Abstract
Remarkable transformations in science and healthcare have resulted in declines in mortality from cardiovascular disease over the past several decades, largely driven by progress in prevention and treatment of persons at risk. However, these trends are now beginning to stall, as our county faces increases in cardiovascular risk factors including overweight and obesity, type 2 diabetes mellitus, and metabolic syndrome. Furthermore, poor long-term adherence to a healthy lifestyle and lifesaving pharmacotherapy have exacerbated these trends, with recent data suggesting unprecedented increases in cardiovascular morbidity and mortality. A paradigm shift is needed to improve the cardiovascular health of our nation. Preventive cardiology, a growing subspecialty of cardiovascular medicine, is the practice of primordial, primary, and secondary prevention of all cardiovascular diseases. Preventive cardiologists and preventive cardiology specialists are well equipped with the knowledge and skill-set necessary to reduce deaths related to the growing burden of heart disease and its risk factors. Despite dedicated efforts, cardiovascular disease remains the leading killer of men and women in the United States. Although there is little debate regarding the importance of prevention, many healthcare professionals question the need for preventive cardiology as a distinct subspecialty. Additionally, the field's growth has been hampered by a lack of organization and standardization, and variability of training within programs across the country. The purpose of this document is to delineate the key attributes that define the field of preventive cardiology according to the American Society for Preventive Cardiology.
Keywords: Preventive cardiology, Atherosclerosis, Primordial prevention, Primary prevention, Secondary prevention, Risk assessment, Cardiovascular disease
Abbreviations: ACC, american college of cardiology; AHA, american heart association; ASPC, american society for preventive cardiology; BMI, body mass index; CAC, coronary artery calcium; CCTA, coronary CT angiography; CMS, centers for medicare and medicaid services; CR, cardiac rehabilitation; CVD, cardiovascular disease; CVH, cardiovascular health; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; FHS, framingham heart study; GLP1-RA, glucagon-like peptide 1 receptor agonists; LDL-C, low-density lipoprotein cholesterol; Mets, metabolic syndrome; NHANES, national health and nutrition examination survey; NIH, national institutes of health; NNT, number needed to treat; OSA, obstructive sleep apnea; PA, physical activity; PCE, pooled cohort equations; PCSK9, proprotein convertase subtilisin kexin 9; PAD, peripheral artery disease; SES, socioeconomic status; SGLT2i, sodium glucose cotransporter 2 inhibitors; T2DM, type 2 diabetes mellitus; US, united states
1. Introduction
Cardiovascular disease (CVD) is eminently preventable, though it remains the leading cause of death and disability in the United States (US) and worldwide [1]. There continues to be debate regarding the clinical utility of preventive cardiology as a distinct subspecialty of cardiovascular medicine, with many arguing that CVD prevention should be undertaken by primary care clinicians or general cardiologists. Additionally, preventive cardiology programs across the country differ in their areas of expertise, approach, and treatment, leading to confusion among healthcare professionals, which has been exacerbated by a general lack of consensus on the definition of this emerging subspecialty.
Some of the confusion regarding the definition of preventive cardiology may stem from inadequate exposure and training during residency and fellowship. While the 2015 American College of Cardiology (ACC) Core Cardiovascular Training Statement (COCATS 4) provided guidance on prevention training during general cardiology fellowship [2], less than 25% of programs meet the requirements for Level I training [3]. Poor awareness among the healthcare community and general public, and the absence of a sub-specialty board examination have also hindered the field. Additionally, many conflate preventive cardiology with general cardiology or clinical lipidology, though the prevention of CVD encompasses a much wider scope that transcends traditional atherosclerosis risk. The practice of preventive cardiology requires specialized knowledge of cardiovascular anatomy and physiology, metabolism, genetics, sex and race/ethnic group specific risk factors, imaging, cardiac rehabilitation (CR), pharmacotherapy, and lifestyle management. To provide clarity regarding the scope of this emerging discipline, this document aims to delineate the key attributes that define the field of preventive cardiology according to the American Society for Preventive Cardiology (ASPC).
2. History of the ASPC
The ASPC was founded in 1985, sprouting out of the National Heart, Lung, and Blood Institute after the organization recommended specific curricula to facilitate medical school teaching and research in the prevention of CVD. Roughly half of US medical schools received 5 year National Institutes of Health (NIH) Preventive Cardiology Academic Awards, which were granted from 1979 to 1996, with many awardees going on to serve in leadership roles throughout the American Heart Association (AHA) and ACC [4]. Joseph Stokes III, a renowned clinical investigator and preventive cardiologist, was the seminal leader and founder of the ASPC whose work transformed the field of CVD prevention.
Richard Carleton, an original recipient of the NIH's Preventive Cardiology Academic Awards, became the first president of the ASPC in 1990. He expanded the organization's mission to include education on population health studies, blood pressure, lipids, exercise, nutrition, and clinical cardiology [4], and broadened collaborations between the ASPC, ACC and AHA. In the modern era, several ASPC leaders expanded the organization's inclusivity, beyond academia, to a broader audience of healthcare professionals which now encompasses multiple disciplines, centered in the mission “to promote the prevention of CVD, advocate for the preservation of cardiovascular health (CVH), and disseminate high-quality, evidence-based information through the education of healthcare professionals and their patients” [4]. In order to implement this mission, the ASPC has proposed a unifying definition of preventive cardiology: a proactive, patient-centered approach in which the clinician, or team of clinicians and non-clinicians, assesses cardiovascular risk and implements a comprehensive strategy of risk mitigation to prevent cardiovascular diseases and its clinical sequelae.
3. Conceptual framework within preventive cardiology
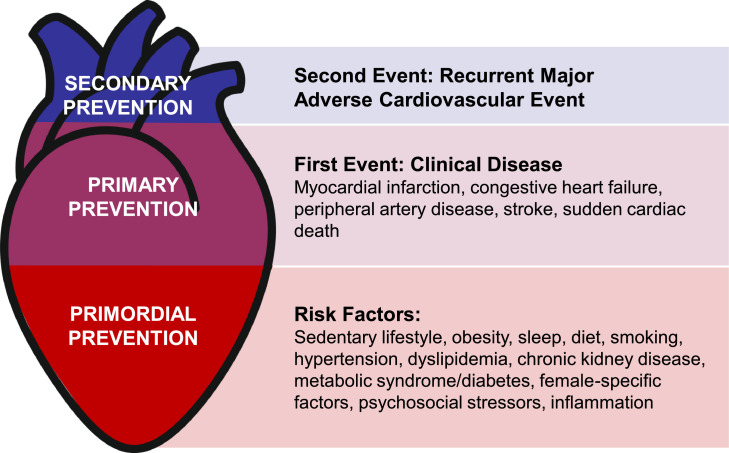
When examining risk from either a population or patient level, preventive cardiologists and preventive cardiology specialists often categorize risk based on the presence or absence of risk factors and/or clinical CVD so that interventions and therapy can be appropriately tailored (Fig. 1). As the burden of heart disease continues to expand, efforts towards upstream prevention will likely yield the most impact in improving CVH. The discussion below provides a general overview of necessary areas of expertise within the field.
Fig. 1.
Primordial, Primary, and Secondary Prevention of Cardiovascualr Disease.
3.1. Primordial prevention
Autopsy studies of young men killed in the First World War, Korean War, and Vietnam War uncovered visible atherosclerotic plaque in coronary arteries, prompting concern that atherosclerosis may begin in childhood [5], [6], [7]. Studies from the 1980s and 1990s went on to uncover the determinants of early atherosclerotic lesions. The landmark Bogalusa Heart Study, which evaluated individuals 2-39 years of age who had autopsies performed, found that every individual had evidence of a fatty streak in the aorta, and half of children 2-15 years old had fatty streaks in their coronary arteries [8]. Serum cholesterol, body mass index (BMI), and blood pressure correlated with the degree of atherosclerosis.
Given these findings, the prevention of CVD should begin early in life with the establishment of a healthy lifestyle. Additionally, with the influence of genetics and the environment on risk, identification and estimation of lifetime risk may have the greatest potential impact on prevention of heart diseases. Once risk factors such as overweight and obesity, type 2 diabetes mellitus (T2DM), sedentary behavior, and unhealthy diets emerge, which are becoming more prevalent at younger ages, it is less likely that individuals will be able to reverse ingrained poor health behaviors and subsequent deleterious downstream cardiovascular effects. Uncontrolled risk factors can lead to the development of subclinical atherosclerotic CVD over time, which may then manifest as a myocardial infarction or stroke. Many of these risk factors also contribute to other cardiovascular and cardiometabolic diseases, such as heart failure, atrial fibrillation, obstructive sleep apnea (OSA), and non-alcoholic fatty liver disease. If these risk factors are identified and treated early, CVD is largely preventable [9]. The relationship between risk factors and CVD is graded and continuous across the life-course; lower cumulative exposure to risk factors remains the most effective path towards a long and heathy life.
Primordial prevention is the prevention of risk factors for CVD, which was conceived in 1978 by Toma Strasser [10] in a paper that acknowledged the need for earlier preventive efforts at younger ages, with the goal of “protecting whole societies from the penetration of risk factor epidemics.” Approaches to primordial prevention focus not only on determinants of CVD risk, but also environmental and social determinants of health. Unfortunately, unhealthy lifestyles continue to plague our society with significant long-term consequences to health and wellbeing. This recognition was highlighted in the Healthy People 2010 goals [11] which included updated objectives for optimizing the health of our country. In order to improve the trajectory of CVH on a national scale, widespread policy changes must be made at the federal, state, and local levels. In order to aid in these efforts, the AHA's 2010 Impact Goals were developed which defined CVH based on Life's Simple 7 [12]. Recently, the addition of sleep has been added to the definition encompassing CVH, which is now based on Life's Essential 8 [13].
3.2. Primary prevention
Preventive cardiologists and preventive cardiology specialists require a deep, comprehensive understanding of risk factors, which should be aggressively managed to prevent incident CVD events, termed primary prevention, and Life's Essential 8 represents an example of a useful construct evaluating modifiable risk factors for individual patients. The metrics include physical activity (PA), diet, smoking, blood pressure, cholesterol, T2DM, BMI, and sleep [13]. Each of the 8 metrics are scored from 0-100 points (a score of 100 is considered optimal). Each component score is averaged to give an overall CVH score, with high CVH defined as a score of 80-100 (Table 1).
Table 1.
Metrics and Definition of Optimal Cardiovascular Health*
| Metric | Definition |
|---|---|
| Nicotine Exposure | Never smoker |
| Body Mass Index | <25 kg/m² |
| Physical Activity | 150 min of moderate or greater intensity activity per week |
| Diet | >95th percentile of DASH-style eating pattern, HEI-2015, or MEPA |
| Blood Lipids | Non-HDL cholesterol <130 mg/dL |
| Blood Pressure | <120/<80 mm Hg |
| Blood Glucose | <100 mg/dL (or HbA1C <5.7) and no history of diabetes |
| Sleep Health | 7 to <9 hours of sleep per night |
Abbreviations: DASH: dietary approaches to stop hypertension, dL: deciliter, HbA1C: hemoglobin A1C, HDL: high-density lipoprotein, HEI: healthy eating index, Hg: mercury, kg: kilograms, m: meters, MEPA: Mediterranean eating pattern for Americans, mg: milligrams, min: minutes, mm: millimeters
Definitions presented here represent the optimal score for each metric (100 points). Each component score can be averaged to obtain a composite cardiovascular health score.
Substantial research demonstrates the impact of CVH and its constituent metrics, which are now tracked continuously for the US population through the National Health and Nutrition Examination Survey (NHANES). For example, attainment of ideal CVH at 50 years of age is associated with substantially lower lifetime risk (5.2% versus 68.9% in men, 8.2% versus 50.2% in women) and strikingly longer survival (>11 years in men, >8 years in women), when compared with individuals without ideal CVH [14]. Increasing numbers of ideal CVH metrics are associated with a lower prevalence and incidence of atherosclerotic CVD, atrial fibrillation, heart failure, and other non-cardiac conditions including cognitive impairment, depression, and cancer [15]. Importantly, risk factor control earlier in the life-course is associated with substantially larger morbidity free survival [16]. Unfortunately, <10% of the US population has ideal CVH, and estimates suggest that 70% of CVD events may be attributable to low and moderate CVH [17]. However, if all US adults were able to attain high levels of CVH, 2 million annual CVD events could be prevented [17].
3.3. Secondary prevention
Secondary prevention aims to reduce disease related events among individuals with established CVD, which is extremely prevalent in the US, affecting 127 million adults, increasing with age in both females and males [18]. Importantly, prevalent CVD remains one of the strongest risk factors for subsequent events and mortality, with a 5-year rate of recurrent myocardial infarction, stroke, heart failure or cardiovascular death between 20-30%. These event rates translate to a roughly 4 to 5-fold increased risk when compared to individuals without prevalent CVD [19]. The 2018 cholesterol guidelines classify risk among patients with atherosclerotic CVD as very high or not at very high risk, which have important treatment implications [20]. Understanding risk categories help further optimize care with pharmacotherapy and lifestyle modification to prevent future CVD events.
CR is an essential component of secondary prevention that provides organized, multifaceted interventions designed to improve social, psychological, and physical functioning, while stabilizing, slowing, or possibly reversing the progression of atherosclerosis [21]. While individualized exercise training and PA counseling remain the focus of CR, programs should also include personalized counseling on nutrition, smoking cessation, psychosocial wellbeing, and management of cardiovascular risk factors. Programs consist of 36 sessions, typically over 12 weeks. Centers for Medicare and Medicaid Services (CMS) covers CR for the following indications: myocardial infarction in the past 12 months, coronary artery bypass graft surgery, stable angina, heart valve repair or replacement, coronary angioplasty or stent, heart or heart-lung transplant, and stable chronic systolic heart failure. CMS also covers 36 sessions of supervised exercise therapy for patients with peripheral artery disease (PAD) [22].
Randomized clinical trials have shown meaningful improvements in clinical outcomes among patients enrolled in CR. In a meta-analysis of patients with coronary heart disease, those who were randomized to CR had a 28% reduction in the risk of fatal or nonfatal myocardial infarction and 23% reduction in CVD mortality [23]. Among patients with heart failure, participation in CR was associated with a significant reduction in heart failure related hospitalization, all-cause hospitalization, and a clinically important improvement in heart failure specific quality of life was observed [24]. CR is also cost effective, although estimates of the incremental cost effectiveness ratios are broad, ranging from $1,065-$71,755 per quality adjusted life year [25].
Despite the robust benefits seen with CR, referral and utilization are consistently low. Participation rates are ∼20% among those who are eligible [26], and significant disparities by race/ethnicity, sex, and socioeconomic factors persist [27]. Automated referrals can be helpful in getting eligible participants into CR programs, and adherence could be improved by providing more flexible scheduling, group sessions for relaxation exercises and psychological counseling, and offering alternatives such as home based and virtual CR [26,28].
4. Risk factors and areas of expertise: Beyond LDL-C
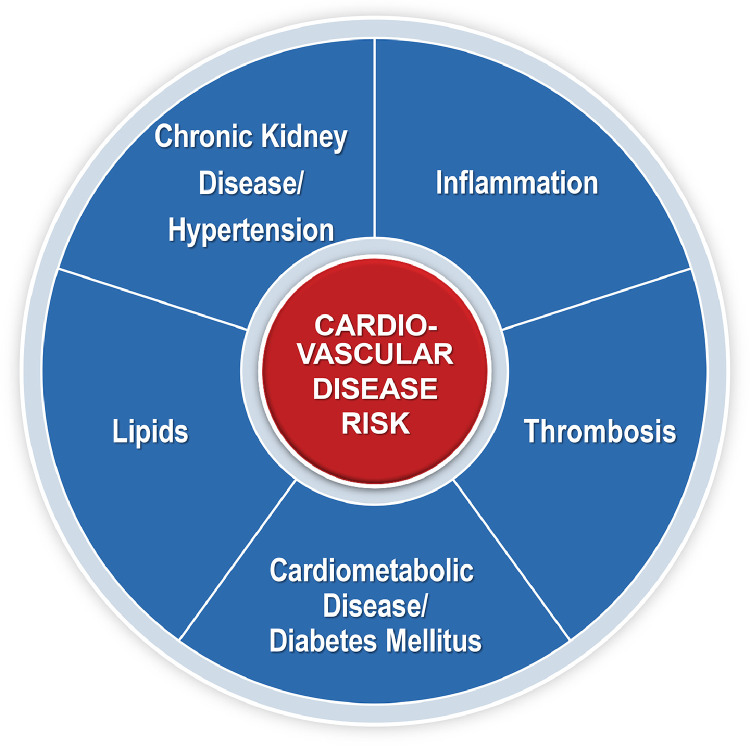
The Framingham Heart Study (FHS) was one of the first longitudinal cardiovascular observational studies that contributed to our understanding of the natural history of heart disease, establishing the major causal risk factors associated with CVD including cigarette smoking [29], high blood pressure, high cholesterol [30], obesity [31], physical inactivity [32], and T2DM [33]. Importantly, this study established that multiple risk factors are responsible for the development of CVD, rather than just a single factor such as low-density lipoprotein cholesterol (LDL-C), which illustrates the need for a more comprehensive approach towards prevention and mitigation of risk. The following provides a brief overview of the major risk factors for CVD, which require expertise among the preventive cardiology community (Fig. 2).
Fig. 2.
Cardiovascular Disease Risk Factors.
4.1. Lipid related risk
The discovery of the LDL receptor and its role in lipid metabolism by Michael Brown and Joseph Goldstein in 1973 [34,35] helped spark a concerted interest in the effects of lipid altering medications on cardiovascular outcomes. As statin therapy became the standard lipid lowering, risk reducing medication, statin-controlled trials were conducted which established the benefits of high vs. low intensity statins. The Improved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT) demonstrated that the addition of ezetimibe to simvastatin is safe and effective, and that this combination not only resulted in further LDL-C lowering, but also lower rates of cardiovascular events when compared with simvastatin alone [36]. This landmark study was one of the first to suggest that, when it comes to LDL-C, “lower is better” [37].
The consistent, reproducible results from the statin mega-trials and other lipid lowering studies laid the groundwork for Lipid Clinics to emerge around the country. The observation that cardiovascular event reduction improves, though events continued to accrue despite significant LDL-C lowering led to the realization that a more comprehensive view on prevention is necessary [38]. Novel and emerging markers of lipid related risk include apolipoprotein B, lipoprotein (a), remnant lipoprotein cholesterol, triglyceride rich lipoproteins, and apolipoprotein CIII, and future studies will help practitioners understand how these markers enhance risk estimation and inform optimal pharmacotherapy when treating patients with dyslipidemias. Special attention should be given to familial hypercholesterolemia given its high prevalence, affecting roughly 1 in 220 individuals [39]. Once an individual is identified, cascade screening, testing for lipoprotein (a), and genetic testing should be offered, while initiating intensive lipid lowering therapy. The fact that this exceedingly common, devastating disease remains underdiagnosed and undertreated highlights the need for preventive cardiologists and preventive cardiology specialists to ensure optimal care in this extremely high-risk population.
4.2. Chronic kidney disease and blood pressure related risk
The relationship between blood pressure and kidney function is complex and interrelated, such that chronic kidney disease, defined as persistent estimated glomerular filtration rate <60 mL/min/1.7 m2 or urine albumin to creatinine ratio ≥30 mg/g, is both a common cause of hypertension and sequelae of long-standing, uncontrolled hypertension. Importantly, each risk factor contributes independently to the development of not only atherosclerotic CVD, but also heart failure, arrhythmias, and sudden cardiac death [40,41]. Blood pressure management continues to be a challenge within our healthcare system, as rates of hypertension control have declined over time to a staggering 19% [42]. Furthermore, albuminuria and proteinuria, common sequelae of hypertension and T2DM that independently increase CVD risk [43], are often unrecognized and undertreated. The US Surgeon General's Call to Action to Control Hypertension has brought this issue into the spotlight, and practitioners of preventive cardiology are primed with the knowledge and tools necessary to respond [44].
4.3. Thrombotic risk
While aspirin for the primary prevention of CVD was previously common, several practice altering randomized controlled trials have demonstrated that the bleeding risk likely outweighs the potential cardiovascular benefit in the vast majority of patients, even among individuals with T2DM [45], [46], [47], [48]. However, coronary artery calcium (CAC) testing facilitates more precise reclassification of risk, with several observational studies suggesting a net benefit for primary prevention aspirin among individuals with a CAC score ≥100 [49], [50], [51]. Additionally, data has emerged suggesting certain subgroups with elevated lipoprotein (a) may also benefit from primary prevention aspirin [52].
The Anti-Xa Therapy to Lower Cardiovascular Events in Addition to Standard Therapy in Subjects with Acute Coronary Syndrome - Thrombolysis in Myocardial Infarction 51 (ATLAS ACS 2-TIMI 51) trial demonstrated that the addition of low dose rivaroxaban to antiplatelet therapy among patients with a recent cardiovascular event reduced the composite endpoint of death from cardiovascular causes, myocardial infarction, or stroke by 16%, though the benefit was offset by significantly increased bleeding [53]. In a comparable study, the Cardiovascular Outcomes for People Using Anticoagulation Strategies (COMPASS) trial demonstrated that the combination of low dose rivaroxaban plus aspirin, compared with aspirin alone, significantly reduced cardiovascular events by 24% among patients with chronic coronary artery disease or PAD [54], [55], [56].
4.4. Inflammatory risk
The immune system and inflammation play a crucial role in the development and pathogenesis of atherosclerosis and most forms of heart disease [57]. The Justification for the Use of Statin in Prevention: an Interventional Trial Evaluating Rosuvastatin (JUPITER) and Pravastatin or Atorvastatin Evaluation and Infection Therapy: Thrombolysis in Myocardial Infarction 22 (PROVE-IT TIMI 22) trial helped establish the link between statins, inflammation (namely high sensitivity C-reactive protein), and CVD [58]. Two additional large cardiovascular outcomes trials, the Colchicine Cardiovascular Outcomes Trial (COLCOT) and the Low-Dose Colchicine 2 (LoDoCo2), demonstrated that colchicine reduces major adverse cardiovascular events in patients with recent myocardial infarction and chronic coronary disease, respectively [59,60]. The 2019 ACC/AHA Primary Prevention Guideline now recognizes chronic inflammatory conditions, in addition to high sensitivity C-reactive protein ≥2 mg/L, as atherosclerotic CVD risk enhancers, which may be used to favor initiation or intensification of statin therapy in certain populations [15].
4.5. Cardiometabolic and diabetes related risk
Metabolic syndrome (MetS) is defined by a group of risk factors underlying metabolic and cardiovascular diseases. Clinically, this condition is diagnosed when ≥3 criteria are present: 1) waist circumstance >40 inches in men or 35 in women; 2) triglycerides ≥150 mg/dL; 3) high-density lipoprotein cholesterol <40 mg/dL in men or <50 mg/dL in women; 4) blood pressure ≥130/85 mmHg; 5) fasting glucose ≥100 mg/dL [61]. In addition, the Internal Diabetes Federation provides a slightly different definition, with ethnic specific waist circumference cut points [62]. While there is overlap among the components that define MetS, these risk factors are causally inter-related, with each independently contributing to increased CVD [63]. However, MetS is more than the sum of its individual parts, with data suggesting that it is a distinct clinical entity associated with an increased risk of cardiovascular events and death, even after controlling for its component risk factors [64].
It is also important to recognize that MetS not only increases atherosclerotic risk but can also lead to multi-organ system dysfunction. Examples include cardiac (ex. atrial fibrillation) endocrine (ex. T2DM), gestational diabetes, polycystic ovarian syndrome), renal (ex. chronic kidney disease, hyperuricemia, cardiorenal syndrome), liver (ex. non-alcoholic fatty liver disease), skeletal (ex. orthopedic and joint disease), psychiatric (ex. depression), sleep (ex. OSA and other sleep disorders), and even malignancy, with estimates suggesting roughly 20% of cancers are related to overweight and obesity [63,65].
Special attention should be given towards assessing sleep given its relationship with cardiometabolic health. Shorter sleep duration is associated with a higher risk of cardiometabolic disease, with racial/ethnic minorities experiencing a greater risk of hypertension, pre-diabetes, T2DM, and obesity [66]. Furthermore, OSA can lead to a host of cardiovascular and cardiometabolic complications, including hypertension, atrial fibrillation, heart failure, coronary artery disease, stroke, MetS, and mortality [67].
When mild insulin resistance progresses in severity to overt T2DM, risk increases substantially, with T2DM accounting for over 87,000 US deaths in 2019 [18]. Poorly controlled T2DM can lead to microvascular complications, including retinopathy, nephropathy, and neuropathy, as well as macrovascular complications which include cerebrovascular disease, peripheral vascular disease, and atherosclerotic CVD. Notably, several practice changing randomized controlled trials have demonstrated significant reductions in cardiovascular and renal outcomes in patients with T2DM treated with sodium glucose cotransporter 2 inhibitors (SGLT2i) and glucagon-like peptide 1 receptor agonists (GLP1-RA), ushering in a new era in T2DM, CVD (atherosclerotic and heart failure), and chronic kidney disease management spearheaded by cardiometabolic and preventive cardiology specialists [63].
5. Risk assessment and atherosclerosis imaging
Heterogeneity exists among individuals with and without established heart disease, and the foundation of preventive cardiology begins with the assessment of CVD risk using global risk scores and atherosclerosis imaging, which enable targeted treatment to reduce cardiovascular outcomes [68].
5.1. Pooled cohort equations
The FHS developed one of the first multivariable risk calculators to assess 10-year risk of coronary heart disease [69]. This risk calculator was then expanded to include stroke, PAD, and heart failure, giving risk to an absolute global CVD risk estimate [70]. However, this risk calculation was hindered by limited generalizability, because participants in the FHS were generally composed of individuals of Caucasians of European ancestry, and not representative of the broader US population [71]. Therefore, a working group from the 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk developed the Pooled Cohort Equations (PCE) [72], which remains the gold standard for quantitative risk assessment in the US.
Although the PCE are able to provide race/ethnic and sex specific estimates of 10-year risk, as well as lifetime risk of atherosclerotic CVD for individuals 20-59 years of age, several limitations should be acknowledged. For example, the PCE underestimates risk in South Asians and overestimates risk in East Asians [20]. Additionally, given the underrepresentation of Hispanic and Latino individuals in many of the cohort studies which were used to create the PCE, risk estimates in these groups are less accurate. Furthermore, socioeconomic status (SES) also seems to modify risk, with data suggesting the PCE overestimates risk in individuals with high SES, and underestimates risk in low SES individuals [73,74].
The PCE provides reasonable discrimination of risk at the population level, though precision is lost at the individual level. Many traditional CVD risk factors including age, race/ethnicity, sex, total and high-density lipoprotein cholesterol, systolic blood pressure, treatment for hypertension, T2DM and smoking status are included in the model, though many other non-traditional risk factors were not incorporated. Thus, the 2018 ACC/AHA Multi-society Guideline on the Management of Blood Cholesterol introduced the concept of risk enhancing factors [75]. When present, these factors may facilitate and enhance shared decision making, a key concept that should not be overlooked when discussing risk reducing strategies and pharmacotherapy.
5.2. Coronary artery calcium
Inherent to the concept of prevention, identification of subclinical atherosclerosis can help overcome the innate limitations of the PCE. CAC, a marker of subclinical coronary artery disease, is now well established in the prevention community, backed by decades of research as well as national [20,76] and international guidelines [77]. It can help reclassify risk when there is uncertainty in the primary prevention setting, informing pharmacotherapy initiation and intensification.
The presence of CAC is strongly associated with CVD risk and has demonstrated predictive value beyond the PCE among a wide range of populations, both young and old [78,79]. Given its superior ability to predict risk, the 2018 ACC/AHA Multi-society Guideline on the Management of Blood Cholesterol states it is reasonable to use a CAC score when considering primary prevention statin therapy among individuals with borderline (5% to <7.5% 10-year) or intermediate (≥7.5% to <20%) risk [75]. If the CAC score is 0, it is reasonable to delay or withhold statin therapy. Recent research has shed light on the appropriate age to consider CAC testing [80], and on the appropriate time interval for repeat CAC testing when initial CAC=0, known as the “warranty period” [81], thereby enhancing the utility in CAC in clinical practice. Notably, statins increase CAC density [82], thereby increasing the CAC score. Thus, the guidelines do not recommend obtaining CAC among individuals already on statin therapy [20].
5.3. Coronary computed tomography angiography
Despite emerging evidence that a CAC score of 0 may serve as a “gatekeeper” for further testing in low to intermediate risk individuals with chest pain [83], current guidelines do not recommend CAC testing in those with symptoms [20]. Instead, coronary CT angiography (CCTA) has emerged as an effective imaging modality that may be useful as an alternative to CAC in certain clinical settings, especially among those with chest pain [84].
CCTA has the ability to quantify plaque characteristics (calcified, non-calcified, or mixed), degree of stenosis, location and distribution throughout the coronary arteries, and can distinguish plaque response to pharmacotherapy and lifestyle interventions. Importantly, these characteristics have prognostic implications. High risk features include a thin fibrous cap, spotty calcifications, positive remodeling, napkin-ring sign, and low attenuation, all of which portend a greater risk of plaque rupture and poor outcomes [85].
The SCOT-HEART (Scottish Computed Tomography of Heart Trial) and PROMISE (Prospective Multicenter Imaging Study for Evaluation of Chest Pain) studies both demonstrated that CCTA provides prognostic information and superior event prediction when compared with standard of care and functional testing (exercise treadmill test, stress myocardial perfusion imaging, stress echocardiography), respectively [86,87]. CCTA allows the clinician the ability to identify plaque before it becomes obstructive, providing the opportunity for aggressive risk factor modification and lifestyle interventions before symptoms and events occur. Future exploration will enable investigators the ability to study the effect of preventive therapies on plaque progression, stabilization, and regression.
5.4. Carotid and lower extremity atherosclerosis assessment
While atherosclerosis imaging of the coronary arteries is important when gauging risk, assessment and imaging of other vascular beds outside of the heart can also aid in CVD risk refinement and enhance medical decision making. The presence of carotid plaque is associated with a higher risk of stroke and cardiovascular events [88], and can be assessed non-invasively with ultrasound. Interestingly, carotid plaque also predicts coronary heart disease even in the absence of CAC [89]. Though carotid intima medial thickness imaging studies were common decades ago, challenges in reproducibility, accuracy, misinterpretation and mischaracterization of results have limited the tests’ clinical utility [90]. Knowledge of carotid intima medial thickness adds little to CVD risk prediction beyond traditional risk factors and CAC, and is no longer recommended in guidelines [72].
Lower extremity PAD, defined by atherosclerosis of the arteries that supply the legs, affects 8.5 million US adults [91], and is associated with high rates of cardiovascular morbidity and mortality [92]. The ankle-brachial index, a ratio of the systolic blood pressure in the ankle (dorsalis pedis artery or posterior tibial artery) over the arm (brachial artery), is a simple, inexpensive, and accurate non-invasive test used for PAD diagnosis. Values <0.9 define significant PAD, warranting high-intensity statin therapy and aspirin [20,93].
6. Lifestyle modification: the foundation of cardiovascular disease prevention
6.1. Healthy dietary patterns
More than half of the top 10 take home messages from the ACC/AHA Primary Prevention Guideline focus on diet, nutrition, and PA [15]. In general, a heart healthy diet includes an emphasis on whole grains, fruits, vegetables, legumes, nuts, fish and seafood. Refined carbohydrates, processed meat, and sugar sweetened beverages should be minimized. Trans and saturated fats should be replaced with dietary mono and polyunsaturated fats, while reductions in sodium and cholesterol are also encouraged to reduce CVD risk [94].
Although individual foods and nutrients may provide specific benefits for CVH, experts suggest an emphasis on dietary patterns, which encompass the variety, balance, and combination of foods and beverages habitually consumed [94]. Evidence based dietary patterns include the Dietary Approaches to Stop Hypertension (DASH), Mediterranean, and plant-based styles, and each has received endorsement from the 2020-2025 Dietary Guidelines for Americans as well as the ASPC [95,96]. Adherence to these dietary patterns are associated with robust improvements in cardiometabolic profiles as well as CVD reduction in both primary and secondary prevention settings [97], [98], [99].
Incorporating a registered dietitian-nutritionists into a preventive cardiology practice is crucial in order to optimize and personalize care based on food preferences, cultural/religious ideals, psychosocial, and economic needs. Data from a systemic review and meta-analysis of 5704 individuals reported that multiple registered dietitian-nutritionist visits for medical nutrition therapy resulted in improved lipids, BMI, hemoglobin A1C, and blood pressure compared with controls [100]. Furthermore, these improvements in cardiometabolic risk factors translated to reductions in medication use and cost savings [100].
6.2. Physical activity
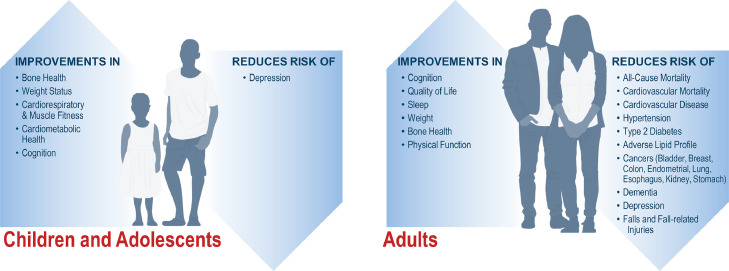
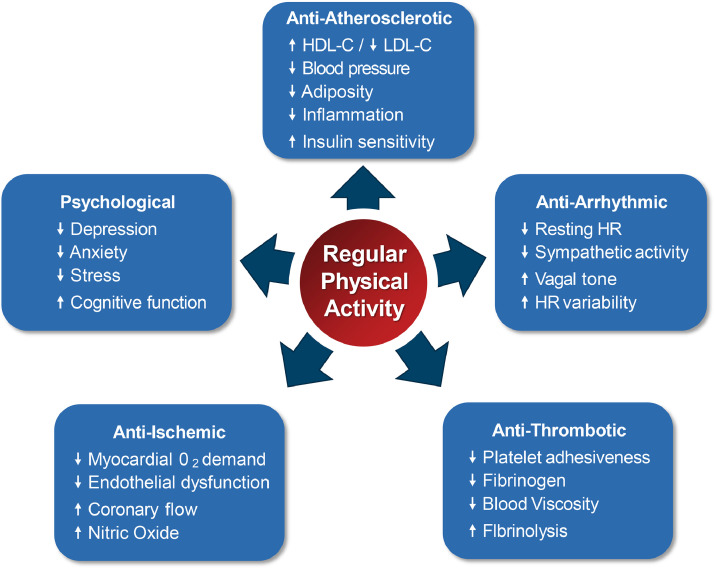
Regular PA has been shown to improve an array of cardiovascular and metabolic risk factors, and reduces the risk of many forms of cancer (Fig. 3) [101]. These cardioprotective benefits are mediated through a variety of mechanisms that ultimately result in reductions in the risk of cardiovascular and all-cause mortality (Fig. 4) [101], [102], [103]. The 2018 Physical Activity Guidelines for Americans recommend ≥150 to 300 minutes of moderate intensity PA, or ≥75 to 150 minutes of vigorous PA a week, in addition to ≥2 days of muscle strengthening exercises for adults. When translating guideline recommendations for patients, the talk test can be useful. During moderate intensity PA, a person can talk but not sing. During vigorous PA, only a few words may be spoken before pausing to take a breath.
Fig. 3.
Benefits of Regular Physical Activity in Children, Adolescents, and Adults.
Fig. 4.
Cardiovascular Benefits of Regular Physical Activity.
*HDL-C= high density lipoprotein cholesterol; HR= heart rate; LDL-C= low density lipoprotein cholesterol.
The 2018 Physical Activity Guideline represents the 2nd edition, and several key points were notable when compared with the initial edition. First, less PA is needed than previously thought to obtain health benefits. In fact, the greatest risk reduction lies in moving from inactivity to light intensity PA [102]. A systematic review of 37 studies from NHANES found that light intensity PA is associated with favorable BMI, blood pressure, hemoglobin A1C, lipid profile, and mortality [104]. Additionally, evidence now suggests that engaging in at least 10 minute sessions (or bouts) of activity is no longer necessary, and healthcare professionals should focus more on cumulative movement of any duration [105]. Furthermore, while the guidelines suggest there is no upper limit of benefit, there is controversy regarding potential negative effects of PA, with studies suggesting very high exercise volumes may be associated with risk of atrial fibrillation, CAC, and myocardial fibrosis [103,106,107]. However, these findings have not been substantiated, and should not discourage clinicians from promoting PA.
Number of steps per day represent a simple, feasible measure of PA that has gained widespread attention with the advent of fitness trackers and smart phones. Although the common goal of 10,000 steps per day is widely promoted for general health, it is not evidence based, and actually originates from a Japanese marketing campaign that was created to promote a pedometer called Manpo-kei, which translates to “10,000 steps meter” in Japanese [108]. Recent data suggests that the optimal number of steps per day varies by age, race/ethnicity, and sex, while stepping rate (or cadence) may not have a strong association with mortality beyond the total number of steps [109,110]. Although step goals were not included in the most recent iteration of PA guidelines, this metric has wide ranging implications and can help inform public health promotion of PA.
Despite the well-known cardiovascular benefits of exercise, very few healthcare professionals either assess or encourage PA in their practice [111]. Counseling has a positive effect on increasing levels of PA, and greater benefits are achieved when behavior-change resources are additionally provided. The number needed to treat (NNT) for 1 sedentary individual to meet the PA guidelines at one year is 12, which is similar to other behavioral counseling interventions including smoking cessation (NNT of 14) and alcohol (NNT of 9) [112,113]. Clinicians should also consider a written exercise prescription. The Exercise is Medicine initiative offers numerous resources, including an exercise prescription template, to aid healthcare professionals in helping their patients adopt and maintain a physically active lifestyle. The evidence now supports the feasibility and effectiveness of PA promotion in routine practice, and practitioners of preventive cardiology must leverage all members of the healthcare community to empower their patients to move more and sit less.
7. Evidence based pharmacotherapy
Statins remain central when considering pharmacotherapy given the causal relationship between LDL-C and atherosclerotic CVD [114], though a variety of adjunctive lipid lowering therapies are also available. If LDL-C goals are not met with statin therapy alone, guidelines recommend the addition of ezetimibe or monoclonal antibodies to proprotein convertase subtilisin kexin 9 (PCSK9) [20]. Additionally, several novel lipid-lowering medications that target various pathways have recently gained FDA approval for LDL-C lowering in specific populations, adding to the preventive cardiology armamentarium. For example, the Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial (REDUCE-IT) established that icosapent ethyl, a highly purified form of eicosapentaenoic acid (EPA), reduced cardiovascular events among patients with fasting triglycerides 135-499 mg/dL. Importantly, patients in this trial were already on background statin therapy, with a median LDL-C of 75 mg/dL [115]. These results were not replicated in studies that used combinations of highly purified EPA and docosahexaenoic acid (DHA) [116], suggesting that the CV benefits of omega 3 fatty acids are driven by EPA and not DHA. Furthermore, scientific advances over the last decade have ushered in exciting novel lipid lowering therapies currently being studied in clinical trials, enabling the ability to target PCSK9, lipoprotein (a), cholesterol ester transfer protein, apolipoprotein CIII, and angiopoetin like 3 protein using monoclonal antibodies, antisense oligonucleotides, small interfering RNA technology, and gene therapy [117], [118], [119], [120]. Inclisiran, a small interfering RNA molecule that catalyzes the breakdown of messenger RNA for PCSK9, was recently approved by the FDA for individuals with heterozygous familial hypercholesterolemia and atherosclerotic CVD. This unique therapy has wide ranging implications for lipid lowering, and cardiovascular outcomes studies are currently underway.
Regarding antihypertensive medications, blockers of the renin-angiotensin system, long-acting calcium channel blockers, and thiazide type diuretics remain first line. Blood pressure that remains above goal despite the concurrent use of these 3 agents at maximally tolerated doses, defines resistant hypertension [121]. Mineralocorticoid receptor antagonists such as spironolactone and eplerenone constitute evidence-based, fourth line therapy [122]. Additional antihypertensive agents may be necessary among those with refractory hypertension, defined as uncontrolled hypertension despite the use of ≥5 different classes of antihypertensive agents, including a long acting thiazide type diuretic and mineralocorticoid receptor antagonist [123]. Ambulatory and home blood pressure monitoring should also be utilized to monitor response to treatment, and may aid in the diagnosis of white coat and masked hypertension.
Anti-diabetes medications including SGLT2i and GLP1-RA have demonstrated reductions in CVD, heart failure, and renal events among patients with T2DM, while SGLT2i specifically are now indicated in patients across the heart failure spectrum of ejection fraction, regardless of diabetes status [124,125]. Although US guidelines still recommend metformin as first line therapy [126]. European guidelines have taken a more progressive approach, recommending SGLT2i and GLP1-RA as first line agents among individuals with T2DM and CVD or at high risk of CVD [127]. Additionally, preventive cardiologists and preventive cardiology specialists should be familiar with Food and Drug Administration approved anti-obesity medications, which are indicated in patients with a BMI ≥30 kg/m2 or ≥27 kg/m2 with at least one obesity related comorbidity [128]. Weight loss surgery in select individuals translates to improvements in cardiometabolic risk factors, and reduces CVD events and mortality [129], [130], [131].
Mentioned above, the COMPASS trial established the efficacy of low dose rivaroxaban plus aspirin among individuals with chronic coronary artery disease or PAD [54], [55], [56]. Smoking cessation medications should also be prescribed to help reduce risk among those that smoke, in addition to other nicotine replacement therapies and behavioral counseling.
Importantly, prescribing appropriate pharmacotherapy alone is not enough; adherence to therapy should also been assessed during each patient encounter to ensure appropriate goals are met. Non-adherence rates to life saving medications are exceedingly high, occurring in >60% of patients with CVD [132]. Full adherence, on the other hand, is associated with a significant reduction in major adverse cardiovascular events and cost savings [133], which should be the standard. Practitioners of preventive cardiology can help improve adherence rates by assessing barriers and providing individualized, culturally appropriate education. Furthermore, mobile health and wearable technology should be leveraged, which helps improve medication compliance [134] and enhance population wide screening and monitoring [135,136].
8. Women's cardiovascular health
CVD continues to be leading cause of death among women, accounting for 420,812 deaths in 2019 in the US [18]. Over 60.8 million US women are living with some form of CVD, and there has been increased recognition of sex-specific responses to traditional CVD risk factors which contribute to the development of atherosclerosis, which must be acknowledged when assessing a woman's risk. There are also differences in the treatment and management of women in both primary and secondary prevention, which should be addressed to achieve the maximal benefit of contemporary therapies and remove sex and gender bias in our cardiovascular care [137,138].
8.1. Sex differences in traditional cardiovascular disease risk factors
Age is part of many CVD risk scores because it is a powerful predictor of cardiovascular events, particularly coronary heart disease [139], which lags by approximately 10 years in women compared with men [140]. Hypertension is equally prevalent in men and women [141], but the presence of hypertension has a more adverse impact on CVD in women compared with men [140]. Women with diabetes have a 3 to 7- fold greater risk of coronary heart disease, compared with a 2 to 3-fold greater risk in men [142,143]. Current cholesterol guidelines focus on LDL-C levels without any sex-specific differences when assessing CVD risk [144], and treatment disparities exist, with women being less likely to be adequately treated compared with men in both primary and secondary prevention [145], [146], [147], [148]. Smoking is also associated with greater atherosclerotic risk in women compared with men [149,150].
8.2. Sex specific and predominant cardiovascular disease risk enhancers
Sex specific risk enhancers need to be investigated when assessing CVD risk. Such risk enhancers should be considered across the lifespan of a biological woman, as risk enhancers may arise at different points in time [151]. Female specific risk enhancers include polycystic ovarian syndrome [152], failure of fertility therapy [153], infertility [154], miscarriage [155], hypertension during pregnancy (which includes gestational hypertension, eclampsia, and pre-eclampsia) [156], gestational diabetes [157], preterm delivery [158], small for gestational age [159], premature menopause [160], premature ovarian insufficiency, [161,162] and functional hypothalamic amenorrhea [161,162]. Early and late onset of menarche is also associated with an increased risk of CVD [163], while breast-feeding has demonstrated a cardioprotective effect [164]. Identifying the presence of risk enhancers at routine prevention visits will better equip the healthcare team and patient to address all risk factors and facilitate shared decision making approaches to CVD preventive strategies.
9. Reducing disparities to achieve health equity
The ASPC supports research and implementation of evidence-based CVD prevention, with therapeutic lifestyle interventions, pharmacotherapy and potential surgical approaches if indicated, to all individuals at increased risk, regardless of race/ethnicity, sex, SES or geography. The ideal practitioner of preventive cardiology must address the impact of the social determinants of health and specific barriers to optimal health in all racial/ethnic populations. This includes implementing regular PA and heart healthy dietary patterns in disadvantaged neighborhoods [165], among individuals with poor access to care. Adoption of healthy levels of PA and nutrition must account for cultural preferences and cost considerations using shared decision making in order to provide tailored treatment.
Hypertension is one of the most prevalent and potent CVD risk factors, especially among African Americans. Preventive cardiologists and preventive cardiology specialists must demonstrate knowledge of the unique aspects of hypertension and suboptimal control, especially in racial/ethnic US populations [166]. Elevated blood pressure must be treated and controlled, with special attention to disparities, including individuals with no or suboptimal healthcare insurance [42]. Moreover, disparities in lowering LDL-C must be addressed. Importantly, cost and prior authorization should be taken into consideration to ensure affordability and medication adherence. There is a similar need for equitable care in the application of novel therapies for individuals with and without T2DM, using SGLT2i and GLP1-RA to reduce obesity, CVD and chronic kidney disease outcomes. Furthermore, addressing inequities among individuals referred for bariatric surgery must be overcome. The ideal preventive cardiologist and preventive cardiology specialist must be knowledgeable on the long-standing, significant and unacceptable disparate outcomes among populations in the US based on race/ethnicity, sex, SES and geography, and must commit to the nuanced aspects of culturally competent, equitable care for all.
10. Conclusion
Since 1980, US life expectancy has steadily fallen behind that of similar countries, resulting in life expectancy declines since 2014 [167]. While some of this trend may stem from communicable diseases, a large proportion is attributed to increases in overweight and obesity, T2DM, hypertension, and metabolic syndrome, all of which are driven by lack of PA, poor dietary patterns, and other poor lifestyle factors. The COVID-19 pandemic has only exacerbated the situation, with projections estimating a ∼3-year decline in life expectancy, with disproportionate declines seen in Black and Hispanic individuals [18].
While the vast majority of CVD related events in the US are related to poor risk factor control, millions of these events could be prevented each year if ideal CVH can be achieved [18]. Our focus must shift from secondary to primary and primordial prevention in order to have the largest impact in improving cardiovascular health. Improvements in screening and detection of risk factors should be undertaken at the community and national level, and collaboration with our allied health colleagues must be undertaken so that we can provide complete care. Additionally, healthcare professionals must partner with policy makers to adopt new strategies and enact regulatory changes [168].
Advances in risk assessment and disease prevention are essential to curtail the approaching tsunami of cardiometabolic disease [169], and our current framework and approach must change. Practitioners of preventive cardiology, a field focused on the practice of primordial, primary, and secondary prevention of all cardiovascular diseases, must take a leadership role to optimize care, while promoting healthy lifestyle and ensuring proper dosing and titration of lifesaving, state-of-the-art pharmacotherapy. Furthermore, a dedicated fellowship and sub-specialty board examination would also help in bringing consensus to the definition of the field. A dramatic reformation is needed to improve the significant gaps in our healthcare system, and practitioners of preventive cardiology are well equipped to fill the void while standing at the forefront of CVD care. It is our hope that this document from the ASPC helps conceptually define the essential set of competencies and knowledge base needed to specialize in this exciting, rapidly expanding, and essential discipline.
Funding
No funding was received for the preparation of this manuscript.
Central Illustration Placeholder
This document is meant to be an official statement from the ASPC and does not fit into any of the prespecified categories. It does not contain a central illustration.
CRediT authorship contribution statement
Charles A. German: Conceptualization, Writing – review & editing. Seth J. Baum: Conceptualization, Writing – review & editing. Keith C. Ferdinand: Writing – review & editing. Martha Gulati: Writing – review & editing. Tamar S. Polonsky: Writing – review & editing. Peter P. Toth: Conceptualization, Writing – review & editing. Michael D. Shapiro: Conceptualization, Writing – review & editing.
Declaration of Competing Interest
Seth Baum: Serves on the advisory board for, provides consulting, and performs clinical research for Amgen, Sanofi/Regeneron, Esperion, Akcea, AstraZeneca, Boehringer Ingelheim/Lilly, Novo Nordisk, and Gemphire. He is a speaker for Amgen, Boehringer Ingelheim/Lilly, Novo Nordisk, and Aralez, and serves as president of Excel Medical Clinical Trials, LLC and Preventive Cardiology, Inc. Keith Ferdinand: Consultant for Amgen, Novartis, Medtronic, and Pfizer. Michael Shapiro: Serves on the advisory board for Amgen, Novartis, and Novo Nordisk. He is a consultant for Regeneron. Peter Toth: Speaker for Amarin, Amgen, Esperion, and Novo Nordisk. He is a consultant for Amarin, Merck, Novartis, and Resverlogix. The rest of the authors do not report any disclosures or competing interests.
References
- 1.Virani SS, Alonso A, Aparicio HJ, et al. Heart disease and stroke statistics-2021 update: a report from the American heart association. Circulation. 2021;143:e254–e743. doi: 10.1161/CIR.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 2.Smith SC, Bittner V, Gaziano JM, et al. COCATS 4 task force 2: training in preventive cardiovascular medicine. J Am Coll Cardiol. 2015;65:1754–1762. doi: 10.1016/j.jacc.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 3.Pack QR, Keteyian SJ, McBride PE, Weaver WD, Kim HE. Current status of preventive cardiology training among united states cardiology fellowships and comparison to training guidelines. Am J Cardiol. 2012;110:124–128. doi: 10.1016/j.amjcard.2012.02.059. [DOI] [PubMed] [Google Scholar]
- 4.Wong ND, Sperling LS, Baum SJ. The American society for preventive cardiology: our 30-year legacy. Clinical cardiology. 2016;39:627–630. doi: 10.1002/clc.22590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mönckeberg J. Über die Atherosklerose der Kombattanten (nach Obduktionsbefunden) Zentralbl Herz Gefasskrankheiten. 1915;7:7–10. [Google Scholar]
- 6.Enos WF, Holmes RH, Beyer J. Coronary disease among United States soldiers killed in action in Korea; preliminary report. J Am Med Assoc. 1953;152:1090–1093. doi: 10.1001/jama.1953.03690120006002. [DOI] [PubMed] [Google Scholar]
- 7.McNamara JJ, Molot MA, Stremple JF, Cutting RT. Coronary artery disease in combat casualties in Vietnam. JAMA. 1971;216:1185–1187. [PubMed] [Google Scholar]
- 8.Berenson GS, Srinivasan SR, Bao W, Newman WP, 3rd, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa heart study. N Engl J Med. 1998;338:1650–1656. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- 9.Weintraub WS, Daniels SR, Burke LE, et al. Value of primordial and primary prevention for cardiovascular disease. Circulation. 2011;124:967–990. doi: 10.1161/CIR.0b013e3182285a81. [DOI] [PubMed] [Google Scholar]
- 10.Strasser T. Reflections on cardiovascular diseases. Interdiscip Sci Rev. 1978;3:225–230. [Google Scholar]
- 11.Health E-UDo, Staff HSD, People H. Healthy people 2010: understanding and improving health: health and human services department, 2000.
- 12.Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American heart association's strategic impact goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 13.Lloyd-Jones DM, Allen NB, Anderson CAM, et al. Life's Essential 8: updating and enhancing the American heart association's construct of cardiovascular health: a presidential advisory from the American heart association. Circulation. 2022;146:e18–e43. doi: 10.1161/CIR.0000000000001078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lloyd-Jones DM, Leip EP, Larson MG, et al. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation. 2006;113:791–798. doi: 10.1161/CIRCULATIONAHA.105.548206. [DOI] [PubMed] [Google Scholar]
- 15.Arnett DK, Blumenthal RS, Albert MA, et al. ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2019 doi: 10.1016/j.jacc.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilkins JT, Ning H, Berry J, Zhao L, Dyer AR, DM Lloyd-Jones. Lifetime risk and years lived free of total cardiovascular disease. JAMA. 2012;308:1795–1801. doi: 10.1001/jama.2012.14312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bundy JD, Zhu Z, Ning H, et al. Estimated impact of achieving optimal cardiovascular health among US adults on cardiovascular disease events. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.120.019681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics—2022 update: a report from the American heart association. Circulation. 2022 doi: 10.1161/CIR.0000000000001052. 0:CIR.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 19.Kerr AJ, Broad J, Wells S, Riddell T, Jackson R. Should the first priority in cardiovascular risk management be those with prior cardiovascular disease? Heart. 2009;95:125–129. doi: 10.1136/hrt.2007.140905. [DOI] [PubMed] [Google Scholar]
- 20.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol. Circulation. 2018 Cir0000000000000625. [Google Scholar]
- 21.Leon AS, Franklin BA, Costa F, et al. Cardiac rehabilitation and secondary prevention of coronary heart disease: an American heart association scientific statement from the council on clinical cardiology (Subcommittee on exercise, cardiac rehabilitation, and prevention) and the council on nutrition, physical activity, and metabolism (Subcommittee on physical activity), in collaboration with the American association of cardiovascular and pulmonary rehabilitation. Circulation. 2005;111:369–376. doi: 10.1161/01.CIR.0000151788.08740.5C. [DOI] [PubMed] [Google Scholar]
- 22.Jensen T, Chin J, Ashby L, Schafer J, Dolan D. Centers for Medicare and Medicaid Services; Baltimore, MD: 2017. Proposed national coverage determination for supervised exercise therapy (SET) for symptomatic peripheral artery disease (PAD) [Google Scholar]
- 23.Dibben G, Faulkner J, Oldridge N, et al. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2021;11 doi: 10.1002/14651858.CD001800.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Long L, Mordi IR, Bridges C, et al. Exercise-based cardiac rehabilitation for adults with heart failure. Cochrane Database Syst Rev. 2019;1 doi: 10.1002/14651858.CD003331.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shields GE, Wells A, Doherty P, Heagerty A, Buck D, Davies LM. Cost-effectiveness of cardiac rehabilitation: a systematic review. Heart. 2018;104:1403–1410. doi: 10.1136/heartjnl-2017-312809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ades PA, Keteyian SJ, Wright JS, et al. Increasing cardiac rehabilitation participation from 20% to 70%: a road map from the million hearts cardiac rehabilitation collaborative. Mayo Clinic Proc. 2017;92:234–242. doi: 10.1016/j.mayocp.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peters AE, Keeley EC. Trends and predictors of participation in cardiac rehabilitation following acute myocardial infarction: data from the behavioral risk factor surveillance system. J Am Heart Assoc. 2017;7 doi: 10.1161/JAHA.117.007664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Content VG, Abraham HM, Kaihoi BH, Olson TP, Brewer LC. Novel virtual world-based cardiac rehabilitation program to broaden access to underserved populations: a patient perspective. JACC Case Reports. 2022;4:911–914. doi: 10.1016/j.jaccas.2022.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dawber TR, Kannel WB, Revotskie N, Stokes J, 3rd, Kagan A, Gordon T. Some factors associated with the development of coronary heart disease: six years' follow-up experience in the Framingham study. Am J Public Health Nations Health. 1959;49:1349–1356. doi: 10.2105/ajph.49.10.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kannel WB, Dawber TR, Kagan A, Revotskie N, Stokes J., 3rd Factors of risk in the development of coronary heart disease–six year follow-up experience. The Framingham study. Ann Intern Med. 1961;55:33–50. doi: 10.7326/0003-4819-55-1-33. [DOI] [PubMed] [Google Scholar]
- 31.Kannel WB, LeBauer EJ, Dawber TR, McNamara PM. Relation of body weight to development of coronary heart disease. The Framingham study. Circulation. 1967;35:734–744. doi: 10.1161/01.cir.35.4.734. [DOI] [PubMed] [Google Scholar]
- 32.Kannel WB. Habitual level of physical activity and risk of coronary heart disease: the Framingham study. Can Med Assoc J. 1967;96:811–812. [PMC free article] [PubMed] [Google Scholar]
- 33.Kannel WB, McGee DL. Diabetes and cardiovascular risk factors: the Framingham study. Circulation. 1979;59:8–13. doi: 10.1161/01.cir.59.1.8. [DOI] [PubMed] [Google Scholar]
- 34.Goldstein JL, Brown MS. Binding and degradation of low density lipoproteins by cultured human fibroblasts: comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974;249:5153–5162. [PubMed] [Google Scholar]
- 35.Goldstein JL, Brown MS. The LDL receptor locus and the genetics of familial hypercholesterolemia. Annu Rev Genet. 1979;13:259–289. doi: 10.1146/annurev.ge.13.120179.001355. [DOI] [PubMed] [Google Scholar]
- 36.Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397. doi: 10.1056/NEJMoa1410489. [DOI] [PubMed] [Google Scholar]
- 37.Jarcho JA, Keaney JF., Jr. Proof that lower is better–LDL cholesterol and IMPROVE-IT. N Engl J Med. 2015;372:2448–2450. doi: 10.1056/NEJMe1507041. [DOI] [PubMed] [Google Scholar]
- 38.Sampson UK, Fazio S, Linton MF. Residual cardiovascular risk despite optimal LDL cholesterol reduction with statins: the evidence, etiology, and therapeutic challenges. Curr Atheroscler Rep. 2012;14:1–10. doi: 10.1007/s11883-011-0219-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGowan MP, Dehkordi SHH, Moriarty PM, Duell PB. Diagnosis and treatment of heterozygous familial hypercholesterolemia. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.013225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019. J Am Coll Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jankowski J, Floege J, Fliser D, Böhm M, Marx N. Cardiovascular disease in chronic kidney disease. Circulation. 2021;143:1157–1172. doi: 10.1161/CIRCULATIONAHA.120.050686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muntner P, Hardy ST, Fine LJ, et al. Trends in blood pressure control among US adults with hypertension, 1999-2000 to 2017-2018. JAMA. 2020;324:1190–1200. doi: 10.1001/jama.2020.14545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gerstein HC, Mann JFE, Yi Q, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421–426. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- 44.Mayfield SK, Foti K, Moran AE, Blakeman DE, Frieden TR. Hypertension call to action: will we respond to the call with action? Am J Hypertens. 2022;35:214–216. doi: 10.1093/ajh/hpab191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McNeil JJ, Wolfe R, Woods RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379:1509–1518. doi: 10.1056/NEJMoa1805819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379:1529–1539. doi: 10.1056/NEJMoa1804988. [DOI] [PubMed] [Google Scholar]
- 47.Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet. 2018;392:1036–1046. doi: 10.1016/S0140-6736(18)31924-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zheng SL, Roddick AJ. Association of aspirin use for primary prevention with cardiovascular events and bleeding events: a systematic review and meta-analysis. JAMA. 2019;321:277–287. doi: 10.1001/jama.2018.20578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ajufo E, Ayers CR, Vigen R, et al. Value of coronary artery calcium scanning in association with the net benefit of aspirin in primary prevention of atherosclerotic cardiovascular disease. JAMA Cardiol. 2021;6:179–187. doi: 10.1001/jamacardio.2020.4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miedema MD, Duprez DA, Misialek JR, et al. Use of coronary artery calcium testing to guide aspirin utilization for primary prevention: estimates from the multi-ethnic study of atherosclerosis. Circ Cardiovasc Qual Outcomes. 2014;7:453–460. doi: 10.1161/CIRCOUTCOMES.113.000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cainzos-Achirica M, Miedema MD, McEvoy JW, et al. Coronary artery calcium for personalized allocation of aspirin in primary prevention of cardiovascular disease in 2019. Circulation. 2020;141:1541–1553. doi: 10.1161/CIRCULATIONAHA.119.045010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lacaze P, Bakshi A, Riaz M, et al. Aspirin for primary prevention of cardiovascular events in relation to lipoprotein (a) genotypes. J Am Coll Cardiol. 2022;80:1287–1298. doi: 10.1016/j.jacc.2022.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mega JL, Braunwald E, Wiviott SD, et al. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med. 2011;366:9–19. doi: 10.1056/NEJMoa1112277. [DOI] [PubMed] [Google Scholar]
- 54.Eikelboom JW, Connolly SJ, Bosch J, et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377:1319–1330. doi: 10.1056/NEJMoa1709118. [DOI] [PubMed] [Google Scholar]
- 55.Anand SS, Bosch J, Eikelboom JW, et al. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet. 2018;391:219–229. doi: 10.1016/S0140-6736(17)32409-1. [DOI] [PubMed] [Google Scholar]
- 56.Connolly SJ, Eikelboom JW, Bosch J, et al. Rivaroxaban with or without aspirin in patients with stable coronary artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet. 2018;391:205–218. doi: 10.1016/S0140-6736(17)32458-3. [DOI] [PubMed] [Google Scholar]
- 57.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 58.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 59.Tardif J-C, Kouz S, Waters DD, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. 2019;381:2497–2505. doi: 10.1056/NEJMoa1912388. [DOI] [PubMed] [Google Scholar]
- 60.Nidorf SM, Fiolet ATL, Mosterd A, et al. Colchicine in patients with chronic coronary disease. N Engl J Med. 2020;383:1838–1847. doi: 10.1056/NEJMoa2021372. [DOI] [PubMed] [Google Scholar]
- 61.Grundy SM, Brewer HB, Cleeman JI, Smith SC, Lenfant C. Definition of metabolic syndrome. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 62.Alberti KGMM, Zimmet P, Shaw J. Metabolic syndrome—a new world-wide definition. A consensus statement from the international diabetes federation. Diabet Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 63.Sperling LS, Mechanick JI, Neeland IJ, et al. The cardiometabolic health alliance: working toward a new care model for the metabolic syndrome. J Am Coll Cardiol. 2015;66:1050–1067. doi: 10.1016/j.jacc.2015.06.1328. [DOI] [PubMed] [Google Scholar]
- 64.Gami AS, Witt BJ, Howard DE, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403–414. doi: 10.1016/j.jacc.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 65.Gallagher EJ, LeRoith D. Epidemiology and molecular mechanisms tying obesity, diabetes, and the metabolic syndrome with cancer. Diabetes Care. 2013;36:S233–S239. doi: 10.2337/dcS13-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tubbs AS, Ghani SB, Valencia D, et al. Racial/ethnic minorities have greater declines in sleep duration with higher risk of cardiometabolic disease: an analysis of the U.S. national health interview survey. Sleep Epidemiol. 2022;2 [Google Scholar]
- 67.Yeghiazarians Y, Jneid H, Tietjens JR et al. Obstructive sleep apnea and cardiovascular disease: a scientific statement from the American heart association. Circulation;0:CIR.0000000000000988. [DOI] [PubMed]
- 68.Wong ND, Budoff MJ, Ferdinand K, et al. Atherosclerotic cardiovascular disease risk assessment: an American society for preventive cardiology clinical practice statement. Am J Prev Cardiol. 2022;10 doi: 10.1016/j.ajpc.2022.100335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 70.D'Agostino RB, Sr., Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham heart study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 71.Tsao CW, Vasan RS. Cohort profile: the Framingham heart study (FHS): overview of milestones in cardiovascular epidemiology. Int J Epidemiol. 2015;44:1800–1813. doi: 10.1093/ije/dyv337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goff DC, Jr., Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American college of cardiology/American heart association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2935–2959. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Colantonio LD, Richman JS, Carson AP, et al. Performance of the atherosclerotic cardiovascular disease pooled cohort risk equations by social deprivation status. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.005676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dalton JE, Perzynski AT, Zidar DA, et al. Accuracy of cardiovascular risk prediction varies by neighborhood socioeconomic position: a retrospective cohort study. Ann Intern Med. 2017;167:456–464. doi: 10.7326/M16-2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Hypertension. 2018;71:e13–e115. doi: 10.1161/HYP.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 76.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation. 2019;140:e596–e646. doi: 10.1161/CIR.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Visseren FLJ, Mach F, Smulders YM, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice: developed by the task force for cardiovascular disease prevention in clinical practice with representatives of the European society of cardiology and 12 medical societies with the special contribution of the European association of preventive cardiology (EAPC) Eur Heart J. 2021;42:3227–3337. doi: 10.1093/eurheartj/ehac458. [DOI] [PubMed] [Google Scholar]
- 78.Tota-Maharaj R, Blaha MJ, McEvoy JW, et al. Coronary artery calcium for the prediction of mortality in young adults <45 years old and elderly adults >75 years old. Eur Heart J. 2012;33:2955–2962. doi: 10.1093/eurheartj/ehs230. [DOI] [PubMed] [Google Scholar]
- 79.Raggi P, Gongora MC, Gopal A, Callister TQ, Budoff M, Shaw LJ. Coronary artery calcium to predict all-cause mortality in elderly men and women. J Am Coll Cardiol. 2008;52:17–23. doi: 10.1016/j.jacc.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 80.Dzaye O, Razavi AC, Dardari ZA, et al. Modeling the recommended age for initiating coronary artery calcium testing among at-risk young adults. J Am Coll Cardiol. 2021;78:1573–1583. doi: 10.1016/j.jacc.2021.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dzaye O, Dardari ZA, Cainzos-Achirica M, et al. Warranty period of a calcium score of zero: comprehensive analysis from MESA. JACC Cardiovasc Imaging. 2021;14:990–1002. doi: 10.1016/j.jcmg.2020.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.German CA, Shapiro MD. Statins and coronary artery calcium: what's the score? Atherosclerosis. 2021;316:71–72. doi: 10.1016/j.atherosclerosis.2020.11.014. [DOI] [PubMed] [Google Scholar]
- 83.Mahmood T, Shapiro MD. Coronary artery calcium testing in low-intermediate risk symptomatic patients with suspected coronary artery disease: an effective gatekeeper to further testing? Plos One. 2020;15 doi: 10.1371/journal.pone.0240539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Budoff MJ, Lakshmanan S, Toth PP, et al. Cardiac CT angiography in current practice: an American society for preventive cardiology clinical practice statement✰. Am J Prev Cardiol. 2022;9 doi: 10.1016/j.ajpc.2022.100318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Williams MC, Moss AJ, Dweck M, et al. Coronary artery plaque characteristics associated with adverse outcomes in the SCOT-HEART study. J Am Coll Cardiol. 2019;73:291–301. doi: 10.1016/j.jacc.2018.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet. 2015;385:2383–2391. doi: 10.1016/S0140-6736(15)60291-4. [DOI] [PubMed] [Google Scholar]
- 87.Hoffmann U, Ferencik M, Udelson JE, et al. Prognostic value of noninvasive cardiovascular testing in patients with stable chest pain: insights from the PROMISE trial (Prospective multicenter imaging study for evaluation of chest pain) Circulation. 2017;135:2320–2332. doi: 10.1161/CIRCULATIONAHA.116.024360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Inaba Y, Chen JA, Bergmann SR. Carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: a meta-analysis. Atherosclerosis. 2012;220:128–133. doi: 10.1016/j.atherosclerosis.2011.06.044. [DOI] [PubMed] [Google Scholar]
- 89.Mehta A, Rigdon J, Tattersall MC, et al. Association of carotid artery plaque with cardiovascular events and incident coronary artery calcium in individuals with absent coronary calcification: the MESA. Circ Cardiovasc Imaging. 2021;14 doi: 10.1161/CIRCIMAGING.120.011701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bays HE, Patel MD, Mavros P, et al. Real-world data to assess changes in low-density lipoprotein cholesterol and predicted cardiovascular risk after ezetimibe discontinuation post reporting of the ezetimibe and simvastatin in hypercholesterolemia enhances atherosclerosis regression trial. J Clin Lipidol. 2017;11:929–937. doi: 10.1016/j.jacl.2017.04.121. [DOI] [PubMed] [Google Scholar]
- 91.Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics—2020 update: a report from the American heart association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 92.Unkart JT, Allison MA, Araneta MRG, Ix JH, Matsushita K, Criqui MH. Burden of peripheral artery disease on mortality and incident cardiovascular events: the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2020;189:951–962. doi: 10.1093/aje/kwaa051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation. 2017;135:e726–e779. doi: 10.1161/CIR.0000000000000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lichtenstein AH, Appel LJ, Vadiveloo M, et al. 2021 Dietary guidance to improve cardiovascular health: a scientific statement from the American heart association. Circulation. 2021;144:e472–e487. doi: 10.1161/CIR.0000000000001031. [DOI] [PubMed] [Google Scholar]
- 95.U.S. Department of Agriculture and U.S. Department of health and human services. Dietary guidelines for Americans -tED.
- 96.Belardo D, Michos ED, Blankstein R, et al. Practical, evidence-based approaches to nutritional modifications to reduce atherosclerotic cardiovascular disease: an American society for preventive cardiology clinical practice statement. Am J Prev Cardiol. 2022;10 doi: 10.1016/j.ajpc.2022.100323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sikand G, Severson T. Top 10 dietary strategies for atherosclerotic cardiovascular risk reduction. Am J Prev Cardiol. 2020;4 doi: 10.1016/j.ajpc.2020.100106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Delgado-Lista J, Alcala-Diaz JF, Torres-Peña JD, et al. Long-term secondary prevention of cardiovascular disease with a mediterranean diet and a low-fat diet (CORDIOPREV): a randomised controlled trial. Lancet. 2022;399:1876–1885. doi: 10.1016/S0140-6736(22)00122-2. [DOI] [PubMed] [Google Scholar]
- 99.Estruch R, Ros E, Salas-Salvadó J, et al. Primary prevention of cardiovascular disease with a mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018;378:e34. doi: 10.1056/NEJMoa1800389. [DOI] [PubMed] [Google Scholar]
- 100.Sikand G, Cole RE, Handu D, et al. Clinical and cost benefits of medical nutrition therapy by registered dietitian nutritionists for management of dyslipidemia: a systematic review and meta-analysis. J Clin Lipidol. 2018;12:1113–1122. doi: 10.1016/j.jacl.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 101.Services USDoHaH. 2018 Physical activity guidelines advisory committee. 2018 Physical activity guidelines advisory committee scientific report. 2018.
- 102.Piercy KL, Troiano RP. Physical activity guidelines for Americans from the US department of health and human services. Circ Cardiovas Qual Outcomes. 2018;11 doi: 10.1161/CIRCOUTCOMES.118.005263. [DOI] [PubMed] [Google Scholar]
- 103.Franklin BA, Thompson PD, Al-Zaiti SS, et al. Exercise-related acute cardiovascular events and potential deleterious adaptations following long-term exercise training: placing the risks into perspective–an update: a scientific statement from the American heart association. Circulation. 2020;141:e705–e736. doi: 10.1161/CIR.0000000000000749. [DOI] [PubMed] [Google Scholar]
- 104.Füzéki E, Engeroff T, Banzer W. Health benefits of light-intensity physical activity: a systematic review of accelerometer data of the national health and nutrition examination survey (NHANES) Sports Med. 2017;47:1769–1793. doi: 10.1007/s40279-017-0724-0. [DOI] [PubMed] [Google Scholar]
- 105.Saint-Maurice PF, Troiano RP, Matthews CE, Kraus WE. Moderate-to-vigorous physical activity and all-cause mortality: do bouts matter? J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.117.007678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Elliott AD, Linz D, Mishima R, et al. Association between physical activity and risk of incident arrhythmias in 402 406 individuals: evidence from the UK Biobank cohort. Eur Heart J. 2020;41:1479–1486. doi: 10.1093/eurheartj/ehz897. [DOI] [PubMed] [Google Scholar]
- 107.Lavie CJ, Wisloff U, Blumenthal RS. Extreme physical activity and coronary artery calcification-running heavily and safely with "Hearts of Stone". JAMA Cardiol. 2019;4:182–183. doi: 10.1001/jamacardio.2018.4647. [DOI] [PubMed] [Google Scholar]
- 108.Lee IM, Shiroma EJ, Kamada M, Bassett DR, Matthews CE, Buring JE. Association of step volume and intensity with all-cause mortality in older women. JAMA Intern Med. 2019 doi: 10.1001/jamainternmed.2019.0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Saint-Maurice PF, Troiano RP, Bassett DR, Jr, et al. Association of daily step count and step intensity with mortality among US adults. JAMA. 2020;323:1151–1160. doi: 10.1001/jama.2020.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Paluch AE, Bajpai S, Bassett DR, et al. Daily steps and all-cause mortality: a meta-analysis of 15 international cohorts. Lancet Public Health. 2022;7:e219–e228. doi: 10.1016/S2468-2667(21)00302-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ma J, Urizar GG, Alehegn T, Stafford RS. Diet and physical activity counseling during ambulatory care visits in the United States. Prev Med. 2004;39:815–822. doi: 10.1016/j.ypmed.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 112.Orrow G, Kinmonth AL, Sanderson S, Sutton S. Effectiveness of physical activity promotion based in primary care: systematic review and meta-analysis of randomised controlled trials. Bmj. 2012;344:e1389. doi: 10.1136/bmj.e1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lobelo F, Rohm Young D, Sallis R, et al. Routine assessment and promotion of physical activity in healthcare settings: a scientific statement from the American heart association. Circulation. 2018;137:e495–e522. doi: 10.1161/CIR.0000000000000559. [DOI] [PubMed] [Google Scholar]
- 114.Borén J, Chapman MJ, Krauss RM, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European atherosclerosis society consensus panel. Eur Heart J. 2020;41:2313–2330. doi: 10.1093/eurheartj/ehz962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bhatt DL, Steg PG, Miller M, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2018;380:11–22. doi: 10.1056/NEJMoa1812792. [DOI] [PubMed] [Google Scholar]
- 116.Nicholls SJ, Lincoff AM, Garcia M, et al. Effect of high-dose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH randomized clinical trial. JAMA. 2020;324:2268–2280. doi: 10.1001/jama.2020.22258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nurmohamed NS, Navar AM, Kastelein JJP. New and emerging therapies for reduction of LDL-cholesterol and apolipoprotein B. J Am Coll Cardiol. 2021;77:1564–1575. doi: 10.1016/j.jacc.2020.11.079. [DOI] [PubMed] [Google Scholar]
- 118.Tsimikas S, Moriarty PM, Stroes ES. Emerging RNA therapeutics to lower blood levels of Lp (a): JACC focus seminar 2/4. J Am Coll Cardiol. 2021;77:1576–1589. doi: 10.1016/j.jacc.2021.01.051. [DOI] [PubMed] [Google Scholar]
- 119.Rosenson RS, Shaik A, Song W. New therapies for lowering triglyceride-rich lipoproteins. J Am Coll Cardiol. 2021;78:1817–1830. doi: 10.1016/j.jacc.2021.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Brandts J, Ray KK. Familial hypercholesterolemia: jacc focus seminar 4/4. J Am Coll Cardiol. 2021;78:1831–1843. doi: 10.1016/j.jacc.2021.09.004. [DOI] [PubMed] [Google Scholar]
- 121.Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American heart association. Hypertension. 2018;72:e53–e90. doi: 10.1161/HYP.0000000000000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bakris G, Sorrentino M. Redefining hypertension - assessing the new blood-pressure guidelines. N Engl J Med. 2018;378:497–499. doi: 10.1056/NEJMp1716193. [DOI] [PubMed] [Google Scholar]
- 123.Dudenbostel T, Siddiqui M, Oparil S, Calhoun DA. Refractory hypertension. Hypertension. 2016;67:1085–1092. doi: 10.1161/HYPERTENSIONAHA.116.06587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Das SR, Everett BM, Birtcher KK, et al. 2020 Expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes: a report of the American college of cardiology solution set oversight committee. J Am Coll Cardiol. 2020;76:1117–1145. doi: 10.1016/j.jacc.2020.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451–1461. doi: 10.1056/NEJMoa2107038. [DOI] [PubMed] [Google Scholar]
- 126.Committee ADAPP. 9 Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2022. Diabetes Care. 2021;45:S125–S143. doi: 10.2337/dc22-S009. [DOI] [PubMed] [Google Scholar]
- 127.Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255–323. doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 128.Garvey WT, Mechanick JI, Brett EM, et al. American association of clinical endocrinologists and American college of endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity executive summary. Endocr Pract. 2016;22:842–884. doi: 10.4158/EP161356.ESGL. https://www.aace.com/publications/guidelines [DOI] [PubMed] [Google Scholar]
- 129.Pareek M, Schauer PR, Kaplan LM, Leiter LA, Rubino F, Bhatt DL. Metabolic surgery. J Am Coll Cardiol. 2018;71:670–687. doi: 10.1016/j.jacc.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 130.Vest AR, Heneghan HM, Agarwal S, Schauer PR, Young JB. Bariatric surgery and cardiovascular outcomes: a systematic review. Heart. 2012;98:1763–1777. doi: 10.1136/heartjnl-2012-301778. [DOI] [PubMed] [Google Scholar]
- 131.Sjöström L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307:56–65. doi: 10.1001/jama.2011.1914. [DOI] [PubMed] [Google Scholar]
- 132.Baroletti S, Dell'Orfano H. Medication adherence in cardiovascular disease. Circulation. 2010;121:1455–1458. doi: 10.1161/CIRCULATIONAHA.109.904003. [DOI] [PubMed] [Google Scholar]
- 133.Bansilal S, Castellano JM, Garrido E, et al. Assessing the impact of medication adherence on long-term cardiovascular outcomes. J Am Coll Cardiol. 2016;68:789–801. doi: 10.1016/j.jacc.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 134.Gandhi S, Chen S, Hong L, et al. Effect of mobile health interventions on the secondary prevention of cardiovascular disease: systematic review and meta-analysis. Can J Cardiol. 2017;33:219–231. doi: 10.1016/j.cjca.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 135.McConnell Michael V, Turakhia Mintu P, Harrington Robert A, King Abby C, Ashley Euan A. Mobile health advances in physical activity, fitness, and atrial fibrillation. J Am Coll Cardiol. 2018;71:2691–2701. doi: 10.1016/j.jacc.2018.04.030. [DOI] [PubMed] [Google Scholar]
- 136.Sana F, Isselbacher Eric M, Singh Jagmeet P, Heist EK, Pathik B, Armoundas Antonis A. Wearable devices for ambulatory cardiac monitoring. J Am Coll Cardiol. 2020;75:1582–1592. doi: 10.1016/j.jacc.2020.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gulati M. Improving the cardiovascular health of women in the nation: moving beyond the bikini boundaries. Circulation. 2017;135:495–498. doi: 10.1161/CIRCULATIONAHA.116.025303. [DOI] [PubMed] [Google Scholar]
- 138.Gulati M. Yentl's Bikini: sex differences in STEMI. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.012873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation. 2019;140:e563–e595. doi: 10.1161/CIR.0000000000000677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics-2022 update: a report from the American heart association. Circulation. 2022;145:e153–e639. doi: 10.1161/CIR.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 141.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2018;71:e127–e248. doi: 10.1016/j.jacc.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 142.Drazner MH. The progression of hypertensive heart disease. Circulation. 2011;123:327–334. doi: 10.1161/CIRCULATIONAHA.108.845792. [DOI] [PubMed] [Google Scholar]
- 143.Regensteiner JG, Golden S, Huebschmann AG, et al. Sex differences in the cardiovascular consequences of diabetes mellitus: a scientific statement from the American heart association. Circulation. 2015;132:2424–2447. doi: 10.1161/CIR.0000000000000343. [DOI] [PubMed] [Google Scholar]
- 144.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation. 2019;139:e1082–e1143. doi: 10.1161/CIR.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Navar AM, Peterson ED, Li S, et al. Prevalence and management of symptoms associated with statin therapy in community practice: insights from the PALM (Patient and provider assessment of lipid management) registry. Circ Cardiovasc Qual Outcomes. 2018;11 doi: 10.1161/CIRCOUTCOMES.117.004249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nanna MG, Wang TY, Xiang Q, et al. Sex differences in the use of statins in community practice. Circ Cardiovasc Qual Outcomes. 2019;12 doi: 10.1161/CIRCOUTCOMES.118.005562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Peters SAE, Colantonio LD, Zhao H, et al. Sex differences in high-intensity statin use following myocardial infarction in the United States. J Am Coll Cardiol. 2018;71:1729–1737. doi: 10.1016/j.jacc.2018.02.032. [DOI] [PubMed] [Google Scholar]
- 148.Ramirez-Morros A, Franch-Nadal J, Real J, Gratacos M, Mauricio D. Sex differences in cardiovascular prevention in type 2: diabetes in a real-world practice database. J Clin Med. 2022;11 doi: 10.3390/jcm11082196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Grundtvig M, Hagen TP, German M, Reikvam A. Sex-based differences in premature first myocardial infarction caused by smoking: twice as many years lost by women as by men. Eur J Cardiovasc Prev Rehabil. 2009;16:174–179. doi: 10.1097/HJR.0b013e328325d7f0. [DOI] [PubMed] [Google Scholar]
- 150.Palmer J, Lloyd A, Steele L, et al. Differential risk of ST-segment elevation myocardial infarction in male and female smokers. J Am Coll Cardiol. 2019;73:3259–3266. doi: 10.1016/j.jacc.2019.03.525. [DOI] [PubMed] [Google Scholar]
- 151.Elder P, Sharma G, Gulati M, Michos ED. Identification of female-specific risk enhancers throughout the lifespan of women to improve cardiovascular disease prevention. Am J Prev Cardiol. 2020;2 doi: 10.1016/j.ajpc.2020.100028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Carmina E, Lobo RA. Is there really increased cardiovascular morbidity in women with polycystic ovary syndrome? J Womens Health (Larchmt) 2018;27:1385–1388. doi: 10.1089/jwh.2018.7162. [DOI] [PubMed] [Google Scholar]
- 153.Udell JA, Lu H, Redelmeier DA. Failure of fertility therapy and subsequent adverse cardiovascular events. CMAJ. 2017;189:E391–E397. doi: 10.1503/cmaj.160744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gleason JL, Shenassa ED, Thoma ME. Self-reported infertility, metabolic dysfunction, and cardiovascular events: a cross-sectional analysis among U.S. women. Fertil Steril. 2019;111:138–146. doi: 10.1016/j.fertnstert.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 155.Wagner MM, Bhattacharya S, Visser J, Hannaford PC, Bloemenkamp KW. Association between miscarriage and cardiovascular disease in a Scottish cohort. Heart. 2015;101:1954–1960. doi: 10.1136/heartjnl-2015-307563. [DOI] [PubMed] [Google Scholar]
- 156.Honigberg MC, Zekavat SM, Aragam K, et al. Long-term cardiovascular risk in women with hypertension during pregnancy. J Am Coll Cardiol. 2019;74:2743–2754. doi: 10.1016/j.jacc.2019.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kramer CK, Campbell S, Retnakaran R. Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta-analysis. Diabetologia. 2019;62:905–914. doi: 10.1007/s00125-019-4840-2. [DOI] [PubMed] [Google Scholar]
- 158.Wu P, Gulati M, Kwok CS, et al. Preterm delivery and future risk of maternal cardiovascular disease: a systematic review and meta-analysis. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.117.007809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ngo AD, Roberts CL, Chen JS, Figtree G. Delivery of a small-for-gestational-age infant and risk of maternal cardiovascular disease–a population-based record linkage study. Heart Lung Circ. 2015;24:696–704. doi: 10.1016/j.hlc.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 160.Peters SA, Woodward M. Women's reproductive factors and incident cardiovascular disease in the UK Biobank. Heart. 2018;104:1069–1075. doi: 10.1136/heartjnl-2017-312289. [DOI] [PubMed] [Google Scholar]
- 161.Daan NM, Muka T, Koster MP, et al. Cardiovascular risk in women with premature ovarian insufficiency compared to premenopausal women at middle age. J Clin Endocrinol Metab. 2016;101:3306–3315. doi: 10.1210/jc.2016-1141. [DOI] [PubMed] [Google Scholar]
- 162.Roeters van Lennep JE, Heida KY, Bots ML, Hoek A. Collaborators of the Dutch multidisciplinary guideline development group on cardiovascular risk management after reproductive D. Cardiovascular disease risk in women with premature ovarian insufficiency: A systematic review and meta-analysis. Eur J Prev Cardiol. 2016;23:178–186. doi: 10.1177/2047487314556004. [DOI] [PubMed] [Google Scholar]
- 163.Lee JJ, Cook-Wiens G, Johnson BD, et al. Age at Menarche and risk of cardiovascular disease outcomes: findings from the national heart lung and blood institute-sponsored women's ischemia syndrome evaluation. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.012406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kirkegaard H, Bliddal M, Stovring H, et al. Breastfeeding and later maternal risk of hypertension and cardiovascular disease - the role of overall and abdominal obesity. Prev Med. 2018;114:140–148. doi: 10.1016/j.ypmed.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 165.Bays HE, Kulkarni A, German C, et al. Ten things to know about ten cardiovascular disease risk factors - 2022. Am J Prev Cardiol. 2022;10 doi: 10.1016/j.ajpc.2022.100342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ogunniyi MO, Commodore-Mensah Y, Ferdinand KC. Race, ethnicity, hypertension, and heart disease: JACC focus seminar 1/9. J Am Coll Cardiol. 2021;78:2460–2470. doi: 10.1016/j.jacc.2021.06.017. [DOI] [PubMed] [Google Scholar]
- 167.Woolf SH, Schoomaker H. Life expectancy and mortality rates in the United States, 1959-2017. Jama. 2019;322:1996–2016. doi: 10.1001/jama.2019.16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Farquhar J, MacLean D, Balram B, et al. Victoria declaration on heart health. CMAJ. 1992;147:1794–1795. [Google Scholar]
- 169.Califf RM. Avoiding the coming tsunami of common, chronic disease. Circulation. 2021;143:1831–1834. doi: 10.1161/CIRCULATIONAHA.121.053461. [DOI] [PubMed] [Google Scholar]