Abstract
Master protocol studies typically use an overarching protocol to answer several questions by guiding a variety of sub-studies. These sub-studies can incorporate multiple diseases, therapies, or both. Although this innovative approach offers many benefits, including the ability to deliver clinical research that is more patient-centric and efficient, several common barriers curtail widespread adoption. The Clinical Trials Transformation Initiative (CTTI) convened industry representatives, regulatory agencies, patient groups, and academic institutions to identify emerging best practices and develop resources designed to help sponsors and other stakeholders overcome these challenges. We first identify some broad changes needed in the clinical trials ecosystem to facilitate mainstream adoption of master protocol studies, and we subsequently summarize CTTI’s resources designed to support this effort.
Keywords: Master protocol studies, clinical trial design, basket trials, umbrella trials, multiple targeted therapies, platform trials, patient-centric clinical trial design, clinical research funding, collaboration
Introduction
Master protocol studies—defined as a group of sub-studies guided by one overarching protocol designed to answer multiple questions about different diseases, therapies, or both—provide a compelling methodological approach well suited to drive efficient patient-centered clinical development. This approach is appealing to stakeholders operating in a clinical trial infrastructure burdened with increasing costs, lengthy investigational medical product development timelines, and a growing demand for therapies that are targeted for increasingly small sub-populations of patients. The US Food and Drug Administration (FDA) has expressed strong support for master protocol studies1 and released guidance for such studies on the treatment and prevention of coronavirus disease 2019 (COVID-19).2 Recently, successful master protocol studies in COVID-19, glioblastoma,3 breast cancer,4 amyotrophic lateral sclerosis,5 and other diseases have amplified enthusiasm. However, the practical experience needed to effectively develop the complex innovative study designs typical of master protocols remains limited.6
The Clinical Trials Transformation Initiative (CTTI), a public-private partnership co-founded by Duke University and the FDA to improve clinical trials, convened a series of multi-stakeholder expert meetings to identify solutions that would facilitate broader adoption of master protocol studies. The CTTI team analyzed the findings from a landscape review and from meetings with industry sponsors, regulatory agencies, patient groups, and academic institutions to identify emerging best practices and develop a robust set of publicly available resources for sponsors and other key stakeholders.7 In the context of this article, sponsor refers to the organization that is ultimately responsible for the initiation and management of the study. This definition takes into account traditional sponsors, such as pharmaceutical companies, as well as non-traditional sponsors, such as non-commercial entities, non-profit patient groups, or academic institutions who may conduct master protocol studies.
CTTI’s multi-stakeholder discussions yielded tools that enable companies, academic research organizations, and patient organizations interested in developing a patient-centric master protocol to initiate their work efficiently. These discussions also provided insight into barriers that have prevented widespread adoption of master protocols. We distilled these conclusions into key areas where ecosystem changes would have the greatest impact: (1) developing a harmonized global vision, (2) mobilizing the clinical trial ecosystem through the leadership of patients and academic groups, and (3) creating readiness with operational partners to catalyze collaborative research. This article details changes that could help strengthen the landscape and outlines examples of innovative work that may advance the use of master protocols. We also summarize CTTI’s resources, which offer solutions to common barriers and help support the development of master protocol studies.
Developing a harmonized global vision
The COVID-19 pandemic occurred during CTTI’s master protocol work, reinforcing the need to accelerate exploration of these studies.8 Despite having the potential to expedite research and delivery on a range of innovative therapies, only 2.5% of COVID-19 intervention trials listed on ClinicalTrials.gov in December 2020 were master protocol studies.9 While the majority of COVID-19 clinical trials yielded no actionable data,10 several master protocol trials, such as the National Institutes of Health (NIH) Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) studies, are producing timely and reliable evidence on investigational therapies.11
Streamlining the clinical trials landscape to create broad, universal access to fit-for-purpose solutions, such as master protocol studies, will require greater harmonization among clinical trial stakeholders and regulators in particular. A synchronized regulatory environment would help drive alignment on key issues, such as appropriate patient populations, efficacy endpoints, safety assessments, trial design, and other key evidentiary standards needed to inform regulatory decisions.
In instances where it is necessary to study specific patient populations that are difficult to enroll, such as pregnant women,12 pediatric patients,13 and people with rare diseases,14 or specific biomarkers,15 researchers often struggle to have a sufficient sample size to base treatment or regulatory recommendations. Master protocols can be particularly effective and efficient in these cases since an internationally coordinated, collaborative approach is often necessary to develop new treatments. Given the iterative nature and potential scope of a master protocol, global coordination will help to prioritize treatments of interest and may aid in limiting duplication of studies, competition for a limited patient population, and unnecessary exposure of patients to investigational treatments.
A key component to supporting wider global development of master protocol studies that meet varied stakeholder and regulatory requirements involves early participation in international multi-stakeholder forums. In a pre-competitive setting, these stakeholder forums convene patient advocates, sponsors, investigators, and regulators to evaluate preclinical and clinical data to inform the best approach for developing treatments, including use of a master protocol.16 Other organizations, such as CTTI, continue to organize multi-stakeholder forums to facilitate knowledge sharing, education, and key stakeholder engagement, to promote master protocol adoption. Initiatives like these are critical to creating stakeholder harmonization to meet the demands of increasingly innovative clinical trial designs.17 Sustained momentum will help scale and broaden the scope of these initiatives to facilitate more efficient exploration of promising study designs.
Mobilizing the clinical trial ecosystem:The need for new leadership
Driving broader adoption of master protocol studies will require stakeholders to demonstrate uncommon vision and leadership. In this article, we describe specific changes suggested by stakeholders during CTTI’s work.
Role of patient groups
Patient advocates play an increasingly visible role in clinical trials,18 and patient communities can be key drivers for developing master protocol studies. When other stakeholders initiate master protocols, patient advocates can be important catalysts who involve reticent stakeholders and motivate alignment across groups with competing interests. For example, patient advocates can influence development strategies for promising investigational agents and identify disease areas where design innovation and collaboration are needed most.
As indicated in Table 1, academic institutions and other non-traditional trial sponsors can also play a critical part in initiating master protocol studies. These non-traditional trial sponsors approach master protocols with a disease-specific focus, and their patient affiliations ensure a patient-centric protocol design. Selection of investigational medicinal products is based on scientific rationale, and these groups then work collaboratively with drug developers to test each product.
Table 1.
| HEALEY ALS platform trial |
| The HEALEY Center for ALS at Massachusetts General Hospital was established in 2018 with a large philanthropic donation. One key objective of the Center is to more efficiently test drug candidates for the treatment of amyotrophic lateral sclerosis (ALS). To meet this objective, they developed the HEALEY ALS Platform Trial to facilitate testing of multiple drug candidates under a single master protocol. It was clear to patients and other stakeholders that the HEALEY ALS Platform Trial offered obvious advantages by providing a way to increase the speed of testing, cut the cost of research, decrease the number of participants exposed to placebo, and bring innovative drugs to patients sooner. By leveraging a platform trial design, they anticipate they will reduce the cost of research by 30% and decrease trial time by 50%. |
| Duchenne Muscular Dystrophy Platform Trial |
| Similar to ALS, the large number of drug candidates in the Duchenne muscular dystrophy (DMD) pipeline reinforced the need for innovative study designs that could speed the development of new therapies. However, perceived loss of patient choice and misalignment on target patient population options and appropriate endpoints introduced challenges in securing buy-in to the platform trial approach. Parent Project Muscular Dystrophy, in partnership with the United States Food and Drug Administration and other key leaders from the community, generated impactful discussion forums that ultimately led to alignment on key patient-centered design considerations that were essential for the progress of the Duchenne Platform Trial and will strengthen future clinical development strategies in DMD. |
Companies interested in overcoming clinical development challenges, such as enrolling patients and creating products that align with patient needs, may find that master protocols in disease areas supported by engaged patient communities are an attractive development pathway. Patient advocates also have a singular interest in advancing patient treatments, which means they can often act as neutral brokers if competing interests among other stakeholders arise. Many patient advocacy groups leverage their affiliate networks to provide broad access to patient communities and the local institutions that serve them. This enables them to assist in site selection and make motivated and informed patients aware of trials.
Role of government and industry
Government agencies and industry stakeholders have demonstrated the viability of public-private partnerships to launch innovative development strategies aimed at accelerating the availability of promising treatments and prophylactics.21
During outbreaks of emerging infectious diseases, government and industry can play a critical leadership role in promoting master protocol studies and engaging diverse groups of stakeholders. For example, as of March 2021, over 2800 COVID-19 clinical trials have been initiated globally.22 This explosion of activity resulted in a glut of redundant and underpowered trials that are unlikely to produce actionable data.8 While the practice of conducting uninformative trials can happen in any disease area, it has significant consequences during a pandemic. Nonetheless, unprecedented collaboration among government, industry, and non-profit organizations happened during the pandemic.21 The NIH ACTIV studies are a prime example of the unique role that government and industry can play in building the infrastructure necessary to support design innovation through public-private partnerships (Table 2). Future use of public-private partnerships to catalyze the development of master protocol studies will require industry stakeholders to embrace new pre-competitive spaces. Consortia organizers—government, patient groups, site networks, or academic institutions—will need to continue reducing the time and cost of collaboration to ensure a compelling value proposition for asset owners.
Table 2.
Potential funding sources for pre-planning, planning, and execution.23
| Potential funding sources | Example |
|---|---|
|
Private philanthropy:
Grants and individual charitable donations |
The HEALEY ALS Adaptive Platform Study was funded in part by a large donation from Sean M. Healey and philanthropic organizations. |
| Government | The National Institutes of Health (NIH) sponsored a number of master protocol studies, including NCI-MATCH and ALCHEMIST. The Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) program and studies are an example of a public-private partnership developed by the NIH to accelerate the prioritization and development of COVID-19 vaccine and therapeutic candidates. |
| Public-private partnership | The European Prevention of Alzheimer’s Dementia is a public-private consortium funded by the Innovative Medicines Initiative. |
| User fees | This strategy, where investigational medical product developers pay for their arm of the trial, is typically used later in the planning stages and for study execution. |
ALS: amyotrophic lateral sclerosis; ALCHEMIST: Adjuvant Lung Cancer Enrichment Marker Identification and Sequencing Trials; NCI-MATCH: National Cancer Institute-Molecular Analysis for Therapy Choice.
Allocate resources to support long-term leadership, collaboration, and infrastructure development
Traditional funding mechanisms that support developing stand-alone, siloed clinical trials do not readily facilitate the development of a robust clinical trial infrastructure to support the design and conduct of master protocol studies. Significant upfront planning costs and extended timelines that characterize master protocol studies challenge conventional public and private grant mechanisms that are designed to distribute funds across many short-term clinical research initiatives in a given disease area. These funding practices can contribute to fragmentation, competition, and redundancy.
Existing master protocol studies have pursued a variety of funding tactics. Many employ innovative public-private funding strategies that are flexible enough to meet the financial demands of intensive upfront planning and can support open-ended execution timelines. CTTI’s Value Proposition Guide23 lists some examples of funding sources (Table 2). These examples demonstrate that there is no one-size-fits-all funding strategy. Sufficient resource allocation to support the development of master protocol studies will require industry, government, and philanthropic groups to generate novel funding mechanisms to incentivize greater coordination, standardization, and collaboration across stakeholders in disease areas with high, unmet need through use of master protocol studies and other complex innovative designs.
Reinventing operational processes and partnerships
Multi-stakeholder insights gained through CTTI’s project activities indicated that implementing innovative solutions that streamline the clinical trials landscape requires challenging the conventional approaches to clinical trial design and implementation while expanding collaboration with partners.
The development and execution of a master protocol is iterative in nature. Increased complexity and limited practical experience designing and conducting master protocols requires sponsors to adopt an approach that considers the input of all stakeholders from the earliest stages of design and prioritizes a streamlined strategy that focuses limited resources on the elements that matter most to participant safety and the credibility of the results.24
Role of operational partners
Contract research organizations, technology providers, and companies that provide other clinical operational services are in a key position to contribute crucial feedback about trial design feasibility when they are engaged early in the planning stages (Figure 1). These operational partners offer important information on how the iterative, cyclical nature of a master protocol study can challenge established contract, budget, and procurement practices. To ensure success, sponsors, and operational partners must communicate frequently to identify challenges and create flexible operating procedures.
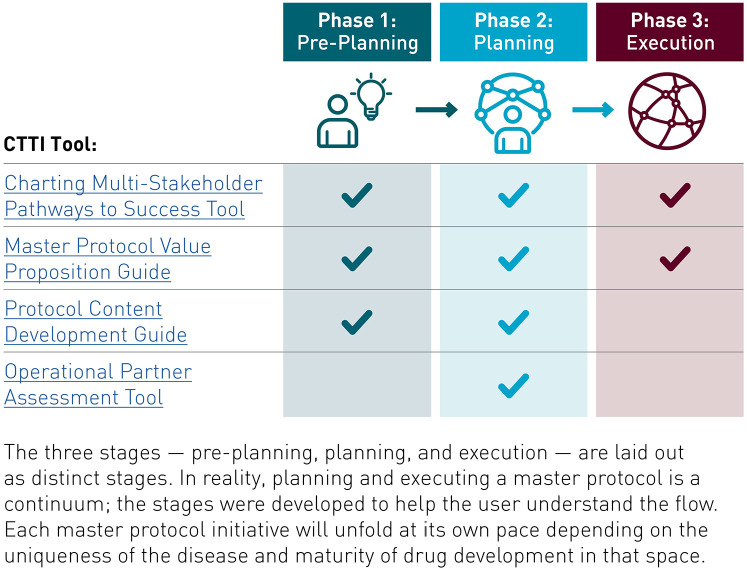
Figure 1.
Pre-planning, planning, and execution stages of master protocol studies.18
Operational partners also need to proactively ensure their tools and processes can support master protocol studies since many have been optimized for use in traditional clinical trials. For example, each investigational medical product tested may have different monitoring needs based on its safety profile; therefore, an operational partner engaged in clinical events classification would need to have broad capabilities to accommodate the profiles of multiple products. A central laboratory partner would need a database with the flexibility to accommodate changes as new study arms are added or dropped.24
Role of sites
Sites should anticipate the need for enhanced communication and education to ensure innovative master protocol study designs are sustainable. Site investigators and study coordinators have a unique perspective and should be engaged early as meaningful partners to develop problem-solving strategies that facilitate flexible practices and long-term sustainability. Working with individual site champions can play a key role in securing site buy-in and enabling strategies that leverage existing site networks for efficient study enrollment.
Sustaining these changes over time will require cultivating a network of champions broadly distributed across multiple stakeholders. Ongoing stakeholder collaboration is especially critical since master protocol studies typically happen over longer periods of time and adapt the design as findings accumulate. As an example, the I-SPY series of trials has been accumulating research for more than 10 years and continues to evolve.4 Existing master protocol study teams should develop educational opportunities to share lessons learned and motivate other organizations to adopt efficient patient-centered study designs and operational practices.25,26
CTTI tools to support master protocol design and operational readiness
Developing a master protocol study requires substantial planning and effort. CTTI’s work on master protocol studies led to the development of tools that support master protocol design and operational readiness by facilitating collaboration and consensus among all stakeholder groups (Figure 1).
The Charting Multi-Stakeholder Pathways to Success Tool provides a high-level roadmap detailing key deliverables, common challenges, and real-world solutions to help stakeholders foster cross-team, cross-institutional problem-solving and strengthen existing planning and operational processes.18
The Value Proposition Guide helps to articulate the value of adopting a master protocol approach during stakeholder engagement and fundraising. This guide provides a high-level overview of resource needs and includes examples of funding models from existing master protocol studies.23
The Protocol Content Development Guide provides a list of key stakeholders that should be engaged in the development of protocols and sub-protocols (protocols that guide the design and conduct of specific study arms). This guide includes a step-by-step tool for creating strategies to engage stakeholders early in the process.27
The Operational Partner Assessment Tool helps to support the operational process by providing a list of key factors that can be used to assess partners’ ability to fulfill key operational functions in the trial.24
Collectively, these tools will support efficient, patient-centric clinical trials that foster collaboration to address major public health threats and ongoing research challenges.
Conclusion
Developing better treatments for patients and advancing the future of health care will involve reexamining current approaches to clinical research and adopting more agile, innovative solutions. Master protocol studies offer the opportunity to conduct research faster and more efficiently, but barriers to planning and execution currently limit implementation. CTTI’s work to analyze current challenges, identify best practices, and create resources to support wider adoption of master protocol studies will empower stakeholders to overcome obstacles to executing this type of solution. Broader adoption of master protocol studies will result in transformative clinical research improvements that have the power to advance the study of innovative therapies faster than ever before and improve the lives of patients on a global scale.
Acknowledgments
The authors would like to thank the experts and key stakeholders from across the clinical trials ecosystem who helped create the resources summarized in this article, including all project team leaders and members, Expert Meeting members, and many others. The authors would also like to recognize Susan Herron of the Duke Clinical Research Institute (DCRI) for medical writing support, Kerry Stenke for design support for tables and figures, Liz Wing for proofreading, Brooke Walker, MS, of the DCRI for editorial assistance in preparing this manuscript, and Zachary Hallinan for project management support and critical review of the manuscript. The HEALEY ALS Platform Trial would like to acknowledge the altruism of the participants and their families and contributions of the research and support staff at each of the participating sites for their contributions to this study.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Daniel Millar and Kimberly Fisher report employment and shareholder interest in Johnson & Johnson. The other authors have no conflicts of interest to disclose.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This article is supported by the Food and Drug Administration (FDA) of the US Department of Health and Human Services (HHS) as part of an award totaling $3,778,241.33 with 15% financed with non-governmental sources. The contents are those of the author(s) and do not necessarily represent the official views of, nor an endorsement, by FDA, HHS, or the US Government. For more information, please visit FDA.gov. Funding for the HEALEY ALS Platform Trial, which provides part of the salary of M. Chase, is supported by The Sean M. Healey and AMG Center for ALS and partially supported by Tackle ALS, The ALS Association, ALS Finding A Cure, ALS One, Muscular Dystrophy Association, I AM ALS, and Tambourine ALS Collaborative.
ORCID iD: Nicholas Richardson https://orcid.org/0000-0001-9378-9909
https://orcid.org/0000-0001-9378-9909
References
- 1. Woodcock J, LaVange LM. Master protocols to study multiple therapies, multiple diseases, or both. N Engl J Med 2017; s377: 62–70. [DOI] [PubMed] [Google Scholar]
- 2. U.S Food and Drug Administration. FDA in brief: FDA provides guidance on master protocols for evaluating prevention, treatment options for COVID-19, https://www.fda.gov/news-events/press-announcements/fda-brief-fda-provides-guidance-master-protocols-evaluating-prevention-treatment-options-covid-19 (2021, accessed 16 March 2022).
- 3. Alexander BM, Ba S, Berger MS, et al. Adaptive global innovative learning environment for glioblastoma: GBM AGILE. Clin Cancer Res 2018; 24(4): 737–743. [DOI] [PubMed] [Google Scholar]
- 4. I-SPY. The I-SPY trials, https://www.ispytrials.org/ (accessed 16 March 2022).
- 5. Paganoni S, Berry JD, Quintana M, et al. Adaptive platform trials to transform amyotrophic lateral sclerosis therapy development. Ann Neurol 2022; 91(2): 165–175. [DOI] [PubMed] [Google Scholar]
- 6. Reitsma D, Song R, Buhler K, et al. How to address—and overcome—operational challenges in master protocol studies. Applied Clinical Trials, https://www.appliedclinicaltrialsonline.com/view/how-to-address-and-overcome-operational-challenges-in-master-protocol-studies (2021, accessed 16 March 2022).
- 7. Clinical Trials Transformation Initiative. Master protocol studies, https://www.ctti-clinicaltrials.org/projects/master-protocol-studies (2020, accessed 16 March 2022).
- 8. Tenaerts P, Woodcock J, Califf RM, et al. The fastest path to effective COVID-19 treatments: using master protocol studies. Master Protocol Public Summit, https://ctti-clinicaltrials.org/the-fastest-path-to-effective-covid-19-treatments-using-master-protocol-studies/ (2021, accessed 16 March 2022).
- 9. Passut C. COVID-19 highlighted master protocol benefits, but challenges remain. CenterWatch RSS, https://www.centerwatch.com/articles/25472-covid-19-highlighted-master-protocol-benefits-but-challenges-remain (2021, accessed 16 March 2022).
- 10. Bugin K, Woodcock J. Trends in COVID-19 therapeutic clinical trials. Nat Rev Drug Discov 2021; 20(4): 254–255. [DOI] [PubMed] [Google Scholar]
- 11. National Institutes of Health. Accelerating COVID-19 therapeutic interventions and vaccines (ACTIV), https://www.nih.gov/research-training/medical-research-initiatives/activ (2020, accessed 16 March 2022).
- 12. U.S. Department of Health and Human Services, Food and Drug Administration; Center for Drug Evaluation and Research; Center for Biologics Evaluation and Research. Pregnant women: scientific and ethical considerations for inclusion in clinical trials guidance for industry, https://www.fda.gov/media/112195/download (2018, accessed 16 March 2022).
- 13. Khan T, Stewart M, Blackman S, et al. Accelerating pediatric cancer drug development: challenges and opportunities for pediatric master protocols. Ther Innov Regul Sci 2019; 53(2): 270–278. [DOI] [PubMed] [Google Scholar]
- 14. Lung-MAP Trial, https://www.lung-map.org/ (accessed 16 March 2022).
- 15. Flaherty K, Gray R, Chen A, et al. The molecular analysis for therapy choice (NCI-MATCH) trial: lessons for genomic trial design. J Natl Cancer Inst 2020; 112(10): 1021–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Clinical Trials Transformation Initiative. CTTI recommendations on effective engagement with patient groups around clinical trials, https://ctti-clinicaltrials.org/wp-content/uploads/2021/06/CTTI_Patient_Group_Engagement_Recs.pdf (2020, accessed 16 March 2022).
- 17. ICH. Reflection on “GCP renovation”: modernization of ICH E8 and subsequent renovation of ICH E6, https://admin.ich.org/sites/default/files/2019-04/ICH_Reflection_paper_GCP_Renovation_Jan_2017_Final.pdf (2017, accessed 16 March 2022).
- 18. Clinical Trials Transformation Initiative. Master protocol design & implementation: charting multi-stakeholder pathways to success, https://ctti-clinicaltrials.org/wp-content/uploads/2021/06/CTTI_Master_Protocol_Roadmap.pdf (2020, accessed 16 March 2022).
- 19. HEALEY ALS. Platform trial. Massachusetts General Hospital, https://www.massgeneral.org/neurology/als/research/platform-trial (accessed 16 March 2022).
- 20. Parent Project Muscular Dystrophy. Master Protocol, 2019, https://www.parentprojectmd.org/research/current-research/our-strategy-impact/master-protocol/ (2019, accessed 16 March 2022).
- 21. Collins FS, Stoffels P. Accelerating COVID-19 therapeutic interventions and vaccines (ACTIV): an unprecedented partnership for unprecedented times. JAMA 2020; 323: 2455–2457. [DOI] [PubMed] [Google Scholar]
- 22. Cytel. Global Coronavirus COVID-19 Clinical Trial Tracker, http://www.covid19-trials.com/ (accessed 28 July 2022).
- 23. Clinical Trials Transformation Initiative. Master Protocol Value Proposition Guide, https://ctti-clinicaltrials.org/wp-content/uploads/2021/06/CTTI_Master_Protocol_Value_Prop_Guide.pdf (2020, accessed 16 March 2022).
- 24. Clinical Trials Transformation Initiative. CTTI master protocols: operations partner assessment, https://www.ctti-clinicaltrials.org/wp-content/uploads/2021/06/CTTI_Operations_Partners_Assessment_Tool.pdf (2020, accessed 16 March 2022).
- 25. Accelerate. ACCELERATE innovation for children and adolescents with cancer. Accelerate Platform, https://www.accelerate-platform.org/ (accessed 16 March 2022).
- 26. Parke T, Pericas J, Posch M, et al. EU-PEARL D2.1. Report on terminology, references and scenarios for platform trials and master protocols, https://eu-pearl.eu/wp-content/uploads/2020/06/EU-PEARL_D2.1_Report-on-Terminology-and-Scenarios-for-Platform-Trials-and-Masterprotocols.pdf (2020, accessed 16 March 2022).
- 27. Clinical Trials Transformation Initiative. Protocol Content Development Guide, https://ctti-clinicaltrials.org/wp-content/uploads/2021/06/CTTI_Master_Protocol_Protocol_Development_Map.pdf (2020, accessed 16 March 2022).