM, Liu X, Huang J, Yuan WP, Zhu XF, Cheng T, Zhao YL, Wang X, Rendtlew Danielsen JM, Liu F, Yang YG. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res 2014;24:177–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tuncel G, Kalkan R. Importance of m N(6)-methyladenosine (m(6)A) RNA modification in cancer. Med Oncol 2019;36:36. [DOI] [PubMed] [Google Scholar]
- 21. Pan Y, Ma P, Liu Y, Li W, Shu Y. Multiple functions of m(6)A RNA methylation in cancer. J Hematol Oncol 2018;11:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stone CA, Jr, Liu Y, Relling MV, Krantz MS, Pratt AL, Abreo A, Hemler JA, Phillips EJ. Immediate hypersensitivity to polyethylene glycols and polysorbates: more common than we have recognized. J Allergy Clin Immunol Pract 2019;7:1533–40.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Choe J, Lin S, Zhang W, Liu Q, Wang L, Ramirez-Moya J, Du P, Kim W, Tang S, Sliz P, Santisteban P, George RE, Richards WG, Wong KK, Locker N, Slack FJ, Gregory RI. mRNA circularization by METTL3-eIF3h enhances translation and promotes oncogenesis. Nature 2018;561: 556–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pendleton KE, Chen B, Liu K, Hunter OV, Xie Y, Tu BP, Conrad NK. The U6 snRNA m(6)A methyltransferase METTL16 regulates SAM synthetase intron retention. Cell 2017;169:824–35.e14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ma H, Wang X, Cai J, Dai Q, Natchiar SK, Lv R, Chen K, Lu Z, Chen H, Shi YG, Lan F, Fan J, Klaholz BP, Pan T, Shi Y, He C. N(6-) methyladenosine methyltransferase ZCCHC4 mediates ribosomal RNA methylation. Nat Chem Biol 2019;15:88–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van Tran N, Ernst FGM, Hawley BR, Zorbas C, Ulryck N, Hackert P, Bohnsack KE, Bohnsack MT, Jaffrey SR, Graille M, Lafontaine DLJ. The human 18S rRNA m6A methyltransferase METTL5 is stabilized by TRMT112. Nucleic Acids Res 2019;47:7719–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jia G, Fu Y, Zhao X, Dai Q, Zheng G, Yang Y, Yi C, Lindahl T, Pan T, Yang YG, He C. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol 2011;7:885–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zheng G, Dahl JA, Niu Y, Fedorcsak P, Huang CM, Li CJ, Vagbo CB, Shi Y, Wang WL, Song SH, Lu Z, Bosmans RP, Dai Q, Hao YJ, Yang X, Zhao WM, Tong WM, Wang XJ, Bogdan F, Furu K, Fu Y, Jia G, Zhao X, Liu J, Krokan HE, Klungland A, Yang YG, He C. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell 2013;49:18–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu J, Ren D, Du Z, Wang H, Zhang H, Jin Y. m(6)A demethylase FTO facilitates tumor progression in lung squamous cell carcinoma by regulating MZF1 expression. Biochem Biophys Res Commun 2018;502:456–64 [DOI] [PubMed] [Google Scholar]
- 30. Chao Y, Shang J, Ji W. ALKBH5-m(6)A-FOXM1 signaling axis promotes proliferation and invasion of lung adenocarcinoma cells under intermittent hypoxia. Biochem Biophys Res Commun 2020;521:499–506 [DOI] [PubMed] [Google Scholar]
- 31. Jin D, Guo J, Wu Y, Yang L, Wang X, Du J, Dai J, Chen W, Gong K, Miao S, Li X, Sun H. m(6)A demethylase ALKBH5 inhibits tumor growth and metastasis by reducing YTHDFs-mediated YAP expression and inhibiting miR-107/LATS2-mediated YAP activity in NSCLC. Mol Cancer 2020;19:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang J, Guo S, Piao HY, Wang Y, Wu Y, Meng XY, Yang D, Zheng ZC, Zhao Y. ALKBH5 promotes invasion and metastasis of gastric cancer by decreasing methylation of the lncRNA NEAT1. J Physiol Biochem 2019;75:379–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yang P, Wang Q, Liu A, Zhu J, Feng J. ALKBH5 holds prognostic values and inhibits the metastasis of colon cancer. Pathol Oncol Res 2020;26:1615–23 [DOI] [PubMed] [Google Scholar]
- 34. Wang J, Wang J, Gu Q, Ma Y, Yang Y, Zhu J, Zhang Q. The biological function of m6A demethylase ALKBH5 and its role in human disease. Cancer Cell Int 2020;20:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liao S, Sun H, Xu C. YTH domain: a family of N(6)-methyladenosine (m(6)A) readers. Genomics Proteomics Bioinformatics 2018;16:99–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang X, Lu Z, Gomez A, Hon GC, Yue Y, Han D, Fu Y, Parisien M, Dai Q, Jia G, Ren B, Pan T, He C. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature 2014;505:117–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Du H, Zhao Y, He J, Zhang Y, Xi H, Liu M, Ma J, Wu L. YTHDF2 destabilizes m(6)A-containing RNA through direct recruitment of the CCR4-NOT deadenylase complex. Nat Commun 2016;7:12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang X, Zhao BS, Roundtree IA, Lu Z, Han D, Ma H, Weng X, Chen K, Shi H, He C. N(6)-methyladenosine modulates messenger RNA translation efficiency. Cell 2015;161:1388–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Alarcon CR, Lee H, Goodarzi H, Halberg N, Tavazoie SF. N6-methyladenosine marks primary microRNAs for processing. Nature 2015;519: 482–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Alarcon CR, Goodarzi H, Lee H, Liu X, Tavazoie S, Tavazoie SF. HNRNPA2B1 is a mediator of m(6)A-dependent nuclear RNA processing events. Cell 2015;162:1299–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu N, Dai Q, Zheng G, He C, Parisien M, Pan T. N(6)-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature 2015;518:560–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wu B, Su S, Patil DP, Liu H, Gan J, Jaffrey SR, Ma J. Molecular basis for the specific and multivariant recognitions of RNA substrates by human hnRNP A2/B1. Nat Commun 2018;9:420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cayir A, Barrow TM, Guo L, Byun HM. Exposure to environmental toxicants reduces global N6-methyladenosine RNA methylation and alters expression of RNA methylation modulator genes. Environ Res 2019;175:228–34 [DOI] [PubMed] [Google Scholar]
- 44. Cheng C, Wu Y, Xiao T, Xue J, Sun J, Xia H, Ma H, Lu L, Li J, Shi A, Bian T, Liu Q. METTL3-mediated m(6)A modification of ZBTB4 mRNA is involved in the smoking-induced EMT in cancer of the lung. Mol Ther Nucleic Acids 2021;23:487–500 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45. Gu S, Sun D, Dai H, Zhang Z. N(6)-methyladenosine mediates the cellular proliferation and apoptosis via microRNAs in arsenite-transformed cells. Toxicol Lett 2018;292:1–11 [DOI] [PubMed] [Google Scholar]
- 46. Dai B, Sun F, Cai X, Li C, Liu H, Shang Y. Significance of RNA N6-methyladenosine regulators in the diagnosis and subtype classification of childhood asthma using the gene expression omnibus database. Front Genet 2021;12:634162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kim H, Lee YS, Kim SM, Jang S, Choi H, Lee JW, Kim TD, Kim VN. RNA demethylation by FTO stabilizes the FOXJ1 mRNA for proper motile ciliogenesis. Dev Cell 2021;56:1118–30.e6 [DOI] [PubMed] [Google Scholar]
- 48. Rabe KF, Watz H. Chronic obstructive pulmonary disease. Lancet 2017; 389:1931–40 [DOI] [PubMed] [Google Scholar]
- 49. Huang X, Lv D, Yang X, Li M, Zhang H. m6A RNA methylation regulators could contribute to the occurrence of chronic obstructive pulmonary disease. J Cell Mol Med 2020;24:12706–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Duffy SP, Criner GJ. Chronic obstructive pulmonary disease: evaluation and management. Med Clin North Am 2019;103:453–61 [DOI] [PubMed] [Google Scholar]
- 51. Xia H, Wu Y, Zhao J, Li W, Lu L, Ma H, Cheng C, Sun J, Xiang Q, Bian T, Liu Q. The aberrant cross-talk of epithelium-macrophages via METTL3-regulated extracellular vesicle miR-93 in smoking-induced emphysema. Cell Biol Toxicol 2021;38:167–83 [DOI] [PubMed] [Google Scholar]
- 52. Britto CJ, Brady V, Lee S, Dela Cruz CS. Respiratory viral infections in chronic lung diseases. Clin Chest Med 2017;38:87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ballester B, Milara J, Cortijo J. Idiopathic pulmonary fibrosis and lung cancer: mechanisms and molecular targets. Int J Mol Sci 2019; 20:593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Han B, Chu C, Su X, Zhang N, Zhou L, Zhang M, Yang S, Shi L, Zhao B, Niu Y, Zhang R. N(6)-methyladenosine-dependent primary microRNA-126 processing activated PI3K-AKT-mTOR pathway drove the development of pulmonary fibrosis induced by nanoscale carbon black particles in rats. Nanotoxicology 2020;14:1–20 [DOI] [PubMed] [Google Scholar]
- 55. Sun W, Li Y, Ma D, Liu Y, Xu Q, Cheng D, Li G, Ni C. ALKBH5 promotes lung fibroblast activation and silica-induced pulmonary fibrosis through miR-320a-3p and FOXM1. Cell Mol Biol Lett 2022;27:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Li X, Peng C, Zhu Z, Cai H, Zhuang Q. The networks of m(6)A-SARS-CoV-2 related genes and immune infiltration patterns in idiopathic pulmonary fibrosis. Aging (Albany NY) 2021;13:6273–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nathan SD, Barbera JA, Gaine SP, Harari S, Martinez FJ, Olschewski H, Olsson KM, Peacock AJ, Pepke-Zaba J, Provencher S, Weissmann N, Seeger W. Pulmonary hypertension in chronic lung disease and hypoxia. Eur Respir J 2019;53:1802148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pullamsetti SS, Kojonazarov B, Storn S, Gall H, Salazar Y, Wolf J, Weigert A, El-Nikhely N, Ghofrani HA, Krombach GA, Fink L, Gattenlohner S, Rapp UR, Schermuly RT, Grimminger F, Seeger W, Savai R. Lung cancer-associated pulmonary hypertension: role of microenvironmental inflammation based on tumor cell-immune cell cross-talk. Sci Transl Med 2017;9:eaai9048 [DOI] [PubMed] [Google Scholar]
- 59. Hu L, Wang J, Huang H, Yu Y, Ding J, Yu Y, Li K, Wei D, Ye Q, Wang F, Shen B, Chen J, Fulton DJR, Chen F. YTHDF1 Regulates Pulmonary Hypertension through Translational Control of MAGED1. Am J Respir Crit Care Med 2021;203:1158–72 [DOI] [PubMed] [Google Scholar]
- 60. Qin Y, Qiao Y, Li L, Luo E, Wang D, Yao Y, Tang C, Yan G. The m(6)A methyltransferase METTL3 promotes hypoxic pulmonary arterial hypertension. Life Sci 2021;274:119366. [DOI] [PubMed] [Google Scholar]
- 61. Lin S, Choe J, Du P, Triboulet R, Gregory RI. The m(6)A methyltransferase METTL3 promotes translation in human cancer cells. Mol Cell 2016;62:335–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jin D, Guo J, Wu Y, Du J, Yang L, Wang X, Di W, Hu B, An J, Kong L, Pan L, Su G. m(6)A mRNA methylation initiated by METTL3 directly promotes YAP translation and increases YAP activity by regulating the MALAT1-miR-1914-3p-YAP axis to induce NSCLC drug resistance and metastasis. J Hematol Oncol 2019;12:135. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 63. Song H, Li H, Ding X, Li M, Shen H, Li Y, Zhang X, Xing L. Long noncoding RNA FEZF1AS1 facilitates nonsmall cell lung cancer progression via the ITGA11/miR516b5p axis. Int J Oncol 2020;57:1333–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wanna-Udom S, Terashima M, Lyu H, Ishimura A, Takino T, Sakari M, Tsukahara T, Suzuki T. The m6A methyltransferase METTL3 contributes to Transforming Growth Factor-beta-induced epithelial-mesenchymal transition of lung cancer cells through the regulation of JUNB. Biochem Biophys Res Commun 2020;524:150–5 [DOI] [PubMed] [Google Scholar]
- 65. Wang H, Deng Q, Lv Z, Ling Y, Hou X, Chen Z, Dinglin X, Ma S, Li D, Wu Y, Peng Y, Huang H, Chen L. N6-methyladenosine induced miR-143-3p promotes the brain metastasis of lung cancer via regulation of VASH1. Mol Cancer 2019;18:181. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 66. Du Y, Hou G, Zhang H, Dou J, He J, Guo Y, Li L, Chen R, Wang Y, Deng R, Huang J, Jiang B, Xu M, Cheng J, Chen GQ, Zhao X, Yu J. SUMOylation of the m6A-RNA methyltransferase METTL3 modulates its function. Nucleic Acids Res 2018;46:5195–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hui L, Zhang S, Wudu M, Ren H, Xu Y, Zhang Q, Qiu X. CBLL1 is highly expressed in non-small cell lung cancer and promotes cell proliferation and invasion. Thorac Cancer 2019;10:1479–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Liu Z, Wu Y, Tao Z, Ma L. E3 ubiquitin ligase Hakai regulates cell growth and invasion, and increases the chemosensitivity to cisplatin in non-small-cell lung cancer cells. Int J Mol Med 2018;42:1145–51 [DOI] [PubMed] [Google Scholar]
- 69. Weng CH, Chen LY, Lin YC, Shih JY, Lin YC, Tseng RY, Chiu AC, Yeh YH, Liu C, Lin YT, Fang JM, Chen CC. Epithelial-mesenchymal transition (EMT) beyond EGFR mutations per se is a common mechanism for acquired resistance to EGFR TKI. Oncogene 2019;38:455–68 [DOI] [PubMed] [Google Scholar]
- 70. Zhao W, Xie Y. KIAA1429 promotes the progression of lung adenocarcinoma by regulating the m6A level of MUC3A. Pathol Res Pract 2021;217:153284. [DOI] [PubMed] [Google Scholar]
- 71. Li J, Han Y, Zhang H, Qian Z, Jia W, Gao Y, Zheng H, Li B. The m6A demethylase FTO promotes the growth of lung cancer cells by regulating the m6A level of USP7 mRNA. Biochem Biophys Res Commun 2019; 512:479–85 [DOI] [PubMed] [Google Scholar]
- 72. Ding Y, Qi N, Wang K, Huang Y, Liao J, Wang H, Tan A, Liu L, Zhang Z, Li J, Kong J, Qin S, Jiang Y. FTO facilitates lung adenocarcinoma cell progression by activating cell migration through mRNA demethylation. Onco Targets Ther 2020;13:1461–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zhu Z, Qian Q, Zhao X, Ma L, Chen P. N(6)-methyladenosine ALKBH5 promotes non-small cell lung cancer progress by regulating TIMP3 stability. Gene 2020;731:144348. [DOI] [PubMed] [Google Scholar]
- 74. Shi Y, Fan S, Wu M, Zuo Z, Li X, Jiang L, Shen Q, Xu P, Zeng L, Zhou Y, Huang Y, Yang Z, Zhou J, Gao J, Zhou H, Xu S, Ji H, Shi P, Wu DD, Yang C, Chen Y. YTHDF1 links hypoxia adaptation and non-small cell lung cancer progression. Nat Commun 2019;10:4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sheng H, Li Z, Su S, Sun W, Zhang X, Li L, Li J, Liu S, Lu B, Zhang S, Shan C. YTH domain family 2 promotes lung cancer cell growth by facilitating 6-phosphogluconate dehydrogenase mRNA translation. Carcinogenesis 2020;41:541–50 [DOI] [PubMed] [Google Scholar]
- 76. Chen Z, Chen X, Lei T, Gu Y, Gu J, Huang J, Lu B, Yuan L, Sun M, Wang Z. Integrative analysis of NSCLC identifies LINC01234 as an oncogenic lncRNA that interacts with HNRNPA2B1 and regulates miR-106b biogenesis. Mol Ther 2020;28:1479–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Muller S, Glass M, Singh AK, Haase J, Bley N, Fuchs T, Lederer M, Dahl A, Huang H, Chen J, Posern G, Huttelmaier S. IGF2BP1 promotes SRF-dependent transcription in cancer in a m6A- and miRNA-dependent manner. Nucleic Acids Res 2019;47:375–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Du M, Zhang Y, Mao Y, Mou J, Zhao J, Xue Q, Wang D, Huang J, Gao S, Gao Y. MiR-33a suppresses proliferation of NSCLC cells via targeting METTL3 mRNA. Biochem Biophys Res Commun 2017;482:582–9 [DOI] [PubMed] [Google Scholar]
- 79. Wei W, Huo B, Shi X. miR-600 inhibits lung cancer via downregulating the expression of METTL3. Cancer Manag Res 2019;11:1177–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Weng L, Qiu K, Gao W, Shi C, Shu F. LncRNA PCGEM1 accelerates non-small cell lung cancer progression via sponging miR-433-3p to upregulate WTAP. BMC Pulm Med 2020;20:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Chen WW, Qi JW, Hang Y, Wu JX, Zhou XX, Chen JZ, Wang J, Wang HH. Simvastatin is beneficial to lung cancer progression by inducing METTL3-induced m6A modification on EZH2 mRNA. Eur Rev Med Pharmacol Sci 2020;24:4263–70 [DOI] [PubMed] [Google Scholar]
- 82. Huang H, Weng H, Chen J. m(6)A modification in coding and non-coding RNAs: roles and therapeutic implications in cancer. Cancer Cell 2020;37:270–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Meng Q, Wang S, Zhou S, Liu H, Ma X, Zhou X, Liu H, Xu C, Jiang W. Dissecting the m(6)A methylation affection on afatinib resistance in non-small cell lung cancer. Pharmacogenomics J 2020;20:227–34 [DOI] [PubMed] [Google Scholar]
- 84. Huang W, Qi CB, Lv SW, Xie M, Feng YQ, Huang WH, Yuan BF. Determination of DNA and RNA methylation in circulating tumor cells by mass spectrometry. Anal Chem 2016;88:1378–84 [DOI] [PubMed] [Google Scholar]
- 85. Zhuang Z, Chen L, Mao Y, Zheng Q, Li H, Huang Y, Hu Z, Jin Y. Diagnostic, progressive and prognostic performance of m(6)A methylation RNA regulators in lung adenocarcinoma. Int J Biol Sci 2020;16:1785–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Su R, Dong L, Li C, Nachtergaele S, Wunderlich M, Qing Y, Deng X, Wang Y, Weng X, Hu C, Yu M, Skibbe J, Dai Q, Zou D, Wu T, Yu K, Weng H, Huang H, Ferchen K, Qin X, Zhang B, Qi J, Sasaki AT, Plas DR, Bradner JE, Wei M, Marcucci G, Jiang X, Mulloy JC, Jin J, He C, Chen J. R-2HG exhibits anti-tumor activity by targeting FTO/m(6)A/MYC/CEBPA signaling. Cell 2018;172:90–105.e23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Cui Q, Shi H, Ye P, Li L, Qu Q, Sun G, Sun G, Lu Z, Huang Y, Yang CG, Riggs AD, He C, Shi Y. m(6)A RNA methylation regulates the self-renewal and tumorigenesis of glioblastoma stem cells. Cell Rep 2017;18:2622–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Huang Y, Yan J, Li Q, Li J, Gong S, Zhou H, Gan J, Jiang H, Jia GF, Luo C, Yang CG. Meclofenamic acid selectively inhibits FTO demethylation of m6A over ALKBH5. Nucleic Acids Res 2015;43:373–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Li Y, Gu J, Xu F, Zhu Q, Chen Y, Ge D, Lu C. Molecular characterization, biological function, tumor microenvironment association and clinical significance of m6A regulators in lung adenocarcinoma. Brief Bioinform 2020;22:bbaa225 [DOI] [PubMed] [Google Scholar]
- 90. Zhang Z, Zhang C, Yang Z, Zhang G, Wu P, Luo Y, Zeng Q, Wang L, Xue Q, Zhang Y, Sun N, He J. m(6)A regulators as predictive biomarkers for chemotherapy benefit and potential therapeutic targets for overcoming chemotherapy resistance in small-cell lung cancer. J Hematol Oncol 2021;14:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Zhang Y, Liu X, Liu L, Li J, Hu Q, Sun R. Expression and Prognostic Significance of m6A-Related Genes in Lung Adenocarcinoma. Med Sci Monit 2020;26:e919644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wu X, Sheng H, Wang L, Xia P, Wang Y, Yu L, Lv W, Hu J. A five-m6A regulatory gene signature is a prognostic biomarker in lung adenocarcinoma patients. Aging 2021;13:10034–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, Viale A, Olshen AB, Gerald WL, Massague J. Genes that mediate breast cancer metastasis to lung. Nature 2005;436:518–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Popper HH. Progression and metastasis of lung cancer. Cancer Metastasis Rev 2016;35:75–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Chen F, Chen Z, Guan T, Zhou Y, Ge L, Zhang H, Wu Y, Jiang GM, He W, Li J, Wang H. N6-methyladenosine regulates mRNA stability and translation efficiency of KRT7 to promote breast cancer lung metastasis. Cancer Res 2021;81:2847–60 [DOI] [PubMed] [Google Scholar]
- 96. Zhang C, Chen L, Liu Y, Huang J, Liu A, Xu Y, Shen Y, He H, Xu D. Downregulated METTL14 accumulates BPTF that reinforces super-enhancers and distal lung metastasis via glycolytic reprogramming in renal cell carcinoma. Theranostics 2021;11:3676–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Selberg S, Blokhina D, Aatonen M, Koivisto P, Siltanen A, Mervaala E, Kankuri E, Karelson M. Discovery of small molecules that activate RNA methylation through cooperative binding to the METTL3-14-WTAP complex active site. Cell Rep 2019;26:3762–71.e5 [DOI] [PubMed] [Google Scholar]
- 98. Bu T, Wang C, Jin H, Meng Q, Huo X, Sun H, Sun P, Wu J, Ma X, Liu Z, Liu K. Organic anion transporters and PI3K-AKT-mTOR pathway mediate the synergistic anticancer effect of pemetrexed and rhein. J Cell Physiol 2020;235:3309–19 [DOI] [PubMed] [Google Scholar]