Abstract
Thromboembolic complications are associated with COVID-19 owing to the hypercoagulable nature of the disease. Although patients with COVID-19 often have higher levels of fibrinogen and D-dimers, hypercoagulability has been attributed to various other factors too. In this prospective observational study conducted between April 2020 and June 2020, we compared coagulation parameters using thromboelastography in COVID-19 patients to non-COVID-19 patients admitted to ICU with respiratory failure. This study demonstrated a significant difference between the cohorts in functional fibrinogen (CFF) progressively from third day of ICU admission whilst there was no difference in the Clauss fibrinogen levels. COVID-19 patients also demonstarted supranormal R time indicating hypocoagulability. These mixed coagulation changes suggest targeting fibrinogen or platelets may prevent thromboembolic complications in COVID-19.
Keywords: COVID-19, Thromboelastography
Introduction
Hypercoagulability in COVID-19 is multifactorial involving increased thrombin generation, inhibition of thrombolysis, platelet hyperactivity and complement activation.1,2 Thromboembolic complications are associated with increased mortality in COVID-19 and have been attributed to a virally induced hypercoagulable state.3,4 Conventional coagulation tests (PT, INR, aPPT) are unable to detect hypercoagulability. We aimed to assess coagulation changes in patients admitted to the Intensive Care Unit (ICU) with COVID-19 compared to those with non-COVID-19 induced lower respiratory tract infections with similar clinical presentation using thromboelastography.
Methods
Ethical approval and the need for written consent were waived by the Trust Research and Development Department. Adult patients admitted to the Royal Free Hospital ICU between April 21st and June 8th, 2020 with a primary diagnosis of type I respiratory failure due to lower respiratory tract infection were included in this prospective observational study. Patients were considered COVID-19 positive if they had either a positive SARS-CoV-2 PCR or radiological features strongly suggestive of COVID-19 on chest computed tomography reported by consultant radiologist. Blood samples for thromboelastography and conventional clotting assays were taken on days 1, 3, 5 and 8 of the ICU stay. Thromboelastography (TEG) was performed using global haemostasis cartridges (TEG®6s, Haemonetics©) that incorporate citrated kaolin (CK), kaolin with heparinase (CKH), rapid TEG and functional fibrinogen (CFF). Data was collected prospectively and analysed using GraphPad Prism 8.4.3 (GraphPad Software, San Diego, USA).
Results
Data were collected on 24 adult patients. Fourteen patients were COVID-19 positive. Demographic characteristics, baseline admission laboratory tests, pre-admission anticoagulation and anticoagulation during ICU admission were comparable between patients with and without COVID-19. (Table 1)
Table 1.
Baseline demographic and admission laboratory data in COVID-19 positive and negative patients.
|
COVID-19 positive |
COVID-19 negative |
||
|---|---|---|---|
| N = 14 | N = 10 | P value | |
| Age in years, mean (±SD) | 55.64 (11.90) | 53.40 (20.35) | 0.737 |
| BMI kg/square meter, median (IQR) | 27.34 (4.75) | 26.59 (4.52) | 0.676 |
| Male, n (%) | 8 (57.14) | 5 (50) | 1.000 |
| Caucasian, n (%) | 5 (35.71) | 6 (60) | 0.408 |
| Fully independent at admission, n (%) | 12 (85.71) | 7 (70) | 0.615 |
| Haemoglobin, g/L, mean (±SD) | 110.10 (26.60) | 101.80 (29.96) | 0.488 |
| White cell count, 109/L, median (IQR) | 8.97 (12.14) | 11.18 (6.78) | 0.518 |
| Neutrophils, 109/L, median (IQR) | 7.31 (11.45) | 9.62 (4.27) | 0.341 |
| Lymphocytes, 109/L, median (IQR) | 0.89 (0.51) | 0.77 (0.53) | 0.428 |
| C reactive protein, mg/L, median (IQR) | 119 (121) | 115 (246) | 0.546 |
| Procalcitonin, µg/L, median (IQR) | 1.14 (3.99) | 0.77 (36.93) | 0.821 |
| Creatinine, µmol/L, median (IQR) | 89.50 (80.25) | 154 (230.50) | 0.118 |
| eGFR, ml/min median (IQR) | 82 (48.75) | 57 (57.00) | 0.177 |
| Ferritin, µg/L, median (IQR) | 564 (692.50) | 254 (969.25) | 0.159 |
| Troponins, ng/L, median (IQR) | 49.50 (90.25) | 82.50 (196.50) | 0.108 |
| NT -proBNP, ng/L, median (IQR) | 1259 (4513) | 3153 (10093) | 0.397 |
| Hypertension, n (%) | 7 (50) | 2 (20) | 0.210 |
| Diabetes mellites, n (%) | 6 (42.86) | 2 (20) | 0.388 |
| Heart disease, n (%) | 3 (21.43) | 4 (40) | 0.393 |
| Chronic respiratory disease, n (%) | 4 (28.57) | 2 (20) | 1.000 |
| Chronic kidney disease, n (%) | 1(7.14) | 1 (10) | 1.000 |
| Chronic liver disease, n (%) | 2 (14.29) | 0 (0) | 0.493 |
| Long term anticoagulation, n (%) | 1 (7.14) | 1 (10) | 1.000 |
| Antiplatelet agents, n (%) | 5 (35.71) | 2 (20) | 0.653 |
| Prophylactic anticoagulation since admission, n (%) | 7 (50) | 8 (50) | 0.210 |
| Treatment dose anticoagulation, n (%) | 6 (42.86) | 1 (10) | 0.172 |
Data shown as mean ± s.d., median [IQR] or n (%).
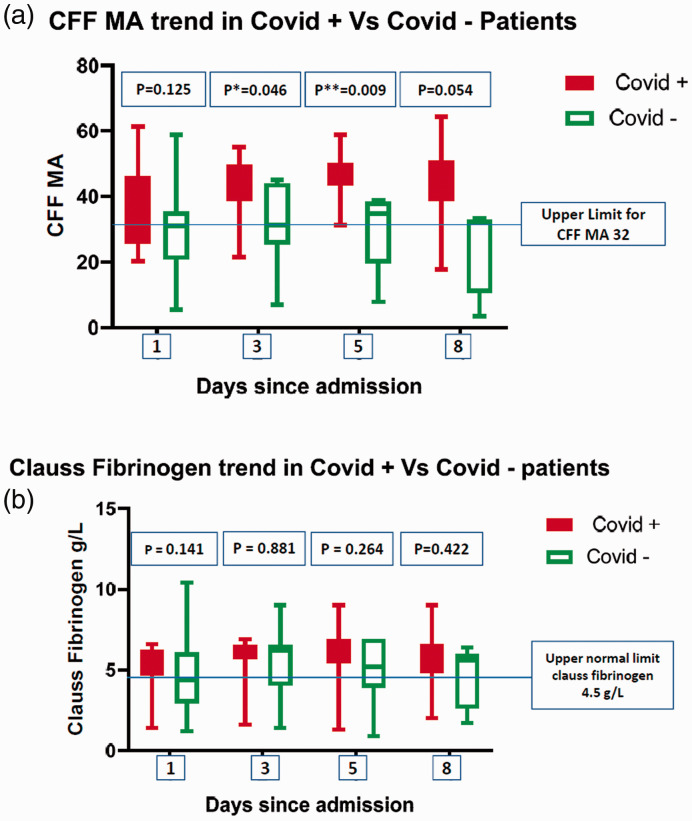
The CKH maximum amplitude (MA) was normal on admission in both cohorts and, while it remained normal in negative patients, in positive patients the MA subsequently became supranormal and was significantly higher than non-COVID-19 patients on day 3 (68.9 vs. 62.5, p = 0.026, Table 2). The CFF MA in COVID-19 patients was supranormal and increased throughout the ICU stay, while in COVID-19 negative patients the CFF MA remained stable at the upper limit of the normal range (Figure 1 and Table 2). There was no statistical difference in the median CFF MA on day 1 between the two cohorts (39.4 vs. 31.0, p = 0.125) however statistical significance was reached by day 3 (41.2 vs. 31.2, p = 0.046) and day 5 (45.5 vs. 34.7, p = 0.009). The CKH R time in COVID-19 patients was supranormal throughout the ICU stay, while it was normal in the non-COVID-19 group, although there was no significant difference.
Table 2.
Thromboelastography TEG®6s assays and conventional laboratory coagulation tests in COVID-19 positive and negative patients.
|
COVID-19 positive |
COVID-19 negative |
|||
|---|---|---|---|---|
| N = 14 | N = 10 | P value | ||
| TEG CFF MA (normal range 15–32) | Day 1 | 39.4 (25.60–46.28) | 31.0 (20.75–35.35) | 0.125 |
| Day 3 | 41.2 (38.78–49.65) | 31.2 (25.50–44.00) | 0.046* | |
| Day 5 | 45.5 (43.40–50.30) | 34.7 (19.65–38.35) | 0.009** | |
| Day 8 | 47.7 (38.65–50.85) | 32.3 (10.63–33.05) | 0.054 | |
| TEG CKH R time (normal range 4.3–8.3) | Day 1 | 9.1 (7.47–15.55) | 7.9 (6.27–9.50) | 0.374 |
| Day 3 | 9.1 (7.72–10.42) | 8.3 (7.17–12.08) | 0.492 | |
| Day 5 | 9.3 (7.00–10.30) | 9.7 (7.55–11.55) | 0.681 | |
| Day 8 | 9.7 (6.22–11.98) | 7.0 (6.62–9.32) | 0.558 | |
| TEG CKH α angle (normal range 64.3–77.1) | Day 1 | 74.7 (70.13–78.03) | 69.8 (46.35–76.00) | 0.175 |
| Day 3 | 75.7 (71.65–77.25) | 72.5 (63.90–76.80) | 0.498 | |
| Day 5 | 77.7 (74.40–79.80) | 71.1 (61.80–76.45) | 0.095 | |
| Day 8 | 77.9 (67.50–79.03) | 76.6 (60.50–78.83) | 0.839 | |
| TEG CKH MA (normal range 52.3–68.9) | Day 1 | 67.2 (62.20–68.95) | 65.2 (58.25–67.50) | 0.328 |
| Day 3 | 68.9 (67.70–70.20) | 62.5 (57.90–68.70) | 0.026* | |
| Day 5 | 69.3 (68.00–70.70) | 68.0 (50.40–69.05) | 0.108 | |
| Day 8 | 69.5 (66.95–72.30) | 67.2 (46.48–69.38) | 0.186 | |
| TEG CK R time (normal range 4.6–9.1) | Day 1 | 10.5 (9.15–17.30) | 8.8 (6.75–10.70) | 0.128 |
| Day 3 | 10.6 (8.22–13.00) | 7.9 (7.00–13.50) | 0.488 | |
| Day 5 | 9.9 (7.20–12.00) | 10.2 (7.25–14.25) | 0.722 | |
| Day 8 | 9.0 (5.70–20.73) | 6.5 (5.87–8.52) | 0.436 | |
| TEG CK α angle (normal range 63–78) | Day 1 | 72.6 (65.15–76.18) | 67.2 (61.65–75.40) | 0.723 |
| Day 3 | 74.2 (60.63–76.78) | 71.7 (66.30–77.30) | 0.975 | |
| Day 5 | 71.0 (50.50–78.20) | 70.4 (65.25–75.80) | 0.743 | |
| Day 8 | 76.9 (57.90–78.65) | 75.4 (64.93–78.23) | 0.945 | |
| TEG CK MA (normal range 52–69) | Day 1 | 65.0 (61.20–68.73) | 64.6 (58.70–68.45) | 0.632 |
| Day 3 | 68.9 (66.83–70.93) | 64.6 (57.90–69.30) | 0.105 | |
| Day 5 | 68.5 (66.30–70.30) | 66.4 (50.3–67.90) | 0.170 | |
| Day 8 | 68.7 (64.33–70.55) | 66.7 (46.85–68.40) | 0.240 | |
| TEG CK Ly30 (normal range 0–2.6) | Day 1 | 0.2 (0.00–0.62) | 0.2 (0.00–0.92) | 0.971 |
| Day 3 | 0.1 (0.00–0.92) | 0.0 (0.00–1.10) | 0.924 | |
| Day 5 | 0.0 (0.00–0.40) | 0.2 (0.00–3.10) | 0.614 | |
| Day 8 | 0.0 (0.00–0.25) | 0.3 (0.05–0.80) | 0.231 | |
| Platelets (normal range 150–450 × 109/L) | Day 1 | 235.3 (81.10) | 192.6 (94.09) | 0.247 |
| Day 3 | 227.8 (94.72) | 183.1 (106.60) | 0.321 | |
| Day 5 | 278.2 (128.50) | 253.1 (142.80) | 0.681 | |
| Day 8 | 322.5 (143.20) | 328.8 (202.00) | 0.939 | |
| INR (normal range 1.0–1.3) | Day 1 | 1.1 (1.07–1.22) | 1.2 (1.10–1.30) | 0.209 |
| Day 3 | 1.0 (1.00–1.10) | 1.1 (1.00–1.17) | 0.767 | |
| Day 5 | 1.1 (1.00–1.10) | 1.0 (1.00–1.30) | 0.792 | |
| Day 8 | 1.1 (1.00–1.10) | 1.1 (1.05–1.30) | 0.792 | |
| aPTT (normal range 22–36) | Day 1 | 48.3 (42.05–52.15) | 37.2 (32.53–46.05) | 0.081 |
| Day 3 | 40.6 (37.80–44.70) | 38.1 (32.63–48.10) | 0.960 | |
| Day 5 | 39.6 (36.3–43.10) | 29.2 (28.30–42.10) | 0.211 | |
| Day 8 | 36.3 (33.80–47.50) | 29.0 (28.30–39.30) | 0.267 | |
| Clauss fibrinogen (normal range 1.5–4.5 g/L) | Day 1 | 5.8 (4.67–6.25) | 4.4 (2.95–6.07) | 0.149 |
| Day 3 | 6.2 (5.70–6.52) | 6.2 (4.02–6.55) | 0.881 | |
| Day 5 | 6.0 (5.40–6.90) | 5.2 (3.90–6.90) | 0.264 | |
| Day 8 | 5.3 (4.80–6.60) | 5.6 (2.60–6.00) | 0.422 | |
| D–dimer (normal range < 500 µg/L) | Day 1 | 1779 (711–3924) | 6047 (1405–13809) | 0.056 |
| Day 3 | 1980 (1032–4222) | 3511 (1594–7646) | 0.210 | |
| Day 5 | 1678 (980–5797) | 3534 (1412–12596) | 0.289 | |
| Day 8 | 2095 (994–6580) | 1809 (1556–59831) | 0.555 |
Figure 1.
Comparision of trends in Functional Fibrinogen (CFF) and Clauss Fibrinogen in COVID-19 positive and COVID-19 negative patients.
Median Clauss fibrinogen was supranormal in both the groups during most of the ICU admission and there was no statistical difference between the groups (Figure 1 and Table 2). Median D-dimer values were elevated in both the groups, although without significant difference. Mean platelet count, median INR and median aPTT in COVID-19 patients throughout the ICU stay were not significantly different to non-COVID-19 patients (Table 2).
Three out of 14 COVID-19 patients developed pulmonary emboli while none were observed in the COVID-19 negative group; there was no association with TEG or clotting results.
Discussion
This study revealed evidence of hypercoagulability in critically ill COVID-19 patients characterised by a supranormal clot strength and fibrinogen activity which increased with illness duration. Fibrinogen concentration did not differ between patient groups indicating a functional, rather than quantitative, hyperfibrinogenaemia in COVID-19 patients. Additionally, a clotting factor deficiency or dysfunction was demonstrated through the prolonged R time.
Similar findings have previously been described in COVID-19 with raised fibrinogen, D-dimer, prothrombin time and lower antithrombin levels. 5 However, there has been little description of a hypocoagulable component to COVID-19 which is key as heparin-based anticoagulation is a mainstay in most ICUs but may be inappropriate in view of the elevated aPTT and R time described here. Furthermore, many centres base their anticoagulation strategies on D-dimer values, which were shown here to be lower in COVID-19 patients.
Additionally, while thromboelastography in COVID-19 patients has shown prothrombotic patterns compared to healthy cohorts,6–8 there is insufficient data comparing thromboelastography in COVID-19 with non-COVID respiratory compromise which appear to drive different coagulopathies.
Conclusion
Thromboelastography can assess the prothrombotic state in COVID-19, unlike conventional clotting tests. The mixed coagulopathy described here with increased MA, functional fibrinogen and prolonged R time suggests targeting platelet or fibrinogen activity may help prevent thromboembolism. Further investigation is required to establish whether TEG can identify patients at risk of thromboembolic complications or help guide individualised anticoagulation strategies.
Acknowledgement
TEG®6s cartridges were provided free of cost by Haemonetics©.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Kunal Joshi https://orcid.org/0000-0001-5602-3592
References
- 1.Ahmed S, Zimba O, Yuri A. Thrombosis in coronavirus disease 2019 (COVID-19) through the prism of Virchow’s triad. Clin Rheumatol 2020; 11: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magro C, et al. , Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res 2020; 220: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Middeldorp S, Coppens M, van Haaps TF, et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost 2020; 18(8): 1995–2002. DOI: 10.1111/jth.14888. pmid:32369666. [DOI] [PMC free article] [PubMed]
- 4.Poissy J, Goutay J, Caplan M, et al. , Pulmonary embolism in COVID-19 patients: awareness of an increased prevalence. Lille ICU Haemostasis COVID-19 group. Circulation 2020; 14: 142(2): 184–186. DOI: 10.1161/CIRCULATIONAHA.120.047430. pmid:32330083. [DOI] [PubMed]
- 5.Han H, et al. , Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med 2020; 58: 1116–1120. [DOI] [PubMed] [Google Scholar]
- 6.Mortus JR, Manek SE, Brubaker LS. Thromboelastographic results and hypercoagulability syndrome in patients with coronavirus disease 2019 who are critically ill. JAMA Netw Open 2020; 3: e2011192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pavoni V, Gianesello L, Pazzi M, et al. Evaluation of coagulation function by rotation thromboelastometry in critically ill patients with severe COVID-19 pneumonia. J Thromb Thrombolysis 2020; 50: 281–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuriditsky E, Horowitz JM, Merchan C, et al. Thromboelastography profiles of critically ill patients with coronavirus disease 2019. Crit Care Med 2020; 48: 1319–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]