Abstract
Purpose
To describe the clinical course and management of a patient with bilateral retinal vasculitis associated with cold agglutinin disease (CAD) treated with obinutuzumab and infliximab.
Observations
A 69-year-old Hispanic woman was referred to a tertiary Uveitis Clinic with progressively worsening blurry vision, right eye (OD) worse than left eye (OS). Past ocular history was significant for epiretinal membranes in both eyes (OU). Past medical history was notable for non-specific joint disease, primarily affecting her knees bilaterally, and pulmonary symptoms (e.g., dyspnea, productive cough) of unclear etiologies one year before presentation. She had been evaluated by rheumatologists and pulmonologists and was placed on low doses of prednisone and methotrexate. Upon examination, her visual acuity was 20/40 in OD and 20/25 in OS. Anterior segment exam was unremarkable with no cell or flare in OU. Dilated fundus examination was notable for 0.5+ vitreous haze in OU and mild vessel attenuation in OU. Wide-angle fluorescein angiography (FA) revealed mild bilateral periphery peri-vasculature leakage in OU. Initial blood evaluations revealed decreased hematocrit, and positive anti-nuclear antibody. Her peripheral smear disclosed 3+ agglutination. She was initially treated with mycophenolate mofetil 1000 mg twice daily and prednisone 20 mg then referred to hematology. Further work up revealed high-titer cold agglutinin and positive thermal amplitude screen at 30 °C. Bone marrow examination demonstrated a chronic lymphocytic leukemia (CLL)-like monoclonal B-cell lymphocytosis. Anti-CD20 monoclonal antibody therapy with obinutuzumab was started in an effort to treat the underlying CLL clone and address the associated ocular vasculitis related to CAD. Three months later, after eight cycles of obinutuzumab, the patient's best- corrected visual acuity (BCVA) continued to be stable at 20/30 in OD and 20/20 in OS. However, FA showed persistent diffuse perivascular leakage. Intravenous infliximab with concurrent intravenous methylprednisolone infusions were started. After two cycles of treatment, FA showed significantly improved perivascular leakage. Visual acuity remained stable at 20/25 in OU.
Conclusions and importance
Ocular involvement in CAD is rare. The index case is the first report of retinal vasculitis in a patient with CAD. Our report not only describes the unique course of CAD-related retinal vasculitis, but also introduces and underscores a successful therapeutic plan.
Keywords: Cold agglutinin disease, Retinal vasculitis, Anti-CD20, Obinutuzumab, Infliximab, Tumor necrosis factor-alpha
1. Introduction
Cold agglutinin disease (CAD) is a rare hemolytic disease accounting for 15% of autoimmune hemolytic anemia (AIHA).1 AIHA is caused by warm-, cold-, or mixed-reactive antibody types directed against antigens on the red blood cell (RBC) surface. Hemolysis mediated by cold autoantibodies, primarily immunoglobulin M (IgM), is known as cold-antibody AIHAs or CAD. At core body temperature, circulating IgM antibodies remain unbound; however, in peripheral circulation, where blood temperature is cooler, IgM binds to the RBC membrane, activating the complement cascade that ultimately results in hemolysis.1 Cold agglutinin IgM molecules can be either polyclonal or monoclonal. Polyclonal antibodies, commonly seen in children, are typically postinfectious and self-resolving, while monoclonal antibodies, seen more often in older adults with a lymphoproliferative disorder, are known as primary CAD and are often treatment-resistant.1,2 Primary CAD represents a spectrum of clonal lymphoproliferative bone marrow disorders, with most patients having either an indolent B-cell lymphoma or plasma-cell neoplasm associated with monoclonal gammopathy.3 In a retrospective study of 86 patients with CAD, Berentsen et al. found an abnormal κ/λ ratio in bone marrow in 90% of patients, lymphoma in 76%, and lymphoplasmacytic lymphoma in 50%.3 Additionally, in another retrospective study of 89 patients with CAD, Swiecicki et al. found that 78% of patients had an underlying hematologic disorder, 47% of which was MGUS1
CAD is typically seen in the seventh decade of life and predominantly affects women.3, 4, 5, 6 Diagnostic criteria for CAD include evidence of chronic hemolysis, positive polyspecific direct antiglobulin test (DAT), positive monospecific DAT for C3d with classically negative IgG, cold agglutinin titer ≥64 at 4 °C, and no overt malignant disease.7 Clinical features vary in severity but include hemolysis, cold-induced circulatory symptoms, livedo reticularis, Raynaud's disease, acrocyanosis, and rarely, cutaneous necrosis.8, 9, 10, 11, 12 Ocular manifestations have rarely been reported.13
To the best of our knowledge, there are no published reports of bilateral retinal vasculitis in CAD patients. We herein report the clinical course and management of bilateral retinal vasculitis associated with CAD that demonstrated minimal response to anti-CD20 monoclonal antibody obinutuzumab and significant improvement following treatment with intravenous (IV) anti-tumor necrosis factor (TNF) infliximab and methylprednisolone.
1.1. Case report
A 69-year-old Hispanic woman was referred to a tertiary Uveitis Clinic with progressively worsening blurry vision, right eye (OD) more than left eye (OS). Past ocular history was significant for epiretinal membranes in both eyes (OU). She has a history of cholecystectomy, oophorectomy, and breast lumpectomy with no malignancy results. The patient's recent medical history was notable for non-specific joint disease, primarily affecting her knees bilaterally, and pulmonary symptoms (e.g., dyspnea, productive cough) of unclear etiologies one year before presentation. She had been evaluated by rheumatologists and pulmonologists and was placed on low doses of prednisone and methotrexate.
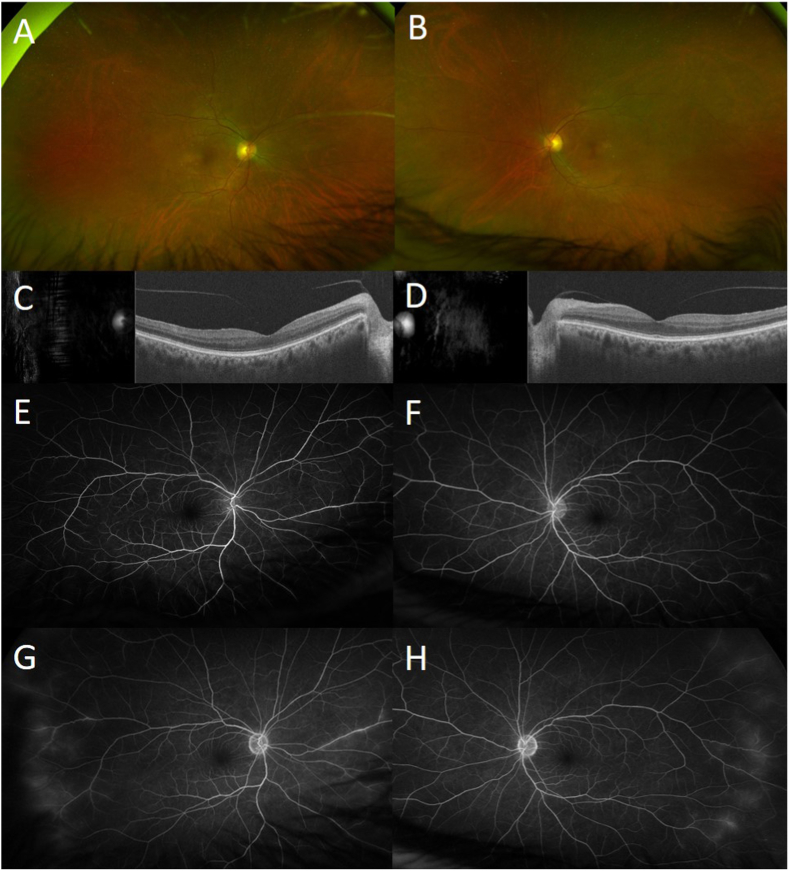
Upon examination, the Snellen best-corrected visual acuity (BCVA) was 20/40 in OD and 20/25 in OS. Intraocular pressure (IOP) was 13 mm Hg in OU. Slit-lamp examination was remarkable only for nuclear sclerotic cataracts in OU (2+ in OD, 1+ in OS), with no cell or flare in OU. Dilated fundus examination was notable for 0.5+ vitreous haze in OU and mild vessel attenuation in OU (Fig. 1A and B). Spectral-domain optical coherence tomography (SD-OCT) showed preserved foveal contour without sub- or intraretinal fluid in OU (Fig. 1C and D). Wide angle fluorescein angiography (FA) was performed and revealed mild bilateral periphery peri-vasculature leakage (Fig. 1E and F). Initial evaluations, including liver function tests, basic metabolic panel, hemoglobin A1c, angiotensin-converting enzyme, lysozyme, QuantiFERON, syphilis titers, anti-dsDNA, and chest X-ray were within normal limits, except for positive antinuclear antibody (ANA) at 1:80. Complete blood count was remarkable for leukocytosis (at 11.6 K/μL) and decreased hemoglobin (at 11.6 g/dl), that had previously been low (at 10.2 g/dl) in the prior year. Initially, methotrexate was discontinued, and the patient was treated with mycophenolate mofetil 1000 mg twice daily and prednisone 20 mg.
Fig. 1.
Wide-field fundus photos at the first visit showing mild vessel attenuation in both eyes (A–B). Spectral domain optical coherence tomography showed preserved foveal contour without sub- or intraretinal fluid (C–D). Wide angle fluorescein angiography revealed mild bilateral periphery peri-vasculature leakage (E–F).
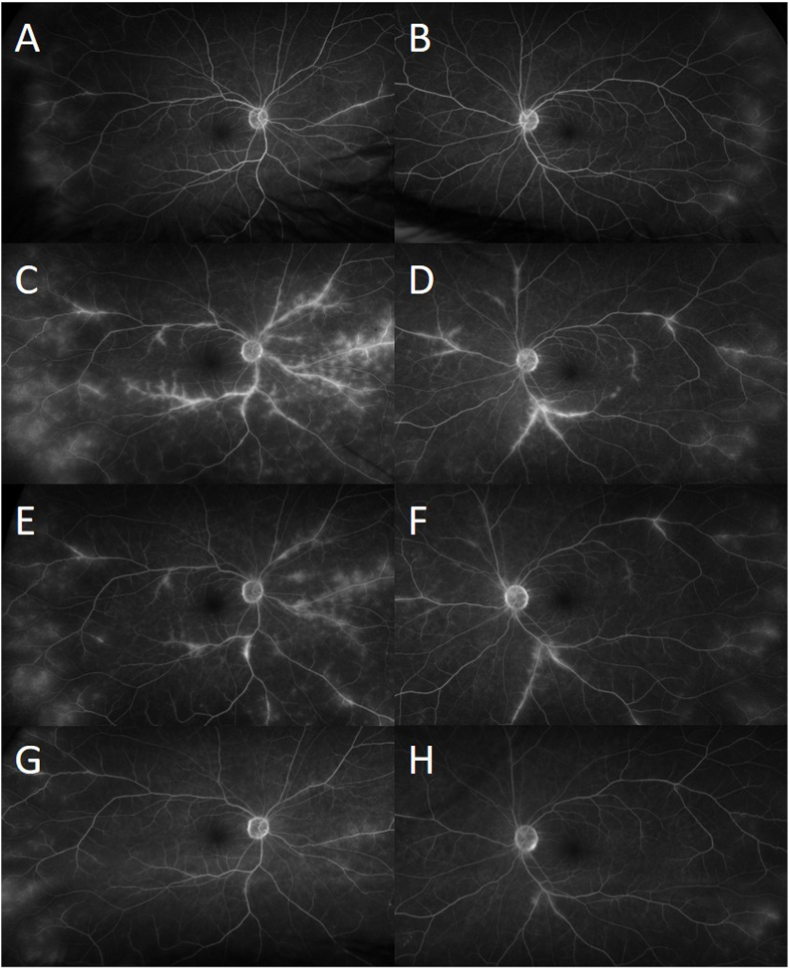
Over the next two follow-up appointments, the patient showed little to no improvement in both functional and anatomic visual findings. While the patient's BCVA continued to be stable at 20/25 in OD and 20/20 in OS, follow-up FA showed worsening of the peri-vascular leakage in the periphery (Fig. 2A). Further attention was paid to her chronic anemia. However, her RBC count, mean corpuscular volume (MCV), and mean corpuscular hemoglobin concentration (MCHC) were unable to be assessed due to 3+ agglutination of RBC. Additional laboratory evaluations were positive for cold antibody screen and direct antiglobulin test (DAT). Evaluations revealed positive DAT with positive C3 and negative IgG, positive cold agglutinin screen, cold agglutinin titer (>1:512) with positive thermal amplitude screen at 30 °C, and negative cryoglobulins, which collectively led to the diagnosis of CAD. Serum protein electrophoresis (SPEP) revealed no monoclonal proteins, and immunoglobulin panel was unremarkable. These hematologic abnormalities led to prompt referral to the Hematology Service. Bone marrow examination demonstrated a chronic lymphocytic leukemia (CLL)-like monoclonal B-cell lymphocytosis. As the retinal vasculitis progressed (Fig. 2C and D), anti-CD20 monoclonal antibody therapy with obinutuzumab was started. Treatment began as weekly infusions and was later lengthened to every 2- and then 3 weeks.
Fig. 2.
At subsequent visits, wide angle fluorescein angiography showed increase leakage of retinal vessels as retinal vasculitis progressed in both eyes (C–D) compared to previous visit (A–B). Three months later, after eight cycles of obinutuzumab, fluorescein angiography showed only minimal improvement with persistent bilateral diffuse perivascular leakage (E–F). After two cycles of monthly intravenous infliximab with intravenous methylprednisolone, fluorescein angiography showed significant improvement in perivascular leakage in both eyes (G–H).
Three months later, after eight cycles of obinutuzumab, the patient's BCVA remained stable at 20/30 in OD and 20/20 in OS. Hematocrit, RBC count, MCV, and MCHC returned to normal, and RBC agglutination was reduced to 1+. However, six months after obinutuzumab therapy, FA showed only mild improvement with persistent diffuse perivascular leakage (Fig. 2E and F). After a discussion of options including additional CLL-targeting agents, therapy was changed to monthly intravenous infliximab (7.5 mg/kg) with concurrent intravenous methylprednisolone (750 mg) for three consecutive days of infusions each month. After two cycles of treatment, FA showed significant improvement in perivascular leakage (Fig. 2G and H), and her BCVA continued to be stable at 20/25 in OU.
2. Discussion
2.1. Generating a diagnosis
We have presented a case of a 69-year-old female with unexplained fatigue who developed blurry vision in OU. When we found that the patient had diffuse leakage affecting all major retinal vessels with extension to the periphery on FA, our primary goal was to determine the underlying etiology of her retinal vasculitis, which was not clear initially. The differential diagnosis for retinal vasculitis is wide-ranging and includes infectious causes (e.g. bacterial, viral, fungal, parasitic), ocular disorders (e.g. Eale's disease, birdshot chorioretinopathy, pars planitis, acute multifocal hemorrhagic retinal vasculitis, Vogt-Koyanagi-Harada syndrome, etc.), systemic inflammatory diseases (Adamantiades-Behçet's disease, sarcoidosis, granulomatosis with polyangiitis, lupus, rheumatoid arthritis, etc.), and malignancy (e.g. leukemia, lymphoma, paraneoplastic syndromes, etc.). No etiology was found on systemic examination and extensive evaluations for infectious uveitis. At subsequent follow-up visits in the Uveitis Clinics, FA showed worsening of the peri-vascular leakage in the periphery. Further attention was directed to her complete blood count to evaluate the patient's chronic anemia. Unexpectedly, her RBC count, MCV, and MCHC were unable to be assessed and reported due to 3+ RBC agglutination. Such findings led to the diagnosis of CAD and prompt referral to the Hematology Service.
The pathophysiology of CAD is related to monoclonal proteins produced in the setting of lymphoproliferative disorders. Because of the monoclonal IgM antibodies seen in CAD, monoclonal gammopathy of undetermined significance (MGUS) and Waldenström macroglobulinemia are included in the differential diagnosis. To evaluate for lymphoproliferative disorders, bone marrow examination and axial imaging are often required.1 CAD has been described in patients with diffuse large B-cell lymphoma, carcinoma, sarcoma, metastatic melanoma, and CLL. Bone marrow evaluations of our patient showed a CLL-like population that could be a potential etiology for her CAD.
Can type II-III hypersensitivity explain the connection between retinal vasculitis and CAD?
Vasculitis is usually the result of immune responses directed against antigens present in the vessel structures. The inflammatory reaction occurs by auto reactive T-lymphocytes, which mount a cellular attack, and B-lymphocytes, which produce auto-antibodies. Auto reactive T-lymphocytes target and damage organs such as the vascular endothelium, arterial wall structure, intracytoplasmic granules, and intranuclear proteins of nucleated cells.14 The underlying pathophysiology of vasculitis is categorized into four basic hypersensitivity reactions. Type I reactions are characterized by elevated IgE levels in the blood and tissues, type II reactions are characterized by ANCA-associated vasculitides, type III reactions are mediated by immune complex formation and deposition in the vessels, and type IV reactions cause granulomatous arteritis.
AIHA is one example of a type II hypersensitivity reaction. The immune hemolytic anemia has two subtypes: warm AIHA is IgG-mediated and cold AIHA is IgM-mediated.15 CAD-related clinical findings occur due to the cold AIHA. CAD-related retinal vasculitis has not yet been reported in the literature. CAD causes extravascular hemolysis and hyperviscosity by an antibody and complement-mediated process. A study showed that AIHA is a risk factor for venous thrombosis and the incidence is 30%.16 Wilson et al. reported a patient who developed CAD after Mycoplasma pneumoniae pneumonia and recurrent arterial thrombosis; the authors suspected that the cause of the thrombosis was related to an autoimmune vasculitis after the Mycoplasma pneumoniae pneumonia infection, but they did not relate it with CAD.17 Otsuka et al. reported a patient who developed neuropathy secondary to necrotizing vasculitis that was suspected to be related to CAD.18 Although no cases of retinal vasculitis have been reported in the context of CAD, one report shared a patient with retinal abnormalities, scattered retinal hemorrhages, and aggregating material visibly flowing in the retinal vessel wall as well as FA findings demonstrated the aggregating material most clearly but without leakage shown.13 Therefore, the etiology of retinal vasculitis in our patient may be associated with CAD-related autoimmunity as a type II reaction targeting the vascular structure.
Although the precise pathophysiology of retinal vasculitis remains unclear, animal models of retinal vasculitis have shown the presence of vascular sheathing and cuffing,19 suggesting that retinal vasculitis might also be due to a type III hypersensitivity reaction, in which immune (antigen-antibody) complex deposition not cleared by innate immune cells could cause an inflammatory response. Given that CAD involves the formation of cold agglutinin antibodies against red blood cells (typically against “I” or “i” antigens), it is plausible that these immune complexes could give rise to an inflammatory response in vessels throughout the body, including the retina.
Currently, there is no explanation for the association between CAD and vasculitis. Salacki et al. shared a case report of myocarditis in a patient with a history of antiphospholipid syndrome and systemic vasculitis in the context of CAD.20 Similarly, İshashiki et al. described a patient who developed acute diffuse retinal phlebitis and placoid lesions in the first stage of disease and choroidal neovascularization (CNV) and hemorrhagic macular detachment in the second stage. During the acute stage of the disease and the late macular complication, the serum cold agglutinin titer showed significant increases as an isolated laboratory finding, though no clinical or other laboratory signs suggested viral infection.21 Our patient showed bilateral periphlebitis with a diagnosis of CAD without any symptoms suggestive of systemic or infectious causes.
2.2. Current treatments for retinal vasculitis associated cold agglutination disease
The management of retinal vasculitis depends largely on the underlying etiology. Achieving adequate control of the underlying inflammation is particularly important, due to the risk of recurrence and possibility that sequelae from untreated retinal vasculitis can lead to lasting and severe visual loss. Before beginning immunosuppressive therapy, it is particularly crucial to rule out infectious causes, as we did for our patient. The mainstay of non-infectious retinal vasculitis treatment includes corticosteroids (local or systemic) and steroid-sparing immunosuppressants.
In our patient, we decided to treat the retinal vasculitis with anti-B-cell therapy, hypothesizing that CAD caused by the CLL-like lymphoproliferation developed retinal vasculitis. Under normal circumstances, the CLL-like population of our patient would prompt annual monitoring rather than intervention. However, because CAD was a potential cause of her visual symptoms, CLL-like therapy started with single-agent obinutuzumab, a humanized anti-CD20 monoclonal antibody. Her CAD condition responded to the treatment; her RBC no longer agglutinated. However, her fluorescein angiography showed only minimal improvement with persistent bilateral diffuse perivascular leakage. Due to the minimal response to this therapy, we changed to infliximab, a TNF-α inhibitor, in conjunction with methylprednisolone, which showed significant improvement.
Recent reports have suggested that TNF receptor signaling is a driver of CLL,22 and that anti-TNF-α therapy may play a role in the clinical management of CLL.23 Given that the patient was already on corticosteroids (prednisone 10 mg/day) and we did not want to increase the dosage, we started the patient on anti-TNF-α therapy; the recommendation was to start IMT when a patient's required prednisone dosage exceeds 10mg/day.24 Moreover, due to the chronic and refractory nature of the retinal vasculitis in this patient, and the need to establish rapid control of the patient's inflammation to prevent permanent damage, we chose to begin concurrent treatment with IV methylprednisolone infusions for 3 days with each monthly cycle of infliximab. AIHA management with infliximab also has been reported in cases associated with ulcerative colitis (UC). Leo-Carnerero et al. reported two cases of UC with AIHA that responded well to infliximab treatment in both conditions. The authors suggested AIHA was caused by the cross-reactivity of red blood cells and antibodies formed against antigens in the colon, and UC patients with AIHA can improve in both conditions with infliximab treatment.25 Additionally, Strik et al. reported a case of a 61‐year‐old male Crohn's disease patient who was diagnosed with hemolytic anemia 6 months after switching from infliximab to CT‐P13, with subsequent improvement after switching back to infliximab. The patient is currently in complete remission while receiving monotherapy with infliximab originator.26
To the best of our knowledge, no case of AIHA with retinal vasculitis has yet been reported; moreover, no treatment for this condition has been described. In this report, we described a patient whose AIHA and retinal vasculitis responded to therapy with infliximab and remained stable. The patient currently remains clinically stable on infliximab therapy, with plan to transit to oral immunomodulatory therapy in the near future.
3. Conclusion
The index case is the first report to our knowledge that demonstrates a possible association between retinal vasculitis and CAD. It remains unclear whether CAD was the definitive cause of the retinal vasculitis. Because the patient did not respond to obinutuzumab but responded to infliximab and methylprednisolone, our case raises the point that ocular manifestations of CAD may not respond to anti-CD20 monotherapy and may require therapy with methylprednisolone and anti-TNF agents such as infliximab. Future studies will be needed to further understand the potential associations between retinal vasculitis and CAD and to guide clinical management.
4. Patient consent
Consent to publish this case report has been obtained from the patient in writing.
Funding
No funding or grant support
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Declaration of competing interest
None of the authors has any relevant conflict of interests pertaining to the index manuscript.
Acknowledgments
The authors acknowledge the medical providers at the Byers Eye Institute at Stanford for their contribution to the evaluation, diagnosis, and management of this patient.
References
- 1.Swiecicki P.L., Hegerova L.T., Gertz M.A. Cold agglutinin disease. Blood. 2013;122:1114–1121. doi: 10.1182/blood-2013-02-474437. [DOI] [PubMed] [Google Scholar]
- 2.Berentsen S., Randen U., Tjonnfjord G.E. Cold agglutinin-mediated autoimmune hemolytic anemia. Hematol Oncol Clin N Am. 2015;29:455–471. doi: 10.1016/j.hoc.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Berentsen S., Ulvestad E., Langholm R., et al. Primary chronic cold agglutinin disease: a population based clinical study of 86 patients. Haematologica. 2006;91:460–466. [PubMed] [Google Scholar]
- 4.Petz L.D. Cold antibody autoimmune hemolytic anemias. Blood Rev. 2008;22:1–15. doi: 10.1016/j.blre.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Hadnagy C. Agewise distribution of idiopathic cold agglutinin disease. Zeitschrift fur Gerontologie. 1993;26:199–201. [PubMed] [Google Scholar]
- 6.Mack P., Freedman J. Autoimmune hemolytic anemia: a history. Transfus Med Rev. 2000;14:223–233. doi: 10.1053/tm.2000.7392. [DOI] [PubMed] [Google Scholar]
- 7.Berentsen S., Tjønnfjord G.E. Diagnosis and treatment of cold agglutinin mediated autoimmune hemolytic anemia. Blood Rev. 2012;26:107–115. doi: 10.1016/j.blre.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Karunarathne S., Weerasinghe S., Govindapala D., Fernando H., Jayaratne B. Cold autoimmune haemolytic anaemia secondary to Epstein Barr virus infection presenting with peripheral gangrene; case report. Thromb J. 2012;10:4. doi: 10.1186/1477-9560-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Läuchli S., Widmer L., Lautenschlager S. Cold agglutinin disease--the importance of cutaneous signs. Dermatology (Basel) 2001;202:356–358. doi: 10.1159/000051682. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell A.B., Pergrum G.D., Gill A.M. Cold agglutinin disease with Raynaud's phenomenon. Proc Roy Soc Med. 1974;67:113–115. [PMC free article] [PubMed] [Google Scholar]
- 11.Oh S.H., Kim D.S., Ryu D.J., Lee K.H. Extensive cutaneous necrosis associated with low titres of cold agglutinins. Clin Exp Dermatol. 2009;34:e229–e230. doi: 10.1111/j.1365-2230.2008.03078.x. [DOI] [PubMed] [Google Scholar]
- 12.Poldre P., Pruzanski W., Chiu H.M., Dotten D.A. Fulminant gangrene in transient cold agglutinemia associated with Escherichia coli infection. Can Med Assoc J. 1985;132:261–263. [PMC free article] [PubMed] [Google Scholar]
- 13.Mahroo O.A., Seshadri N., Whitefield L.A. Case report: retinopathy in a patient with cold hemagglutinin disease. Retin Cases Brief Rep. 2011;5:254–255. doi: 10.1097/ICB.0b013e3181f04725. [DOI] [PubMed] [Google Scholar]
- 14.Perez V.L., Chavala S.H., Ahmed M., et al. Ocular manifestations and concepts of systemic vasculitides. Surv Ophthalmol. 2004;49:399–418. doi: 10.1016/j.survophthal.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Justiz Vaillant A.A., Zito P.M. StatPearls. Treasure Island (FL); 2020. Immediate Hypersensitivity Reactions. [PubMed] [Google Scholar]
- 16.Hendrick A.M. Auto-immune haemolytic anaemia--a high-risk disorder for thromboembolism? Hematology. 2003;8:53–56. doi: 10.1080/1024533021000059474. [DOI] [PubMed] [Google Scholar]
- 17.Wilson M.L., Menjivar E., Kalapatapu V., Hand A.P., Garber J., Ruiz M.A. Mycoplasma pneumoniae associated with hemolytic anemia, cold agglutinins, and recurrent arterial thrombosis. South Med J. 2007;100:215–217. doi: 10.1097/01.smj.0000254212.35432.99. [DOI] [PubMed] [Google Scholar]
- 18.Otsuka R., Umehara F., Arimura K., Maruyama Y., Arimura Y., Osame M. Necrotising vasculitis with conduction block in mononeuropathy multiplex with cold agglutinins. J Neurol Neurosurg Psychiatry. 1999;67:556–557. doi: 10.1136/jnnp.67.4.556a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stanford M.R., Graham E.M., Kasp E., Brown E.C., Dumonde D.C., Sanders M.D. Retinal vasculitis: correlation of animal and human disease. Eye. 1987;1:69–77. doi: 10.1038/eye.1987.11. [DOI] [PubMed] [Google Scholar]
- 20.Salacki A.J., Wysokinski A.P. Myocarditis suggesting acute myocardial ischemia, without occlusion of the coronary artery, in a patient with antiphospholipid syndrome and systemic vasculitis in the course of cold agglutinin disease. Arch Med Sci Atheroscler Dis. 2016;1:e32–e35. doi: 10.5114/amsad.2016.60026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Isashiki M., Koide H., Yamashita T., Ohba N. Acute posterior multifocal placoid pigment epitheliopathy associated with diffuse retinal vasculitis and late haemorrhagic macular detachment. Br J Ophthalmol. 1986;70:255–259. doi: 10.1136/bjo.70.4.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dürr C., S H.B., Schulz A., et al. Tumor necrosis factor receptor signaling is a driver of chronic lymphocytic leukemia that can be therapeutically targeted by the flavonoid wogonin. Haematologica. 2018;103:688–697. doi: 10.3324/haematol.2017.177808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balato A., Lembo S., Cirillo T., Megna M., Raimondo A., Costanzo L.D. Anti-tumor necrosis factor-α therapy in the management of psoriasis and B-chronic lymphocytic leukemia. Case Rep Dermatol. 2011;3:60–63. doi: 10.1159/000324344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jabs D.A., Rosenbaum J.T., Foster C.S., et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: recommendations of an expert panel. Am J Ophthalmol. 2000;130:492–513. doi: 10.1016/s0002-9394(00)00659-0. [DOI] [PubMed] [Google Scholar]
- 25.Leo-Carnerero E., Araujo-Míguez A., Trigo-Salado C., De-la-Cruz-Ramírez M.D., Herrera-Justiniano J.M., Márquez-Galán J.L. The effect of controlling inflammatory activity in the colon on the response to infliximab of autoimmune haemolytic anaemia associated with ulcerative colitis. Rev Esp Enferm Dig. 2014;106:295–296. [PubMed] [Google Scholar]
- 26.Strik A.S., D'Haens G.R., Löwenberg M. Hemolytic anemia after switching from infliximab originator to biosimilar CT-P13 in a patient with inflammatory bowel disease: a case report. Clinical case reports. 2019;7:2049–2053. doi: 10.1002/ccr3.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]