Abstract
Human susceptibility to Schistosoma mansoni infections is controlled by the SM1 locus on chromosome 5 in q31-q33. This genetic region encodes cytokines which regulate the development of helper T lymphocytes. In the present work, a clonal analysis of CD4+ T lymphocytes of homozygous resistant and homozygous susceptible subjects was undertaken to evaluate whether SM1 controls helper T-cell differentiation. Of 121 CD4+ T-cell clones (TCC) from three susceptible (S) and three resistant (R) subjects, 68 proliferated when stimulated by parasite antigens. Parasite-specific TCC derived from susceptible subjects (33 STCC) produced 10- to 1,000-fold less interleukin-4 and -5 than TCC from resistant subjects (25 RTCC). Clones from both patient groups produced, however, the same amount of gamma interferon. Parasite-specific STCC were type 1 helper (Th1) or Th0/1, whereas RTCC were either Th2 or Th0/2. These results, together with the localization of SM1 in 5q31-q33, indicate that the SM1 locus controls the differentiation of Th2 lymphocytes.
In an area where schistosomiasis is endemic, infections are heterogeneous, low and high levels of infection can be recorded in subjects living in similar conditions of exposure to the parasite. Since high levels of infection are a factor in disease development, we and others have attempted to identify the reasons for high levels of infection. It was shown that exposure to infection and environmental factors cannot fully account for the heterogeneity of infection levels in an endemic population, and several studies have indicated that infection intensities depended on individual resistance and susceptibility levels (4, 9, 10, 30, 31). At least two important components of the mechanisms of immune resistance, immunoglobulin E (8, 11, 14, 24) and eosinophils (6, 15, 28) were identified, and resistance has been associated with an increased production of interleukin-4 (IL-4) and IL-5 (7, 13, 20, 25). It was also observed that resistance depended on at least two major factors: the age of the subjects (4, 5, 9, 10) and some inherited factor(s) that segregated in Brazilian families as a major locus (SM1), which was mapped to chromosome 5 in the q31-q33 region (2, 19). This result was confirmed in a Senegalese population (21).
The 5q31-q33 region comprises a number of cytokine genes or genes encoding cytokine receptors which are critical for helper T-cell (Th) differentiation. This and the various reports showing that Th1 or Th2 lymphocytes play a central role in immunity to infectious pathogens (1, 18) led us to test whether SM1 affected the differentiation of Th1 or Th2 subsets. In a previous work, we showed that resistance to infection was associated with Th2 or Th0/2 cells (7); the aim of the present study was to evaluate Th-cell subsets in the most susceptible subjects. The data indicate that susceptibility is associated with a reduced ability to develop Th2 lymphocytes.
MATERIALS AND METHODS
Patients.
This work was performed on Brazilians described previously (9, 10) who were born and who lived in Caatinga do Moura, a village of an area where S. mansoni is endemic. These subjects had average or high levels of water contact; resistant subjects (ages 10, 21, and 33 years) excreted fewer than 100 eggs/g of feces, whereas susceptible subjects (ages 15, 16, and 18 years) excreted more than 2,500 eggs/g. Parasitological and epidemiological data, including water contact, have been measured for several years and are recorded as described previously (9, 10). Genotypes were defined according to the transmission biallelic gene model predicted by segregation analysis (2); this model takes into account the influences of covariates such as age, sex, and water contacts. Genotypes were confirmed by the linkage analysis data (19). These subjects were either homozygous for the allele that determines resistance to infection or were homozygous for the allele that determines high rates of infection.
Preparation and characterization of properties of TCC.
T-cell clones (TCC) were derived from peripheral blood mononuclear cells (PBMC) as previously described (7). Briefly, blood mononuclear cells were purified by centrifugation on a Ficoll-Hypaque cushion and cultured for 10 days with 10 U of recombinant human IL-2 (Chiron, Amsterdam, Holland) per ml and 10 μg of schistosome larval extracts per ml prepared from mechanically transformed schistosomula (9). T lymphocytes were cloned by limiting dilution with irradiated PBMC, as feeder cells, in the presence of 5 μg of phytohemagglutinin and 10 U of IL-2 per ml (7). The specificity of TCC for schistosome extracts was tested in a blastogenesis assay by using autologous PBMC as antigen-presenting cells. TCC with a stimulation index of >2 were considered specific (spTCC), whereas TCC with a lower stimulation index were considered to be of unknown specificity (uspTCC). To assess lymphokine production, TCC were stimulated with 10 ng of phorbol myristate acetate (PMA; Sigma, St. Quentin Fallavier, France) and 1 μg of anti-CD3 monoclonal antibody (MAb; Immunotech, Marseille, France) per ml. Because it was not possible to bleed patients several times, stimulations with parasite extracts were not used. Previous works have shown that polyclonal stimulations do not modify the lymphokine secretion pattern of established TCC (29).
Titration of cytokines.
Cytokines were measured by enzyme-linked immunosorbent assay in 24-h culture supernatants. Anti-IL-4 MAb, anti-gamma interferon (IFN-γ) MAb (Mabtech), and anti-IL-5 (Pharmingen) coated plates (Nunc) were incubated overnight with dilutions of culture supernatants. Plates were then washed four times with phosphate-buffered saline with 0.05% Tween and then incubated with biotinylated MAb anti-IL-4, anti-IL-5, or anti-IFN-γ antibody for 4 h. At the end of this period, the plates were washed four times and incubated for 2 h with a dilution of alkaline phosphatase-conjugated streptavidin. Finally, plates were washed again four times, and the alkaline phosphatase activity was revealed by adding p-nitrophenyl phosphate (Sigma). The absorbancy was read at 495 nm in a plate reader (Bio-Rad). The sensitivity of the titration assays were 20 pg/ml.
Statistical analysis.
The statistical analyses were performed by using the Student’s t test on the logarithm of the cytokine concentrations (to approximate a normal distribution).
RESULTS
Schistosome-specific TCC from homozygous resistant subjects produce more IL-4 and more IL-5 than specific TCC from homozygous susceptible individuals.
TCC were derived from PBMC from three patients homozygous for the allele(s) that determines resistance to infection and from three patients homozygous for the allele(s) that determines high-level infections. Of 121 CD4+ TCC, 68 (56%) proliferated when stimulated with parasite larval extracts and were termed specific TCC (spTCC); the others, referred to as clones of unknown specificity (uspTCC), did not. There was no association between clone proliferation and the resistant (R) or susceptible (S) status of the donor.
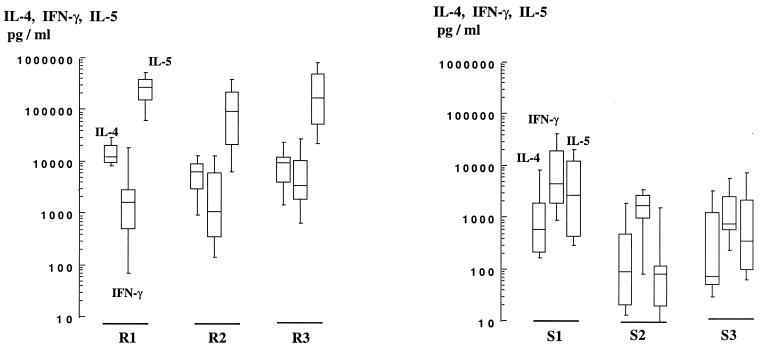
Production of IL-4, IL-5, and IFN-γ by these clones was assessed. Almost all clones produced IL-4, IL-5, and IFN-γ (Fig. 1); thus, most of them did not exhibit a fully polarized Th1 or Th2 phenotype; however, spTCC from the two study groups differed markedly in two aspects: first, TCC from homozygous resistant subjects produced significantly (P < 0.01) more IL-4 (4,700 to 14,000 pg/ml versus 120 to 760 pg/ml; all geometric means) and more IL-5 (66,000 to 226,000 pg/ml versus 70 to 2,560 pg/ml) than did spTCC from homozygous susceptible individuals (Fig. 1). Second, the pattern of IL-4, IL-5, and IFN-γ secretion was characteristic of each group: resistant subjects yielded clones producing more IL-4 and IL-5 than IFN-γ, whereas IFN-γ was produced in greater quantities than IL-4 and IL-5 by spTCC from susceptible subjects. No consistent difference was recorded between the amounts of IFN-γ produced by TCC from the two study groups. No significant correlation was observed between the ability of TCC to proliferate and their cytokine secretion profiles.
FIG. 1.
Production of IL-4, IL-5, and IFN-γ by parasite-specific CD4+ TCC from resistant (left) and susceptible (right) individuals. Boxes represent the 25th to 75th centiles, and vertical bars represent the 10th to the 90th centiles of the mean of one or two duplicate determinations performed on culture supernatants of each TCC stimulated by anti-CD3 plus PMA. Horizontal lines are the median values. The numbers of clones were 7, 11, 7, 13, 11, and 9 for subjects R1, R2, R3, S1, S2, and S3, respectively.
Genetic susceptibility to schistosome infection is associated with Th0/1 or Th1 TCC.
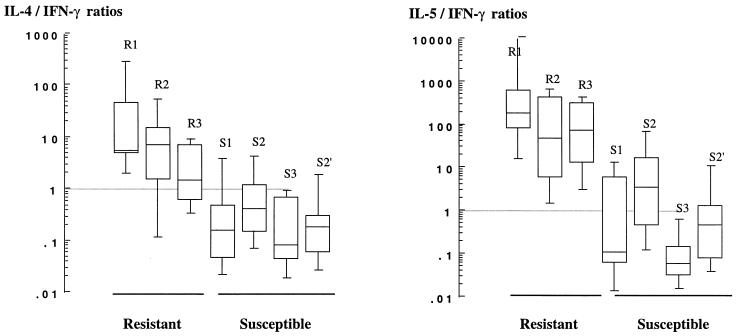
Cytokine ratios are presented in Fig. 2 for spTCC from resistant and susceptible subjects. On average IL-4/IFN-γ ratios of spTCC from susceptible subjects varied from 0.12 to 0.44 (geometric mean) and were 10 to 100 times lower than those of resistant subjects, which varied from 3.7 to 12. Likewise, IL-5/IFN-γ ratios for spTCC from susceptible subjects varied from 3.6 to 12, whereas the average values for spTCC from resistant subjects varied from 40 to 188. Tumor necrosis factor beta (TNF-β) levels measured in the supernatants of spTCC did not differ consistently between TCC from both study groups (data not show).
FIG. 2.
IL-4/IFN-γ (left) and IL-5/IFN-γ (right) ratios of S. mansoni-specific CD4+ TCC derived from resistant and susceptible individuals (see legend to Fig. 1). Results of two separate clonings performed on cells of patients S2 are presented (S2, 11 clones; S2′, 9 clones).
Phenotypes of TCC of unknown specificity.
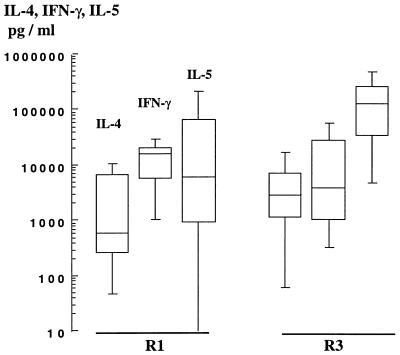
The cloning procedure allows for the emergence of clones that are not specific for parasite antigen (uspTCC); the phenotypes of uspTCC from two resistant (R1 and R3) and two susceptible (S1 and S2) subjects were studied. Levels of IL-4, IL-5, and IFN-γ production by uspTCC from S1 and S2 were comparable to the observations made with spTCC from the same susceptible subjects (data not shown). TCC of unknown specificity from R3 were comparable to spTCC from the same subjects; they produced comparable (P > 0.2) amounts of IL-4 (2,400 versus 7,000 pg/ml) and IL-5, (85,000 versus 158,000 pg/ml). However, uspTCC from the R1 subject exhibited a phenotype closer to that of TCC from the susceptible subjects: they produced less IL-4 (930 versus 14,400 pg/ml; P = 0.003) and less IL-5 (3,700 versus 226,000 pg/ml; P = 0.04) than spTCC from the same subjects (Fig. 3). Specific TCC and uspTCC from R1 and R3 produced, however, comparable amounts of TNF-β and IFN-γ (data not shown). These findings are consistent with the conclusion that spTCC of resistant and susceptible individuals differ in IL-4 and IL-5 production, but not in IFN-γ production.
FIG. 3.
IL-4, IL-5, and IFN-γ production by CD4+ TCC of unknown specificity derived from subjects R1 and R3. The numbers of uspTCC were 11 and 9 for subjects R1 and R3, respectively.
DISCUSSION
Human susceptibility to S. mansoni infections is controlled by the SM1 locus in the 5q31-q33 region (2, 10, 19). This genetic region encodes several cytokines that regulate the differentiation of Th1 and Th2 lymphocytes. A clonal analysis of CD4+ T lymphocytes in resistant and susceptible subjects has been undertaken to evaluate whether this genetic control is acting on Th1/Th2 pathways.
Of 121 CD4+ TCC from three susceptible and three resistant subjects, 68 proliferated when stimulated by parasite antigen. Parasite-specific TCC derived from susceptible subjects (33 STCC) produced 10- to 1,000-fold less IL-4 and IL-5 than TCC from resistant subjects (25 RTCC). Clones from both patient groups, however, produced the same amount of IFN-γ. Parasite-specific STCC were Th1 or Th0/1, whereas RTCC were Th2 or Th0/2.
Precursors of Th cells require several signals to engage a Th2 development program; critical signals are delivered by antigen and by IL-4 (reviewed in reference 22). The effect of IL-4 is essential during the initiation of the immune response. Helminth antigens and allergens have a selective ability to release IL-4 from various sources, including from NK1+ subsets of CD4+ T cells (reviewed in reference 3), mast cells, eosinophils, and basophils (reviewed in reference 23), and Lack-specific CD4+ T cells (17). The IL-4 polarizing effect on the Th lymphocyte response is dominant over the effects of the Th1 polarizing cytokines, IL-12 and IFN-γ (16, 27). Thus, when IL-4 levels are below a certain threshold, Th cells will proceed into the Th1 pathway.
The observation that R1 TCC are Th2 when they are specific for schistosome antigen and are Th1 when they are not specific for parasite antigen further confirms that schistosome antigen has a selective ability to turn on the Th2 pathway during the course of the infection, thus indicating that infections by schistosome produce the appropriate cytokine environment for Th2 development. This effect may be so marked that a normal Th1 response to a conventional antigen may, in certain subjects, deviate toward a Th2 response (26). This is probably the case in R3 subjects, who produced uspTCC with a Th0/2 phenotype. The observation that spTCC from susceptible subjects are Th0/1 or Th1 indicate that Th cells from these subjects fail to engage in Th2 development when they are stimulated by antigens that initiate the Th2 development program in most individuals. Since we linked this property to the 5q31-q33 region, it is most likely that this phenomenon is related to the cytokine environment in which T cells develop in susceptible subjects. Differences in the cytokine environment in resistant and susceptible subjects could be produced by certain polymorphisms in IL-4 or IL-12 (eventually IL-13) genes that could modify the level of production of these cytokines.
The genetic model obtained by segregation analysis is that of major locus with biallellic gene(s). Until the molecular analysis of the susceptibility locus is completed, it is not possible to decide between a simple biallellic model and more-complex models involving more than two alleles at the susceptibility locus. The fact that cytokine ratios follow a rather continuous distribution between resistant and susceptible subjects could indicate the presence of several alleles of susceptibility producing more than one susceptible genotype. It is also possible that the penetrance of the susceptible allele(s) may vary between study subjects, depending on the effects of covariates such as age or exposure.
In conclusion, the results presented in this study, together with the mapping of the SM1 locus to the human 5q31-q33, indicate that genetic control of infection by schistosomes is linked to the differentiation of Th2 lymphocytes. These findings, taken together, suggest that SM1 could play a key role in other infectious diseases that are dependent on the balance between type 1 and type 2 cytokines. Along this line, we have reported that the 5q31-33 region is also involved in the control of blood parasitemia by Plasmodium falciparum (12).
ACKNOWLEDGMENTS
This work received financial assistance from INSERM, FAPEMIG, and CNPq; from the UNDP/World Bank/WHO special program for research and training in parasitic diseases, and from the STD European program.
V.R. and K.P. contributed equally to this work.
REFERENCES
- 1.Abbas A K, Murphy K M, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 2.Abel L, Demenais F, Prata A, Souza A E, Dessein A. Evidence for the segregation of a major gene in human susceptibility/resistance to infection by Schistosoma mansoni. Am J Hum Genet. 1991;48:959–970. [PMC free article] [PubMed] [Google Scholar]
- 3.Bendelac A, Rivera M N, Park S H, Roark J H. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu Rev Immunol. 1997;15:535–562. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- 4.Butterworth A E, Capron M, Cordingley J S, Dalton P R, Dunne D W, Kariuki H C, Kimani G, Koech D, Mugambi M, Ouma J H, et al. Immunity after treatment of human schistosomiasis mansoni. II. Identification of resistant individuals, and analysis of their immune responses. Trans R Soc Trop Med Hyg. 1985;79:393–408. doi: 10.1016/0035-9203(85)90391-8. [DOI] [PubMed] [Google Scholar]
- 5.Butterworth A E, Dalton P R, Dunne D W, Mugambi M, Ouma J H, Richardson B A, Siongok T K, Sturrock R F. Immunity after treatment of human schistosomiasis mansoni. I. Study design, pretreatment observations and the results of treatment. Trans R Soc Trop Med Hyg. 1984;78:108–123. doi: 10.1016/0035-9203(84)90190-1. [DOI] [PubMed] [Google Scholar]
- 6.Butterworth A E, Sturrock R F, Houba V, Mahmoud A A, Sher A, Rees P H. Eosinophils as mediators of antibody-dependent damage to schistosomula. Nature. 1975;256:727–729. doi: 10.1038/256727a0. [DOI] [PubMed] [Google Scholar]
- 7.Couissinier-Paris P, Dessein A J. Schistosoma-specific helper T cell clones from subjects resistant to infection by Schistosoma mansoni are Th0/2. Eur J Immunol. 1995;25:2295–2302. doi: 10.1002/eji.1830250827. [DOI] [PubMed] [Google Scholar]
- 8.Demeure C E, Rihet P, Abel L, Ouattara M, Bourgois A, Dessein A J. Resistance to Schistosoma mansoni in humans: influence of the IgE/IgG4 balance and IgG2 in immunity to reinfection after chemotherapy. J Infect Dis. 1993;168:1000–1008. doi: 10.1093/infdis/168.4.1000. [DOI] [PubMed] [Google Scholar]
- 9.Dessein A J, Begley M, Demeure C, Caillol D, Fueri J, dos Reis M G, Andrade Z A, Prata A, Bina J C. Human resistance to Schistosoma mansoni is associated with IgG reactivity to a 37-kDa larval surface antigen. J Immunol. 1988;140:2727–2736. [PubMed] [Google Scholar]
- 10.Dessein A J, Couissinier P, Demeure C, Rihet P, Kohlstaedt S, Carneiro-Carvalho D, Ouattara M, Goudot-Crozel V, Dessein H, Bourgois A, et al. Environmental, genetic and immunological factors in human resistance to Schistosoma mansoni. Immunol Invest. 1992;21:423–453. doi: 10.3109/08820139209069383. [DOI] [PubMed] [Google Scholar]
- 11.Dunne D W, Butterworth A E, Fulford A J, Ouma J H, Sturrock R F. Human IgE responses to Schistosoma mansoni and resistance to reinfection. Mem Inst Oswaldo Cruz. 1992;87:99–103. doi: 10.1590/s0074-02761992000800014. [DOI] [PubMed] [Google Scholar]
- 12.Garcia A, Marquet S, Bucheton B, Hillaire D, Cot M, Fievet N, Dessein A J, Abel L. Linkage analysis of blood Plasmodium falciparum levels: interest of the 5q31-q33 chromosome region. Am J Trop Med Hyg. 1998;58:705–709. doi: 10.4269/ajtmh.1998.58.705. [DOI] [PubMed] [Google Scholar]
- 13.Grogan J L, Kremsner P G, Deelder A M, Yazdanbakhsh M. Antigen-specific proliferation and interferon-gamma and interleukin-5 production are down-regulated during Schistosoma haematobium infection. J Infect Dis. 1998;177:1433–1437. doi: 10.1086/517832. [DOI] [PubMed] [Google Scholar]
- 14.Hagan P, Blumenthal U J, Dunn D, Simpson A J, Wilkins H A. Human IgE, IgG4 and resistance to reinfection with Schistosoma haematobium. Nature. 1991;349:243–245. doi: 10.1038/349243a0. [DOI] [PubMed] [Google Scholar]
- 15.Hagan P, Wilkins H A, Blumenthal U J, Hayes R J, Greenwood B M. Eosinophilia and resistance to Schistosoma haematobium in man. Parasite Immunol. 1985;7:625–632. doi: 10.1111/j.1365-3024.1985.tb00106.x. [DOI] [PubMed] [Google Scholar]
- 16.Hsieh C S, Macatonia S E, Tripp C S, Wolf S F, O’Garra A, Murphy K M. Development of Th1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 17.Julia V, Rassoulzadegan M, Glaichenhaus N. Resistance to Leishmania major induced by tolerance to a single antigen. Science. 1996;274:421–423. doi: 10.1126/science.274.5286.421. [DOI] [PubMed] [Google Scholar]
- 18.King C L, Nutman T B. Biological role of helper T-cell subsets in helminth infections. Chem Immunol. 1992;54:136–165. [PubMed] [Google Scholar]
- 19.Marquet S, Abel L, Hillaire D, Dessein H, Kalil J, Feingold J, Weissenbach J, Dessein A J. Genetic localization of a locus controlling the intensity of infection by Schistosoma mansoni on chromosome 5q31-q33. Nat Genet. 1996;14:181–184. doi: 10.1038/ng1096-181. [DOI] [PubMed] [Google Scholar]
- 20.Medhat A, Shehata M, Bucci K, Mohamed S, Dief A D, Badary S, Galal H, Nafeh M, King C L. Increased interleukin-4 and interleukin-5 production in response to Schistosoma haematobium adult worm antigens correlates with lack of reinfection after treatment. J Infect Dis. 1998;178:512–519. doi: 10.1086/515630. [DOI] [PubMed] [Google Scholar]
- 21.Muller-Myhsok B, Stelma F F, Guisse-Sow F, Muntau B, Thye T, Burchard G D, Gryseels B, Horstmann R D. Further evidence suggesting the presence of a locus, on human chromosome 5q31-q33, influencing the intensity of infection with Schistosoma mansoni. Am J Hum Genet. 1997;61:452–454. doi: 10.1016/S0002-9297(07)64073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Garra A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 1998;8:275–283. doi: 10.1016/s1074-7613(00)80533-6. [DOI] [PubMed] [Google Scholar]
- 23.Paul W E, Seder R A, Plaut M. Lymphokine and cytokine production by Fc epsilon R1+ cells. Adv Immunol. 1993;53:1–29. [PubMed] [Google Scholar]
- 24.Rihet P, Demeure C E, Bourgois A, Prata A, Dessein A J. Evidence for an association between human resistance to Schistosoma mansoni and high anti-larval IgE levels. Eur J Immunol. 1991;21:2679–2686. doi: 10.1002/eji.1830211106. [DOI] [PubMed] [Google Scholar]
- 25.Roberts M, Butterworth A E, Kimani G, Kamau T, Fulford A J, Dunne D W, Ouma J H, Sturrock R F. Immunity after treatment of human schistosomiasis: association between cellular responses and resistance to reinfection. Infect Immun. 1993;61:4984–4993. doi: 10.1128/iai.61.12.4984-4993.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sabin E A, Araujo M I, Carvalho E M, Pearce E J. Impairment of tetanus toxoid-specific Th1-like immune responses in humans infected with Schistosoma mansoni. J Infect Dis. 1996;173:269–272. doi: 10.1093/infdis/173.1.269. [DOI] [PubMed] [Google Scholar]
- 27.Seder R A, Paul W E. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu Rev Immunol. 1994;12:635–673. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- 28.Vadas M A, David J R, Butterworth A E, Houba V, Sturrock R F, David L, Herson R, Siongok T A, Kimani R. Functional studies on purified eosinophils and neutrophils from patients with Schistosoma mansoni infections. Clin Exp Immunol. 1980;39:683–694. [PMC free article] [PubMed] [Google Scholar]
- 29.Wierenga E A, Snoek M, Jansen H M, Bos J D, van Lier R A, Kapsenberg M L. Human atopen-specific types 1 and 2 T helper cell clones. J Immunol. 1991;147:2942–2949. [PubMed] [Google Scholar]
- 30.Wilkins H A, Blumenthal U J, Hagan P, Hayes R J, Tulloch S. Resistance to reinfection after treatment of urinary schistosomiasis. Trans R Soc Trop Med Hyg. 1987;81:29–35. doi: 10.1016/0035-9203(87)90273-2. [DOI] [PubMed] [Google Scholar]
- 31.Wilkins H A, Goll P H, Marshall T F, Moore P J. Dynamics of Schistosoma haematobium infection in a Gambian community. III. Acquisition and loss of infection. Trans R Soc Trop Med Hyg. 1984;78:227–232. doi: 10.1016/0035-9203(84)90283-9. [DOI] [PubMed] [Google Scholar]