Abstract
Background and Objectives
Acute inflammatory CNS diseases include neuromyelitis optica spectrum disorders (NMOSDs) and myelin oligodendrocyte glycoprotein antibody–associated disease (MOGAD). Both MOGAD and acute disseminated encephalomyelitis (ADEM) have been reported after vaccination. Consequently, the mass SARS-CoV-2 vaccination program could result in increased rates of these conditions. We described the features of patients presenting with new acute CNS demyelination resembling NMOSDs or MOGAD within 8 weeks of SARS-CoV-2 vaccination.
Methods
The study included a prospective case series of patients referred to highly specialized NMOSD services in the UK from the introduction of SARS-CoV-2 vaccination program up to May 2022. Twenty-five patients presented with new optic neuritis (ON) and/or transverse myelitis (TM) ± other CNS inflammation within 8 weeks of vaccination with either AstraZeneca (ChAdOx1S) or Pfizer (BNT162b2) vaccines. Their clinical records and paraclinical investigations including MRI scans were reviewed. Serologic testing for antibodies to myelin oligodendrocyte glycoprotein (MOG) and aquaporin 4 (AQP4) was performed using live cell–based assays. Patients' outcomes were graded good, moderate, or poor based on the last clinical assessment.
Results
Of 25 patients identified (median age 38 years, 14 female), 12 (48%) had MOG antibodies (MOGIgG+), 2 (8%) had aquaporin 4 antibodies (AQP4IgG+), and 11 (44%) had neither. Twelve of 14 (86%) antibody-positive patients received the ChAdOx1S vaccine. MOGIgG+ patients presented most commonly with TM (10/12, 83%), frequently in combination with ADEM-like brain/brainstem lesions (6/12, 50%). Transverse myelitis was longitudinally extensive in 7 of the 10 patients. A peak in new MOGAD cases in Spring 2021 was attributable to postvaccine cases. Both AQP4IgG+ patients presented with brain lesions and TM. Four of 6 (67%) seronegative ChAdOx1S recipients experienced longitudinally extensive TM (LETM) compared with 1 of 5 (20%) of the BNT162b2 group, and facial nerve inflammation was reported only in ChAdOx1S recipients (2/5, 40%). Guillain-Barre syndrome was confirmed in 1 seronegative ChAdOx1S recipient and suspected in another.
Discussion
ChAdOx1S was associated with 12/14 antibody-positive cases, the majority MOGAD. MOGAD patients presented atypically, only 2 with isolated ON (1 after BNT162b2 vaccine) but with frequent ADEM-like brain lesions and LETM. Within the seronegative group, phenotypic differences were observed between ChAdOx1S and BNT162b2 recipients. These observations might support a causative role of the ChAdOx1S vaccine in inflammatory CNS disease and particularly MOGAD. Further study of this cohort could provide insights into vaccine-associated immunopathology.
Antibody-mediated inflammatory diseases of the CNS include neuromyelitis optica spectrum disorders (NMOSDs), which were initially considered diseases of the optic nerves and spinal cord. The identification of aquaporin 4 (AQP4) IgG in the serum of most patients has expanded the clinical phenotype to include involvement of the brainstem and area postrema, mesencephalon, and cerebral hemispheres.1 It is a rare disease, with a prevalence of 0.5–4 per 100,000 worldwide.2
More recently, antibodies to myelin oligodendrocyte glycoprotein (MOG) have been associated with a similar disease phenotype in adults3 but more often with acute disseminated encephalomyelitis (ADEM) in children.4,5 MOG antibody–associated disease (MOGAD) is also rare, with a prevalence of 0.16–3.42 per 100,000.6,7
The severity and frequency of relapses in untreated NMOSD lead to high levels of disability and even death.8 Although the prognosis of MOGAD is better, significant physical disability occurs in almost 50%, with 28% reporting long-term bladder and erectile dysfunction, and 40% of patients with ADEM-like disease experience cognitive disability.5
The National Health Service for England–commissioned services for NMOSD at the John Radcliffe Hospital in Oxford and the Walton Centre in Liverpool were referred cases of NMOSD-like disorders arising de novo in the 8 weeks after vaccination against SARS-CoV-2. In this study, we report these cases according to antibody status and vaccine type.
Methods
Patient Cohort
Patients who presented with CNS inflammation (including at least one of optic neuritis [ON] or transverse myelitis [TM]) within 8 weeks of receiving a vaccination against SARS-CoV-2 were included in the analysis. Only those referred up to May 2022 were included. Those with preexisting inflammatory CNS diseases were excluded. Patients were classified according to self-identified race.
Assay
In-house live cell–based assays were used to quantify immunofluorescence detection of antibodies to AQP49 and MOG protein10 for all patients.
MRI
All MRI scans were reviewed by the NMOSD neurologists and neuroradiologists. ADEM-like appearances were defined as multiple acute inflammatory lesions involving the supratentorial brain and/or brainstem without MS-like features, with or without optic nerve or spinal cord involvement.
Clinical Assessment
All patients were examined by the Oxford or Liverpool NMO Service clinicians except 4; 3 were reviewed by remote consultation due to reluctance to travel during the SARS-CoV-2 pandemic and 1 whose disease severity precluded travel and who subsequently died.
Outcome
Residual disability at the latest clinical assessment was classified according to the following criteria: “good” for full recovery, minor sensory symptoms not interfering with normal function, or visual acuity equal to or better than 6/7.5 in both eyes; “moderate” for residual motor deficit but mobile with or without unilateral assistance, sensory disturbance significantly disrupting normal function, sphincter disturbance, erectile dysfunction, or visual acuities better than 6/36 but poorer than 6/7.5 in at least 1 eye; and “poor” if requiring bilateral assistance to mobilize or wheelchair user, plegic in 1 or more limbs, or visual impairment poorer than 6/36 in at least 1 eye.
Seasonality of MOGAD-Onset Attacks
Monthly frequency of MOGAD-onset attacks was calculated since 1980. Expected frequencies were calculated assuming a uniform distribution throughout the year, adjusting for month length and patient numbers under follow-up. Any deviation from a uniform distribution was analyzed in the periods before the SARS-CoV-2 pandemic started (until February 29, 2020) and in the 2 years after (between March 1, 2020, and February 28, 2021, and between March 1, 2021, and February 28, 2022). Seasonal peaks were assessed using the Friedman test and the Ratchet circular scan test with WINPEPI software package v.11.65.
Standard Protocol Approvals, Registrations, and Patient Consents
Data were collected according to the NHS Research Ethics Committee protocols 16/SC/0224 and 15/LO/1433. All living patients provided consent for inclusion of their anonymized data and MR images. Four MOGIgG+ patients were included in a short Correspondence to Lancet Neurology.11
Data Availability
Data not provided in the article may be shared (anonymized) at the request of any qualified investigator for purposes of replicating procedures and results.
Results
Twenty-six patients were referred (Table), 5 to the Walton Centre and 21 to the Oxford service. One patient did not consent to the use of their data, and 1 patient died. Of the 25 patients included, 18 received the ChAdOx1S vaccine (median age 42.5 years, range 28–65) and 7 received the BNT162b2 vaccine (median age 27 years, range 18–36). Twenty-three patients (92%) presented after their first dose of vaccine. The majority (15/25; 67%) received vaccinations between February and April 2021 (eFigure 1, links.lww.com/NXI/A771). All patients presented within 6 weeks of vaccination.
Table.
Comparison of Demographic, Clinical, and Paraclinical Data According to Serostatus and Vaccine Type
Of 14 seropositive patients, 12 (86%) had antibodies to MOG (MOGIgG+) and 2 (14%) had antibodies to AQP4 (AQP4IgG+). No patients tested positive for both antibodies. Both AQP4IgG+ patients and all but 2 MOGIgG+ patients received ChAdOx1S. Among the 11 seronegative patients, 6 received ChAdOx1S and 5 received BNT162b2 (Table).
MOGIgG+ Patients
Twelve MOGIgG+ patients, half of whom were male and with a median age of 40.5 years (range 22–65), developed symptoms at a median time from ChAdOx1S vaccine of 15 days (range 6–41 days) (Table). Eleven were British White, and 1 was of White Greek descent. For demographic and clinical details, see Table and eTable 1 (links.lww.com/NXI/A771).
Ten patients (83%) experienced TM, 8 of which were longitudinally extensive TM (LETM) (Figure 1A), and 2 experienced isolated ON, 1 of whom received BNT162b2 vaccination.
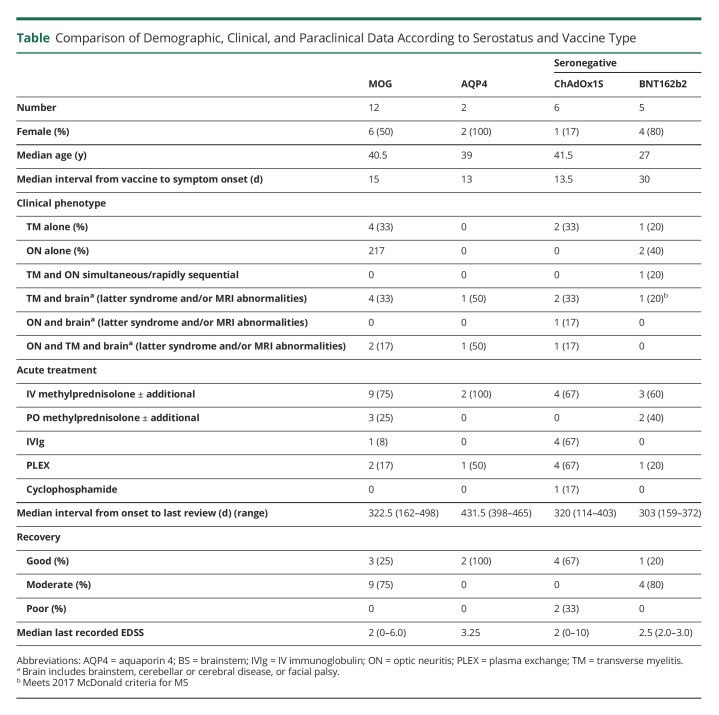
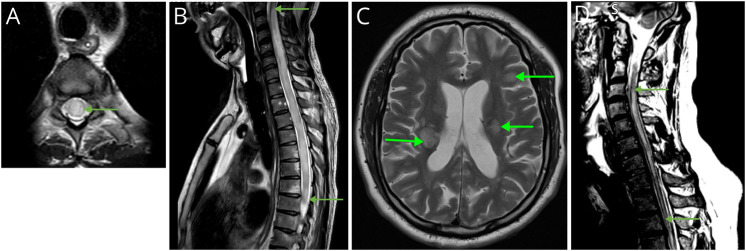
Figure 1. MOGIgG+ MRI.
(A) T2-weighted cervical spinal cord MRI. LETM (arrows), typical of MOGAD. Patient also tested positive for Borrelia burgdorferi. (B) Axial T2-weighted MRI. Bilateral asymmetric T2 hyperintensities in the middle cerebellar peduncles (arrows), typical of cerebral MOGAD. (C) Axial FLAIR MRI. Multiple hyperintensities involving left trigone, posterior limb of left internal capsule, and frontal horn right lateral ventricle (arrows). Abbreviations: LETM = longitudinally extensive TM; MOGAD = myelin oligodendrocyte glycoprotein antibody–associated disease
Of 10 patients with TM, 6 had additional brain involvement including the cerebellar peduncles in 4 (Figure 1B). Brain lesions were symptomatic in 2 patients: 1 patient with multiple lesions in the posterior fossa developed encephalopathy, headache, and fever; the other had lesions in the left middle cerebellar peduncle, internal capsule, and splenium of the corpus callosum with hemifacial numbness, diplopia, and vomiting (Figure 1C). Another experienced lower limb ataxia that may have been attributable to lesions of the left middle cerebellar peduncle or spinal cord. All patients with TM at any spinal level developed urinary retention requiring catheterization.
One patient tested positive for Borrelia sp. with antibodies in serum and CSF and was treated with IV corticosteroids followed by antibiotics once Borrelia antibodies were confirmed. In the presence of LETM with strongly and persistently positive MOG antibodies, a 3- to 4-month delay between erythema migrans and neurologic symptoms, and a rapid response to steroid treatment, the cause of his presentation was attributed to MOGAD.
Of the 11 patients who had CSF sampled, the median CSF protein was slightly elevated (0.63g/L, range 0.33–2.25). The median CSF lymphocyte count was 36 × 106/L (range 0–248), and none of the 11 tested had unmatched oligoclonal bands (OCBs) (see eTable 1 for additional test results, links.lww.com/NXI/A771).
Nine (75%) MOGIgG+ patients received acute therapy with IV methylprednisolone (IVMP). Two patients had additional plasma exchange (PLEX), and 1 had additional IV immunoglobulin (IVIg). One patient with TM, ON, and brainstem lesions received concomitant oral prednisolone and PLEX. The 2 patients with isolated ON received oral prednisolone only. All except 1 patient had a prolonged course of steroids, tapering over between 8 and 24 months.
The median follow-up was 322.5 days (range 162–498). Three (25%) had good outcomes, including 2 patients with isolated ON and 1 with LETM and brain lesions. Nine (75%) had moderate outcomes due to bladder and/or erectile dysfunction. Those with moderate outcomes had a longer median follow-up time (335 days) than those with good outcomes (257 days), so a longer observation does not explain better prognosis.
The only patient not given a prolonged course of oral steroids after acute treatment had a moderate outcome but relapsed 5 months after the initial episode with a new lesion on MRI. They had a moderate outcome thereafter. No other relapses have been reported.
In the seasonality analysis of MOGAD-onset attacks, 286 patients were included. No significant seasonal pattern was identified before the SARS-CoV-2 pandemic (n = 233, the Friedman test v(N) = 0.054, p > 0.1) or in the first year of the pandemic (before vaccine rollout; n = 24, the Friedman test v(N) = 0.106, p > 0.1). In the period from March 2021 to February 2022, a seasonal peak was identified from March to May (n = 29, the Friedman test v(N) = 0.256, p < 0.05, the Ratchet circular scan test, p < 0.025). When postvaccine MOGIgG+ patients were excluded, the seasonal peak disappeared (n = 18, the Friedman test v(N) = 0.166, p > 0.1; eFigure 1, links.lww.com/NXI/A771).
AQP4IgG+ Patients
Two AQP4IgG+ patients, both female and identifying as Afro-Caribbean, presented with brainstem symptoms (Table, eTable 1, links.lww.com/NXI/A771). Patient 1, aged 28 years, developed neck and facial dysesthesia 1 day after receiving the ChAdOx1S vaccine and 12 days later experienced symptoms of TM. Patient 2, aged 50 years, presented with area postrema syndrome and reduced visual acuity 25 days after receiving the ChAdOx1S vaccine.
An MRI examination of patient 1 revealed cervical LETM, a discrete lesion in the left hemipons (Figure 2, A and B), and subtle periependymal FLAIR hyperintensities. Patient 2 had lesions in the brainstem around the fourth ventricle, right middle cerebellar peduncle, and the midbrain involving the periaqueductal gray (Figure 2, C and D), with an additional short cervical TM.
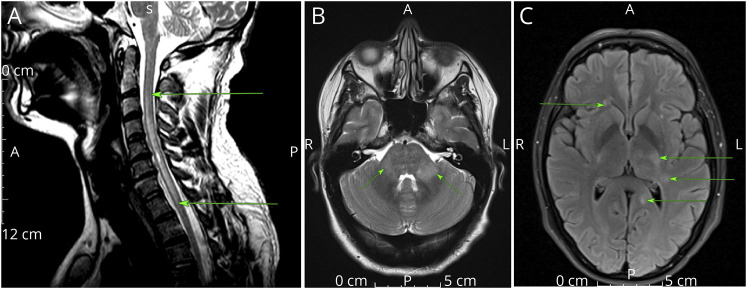
Figure 2. AQP4IgG+ MRI.
(A) T2-weighted sagittal MRI patient 1. Typical bright, spotty appearance of AQP4+ NMOSD (arrows). (B) T2-weighted axial MRI patient 1. Ill-defined right pontine lesion (arrow). (C and D) Sagittal (C) and axial (D) T2-weighted MRI. Hyperintensity in posterior medulla abutting fourth ventricle (arrows), typical of AQP4+ NMOSD. Abbreviations: AQP4 = aquaporin 4; NMOSD = neuromyelitis optica spectrum disorder
Both patients had other antibodies, and CSF analyses were normal, other than an elevated lymphocyte count in patient 1 at 41 × 106/L (eTable 1, links.lww.com/NXI/A771). Patient 2 was on antiviral therapy (lamuvidine) for chronic Hepatitis B infection with an undetectable viral load. Both patients were given IVMP, and patient 2 was also treated with PLEX.
At a follow-up of 398 and 465 days, respectively, both had good outcomes with mild sensory deficits and neuropathic pain as the main residual symptom. Neither had relapsed while on immunomodulatory therapy.
Seronegative Patients
Eleven patients were negative for both AQP4 and MOG antibodies, 9 identifying as White and 2 as South Asian, with a median age of 36 years. Five received the BNT162b2 vaccine and 6 the ChAdOx1S vaccine (Table, eTables 2–3, links.lww.com/NXI/A771).
The median age of disease onset in the BNT162b2 group was 27 years (range 18–36), compared with 41.5 years (range 35–50) in the ChAdOx1S group. Four of 5 (80%) in the BNT162b2 group were female, compared with 1 of 6 (16.7%) in the ChAdOx1S group. The median interval between vaccination and symptoms was longer in the BNT162b2 group (median 30 days, range 6–39) than in the ChAdOx1S group (median 13.5 days, range 6–42).
Four of the 6 (67%) ChAdOx1S recipients experienced LETM, compared with only 1 of 5 (20%) of the BNT162b2 patients (Figure 3). Two patients (33%) who received ChAdOx1S experienced facial nerve palsy with enhancement on MRI, but facial nerve involvement was not reported among the BNT162b2 group. One ChAdOx1S recipient had multifocal demyelinating neuropathy with conduction block, suggestive of Guillain-Barre syndrome (GBS) and another had flaccid paraplegia and areflexia, clinically suggestive of GBS, but no BNT162b2 recipient presented with a peripheral neuropathy. No patient in the ChAdOx1S group presented with isolated ON, compared with 2 of 5 (40%) in the BNT162b2 group.
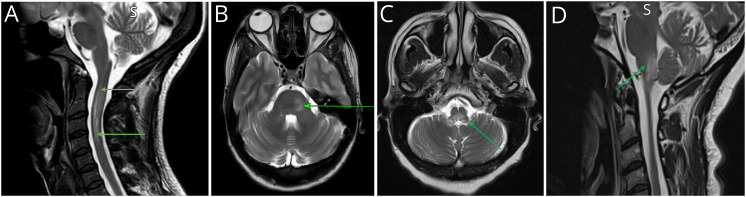
Figure 3. MRI Spinal Cord of Seronegative Patients.
(A) Gadolinium-enhanced T1-weighted sagittal MRI. Patchy enhancement throughout cervical cord (arrows). (B) T2-weighted sagittal MRI. LETM from level of C5 to T3 (long arrows) with discrete lesions throughout thoracic cord (short arrows). (C) T2-weighted axial MRI at level of T3. Centrally located hyperintensity affecting more than half the cord diameter (arrow). Abbreviation: LETM = longitudinally extensive TM.
eTables 2–3 (links.lww.com/NXI/A771) summarize the clinical syndromes, investigations, and treatments. Unmatched OCBs were detected in CSF samples from 2 of 3 BNT162b2 recipients tested, one of whom had multiple periventricular lesions on MRI and is now on natalizumab after diagnosis of MS. The other developed a silent peritrigonal lesion on MRI 6 months after symptom onset, suggestive of MS. None of the 5ChAdOx1S recipients tested had unmatched CSF OCBs.
The median duration of follow-up was similar—303 days (range 159–372) in the BNT162b2 group and 320 days (range 114–403) in the ChAdOx1S group.
Four of 6 (67%) ChAdOx1S recipients achieved good outcomes. One patient in the ChAdOx1S group with LETM (Figure 4, A and B) and biparietal lesions remained bedbound and incontinent at day 186 despite IVMP, PLEX, IVIg, and cyclophosphamide. Another patient in the ChAdOx1S group with cerebral palsy presented with rapidly progressive paraparesis and brainstem signs and died on day 25 after receiving IVMP and PLEX. MRI showed LETM and widespread ADEM-like lesions (Figure 4, C and D). Postmortem studies confirmed multifocal areas of demyelination associated with foamy macrophages, consistent with ADEM.
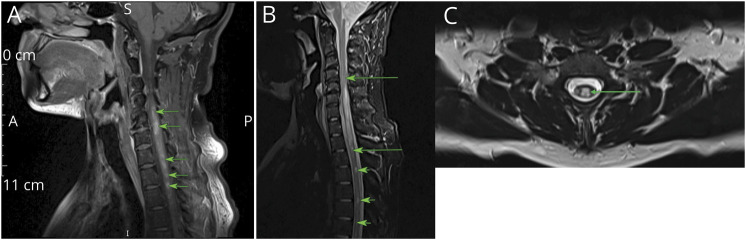
Figure 4. MRI Scans of Seronegative Patients With Poor Outcomes.
(A and B) Axial (A) and sagittal (B) T2-weighted spinal cord MRI. Diffuse swelling and T2 hyperintensity affecting full cord diameter (arrows). (C) Axial T2-weighted MRI. Multiple, rounded lesions in deep white matter (arrows). (D) Sagittal T2-weighted MRI. LETM (arrows). Abbreviation: LETM = longitudinally extensive TM.
One of 5 (20%) BNT162b2 recipients with a subsequent diagnosis of MS achieved a good outcome, while the 3 recipients with ON had moderate visual impairment. The remaining patient with TM had moderate outcome due to sphincter involvement and lower limb sensory impairment. No patient relapsed.
Discussion
We reported 25 cases of acute CNS inflammation, all occurring within 6 weeks of SARS-CoV-2 vaccination, although our a priori maximum interval was 8 weeks. Almost half were seropositive for MOG or AQP4 antibodies, and all but 2 of them received the ChAdOx1S vaccine. A peak in first attacks of MOGAD in Spring 2021 was attributable to postvaccine patients, supporting the hypothesis that vaccination can precipitate MOGAD. Most of the postvaccine MOGAD cases presented with the rarer phenotype of ADEM-like brain/brainstem inflammation and/or LETM, and none exhibited the typical isolated ON of adult-onset MOGAD. Seronegative ChAdOx1S recipients presented with a similar phenotype, and GBS and facial palsy were seen only in those who received the ChAdOx1S vaccine. Poor outcome was observed only among seronegative ChAdOx1S recipients with ADEM-like lesions.
ADEM has long been associated with preceding vaccination or infection (for review, see Refs. 12 and 13). MOG antibodies have been detected in 44–57% childhood ADEM cases.14,15 MOGAD has also been reported to follow an infection or vaccination,16-19 suggesting a proportion of vaccine-triggered childhood ADEM is caused by MOG antibodies. MOGAD presentation varies with age. ADEM is the presenting phenotype in 68% of children younger than 12 years but less than 15% of adult-onset MOGAD. By contrast, more than 50% of adults present with isolated ON (unilateral or bilateral).6,20,21 The absence of isolated ON and high proportion of our patients presenting with ADEM-like disease, often in combination with LETM and similar to that seen in children postvaccine, supports the hypothesis that these cases are vaccine triggered.
Although brain lesions are an atypical first presentation of MOGAD in adults, when they occur, they are typically large and ill-defined, often involving the cerebellar peduncles and pons.19,22 Asymptomatic brain and brainstem lesions such as those reported here are also well described in MOGAD attacks.19,23 Thus, the phenotypes in this study are infrequent but recognized sequelae of adult MOGAD attacks.
In incident MOGAD cohorts, relapses are reported in 25.1–36.0% over a median follow-up of 15.5–24.4 months,5,20,21 although higher relapse rates have been reported in nonincident cohorts.16 The short follow-up period and small number of patients prevent us determining whether vaccine-related MOGAD is more likely to be monophasic. However, 1 of 12 (8%) MOGIgG+ patients relapsed at 5 months, which may raise some concern.
The 1 MOGIgG+ patient who showed positive test results for Borrelia testing had neurologic symptoms felt to be compatible with MOGAD because erythema migrans occurred 3–4 months before neurologic symptoms (a longer interval than typical neuroborreliosis24), the MOG antibody titers were persistently high up to 12 months after onset, and the patient improved clinically after steroid treatment and before antibiotic therapy. They did not have the typical meningoradiculitis or facial nerve palsy associated with neuroborreliosis.24
AQP4 IgG was detected in only 2 patients with typical demographic features, both being female and of Afro-Caribbean descent.2 Both had some typical imaging features, such as lesions around the periaqueductal gray and third and lateral ventricles and bright spotty TM lesions.25,26
Both AQP4IgG+ patients also had large, hazy brainstem lesions, reminiscent of those seen in MOGIgG+ ADEM and our MOGIgG+ group. Both had good outcomes, whereas recovery from AQP4IgG+ relapses is typically poor, with 1 study showing EDSS ≥6 in almost half of patients and bilateral blindness in more than 10%.27 It is uncertain whether the short time interval between vaccination and symptom onset for patient 1 is compatible with a vaccine-associated autoimmune disease.
Although both vaccines were equally implicated in antibody-negative cases, we identified differences in the clinicoradiologic syndromes and outcomes associated with the BNT162b2 and the ChAdOx1S vaccines. BNT162b2 recipients were younger and developed symptoms after a longer postvaccine interval than ChAdOx1S recipients. The most striking radiologic findings were reported in 2 ChAdOx1S recipients with large ADEM-like lesions, one of whom died and the other remained quadriplegic at the last follow-up. LETM was also more frequently observed following ChAdOx1S than BNT162b2. Radiologic similarities between seronegative and MOGIgG+ ChAdOx1S recipients may suggest a common mechanism underlying the 2 postvaccine serotypes. In addition, facial nerve involvement and GBS-like syndromes were unique to seronegative ChAdOx1S recipients. This may not be coincidental because peripheral nerve problems, such as Bell palsy and GBS, have been associated with the ChAdOx1S vaccine.28 Two seronegative BNT162b2 recipients had features suggestive of MS, and these events may represent first clinical episodes of previously quiescent disease provoked by vaccination, rather than de novo CNS inflammation. In this case, instances of new CNS inflammation following BNT162b2 are even less frequent than suggested in this study, supporting the hypothesis that ChAdOx1S specifically triggers acute CNS inflammation.
We have a wealth of experience with standard vaccine approaches and their consequences, but mRNA vaccine technology is new, and thus, our experience of the consequences is limited. However, the vast numbers of patients vaccinated over a short period may allow us to detect even rare side effects. Several observations suggest ChAdOx1S vaccination may be more likely to trigger autoimmune CNS disease than BNT162b2. First, ChAdOx1S vaccine is overrepresented in our cohort (18/25; 72%); the earliest vaccination in the current group was in February 2021, and by May 2021, only 42% of vaccine recipients aged 20–40 years (encompassing the median age of our cohort) had received ChAdOx1S.28 After May 2021, BNT162b2 was recommended for people younger than 40 years. Therefore, our sample contains an unexpectedly high proportion of young ChAdOx1S recipients.
Second, almost all patients with detectable autoantibodies received ChAdOx1S. This is possibly the most compelling evidence that this vaccine specifically triggers CNS autoimmune inflammation. Third, both AQP4IgG+, half MOGIgG+, and half seronegative ChAdOx1S patients shared TM and brain involvement, mimicking the childhood-onset vaccine-triggered phenotypes.
Fourth, the median interval between vaccination and symptom onset was shorter in the ChAdOx1S group; only 3 of 18 (17%) patients in the ChAdOx1S group developed symptoms beyond 3 weeks after vaccination, compared with 4 of 7 (57%) patients in the BNT162b2 group. This shorter time course is more typical of a vaccine-related autoimmune response29 and is consistent with the time course of other SARS-CoV-2 vaccine–related neurologic diseases.28 This further supports our hypothesis that vaccination may trigger CNS inflammation.
Finally, ChAdOx1S is associated with other antibody-mediated diseases, including vaccine-induced thrombosis and thrombocytopenia (VITT), caused by antibodies to platelet factor 4 (PF4).30-32 A large study identified significant increases in risk of the antibody-mediated peripheral nerve disease GBS and Bell palsy in the 3 weeks after ChAdOx1S vaccine,28 both in line with our findings. Moreover, a trend was observed toward an increased risk of a combined outcome of meningitis, encephalitis, and myelitis between 8 and 14 days after ChAdOx1S vaccine.
Vaccination associated with acute CNS demyelination, particularly ADEM, was well recognized before MOG IgG was described12,13,29 and at least half of patients with ADEM have MOG IgG.4,14,15 In addition, there are several case reports of ON and LETM after vaccination (for review, see Ref. 29). There are also rare reports of and both AQP4-NMOSD17,33 and MOGAD occurring postvaccination.16-19 This supports our hypothesis that some postvaccine CNS inflammation may be caused by vaccine-triggered MOG IgG.
The pathophysiology underlying vaccine-induced CNS inflammation is uncertain. The existence of MOG-specific B cells in healthy individuals34 suggests preexisting susceptibility in patients, and that vaccination somehow leads to their activation, although de novo antibody synthesis may also occur.35
Clues to the underlying immunopathology may come from studying the consequences of SARS-CoV-2 infection. Patients' serum has been found to contain a range of autoantibodies that attack different tissues,35 and cases of AQP4IgG+ NMOSD and MOGAD have been reported in the context of SARS-CoV-2 infection.36-39
It is possible that the host immune response to a particular vaccine or viral epitope cross-reacts with CNS autoantigens because of “molecular mimicry.” If molecular mimicry is responsible for autoantibody synthesis after infection and vaccination, a spike protein epitope must share structural homology with CNS antigens because this is the only component shared by the vaccines and the virus.
While this explains acute CNS inflammation after both infection and vaccination, it fails to account for the higher CNS autoimmunity associated with the ChAdOx1S vaccine compared with BNT162b2. The former consists of spike protein–encoding DNA in a replication-deficient chimpanzee adenovirus vector. The latter is composed of spike protein–encoding mRNA encased in lipid nanoparticles.40,41 The greater autoreactive potential may be attributable to the viral vector in the ChAdOx1S vaccine, as exemplified by VITT, where adenovirus binding to cellular heparan has been proposed to lead to PF4 antibody production.42
Alternative theories to molecular mimicry include bystander activation, antigen spreading, or a combination of these mechanisms. Epitope spreading involves B-cell cocapture of vaccine antigen and autoantigen, leading to expansion and stimulation of autoantibody-producing B cells.43 This mechanism has been demonstrated in mice, where simultaneous internalization of MOG protein and viral antigen by MOG-specific B cells stimulates T-cell proliferation and robust MOG IgG production in vitro.44 Furthermore, both infection and vaccination can produce localized and systemic proinflammatory environments and release of self-antigens from damaged tissue. These self-antigens might trigger previously unresponsive, autoreactive “bystander” T cells and/or B cells that on activation “spread” to target additional self-epitopes.
These hypotheses depend on the entry of MOG antibodies or MOG IgG–producing B cells to the CNS. This requires an increased permeability of the blood-brain barrier, a phenomenon suggested by the detection of antibodies to CNS-restricted antigens in the CSF and serum of patients with severe SARS-CoV-2 infection,45 and similar changes in barrier function are observed after vaccination.46
Owing to the rarity of the conditions under investigation and the lack of systematic UK-wide sampling, we are unable to determine whether the UK incidence of MOGAD increased after introduction of the ChAdOx1S vaccine. However, the observed peak in onset MOGAD in the Spring of 2021 was not observed in preceding years and followed the peak of first vaccine administration, supporting an association between vaccination and new MOGAD attacks.
We do not have experience with DNA vaccine technology, but the 1-day interval between vaccination and symptom onset in 1 AQP4IgG+ case may be too short for a vaccine-mediated autoimmune reaction, although if the AQP4IgG were present already, the vaccination might have triggered a surge of antibodies.47
In 8 seronegative cases, antibodies were tested after treatment initiation or the order of sampling and treatment was unknown, potentially falsely reducing the proportion of seropositive cases. However, because repeat samples in 4 of 8 patients were persistently negative (including 3 sampled after treatment), there are likely true seronegative patients.
One AQP4IgG+ patient had low antibody titers and their serum was not retested, although in our laboratory, a titer of 25 is specific for the NMOSD phenotype. Finally, 4 cases were previously mentioned in a correspondence to Lancet Neurology, and it is important to note the cases are not additive.
Strengths of the study include our standing as a tertiary referral service enables comprehensive data collection and access to specialist neuroradiologists and neuroimmunologists. Furthermore, we had access to a postmortem specimen, which confirmed the diagnosis of ADEM. Although the short follow-up period limits our ability to predict prognosis of these patients, our service provides long-term care that will permit us to compare long-term outcomes with those of nonvaccine-associated NMOSD and MOGAD.
ChAdOx1S vaccine was associated with antibody-mediated CNS inflammation and mainly with MOGAD. Adult patients who developed MOGAD after vaccination presented atypically with brain lesions and LETM, more reminiscent of MOGIgG+ ADEM seen in children, supporting a causative role. This suggests vaccination may influence the phenotypic presentation of MOGAD, and some adult-onset ADEM-like MOGAD cases might be triggered by environmental factors. Further study is required to determine the sequence of immunologic events that link vaccination and autoimmune CNS disease. We stress that SARS-CoV-2 infection is associated with high morbidity and mortality, and neurologic sequelae are more frequent after infection than vaccination.28 Consequently, physicians should continue to promote vaccination against SARS-CoV-2, and we have encouraged our postvaccine group to pursue further vaccination with an alternative formulation to that which preceded their illness.
Acknowledgment
The authors acknowledge NHS Highly Specialised Services and the Oxford Neuroimmunology laboratory.
Glossary
- ADEM
acute disseminated encephalomyelitis
- AQP4
aquaporin 4
- IVIg
IV immunoglobulin
- IVMP
IV methylprednisolone
- LETM
longitudinally extensive TM
- GBS
Guillain-Barre syndrome
- MOGAD
myelin oligodendrocyte glycoprotein antibody–associated disease
- NMOSD
neuromyelitis optica spectrum disorder
- OCB
oligoclonal band
- ON
optic neuritis
- PF4
platelet factor 4
- PLEX
plasma exchange
- TM
transverse myelitis
- VITT
vaccine-induced thrombosis and thrombocytopenia
Appendix. Authors
Study Funding
The authors report no targeted funding.
Disclosure
A. Francis, K. Elhadd, V. Camera, P. Adib-Samii, B. Athwal, K. Attfield, A. Barritt, M. Craner, L. Fisniku, A.K.N. Iversen, O Leach, L. Matthews, J. O'Riordan, A. Scalfari, R. Tanasescu, D. wren, S. Huda, M.I. Leite, L. Fugger, and J. Palace report no disclosures relevant to this manuscript. Addendum: Dr. Chiara Rocchi, Dr. Monica dos Santos, and Dr. Ian Redmond report no disclosures relevant to this manuscript. Go to Neurology.org/NN for full disclosures.
References
- 1.Wingerchuk DM, Banwell B, Bennett JL, et al. . International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85(2):177-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hor JY, Asgari N, Nakashima I, et al. . Epidemiology of neuromyelitis optica spectrum disorder and its prevalence and incidence worldwide. Front Neurol. 2020;11:501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kitley J, Woodhall M, Waters P, et al. . Myelin-oligodendrocyte glycoprotein antibodies in adults with a neuromyelitis optica phenotype. Neurology. 2012;79(12):1273-1277. [DOI] [PubMed] [Google Scholar]
- 4.Baumann M, Sahin K, Lechner C, et al. . Clinical and neuroradiological differences of paediatric acute disseminating encephalomyelitis with and without antibodies to the myelin oligodendrocyte glycoprotein. J Neurol Neurosurg Psychiatry. 2015;86(3):265-272. [DOI] [PubMed] [Google Scholar]
- 5.Jurynczyk M, Messina S, Woodhall MR, et al. . Clinical presentation and prognosis in MOG-antibody disease: a UK study. Brain. 2017;140(12):3128-3138. [DOI] [PubMed] [Google Scholar]
- 6.de Mol C, Wong Y, van Pelt E, et al. . The clinical spectrum and incidence of anti-MOG-associated acquired demyelinating syndromes in children and adults. Mult Scler Houndmills Basingstoke Engl. 2020;26(7):806-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inc MG. The epidemiology of myelin oligodendrocyte glycoprotein antibody. Dr. Eoin Flanagan [Internet]; 2022. Available at: onlinelibrary.ectrims-congress.eu/ectrims/2019/stockholm/278755/eoin.flanagan.the.epidemiology.of.myelin.oligodendrocyte.glycoprotein.antibody.html
- 8.Kitley J, Leite MI, Nakashima I, et al. . Prognostic factors and disease course in aquaporin-4 antibody-positive patients with neuromyelitis optica spectrum disorder from the United Kingdom and Japan. Brain J Neurol. 2012;135(Pt 6):1834-1849. [DOI] [PubMed] [Google Scholar]
- 9.Waters P, Reindl M, Saiz A, et al. . Multicentre comparison of a diagnostic assay: aquaporin-4 antibodies in neuromyelitis optica. J Neurol Neurosurg Psychiatry. 2016;87(9):1005-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waters P, Woodhall M, O'Connor KC, et al. . MOG cell-based assay detects non-MS patients with inflammatory neurologic disease. Neurol - Neuroimmunol Neuroinflammation. 2015;2(3):e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Francis A, Palace J, Fugger L. MOG antibody-associated disease after vaccination with ChAdOx1 nCoV-19. Lancet Neurol. 2022;21(3):217-218. [DOI] [PubMed] [Google Scholar]
- 12.Torisu H, Okada K. Vaccination-associated acute disseminated encephalomyelitis. Vaccine. 2019;37(8):1126-1129. [DOI] [PubMed] [Google Scholar]
- 13.Huynk W, Cordato D, Kehdi E, Masters L, Dedousis C. Post-vaccination encephalomyelitis: literature review and illustrative case. J Clin Neurosci. 2008;15:1315-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hennes EM, Baumann M, Schanda K, et al. . Prognostic relevance of MOG antibodies in children with an acquired demyelinating syndrome. Neurology. 2017;89(9):900-908. [DOI] [PubMed] [Google Scholar]
- 15.Di Pauli F, Mader S, Rostasy K, et al. . Temporal dynamics of anti-MOG antibodies in CNS demyelinating diseases. Clin Immunol. 2011;138(3):247-254. [DOI] [PubMed] [Google Scholar]
- 16.Jarius S, Ruprecht K, Kleiter I, et al. . MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflammation. 2016;13(1):280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar N, Graven K, Joseph NI, et al. . Case report: postvaccination anti-myelin oligodendrocyte glycoprotein neuromyelitis optica spectrum disorder: a case report and literature review of postvaccination demyelination. Int J MS Care. 2020;22(2):85-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramanathan S, Mohammad S, Tantsis E, et al. . Clinical course, therapeutic responses and outcomes in relapsing MOG antibody-associated demyelination. J Neurol Neurosurg Psychiatry. 2018;89(2):127-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banks SA, Morris PP, Chen JJ, et al. . Brainstem and cerebellar involvement in MOG-IgG-associated disorder versus aquaporin-4-IgG and MS. J Neurol Neurosurg Psychiatry. 2021;92(4):384-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cobo-Calvo A, Ruiz A, Rollot F, et al. . Clinical features and risk of relapse in children and adults with myelin oligodendrocyte glycoprotein antibody–associated disease. Ann Neurol. 2021;89(1):30-41. [DOI] [PubMed] [Google Scholar]
- 21.Satukijchai C, Mariano R, Messina S, et al. . Factors associated with relapse and treatment of myelin oligodendrocyte glycoprotein antibody–associated disease in the United Kingdom. JAMA Netw Open. 2022;5(1):e2142780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jurynczyk M, Geraldes R, Probert F, et al. . Distinct brain imaging characteristics of autoantibody-mediated CNS conditions and multiple sclerosis. Brain. 2017;140(3):617-627. [DOI] [PubMed] [Google Scholar]
- 23.Camera V, Holm-Mercer L, Ali AAH, et al. . Frequency of new silent MRI lesions in myelin oligodendrocyte glycoprotein antibody disease and aquaporin-4 antibody neuromyelitis optica spectrum disorder. JAMA Netw Open. 2021;4(12):e2137833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russell ALR, Dryden M, Pinto AA, Lovett J. Lyme disease: diagnosis and management. Pract Neurol. 2018;18:455-464. [DOI] [PubMed] [Google Scholar]
- 25.Salama S, Levy M. Bright spotty lesions as an imaging marker for neuromyelitis optica spectrum disorder. Mult Scler J. 2021;26:1352458521994259. [DOI] [PubMed] [Google Scholar]
- 26.Kim HJ, Paul F, Lana-Peixoto MA, et al. . MRI characteristics of neuromyelitis optica spectrum disorder. Neurology. 2015;84(11):1165-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palace J, Lin DY, Zeng D, et al. . Outcome prediction models in AQP4-IgG positive neuromyelitis optica spectrum disorders. Brain. 2019;142(5):1310-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patone M, Handunnetthi L, Saatci D, et al. . Neurological complications after first dose of COVID-19 vaccines and SARS-CoV-2 infection. Nat Med. 2021;27(12):2144-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karussis D, Petrou P. The spectrum of post-vaccination inflammatory CNS demyelinating syndromes. Autoimmun Rev. 2014;13(3):215-224. [DOI] [PubMed] [Google Scholar]
- 30.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384(22):2092-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.COVID-19 Vaccine (Temporary Authorisation—Review Product Information Tab before Reading)—Summary of Product Characteristics (SmPC)- (Emc) [Internet]. 2021. Available at: medicines.org.uk/emc/product/12333/smpc#CLINICAL_PRECAUTIONS [Google Scholar]
- 32.Schulz JB, Berlit P, Diener HC, et al. . COVID-19 vaccine-associated cerebral venous thrombosis in Germany. Ann Neurol. 2021;90(4):pp627-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menge T, Cree B, Saleh A, et al. . Neuromyelitis optica following human papillomavirus vaccination. Neurology. 2012;79(3):285-287. [DOI] [PubMed] [Google Scholar]
- 34.Reindl M, Waters P. Myelin oligodendrocyte glycoprotein antibodies in neurological disease. Nat Rev Neurol. 2019;15(2):89-102. [DOI] [PubMed] [Google Scholar]
- 35.Wang EY, Mao T, Klein J, et al. . Diverse functional autoantibodies in patients with COVID-19. Nature. 2021;595(7866):283-288. [DOI] [PubMed] [Google Scholar]
- 36.Ismail II, Salama S. Association of CNS demyelination and COVID-19 infection: an updated systematic review. J Neurol. 2021:1-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kogure C, Kikushima W, Fukuda Y, et al. . Myelin oligodendrocyte glycoprotein antibody-associated optic neuritis in a COVID-19 patient: a case report. Medicine (Baltimore). 2021;100(19):e25865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peters J, Alhasan S, Vogels CBF, Grubaugh ND, Farhadian S, Longbrake EE. MOG-associated encephalitis following SARS-COV-2 infection. Mult Scler Relat Disord. 2021;50:102857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou S, Jones-Lopez EC, Soneji DJ, Azevedo CJ, Patel VR. Myelin oligodendrocyte glycoprotein antibody–associated optic neuritis and myelitis in COVID-19. J Neuroophthalmol. 2020;40(3):398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kyriakidis NC, López-Cortés A, González EV, Grimaldos AB, Prado EO. SARS-CoV-2 vaccines strategies: a comprehensive review of phase 3 candidates. Npj Vaccin. 2021;6(1):1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arashkia A, Jalilvand S, Mohajel N, et al. . Severe acute respiratory syndrome-coronavirus-2 spike (S) protein based vaccine candidates: state of the art and future prospects. Rev Med Virol. 2020;31(3):e2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McLean‐Tooke A, Lucas M, French M. Autoimmunity elicited by the chemokine response to adenovirus vector vaccines may underlie vaccine-induced immune thrombotic thrombocytopenia: a hypothesis. Clin Transl Immunol. 2021;10(10):e1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prüss H. Autoantibodies in neurological disease. Nat Rev Immunol. 2021;21(12):798-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanderson NSR, Zimmermann M, Eilinger L, et al. . Cocapture of cognate and bystander antigens can activate autoreactive B cells. Proc Natl Acad Sci. 2017;114(4):734-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Franke C, Ferse C, Kreye J, et al. . High frequency of cerebrospinal fluid autoantibodies in COVID-19 patients with neurological symptoms. Brain Behav Immun. 2021;93:415-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hervé C, Laupèze B, Del Giudice G, Didierlaurent AM, Tavares Da Silva F. The how's and what's of vaccine reactogenicity. Npj Vaccin. 2019;4(1):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leite MI, Coutinho E, Lana-Peixoto M, et al. . Myasthenia gravis and neuromyelitis optica spectrum disorder: a multicenter study of 16 patients. Neurology. 2012;78(20):1601-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data not provided in the article may be shared (anonymized) at the request of any qualified investigator for purposes of replicating procedures and results.