Abstract
Background and Objectives
Cladribine tablets cause a reduction in lymphocytes with a predominant effect on B-cell and T-cell counts. The MAGNIFY-MS substudy reports the dynamic changes on multiple peripheral blood mononuclear cell (PBMC) subtypes and immunoglobulin (Ig) levels over 12 months after the first course of cladribine tablets in patients with highly active relapsing multiple sclerosis (MS).
Methods
Immunophenotyping was performed at baseline (predose) and at the end of months 1, 2, 3, 6, and 12 after initiating treatment with cladribine tablets. Assessments included lymphocyte subtype counts of CD19+ B cells, CD4+ and CD8+ T cells, CD16+ natural killer cells, plasmablasts, and Igs. Immune cell subtypes were analyzed by flow cytometry, and serum IgG and IgM were analyzed by nephelometric assay. Absolute cell counts and percentage change from baseline were assessed.
Results
The full analysis set included 57 patients. Rapid reductions in median CD19+, CD20+, memory, activated, and naive B-cell counts were detected, reaching nadir by month 2. Thereafter, total CD19+, CD20+, and naive B-cell counts subsequently reconstituted, but memory B cells remained reduced by 93%–87% for the remainder of the study. The decrease in plasmablasts was slower, reaching nadir at month 3. Decrease in T-cell subtypes was also slower and more moderate compared with B-cell subtypes, reaching nadir between months 3 and 6. IgG and IgM levels remained within the normal range over the 12-month study period.
Discussion
Cladribine tablets induce a specific pattern of early and sustained PBMC subtype dynamics in the absence of relevant Ig changes: While total B cells were reduced dramatically, T cells were affected significantly less. Naive B cells recovered toward baseline, naive CD4 and CD8 T cells did not, and memory B cells remained reduced. The results help to explain the unique immune depletion and repopulation architecture regarding onset of action and durability of effects of cladribine tablets while largely maintaining immune competence.
Trial Registration Information
ClinicalTrials.gov Identifier: NCT03364036. Date registered: December 06, 2017.
Multiple sclerosis (MS) is a chronic inflammatory disease of the CNS for which several disease-modifying therapies (DMTs) are available to reduce the risk of relapse and worsening disability. Although the pathogenesis of MS is not fully established, numerous immune cell subtypes are believed to play a role in demyelination and neuroaxonal damage.1,2 Indeed, MS has been historically associated with T cells, specifically T helper cells.3 However, more recently there is increasing evidence of the contribution of B cells,4-6 microglial cells,7,8 and potentially natural killer (NK) cells9-11 in the pathogenesis of MS.
Current generation DMTs for MS include a range of immune depletion and repopulating agents.12 Such treatments include cladribine tablets 10 mg (MAVENCLAD, the healthcare business of Merck KGaA, Darmstadt, Germany; 3.5 mg/kg cumulative dose over 2 years), a highly efficacious DMT that is approved for the treatment of MS in >80 countries worldwide. Evidence suggests that cladribine tablets act as an immune reconstitution therapy, causing a reduction of lymphocytes with predominance in B-cell and T-cell counts followed by reconstitution occurring at different times and rates according to cell subtype.13-15 While cladribine tablets selectively reduce adaptive immune cell counts, the effects on the innate immune system are negligible.16
Some long-term evaluations of the effect of treatment with cladribine tablets on peripheral blood mononuclear cell (PBMC) subtype dynamics have been completed in previous clinical trials (CLARITY [CLAdRIbine Tablets treating multiple sclerosis orallY], CLARITY Extension, Prospective observational long-term safety registry of multiple sclerosis patients who have parti-cipated in cladribine clinical trials [PREMIERE] and the oral cladribine for early multiple sclerosis trial [ORACLE-MS]).17,18 However, the results described only major cell types and effects of cladribine tablets on a range of immune cell subtypes while a deeper immune phenotyping was not determined in these studies.
In this study, as part of an exploratory substudy, our research question concerned PBMC subtype dynamics and immunoglobulin (Ig) levels during the first 12 months after patients had initiated treatment with cladribine tablets for highly active relapsing MS. The substudy was part of MAGNIFY-MS (NCT03364036), which has previously shown significant reductions in active MRI lesions from month 2 (day 60) onward after treatment initiation with cladribine tablets.19
Methods
Study Design and Participants
A 2-year Prospective Study to Evaluate the Onset of Action of Mavenclad in Subjects With Highly Active Relapsing Multiple Sclerosis (MAGNIFY-MS) (NCT03364036) was a 2-year, phase IV, open-label, single-arm study in which eligible patients are scheduled to receive cladribine tablets 3.5 mg/kg cumulative dose over 2 years (eFigure 1, http://links.lww.com/NXI/A759). Patients receive 2 weeks of active treatment per course (week 1 and week 5 of each year), the start of the first week of treatment in year 1 being considered baseline.
Patients aged 18 years or older, with an Expanded Disability Status Scale (EDSS) score ≤5 and a diagnosis of highly active relapsing MS were enrolled between May 28, 2018, and April 23, 2019. In this study, highly active relapsing MS was defined as 1 relapse in the previous year and ≥1 T1 gadolinium enhancing (Gd+) lesion or ≥9 T2 lesions while on treatment with another DMT or ≥2 relapses in the previous year whether on DMT or not.
Patients were excluded if they had previous exposure to DMT (fingolimod, natalizumab, alemtuzumab, mitoxantrone, or ocrelizumab); a lymphocyte count not within normal laboratory limits; presence of signs of progressive multifocal leukoencephalopathy; tested positive for HIV, hepatitis B or C, or active/latent tuberculosis; had an active malignancy or had an allergy or hypersensitivity to gadolinium; and/or any other contraindication to perform MRI.
Peripheral Blood Sampling
This exploratory substudy of MAGNIFY-MS involved a longitudinal evaluation of PBMCs, as summarized in eFigure1, http://links.lww.com/NXI/A759. Immunophenotyping was performed on blood samples collected at baseline (predose) and at the end of months 3, 6, and 12. Further immunophenotyping was completed at the end of months 1 and 2 for the full B-cell panel, CD4+ and CD8+ T cells, and CD16+ NK cells. Immune cell subtypes were analyzed by flow cytometry (Becton Dickinson FACSCanto II cell analyzer) and were detected using surface cell markers; details are provided in eAppendix1, http://links.lww.com/NXI/A759. For the purposes of immunophenotyping, CD19+ B cells were analyzed as part of the TBNK cell panel while B cell subtypes were analyzed as part of the full B-cell panel.
In parallel, serum levels of IgG and IgM were analyzed by nephelometric assay (eAppendix1, http://links.lww.com/NXI/A759).
Absolute cell counts and median percentage change from baseline were assessed for cell subtypes and Igs.
Statistical Analysis
The analysis of PBMC cell subtypes was an exploratory substudy of MAGNIFY-MS, and thus, demographical and safety data for the full study cohort were analyzed descriptively with no formal statistical testing. All patients who received ≥1 dose of cladribine tablets were included in this analysis.
The study protocol and statistical analysis plan for MAGNIFY-MS have been published on ClinicalTrials.gov (NCT03364036).20
Standard Protocol Approvals, Registrations, and Patient Consents
Ethical approval for the MAGNIFY-MS study (NCT03364036) was obtained from independent ethics committees for each trial site, and the study was performed in line with the principles of the Declaration of Helsinki. All participants provided written informed consent before participation in the study.
Data Availability
Any requests for data by qualified scientific and medical researchers for legitimate research purposes will be subject to the Data Sharing Policy of the healthcare business of Merck KGaA, Darmstadt, Germany. All requests should be submitted in writing to the data sharing portal of the healthcare business of Merck KGaA, Darmstadt, Germany, emdgroup.com/en/research/our-approach-to-research-and-development/healthcare/clinical-trials/commitment-responsible-data-sharing.html. When the healthcare business of Merck KGaA, Darmstadt, Germany, has a coresearch, codevelopment, or comarketing or copromotion agreement, or when the product has been out-licensed, the responsibility for disclosure might be dependent on the agreement between parties. Under these circumstances, the healthcare business of Merck KGaA, Darmstadt, Germany, will endeavor to gain agreement to share data in response to requests.
Results
Between May 28, 2018, and April 23, 2019, a total of 70 patients were screened for enrollment to this MAGNIFY-MS substudy and 57 of these patients initiated treatment with cladribine tablets (13 patients were screening failures). The treated patient population were mostly aged 40 years or younger (61.4%; median 37.0 [range 20–60] years), were predominantly female (61.4%), and had a median EDSS score of 2.5 at baseline. Patient demographics and characteristics from this MAGNIFY-MS substudy are shown in Table 1 and indicate that the population from this substudy was similar to the overall population of the MAGNIFY-MS study.
Table 1.
Demographics and Characteristics of Patients Included in This Substudy of MAGNIFY-MS and the Overall Population From the MAGNIFY-MS Study
Changes in the Adaptive Immune Components
B-Cell Subtypes
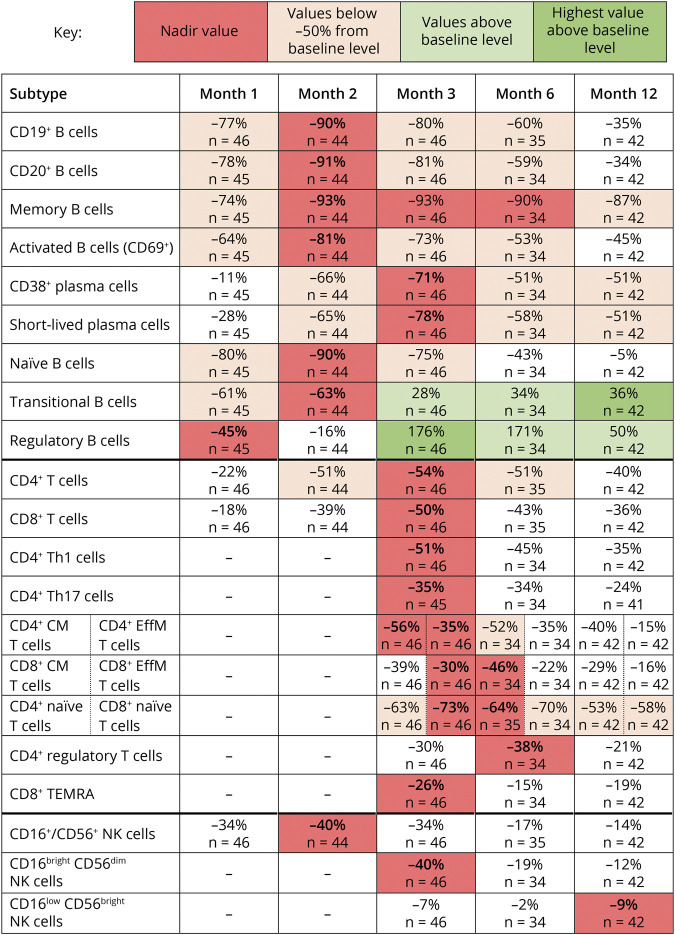
CD19+, CD20+, memory, naive, and activated (CD69+) B cells were reduced early on during treatment, as shown by median percentage changes from baseline to month 1 of −77%, −78%, −74%, −80%, and −64%, respectively (Figures 1–3). Following nadir at month 2, median percentage change of CD19+ (−90%), CD20+ (−91%), and CD69+ (−81%) B cells showed recovery toward baseline from month 3 onward and largely shared similar repopulation profiles, with a reduction in median percentage changes between −34% and −45% at month 12. Naive B cells also reached nadir at month 2 (median percentage change: −90%) but recovered to near baseline levels at month 12 (median percentage change: −5%).
Figure 1. Median Percentage Change From Baseline of B-Cell and T-Cell Subtypes.
CM = central memory; EffM = effector memory; NK = natural killer; TEMRA = terminally differentiated effector memory RA+; Th = T helper.
Figure 2. Median Values of CD19+ and CD20+ B Cell, CD4+ and CD8+ T Cell, and CD16+/CD56+ Natural Killer (NK) Cell Counts Where Additional Immunophenotyping Was Performed at Months 1 and 2, in Patients Treated With Cladribine Tablets.
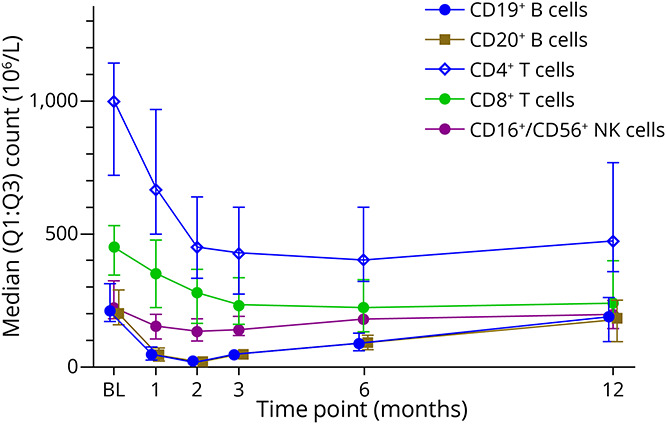
The first treatment course of cladribine tablets was administered at baseline and month 1. Data for CD19+ and CD20+ B cells have been slightly offset at each time point to improve readability as data points are overlapping. BL = baseline; Q = quartile.
Figure 3. Median Values of B-Cell and T-Cell Counts in Patients Treated With Cladribine Tablets.
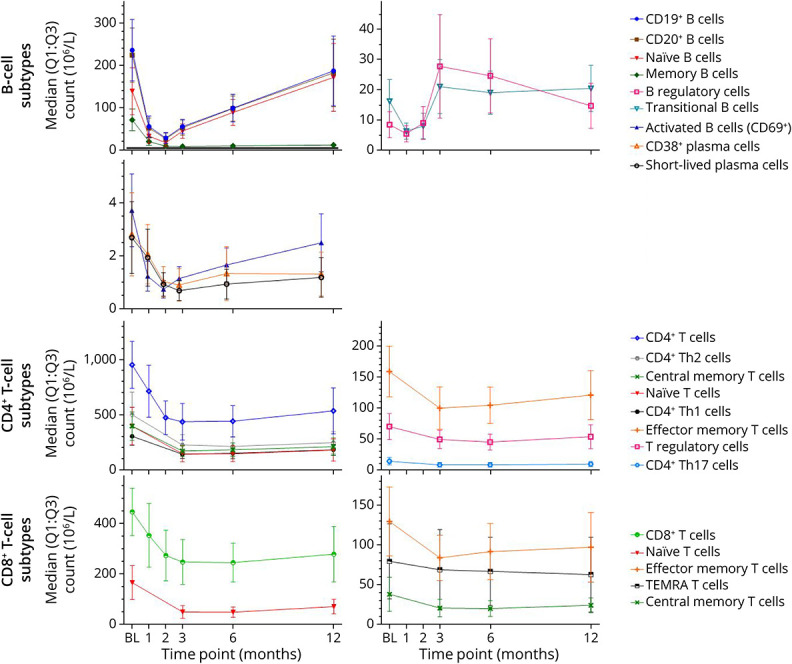
The first treatment course of cladribine tablets was administered at baseline and month 1. BL = baseline; Q = quartile; TEMRA = terminally differentiated effector memory RA+; Th = T helper.
While most B-cell subtypes reached nadir by month 2 after treatment, CD38+ plasmablasts and short-lived plasma cells reached nadir at month 3 (median percentage change: −71% and −78%, respectively) with both subtypes reaching a median percentage change of −51% at month 12.
After the 3-month time point, the reduction in memory B cells was sustained to month 12 (median percentage change: −87%). Reductions in B regulatory (Breg) and B transitional (Btrans) cells were relatively rapid but less profound at month 1 (median percentage change: −45% and −61%, respectively), with Breg beginning to repopulate from month 2 (median percentage change: −16%). By month 3, cell counts for both Breg and Btrans cell subtypes had recovered and were increased over baseline levels (median percentage change: Bregs +176%; Btrans +28%). Breg and Btrans cell numbers continued to show a sustained increase over baseline levels up to month 12, potentially increasing the ratio of regulatory vs effector B-cell subtypes from month 2 and 3 onward.
T-Cell Subtypes
While total B cells were reduced dramatically, T cells were less affected. CD4+ and CD8+ T cells showed a slower and more moderate reduction in cell counts compared with B-cell subtypes, as represented by a smaller median percentage change from baseline to month 1 (−22% and −18%, respectively) while nadir was reached at month 3 followed by a slight recovery toward baseline (Figures 1–3).
For CD4+ and CD8+ central memory and effector memory T cells, the greatest median percentage change from baseline was reached between months 3 and 6. CD4+ central and effector memory cells showed the greatest median percentage change from baseline of −56% and −35% at month 3, respectively, with nadir for effector memory cells sustained into month 6. CD8+ central and effector memory cells showed the greatest median percentage change from baseline of −46% and −22% at month 6, respectively. All CD4+ and CD8+ central and effector memory T-cell subtypes showed recovery toward baseline at month 12.
The greatest reduction in cell count for CD4+ T helper (Th)1 and CD4+ Th17 cells occurred at month 3, with median percentage changes from baseline of −51% and −35%, respectively.
While we have seen naive B cells recover toward baseline, naive CD4 and CD8 T cells did not. Naive CD4+ and CD8+ T cells showed the greatest median percentage change from baseline at month 6 (−64% and −70%, respectively), followed by some recovery toward baseline at month 12.
CD4+ T regulatory (Treg) cells showed a slower and less profound decrease from baseline compared with other T-cell subtypes, reaching the lowest level of median percentage change of −38% at month 6. Thereafter, Treg counts showed a slight recovery toward baseline at month 12.
CD8+ terminally differentiated effector memory RA+ (TEMRA) T cells showed the greatest median percentage change from baseline at month 3 (−26%), followed by a recovery of +11% from month 3 to 6. This was followed by a slight decline of −4% at month 12.
Changes in NK Cells
Changes in CD16+/CD56+ NK cell counts showed a median percentage decrease at month 1 of −34% with nadir reached at month 2, as represented by a median percentage change from baseline of −40% (Figures 1, 3, and 4). Recovery of these NK cells toward baseline occurred between months 3 and 12. CD16bright CD56dim NK cell counts were decreased at month 3 (median percentage change: −40%) and remained below baseline level at month 12 (median percentage change: −12%), while no effect on CD16low CD56bright NK cell counts was noted.
Figure 4. Median Values of Natural Killer (NK) Cell Counts in Patients Treated With Cladribine Tablets.
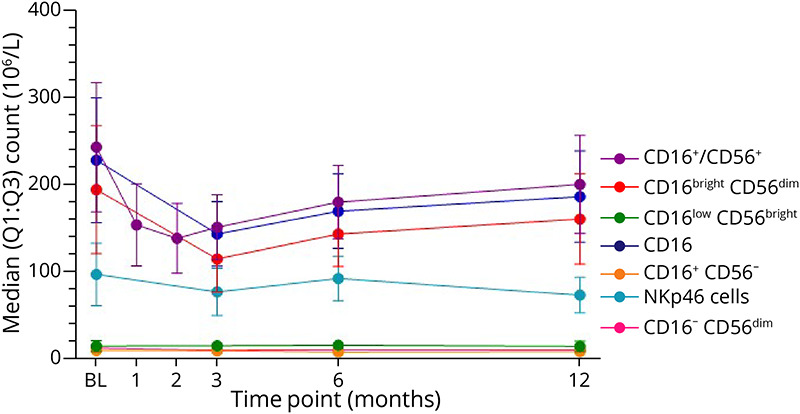
The first treatment course of cladribine tablets was administered at baseline and month 1. BL = baseline; Q = quartile.
Impact on Igs
Throughout the study period, serum IgG and IgM levels remained within the normal ranges of 5.65–17.65 g/L and 0.40–2.30 g/L, respectively (eFigure 2, http://links.lww.com/NXI/A759); however, it should be noted that significant differences were not expected during the short 12-month observation period.
Discussion
The primary analysis of the MAGNIFY-MS study, reported elsewhere, was conducted to determine the onset of action of cladribine tablets by observing changes in MRI lesion counts.19 In this study, we studied dynamic changes of various cellular and noncellular immune components. Cladribine induces a specific pattern of early and sustained PBMC subtype dynamics in the absence of relevant Ig changes: While total B cells were reduced dramatically, T cells were affected significantly less. While naive B cells recovered toward baseline, naive CD4 and CD8 T cells did not and memory B cells remained reduced until month 12. The results from this substudy help to explain the unique immune depletion and repopulation architecture regarding the onset of action and durability of effects of cladribine tablets while largely maintaining immune competence. The immunologic durability of cladribine tablets beyond this first year is still to be assessed as part of the full 2-year study findings of MAGNIFY-MS. Such results will add to previous studies that have reported on dynamics of major cell types during treatment with cladribine tablets 3.5 mg/kg cumulative dose over 2 years.17,18
While we observed a fast and profound reduction in B-cell subtype counts with cladribine tablets (months 1 and 2), these cell counts recovered well in the following months. By contrast, memory B cells remained close to their lower nadir level to month 12. This confirms the findings from a small observational study of Italian patients treated with cladribine tablets21 and those from a cross-sectional study of people with MS treated with a different preparation of cladribine.22 The strong association between the time course of B-cell subtype depletion and the apparent inhibition of new lesion evolution on MRI demonstrated in MAGNIFY-MS, as well as the sustained and significant reduction in memory B cells over at least 12 months, suggest an important role of this specific B-cell subset for sustained disease control in people with MS.5,23 Monoclonal B-cell depleting antibodies such as alemtuzumab,24 ocrelizumab,25 or rituximab26 (although the latter is not licensed, it is commonly used off-label in people with MS)27 lead to similar patterns of rapid and general B-cell depletion followed by reconstitution of B-cell subsets, except for memory B cells. Even with alemtuzumab, although targeting a broad range of T and B lymphocyte subsets, a distinct pattern of B-cell lysis followed by reconstitution (again, with the exception of memory B cells) can be observed.24
The PBMC subtype dynamics identified in the MAGNIFY-MS study may not only explain how cladribine tablets effectively reduce MS disease activity and disability accrual but also indicate why patients treated with cladribine tablets largely retain immune competence and thus may be able to mount a relevant vaccine response, such as that seen in response to COVID-19 mRNA vaccines.28 While there was a decrease in B-cell subtypes as early as month 1 after initiating treatment with cladribine tablets, naive B cells started to recover from month 2 and reached near baseline levels at month 12. It is these naive B cells that are associated with early immune responses and the generation of antibodies in response to new immune challenges (e.g., infections, vaccination). Indeed, another substudy of the MAGNIFY-MS population has reported a favorable humoral response to varicella zoster virus and influenza vaccines, including patients vaccinated against influenza shortly after initiating treatment with cladribine tablets.29
The results also show that cladribine is able to directly affect and reduce plasmablasts, which may be associated with disease pathology, by means of autoantibody production and direct cellular toxicity within the CSF.30 Most current DMTs for the treatment of MS do not target the plasmablast and plasma cell subtypes directly (including CD20 B cell depleting agents),25 a cell subset potentially indicative of more severe MS disease.31
Despite the sustained decrease of memory B cells and a decrease in CD38+ plasmablasts in the first 12 months of MAGNIFY-MS, serum IgG and IgM levels remained within the normal range over this period. Atacicept, which was shown to target long-lived plasma cells, shows a fast onset of Ig reduction at 4 weeks.32 Consequently, it is likely that long-lived CD138+ plasma cells would not be affected by treatment with cladribine tablets given the unchanged profiles of total serum IgG and IgM up to 12 months, although this subtype was not evaluated in our substudy.
Breg and Btrans cell subtypes contribute to the maintenance of immune tolerance and the modulation of immune responses.33,34 A variety of B-cell subtypes have been documented as Breg cells, with CD19+CD24hiCD38hi B cells being identified as an interleukin (IL)-10–expressing subtype that may participate in suppressing the increased autoimmune responses observed in MS. Breg cells have not yet been studied for all MS DMTs; however, some recent data have shown that after alemtuzumab treatment levels of CD19+CD24hiCD38hi, Breg cells are reduced after severe relapses and subsequently repopulate during recovery.35,36 In this substudy, Btrans cells showed a similar repopulation profile to Breg cells. These Btrans cells represent one of the Breg cell subpopulations in healthy individuals, but the frequency of such cells in circulation may be altered in individuals with MS.34 Btrans cells can also produce IL-10, as well as transforming growth factor-β, and can regulate CD4+ T-cell proliferation and differentiation toward Th effector cells. Given the sustained decrease of mature B-cell subtypes, especially memory B cells, along with an increase in Breg and Btrans cells in the first year after treatment with cladribine tablets, it is believed that an increased Breg/B cell (Btrans/B cell) or Breg/B memory (Btrans/B memory) ratio might contribute to the prolonged clinical efficacy of cladribine tablets. Future analyses may determine the intracellular cytokine expression and the Breg vs B effector subtype ratios and their correlation with clinical efficacy end points.
At month 12, the total B-cell count after treatment with cladribine tablets was generally more than 30% below baseline levels. At this same time point, an overshoot of the total B-cell count of about 30% above baseline has been reported in the pivotal studies of alemtuzumab.24,37 However, our analysis of the MAGNIFY-MS data reported here revealed that while B memory cells remain depleted, Breg and Btrans cells exceed their baseline values by month 3 and remain elevated above baseline at month 12 at +50% and +36%, respectively. Hence, rebound and overshoot of B-cell subsets alone may not sufficiently explain why secondary B-cell autoimmunities do not seem to occur with cladribine tablets, yet are so common after treatment with alemtuzumab. The reported secondary autoimmunities reported for alemtuzumab include glomerulonephritis, immune thrombocytopenia, sarcoidosis, thyroid autoimmunity, and vitiligo.38
As previously suggested, the less pronounced effects of cladribine on T cells, particularly during the first few months after treatment, may be key, alongside the described immune cell dynamics of specific B-cell subsets, for the protection from secondary autoimmunity with cladribine tablets.24 A recent in-depth immune phenotyping study38 provided further evidence of alemtuzumab-induced changes to lymphocytes and the underlying immunologic processes involved in secondary autoimmunity. Autoimmunity after treatment with alemtuzumab is associated with homeostatic proliferation of T cells, and it has been suggested that this occurs in the context of a defective thymus.39 However, antibody-mediated T-cell depletion typically leads to expansion of memory cells and is not associated with the development of B-cell autoimmunities.24,40,41 It is therefore hypothesized that in the context of B-cell depletion and recovery with alemtuzumab, the associated depletion of Treg cells, normally silencing autoreactive immature B cells exiting the bone marrow as they mature into naive B cells,42,43 facilitates secondary autoimmunity.24,44,45 Because autoantibody production is likely to be CD4+ T-cell–dependent, autoimmunity will require T-cell support, and thus, autoimmunity is unlikely to occur until CD4+ T-cell numbers recover, explaining the delay between the time of B-cell hyper-reactivity and the development of secondary autoimmunity.24
The reduction and repopulation profile for CD8+ T cells was comparable with CD4+ T cells for both alemtuzumab and cladribine, respectively. A further, more recent study has also shown alemtuzumab to decrease CD4+ and CD8+ T-cell counts followed by a period of repopulation, with CD8+ T cells increasing above the lower limit of normal at 12 months after treatment.36 For CD8+ T cells, there was a small percentage decrease from baseline to week 2, which was then maintained throughout the study period. In addition to the greater reduction of T cells with alemtuzumab, CD4+ effector memory and CD8+ TEMRA T cells have been shown to be substantially expanded during the first 6 months after treatment.39 Such clonal expansion has not been observed with cladribine tablets.
Initial analysis of the full 2-year safety findings from the present MAGNIFY-MS study have not raised such concerns for cladribine tablets; indeed, no treatment-related serious AEs were reported (data on file; the healthcare business of Merck KGaA, Darmstadt, Germany). Regarding lymphopenia, very few patients experienced grade 4 events (0.7%), with 24.4% and 54.4% of patients, respectively, experiencing grade 3 and grade 1 or 2 lymphopenia (data on file; the healthcare business of Merck KGaA, Darmstadt, Germany).
While PBMC counts in this study were measured in peripheral blood samples, it should be remembered that cladribine penetrates into other tissues including the CNS shortly after administration,46 raising the possibility of direct effects on CNS resident lymphocyte subtypes. Further studies to explore these potential effects are under way.47
One shortcoming of this study is that we did not directly correlate the MRI findings with the PBMC subtype dynamics reported here. Our data, however, suggest an early and subsequent sustained effect of cladribine tablets in a specific pattern of depletion and reconstitution of B-cell and T-cell subtypes. The pronounced reduction of B-cell subsets from month 2 and the sustained depletion of memory B cells seem most closely associated with the effects of cladribine detected on MRI and clinical outcomes.13,17 The contribution of moderate reductions across T-cell subtypes, as well as the sustained increase of regulatory and transitional B cells from month 3 onward, are less clear. While they might contribute to the long-term therapeutic effect of cladribine tablets, the limited reduction of T-cell subsets is likely advantageous for safety. In the context of safety, it should also be noted that, despite the sustained decrease of memory B cells and a decrease in CD38+ plasma cells, no relevant changes in serum IgG and IgM concentrations were observed over the 12-month study period.
Acknowledgment
The authors thank patients and their families, investigators, co-investigators, and study teams at each of the participating centers.
Glossary
- DMTs
disease-modifying therapies
- EDSS
Expanded Disability Status Scale
- Ig
immunoglobulin
- MS
multiple sclerosis
- NK
natural killer
- PBMC
peripheral blood mononuclear cell
- TEMRA
terminally differentiated effector memory RA+
- Th
T helper
- Treg
T regulatory
Appendix. Authors
Study Funding
This work was supported by the healthcare business of Merck KGaA, Darmstadt, Germany (CrossRef Funder ID: 10.13039/100009945).
Disclosure
H. Wiendl is member of scientific advisory boards/steering committees for Bayer, Biogen, the healthcare business of Merck KGaA (Darmstadt, Germany), Novartis, Roche, Sanofi, and Teva. He received speaker honoraria and travel support from Bayer, Biogen, CSL Behring, EMD Serono, Fresenius Medical Care, Genzyme, the healthcare business of Merck KGaA (Darmstadt, Germany), Omniamed, Novartis, Sanofi, and Teva. He received compensation as a consultant from Biogen, the healthcare business of Merck KGaA (Darmstadt, Germany), Novartis, Omniamed, Roche, and Sanofi. He has received research support from Bayer, Biogen, the healthcare business of Merck KGaA (Darmstadt, Germany), Novartis, Sanofi, and Teva, as well as German Ministry for Education and Research (BMBF), German Research Foundation (DFG), Else Kröner Fresenius Foundation, Fresenius Foundation, Hertie Foundation, NRW Ministry of Education and Research, Interdisciplinary Center for Clinical Studies (IZKF) Muenster, and RE Children's Foundation; K. Schmierer has received research support from Biogen, the healthcare business of Merck KGaA (Darmstadt, Germany), and Novartis; speaking honoraria from, and/or served in an advisory role for, Amgen, Biogen, EMD Serono, the healthcare business of Merck KGaA (Darmstadt, Germany), Novartis, Roche, Sanofi, and Teva; and remuneration for teaching activities from AcadeMe, Medscape, and the Neurology Academy; S. Hodgkinson serves on advisory boards for Bayer, Biogen, the healthcare business of Merck KGaA (Darmstadt, Germany), Novartis, Roche, and Sanofi; she has received money for travel and speaker honoraria from Bayer, Biogen, the healthcare business of Merck KGaA (Darmstadt, Germany), Novartis, Roche, and Sanofi; T. Derfuss serves on scientific advisory boards for Bayer, Biogen, GeNeuro, MedDay, the healthcare business of Merck KGaA (Darmstadt, Germany), Mitsubishi Pharma, Novartis Pharmaceuticals, Roche, and Sanofi; has received funding for travel and/or speaker honoraria from Bayer, Biogen, the healthcare business of Merck KGaA (Darmstadt, Germany), Novartis Pharmaceuticals, Roche, and Sanofi; and receives research support from Biogen, the European Union, Novartis Pharma, the Swiss MS Society, and the Swiss National Foundation; A. Chan has received speakers'/board honoraria from Actelion (Janssen/J&J), Almirall, Bayer, Biogen, Celgene (BMS), the healthcare business of Merck KGaA (Darmstadt, Germany), Novartis, Roche, Sanofi, and Teva, all for hospital research funds and research support from Biogen, Sanofi, and UCB; F. Sellebjerg has served on scientific advisory boards, been on the steering committees of clinical trials, served as a consultant, received support for congress participation, received speaker honoraria, or received research support for his laboratory from Biogen, EMD Serono, the healthcare business of Merck KGaA (Darmstadt, Germany), Novartis, Roche, Sanofi, and Teva; A. Achiron has received over the last 5 years' honoraria or consulting fees for participating in advisory boards related to clinical trials design, trial steering committees, and data and safety monitoring committees from Biogen, BMS, Novartis, the healthcare business of Merck KGaA (Darmstadt, Germany), Roche, and Sanofi and research support for investigator-initiated trials and MS patients' benefits activities from Biogen, BMS, the healthcare business of Merck KGaA (Darmstadt, Germany), Novartis, Roche, and Sanofi; X. Montalban has received speaking honoraria and travel expenses for participation in scientific meetings, has been a steering committee member of clinical trials or participated in advisory boards of clinical trials in the past years with Abbvie, Actelion (Janssen/J&J), Alexion, Bayer, Biogen, Celgene (BMS), EMD Serono, Immunic, Janssen (J&J), MedDay, the healthcare business of Merck KGaA (Darmstadt, Germany), Mylan, Nervgen, Novartis, Roche, Sandoz, Sanofi, Teva, TG Therapeutics, Excemed, MSIF, and NMSS; A. Prat has received speaking honoraria and travel expenses for participation in scientific meetings, has been a steering committee member of clinical trials or participated in advisory boards of clinical trials in the past years, and/or received operating grants from Alexion, Bayer, Biogen, Celgene (BMS), EMD Serono, Billerica, MA, Novartis, Roche, Sanofi, and Teva; N. De Stefano is a consultant for Bayer, Biogen, the healthcare business of Merck KGaA (Darmstadt, Germany), Novartis, Roche, Sanofi, and Teva; has grants or grants pending from FISM and Novartis; is on the speakers' bureaus of Biogen, the healthcare business of Merck KGaA (Darmstadt, Germany), Novartis, Roche, Sanofi, and Teva; and has received travel funds from the healthcare business of Merck KGaA (Darmstadt, Germany), Novartis, Roche, Sanofi, and Teva; F. Barkhof is supported by the NIHR Biomedical Research Centre at UCLH and is a consultant to Biogen, Combinostics, IXICO, the healthcare business of Merck KGaA (Darmstadt, Germany), and Roche; L. Leocani has received honoraria for consulting services or speaking activities from Biogen, Novartis, the healthcare business of Merck KGaA (Darmstadt, Germany), and Roche and research support from Biogen, the healthcare business of Merck KGaA (Darmstadt, Germany), and Novartis; P. Vermersch has received honoraria or consulting fees from AB Science, Biogen, Celgene (BMS), Imcyse, the healthcare business of Merck KGaA (Darmstadt, Germany), Novartis, Roche, Sanofi, and Teva and research support from Novartis, Roche, and Sanofi; A. Chudecka is an employee of Cytel Inc., Geneva Branch, Switzerland, and was funded by the healthcare business of Merck KGaA (Darmstadt, Germany) to perform statistical analyses for this study; K.H. Holmberg was an employee of EMD Serono, Billerica, MA, at the time the study was conducted and is currently an employee of Biogen, Cambridge, MA; C. Mwape is an employee of inScience Communications, Springer Healthcare Ltd., Chester, UK. inScience Communications received funding from the healthcare business of Merck KGaA Darmstadt, Germany, for medical writing assistance for this article; U. Boschert and S. Roy are employees of Ares Trading SA, Eysins, Switzerland, an affiliate of Merck KGaA, Darmstadt, Germany. Go to Neurology.org/NN for full disclosure.
References
- 1.Filippi M, Bar-Or A, Piehl F, et al. Multiple sclerosis. Nat Rev Dis Primers. 2018;4(1):43. doi: 10.1038/s41572-018-0041-4. [DOI] [PubMed] [Google Scholar]
- 2.Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. New Engl J Med. 2018;378(2):169-180. doi: 10.1056/NEJMra1401483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zozulya AL, Wiendl H. The role of regulatory T cells in multiple sclerosis. Nat Rev Neurol. 2008;4(7):384-398. doi: 10.1038/ncpneuro0832. [DOI] [PubMed] [Google Scholar]
- 4.Cencioni MT, Mattoscio M, Magliozzi R, Bar-Or A, Muraro PA. B cells in multiple sclerosis–from targeted depletion to immune reconstitution therapies. Nat Rev Neurol. 2021;17(7):399-414. doi: 10.1038/s41582-021-00498-5. [DOI] [PubMed] [Google Scholar]
- 5.Baker D, Marta M, Pryce G, Giovannoni G, Schmierer K. Memory B cells are major targets for effective immunotherapy in relapsing multiple sclerosis. EBioMedicine. 2017;16:41-50. doi: 10.1016/j.ebiom.2017.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bar-Or A, Li R. Cellular immunology of relapsing multiple sclerosis: interactions, checks, and balances. Lancet Neurol. 2021;20(6):470-483. doi: 10.1016/s1474-4422(21)00063-6. [DOI] [PubMed] [Google Scholar]
- 7.Luo C, Jian C, Liao Y, et al. The role of microglia in multiple sclerosis. Neuropsychiatr Dis Treat. 2017;13:1661-1667. doi: 10.2147/ndt.S140634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guerrero BL, Sicotte NL. Microglia in multiple sclerosis: friend or foe? Front Immunol. 2020;11:374. doi: 10.3389/fimmu.2020.00374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laroni A, Armentani E, Kerlero de Rosbo N, et al. Dysregulation of regulatory CD56(bright) NK cells/T cells interactions in multiple sclerosis. J Autoimmun. 2016;72:8-18. doi: 10.1016/j.jaut.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Gross CC, Schulte-Mecklenbeck A, Rünzi A, et al. Impaired NK-mediated regulation of T-cell activity in multiple sclerosis is reconstituted by IL-2 receptor modulation. Proc Natl Acad Sci. 2016;113(21):E2973-E2982. doi: 10.1073/pnas.1524924113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gross CC, Schulte-Mecklenbeck A, Wiendl H, et al. Regulatory functions of natural killer cells in multiple sclerosis. Mini Rev Front Immunol. 2016;7(606):606. doi: 10.3389/fimmu.2016.00606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lünemann JD, Ruck T, Muraro PA, Bar-Or A, Wiendl H. Immune reconstitution therapies: concepts for durable remission in multiple sclerosis. Nat Rev Neurol. 2020;16(1):56-62. doi: 10.1038/s41582-019-0268-z. [DOI] [PubMed] [Google Scholar]
- 13.Giovannoni G. Cladribine to treat relapsing forms of multiple sclerosis. Neurotherapeutics. 2017;14(4):874-887. doi: 10.1007/s13311-017-0573-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiendl H. Cladribine–an old newcomer for pulsed immune reconstitution in MS. Nat Rev Neurol. 2017;13(10):573-574. doi: 10.1038/nrneurol.2017.119. [DOI] [PubMed] [Google Scholar]
- 15.Baker D, Herrod SS, Alvarez-Gonzalez C, Zalewski L, Albor C, Schmierer K. Both cladribine and alemtuzumab may effect MS via B-cell depletion. Neurol Neuroimmunol Neuroinflamm. 2017;4(4):e360. doi: 10.1212/nxi.0000000000000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soelberg Soerensen P, Dangond F, Hicking C, Giovannoni G. 1141: innate immune cell counts in patients with relapsing-remitting multiple sclerosis (RRMS) treated with cladribine tablets 3.5 mg/kg in CLARITY and CLARITY Extension. Mult Scler J. 2017;23(S3):598. [Google Scholar]
- 17.Comi G, Cook S, Giovannoni G, et al. Effect of cladribine tablets on lymphocyte reduction and repopulation dynamics in patients with relapsing multiple sclerosis. Mult Scler Relat Disord. 2019;29:168-174. doi: 10.1016/j.msard.2019.01.038. [DOI] [PubMed] [Google Scholar]
- 18.Stuve O, Soelberg Soerensen P, Leist T, et al. Effects of cladribine tablets on lymphocyte subsets in patients with multiple sclerosis: an extended analysis of surface markers. Ther Adv Neurol Disord. 2019;12:175628641985498. doi: 10.1177/1756286419854986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Stefano N, Barkhof F, Montalban X, et al. Early reduction of MRI activity during six months of cladribine tablets treatment for highly active relapsing multiple sclerosis: MAGNIFY-MS. Neurol Neuroimmunol Neuroinflamm. 2022;9(4):e1187. doi: 10.1212/nxi.0000000000001187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clinicaltrials.gov [Internet]. Identifier NCT03364036, Evaluation of the Onset of Action in Highly Active MS (MAGNIFY); 2017; clinicaltrials.gov/ct2/show/NCT03364036. [Google Scholar]
- 21.Spiezia AL, Cerbone V, Molinari EA, et al. Changes in lymphocytes, neutrophils and immunoglobulins in year-1 cladribine treatment in multiple sclerosis. Mult Scler Relat Disord. 2022;57(0):103431. doi: 10.1016/j.msard.2021.103431. [DOI] [PubMed] [Google Scholar]
- 22.Ceronie B, Jacobs BM, Baker D, et al. Cladribine treatment of multiple sclerosis is associated with depletion of memory B cells. J Neurol. 2018;265(5):1199-1209. doi: 10.1007/s00415-018-8830-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DiSano KD, Gilli F, Pachner AR. Memory B cells in multiple sclerosis: emerging players in disease pathogenesis. Front Immunol. 2021;12:676686. doi: 10.3389/fimmu.2021.676686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baker D, Herrod SS, Alvarez-Gonzalez C, Giovannoni G, Schmierer K. Interpreting lymphocyte reconstitution data from the pivotal phase 3 trials of alemtuzumab. JAMA Neurol. 2017;74(8):961-969. doi: 10.1001/jamaneurol.2017.0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernández-Velasco JI, Kuhle J, Monreal E, et al. Effect of ocrelizumab in blood leukocytes of patients with primary progressive MS. Neurol Neuroimmunol Neuroinflamm. 2021;8(2):e940. doi: 10.1212/nxi.0000000000000940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palanichamy A, Jahn S, Nickles D, et al. Rituximab efficiently depletes increased CD20-expressing T cells in multiple sclerosis patients. J Immunol. 2014;193(2):580-586. doi: 10.4049/jimmunol.1400118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spelman T, Magyari M, Piehl F, et al. Treatment escalation vs immediate initiation of highly effective treatment for patients with relapsing-remitting multiple sclerosis: data from 2 different national strategies. JAMA Neurol. 2021;78(10):1197. doi: 10.1001/jamaneurol.2021.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Achiron A, Mandel M, Dreyer-Alster S, et al. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther Adv Neurol Disord. 2021;14:175628642110128. doi: 10.1177/17562864211012835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmierer K, Wiendl H, Oreja-Guevara C, et al. Varicella zoster virus and influenza vaccine antibody titres in patients from MAGNIFY-MS who were treated with cladribine tablets for highly active relapsing multiple sclerosis. Mult Scler. 2022;28(13):2151-2153. doi: 10.1177/13524585221099413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blauth K, Owens GP, Bennett JL. The ins and outs of B cells in multiple sclerosis. Front Immunol. 2015;6:565. doi: 10.3389/fimmu.2015.00565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Telesford KM, Kaunzner UW, Perumal J, et al. Black African and Latino/a identity correlates with increased plasmablasts in MS. Neurol Neuroimmunol Neuroinflamm. 2020;7(1):e634. doi: 10.1212/nxi.0000000000000634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kappos L, Hartung H-P, Freedman MS, et al. Atacicept in multiple sclerosis (ATAMS): a randomised, placebo-controlled, double-blind, phase 2 trial. Lancet Neurol. 2014;13(4):353-363. doi: 10.1016/S1474-4422(14)70028-6. [DOI] [PubMed] [Google Scholar]
- 33.Ran Z, Yue-Bei L, Qiu-Ming Z, Huan Y. Regulatory B cells and its role in central nervous system inflammatory demyelinating diseases. Front Immunol. 2020;11:1884. doi: 10.3389/fimmu.2020.01884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou Y, Zhang Y, Han J, Yang M, Zhu J, Jin T. Transitional B cells involved in autoimmunity and their impact on neuroimmunological diseases. J Transl Med. 2020;18(1):131. doi: 10.1186/s12967-020-02289-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim Y, Kim G, Shin HJ, et al. Restoration of regulatory B cell deficiency following alemtuzumab therapy in patients with relapsing multiple sclerosis. J Neuroinflamm. 2018;15(1):300. doi: 10.1186/s12974-018-1334-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wiendl H, Carraro M, Comi G, et al. Lymphocyte pharmacodynamics are not associated with autoimmunity or efficacy after alemtuzumab. Neurol Neuroimmunol Neuroinflamm. 2020;7(1):e635. doi: 10.1212/nxi.0000000000000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thompson SAJ, Jones JL, Cox AL, Compston DAS, Coles AJ. B-cell reconstitution and BAFF after alemtuzumab (Campath-1H) treatment of multiple sclerosis. J Clin Immunol. 2010;30(1):99-105. doi: 10.1007/s10875-009-9327-3. [DOI] [PubMed] [Google Scholar]
- 38.Ruck T, Barman S, Schulte-Mecklenbeck A, et al. Alemtuzumab-induced immune phenotype and repertoire changes: implications for secondary autoimmunity. Brain. 2022;145(5):1711-1725. doi: 10.1093/brain/awac064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones JL, Thompson SAJ, Loh P, et al. Human autoimmunity after lymphocyte depletion is caused by homeostatic T-cell proliferation. Proc Natl Acad Sci U S A. 2013;110(50):20200-20205. doi: 10.1073/pnas.1313654110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Oosten BW, Lai M, Hodgkinson S, et al. Treatment of multiple sclerosis with the monoclonal anti-CD4 antibody cM-T412: results of a randomized, double-blind, placebo-controlled, MR-monitored phase II trial. Neurology. 1997;49(2):351-357. doi: 10.1212/wnl.49.2.351. [DOI] [PubMed] [Google Scholar]
- 41.Llewellyn-Smith N, Lai M, Miller DH, Rudge P, Thompson AJ, Cuzner ML. Effects of anti-CD4 antibody treatment on lymphocyte subsets and stimulated tumor necrosis factor alpha production: a study of 29 multiple sclerosis patients entered into a clinical trial of cM-T412. Neurology. 1997;48(4):810-816. doi: 10.1212/wnl.48.4.810. [DOI] [PubMed] [Google Scholar]
- 42.Kinnunen T, Chamberlain N, Morbach H, et al. Accumulation of peripheral autoreactive B cells in the absence of functional human regulatory T cells. Blood. 2013;121(9):1595-1603. doi: 10.1182/blood-2012-09-457465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meffre E, Wardemann H. B-cell tolerance checkpoints in health and autoimmunity. Curr Opin Immunol. 2008;20(6):632-638. doi: 10.1016/j.coi.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 44.Sakuraba K, Oyamada A, Fujimura K, et al. Interleukin-21 signaling in B cells, but not in T cells, is indispensable for the development of collagen-induced arthritis in mice. Arthritis Res Ther. 2016;18(1):188. doi: 10.1186/s13075-016-1086-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krupica T Jr, Fry TJ, Mackall CL. Autoimmunity during lymphopenia: a two-hit model. Clin Immunol. 2006;120(2):121-128. doi: 10.1016/j.clim.2006.04.569. [DOI] [PubMed] [Google Scholar]
- 46.Hermann R, Karlsson MO, Novakovic AM, Terranova N, Fluck M, Munafo A. The clinical pharmacology of cladribine tablets for the treatment of relapsing multiple sclerosis. Clin Pharmacokinet. 2019;58(3):283-297. doi: 10.1007/s40262-018-0695-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Europe PMC. Does cladribine target CNS plasma cells and reduce nerve damage in people with MS (CLADRIPLAS)? Accessed September 17, 2021. europepmc.org/grantfinder/grantdetails?query=pi%3A%22Schmierer%20K%22%20gid%3A%2269%22%20ga%3A%22Multiple%20Sclerosis%20Society%22&cat=.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Any requests for data by qualified scientific and medical researchers for legitimate research purposes will be subject to the Data Sharing Policy of the healthcare business of Merck KGaA, Darmstadt, Germany. All requests should be submitted in writing to the data sharing portal of the healthcare business of Merck KGaA, Darmstadt, Germany, emdgroup.com/en/research/our-approach-to-research-and-development/healthcare/clinical-trials/commitment-responsible-data-sharing.html. When the healthcare business of Merck KGaA, Darmstadt, Germany, has a coresearch, codevelopment, or comarketing or copromotion agreement, or when the product has been out-licensed, the responsibility for disclosure might be dependent on the agreement between parties. Under these circumstances, the healthcare business of Merck KGaA, Darmstadt, Germany, will endeavor to gain agreement to share data in response to requests.