Abstract
Background
Typing on smartphones, which has become a near daily activity, requires both upper limb and cognitive function. Analysis of keyboard interactions during regular typing, that is, keystroke dynamics, could therefore potentially be utilized for passive and continuous monitoring of function in patients with multiple sclerosis.
Objective
To determine whether passively acquired smartphone keystroke dynamics correspond to multiple sclerosis outcomes, we investigated the association between keystroke dynamics and clinical outcomes (upper limb and cognitive function). This association was investigated longitudinally in order to study within-patient changes independently of between-patient differences.
Methods
During a 1-year follow-up, arm function and information processing speed were assessed every 3 months in 102 patients with multiple sclerosis with the Nine-Hole Peg Test and Symbol Digit Modalities Test, respectively. Keystroke-dynamics data were continuously obtained from regular typing on the participants’ own smartphones. Press-and-release latency of the alphanumeric keys constituted the fine motor score cluster, while latency of the punctuation and backspace keys constituted the cognition score cluster. The association over time between keystroke clusters and the corresponding clinical outcomes was assessed with linear mixed models with subjects as random intercepts. By centering around the mean and calculating deviation scores within subjects, between-subject and within-subject effects were distinguished.
Results
Mean (SD) scores for the fine motor score cluster and cognition score cluster were 0.43 (0.16) and 0.94 (0.41) seconds, respectively. The fine motor score cluster was significantly associated with the Nine-Hole Peg Test: between-subject β was 15.9 (95% CI 12.2-19.6) and within-subject β was 6.9 (95% CI 2.0-11.9). The cognition score cluster was significantly associated with the Symbol Digit Modalities Test between subjects (between-subject β –11.2, 95% CI –17.3 to –5.2) but not within subjects (within-subject β –0.4, 95% CI –5.6 to 4.9).
Conclusions
Smartphone keystroke dynamics were longitudinally associated with multiple sclerosis outcomes. Worse arm function corresponded with longer latency in typing both across and within patients. Worse processing speed corresponded with higher latency in using punctuation and backspace keys across subjects. Hence, keystroke dynamics are a potential digital biomarker for remote monitoring and predicting clinical outcomes in patients with multiple sclerosis.
Trial Registration
Netherlands Trial Register NTR7268; https://trialsearch.who.int/Trial2.aspx?TrialID=NTR7268
Keywords: multiple sclerosis, smartphone, mobile app, digital technology, keystroke dynamics, typing, upper extremity, cognition, outpatient monitoring
Introduction
In multiple sclerosis (MS), a vast number of disease-modifying therapies targeting disease activity are available, and therapies preventing (and potentially counteracting) disease progression are emerging [1-3]. Additional treatment modalities include nonpharmacological therapies, such as rehabilitative and cognitive therapies [4,5]. This wide array of expanding treatment options will increasingly lead to patient-centered disease management. The personalized treatment of MS would strongly benefit from early and improved recognition of disability progression or symptom onset. However, disease progression (ie, deterioration of neurological function independent of relapses) and newly occurring symptoms are often subtle in MS [6]. Additionally, the currently most widely used clinical measure in MS, the Expanded Disability Status Scale (EDSS) [7], assesses neurological function over a period spanning a year or almost a year and may need reassessment over time to confirm deterioration [6]. The Multiple Sclerosis Functional Composite (MSFC) consists of brief objective measurements in 3 important domains in MS: ambulatory, upper limb, and cognitive function. It was designed to complement the EDSS and improve sensitivity in capturing disease status [8]. Compared to the extensive implementation of the MSFC in clinical trials, it has been poorly incorporated into clinical practice, as clinical evaluations are too sporadic for the measure to be sensitive or provide meaningful temporal information for monitoring patients on the individual level [9].
The advent of digital devices allows for more continuous and more fine-grained measurements of biometrics that could be related to functioning in patients with MS. With the digitalization of society, smartphones have become widespread and part of everyday living. Consequently, keystroke dynamics (KD) from typing on smartphones has been investigated for quantifying disability in MS. KD encompasses quantitative metrics of keyboard interactions during regular typing. In our previous work, KD was found to be correlated with upper limb and cognitive function, and, to a lesser extent, overall disability, as measured with the EDSS [10]. Across a wide range of KD features and aggregation methods, KD was also found to reach adequate responsiveness to meaningful change in radiological disease activity, ambulatory function, and upper limb function over a period of 3 months [11]. Additionally, analysis of KD data using a nonlinear time-series approach identified potential indicators of clinical change [12]. Based on these previous findings, 2 keystroke clusters were derived, one specific to upper limb function and the other to cognition, since these 2 domains are most directly related to typing. In order to translate this new biomarker into clinical practice for monitoring upper limb function and cognition in MS, the association with clinical measures over time and within individual patients needs investigation.
Our objective was to investigate the longitudinal associations between KD features, passively derived from regular typing on a smartphone, and upper limb function and cognition in patients with MS. Additionally, we sought to differentiate these longitudinal associations for both between-subject differences and within-subject changes in order to enable disease monitoring on the individual-patient level.
Methods
Study Design and Participants
This was a prospective cohort study at the MS Center of the Amsterdam University Medical Centers (VU University Medical Center location). The study design and interim analyses have been reported previously [10,11]. In brief, after a baseline assessment (M0), we followed the patients for 1 year, with clinical visits every 3 months (M3, M6, M9, and M12). During the study, participants used the Neurokeys keyboard app on their own smartphones [13]. Participants were patients with MS and were consecutively included in the study between August 2018 and December 2019 until a cohort size of 100 participants was reached. Patients were eligible if they were aged between 18 and 65 years, had a definite diagnosis of MS, had an EDSS score below 7.5, had access to a smartphone with the Android (5.0 or higher) or iOS (10 or higher) operating systems, had no visual or upper extremity deficits affecting regular smartphone use, and had no mood or sleep disorders impacting daily living (based on medical history-taking by a screening physician).
Ethical Considerations
The study received ethical approval from the Medisch Ethische Toetsingscommissie Vrije Universiteit medisch centrum (reference 2017.576) and conformed to legislation regarding data privacy and medical devices (Dutch Health and Youth Care Inspectorate; reference VGR2006948). All patients gave written informed consent. The study was registered as trial number NTR7268 at the Netherlands Trial Register.
Clinical Outcomes
Clinical outcomes for important aspects of MS were assessed, including clinically reported relapses, conventional magnetic resonance imaging (MRI) for disease activity, the EDSS, the MSFC, patient-reported outcomes, quantitative MRI, and optical coherence tomography for evaluation of domain-specific and overall disease severity and disease progression over time. As KD is most directly related to upper limb function and cognition, the current analysis focuses on the clinical assessments made every 3 months with the Nine-Hole Peg Test (NHPT) and Symbol Digit Modalities Test (SDMT). The NHPT is a measure of upper limb function that records the time needed to place, with a single hand, 9 pegs into 9 holes and then remove them [14]. The task is performed twice for each hand, and the 4 trials are averaged into a single score, with a higher score reflecting worse performance. The SDMT is a measure of information processing speed, the cognitive domain that is most commonly affected in MS and indicates overall cognitive functioning [15]. Using a key with 9 symbol-digit pairings, the number of correct digits corresponding to symbols during a 90-second trial is recorded as the total score [16]. A higher score reflects better performance.
KD and Keystroke Features
During the 1-year follow-up period, patients used the Neurokeys app (Neurocast BV) on their own smartphones [13]. The Neurokeys app replaces the native keyboard with a similar-looking keyboard (Figure 1A) that passively and continuously collects data on press-and-release typing events during everyday typing. From these keyboard interactions, keystroke features are derived based on key type (Figure 1B). For alphanumeric keys, the features include the latency between presses (press-press latency) and releases (release-release latency), the keypress time (hold time), and the time between keys (flight time). For the backspace key, derived features include latency prior to the use of the key (precorrection slowing), during use (correction duration) and after use (postcorrection slowing). Lastly, the time after a punctuation key was used was also derived (after-punctuation pause). A keystroke event count threshold of 50 events was used to remove days with insufficient data.
Figure 1.
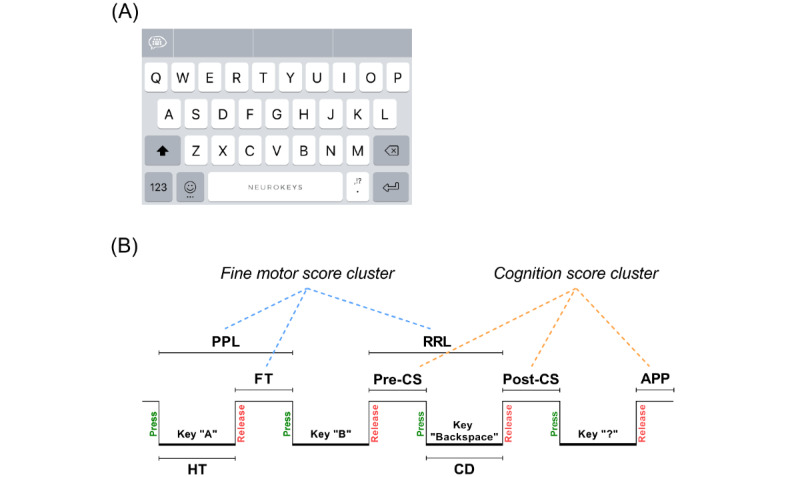
Overview of the Neurokeys keyboard (A) and a schematic representation of the keystroke dynamics features and clusters (B). APP: after-punctuation pause; CD: correction duration; FT: flight time; HT: hold time; post-CS: postcorrection slowing; PPL: press-press latency; pre-CS: precorrection slowing; RRL: release-release latency.
Construction of Keystroke Clusters
To compare the continuously collected keystroke data with clinical outcomes, the keystroke features were aggregated and clustered. First, the keystroke features were aggregated per day by the mean and median values, as both statistical measures summarize the data well and remain on the same unit scale (ie, seconds) to retain interpretability. Since mean and median values of the keystroke features were highly correlated, rather than discarding one, both summary values were averaged to reduce potential multicollinearity. Second, the fine motor score cluster (FMSC) and cognition score cluster (CSC) were derived based on the hypothesis that timing-related features (press-press latency, release-release latency, hold time, and flight time) are more related to fine motor skills, while error-related (precorrection slowing, correction duration, and postcorrection slowing) and paralinguistic (after-punctuation pause) features are more specific to events reflecting cognitive function. This concept-based clustering was then analyzed with principal component analysis and correlation analysis (Multimedia Appendices 1 - 3). Only features that contributed equally in the component analysis and were highly correlated (r>0.50) were included in the final cluster [17]. Finally, near the time of each clinical visit, 28-day (the 14 days before and after the clinical visit) and 14-day (the 7 days before and after the clinical visit) aggregation periods for the keystroke clusters was chosen for FMSC and CSC, respectively, since fine motor function can be considered more stable over time than cognitive function. The 28-day and 14-day periods for the keystroke clusters were aggregated by the mean value. Using these criteria, FMSC included press-press latency, release-release latency, and flight time, whereas CSC included precorrection slowing, postcorrection slowing, and after-punctuation pause.
Statistical Analysis
Analysis was performed with SPSS (version 26; IBM Corp) and R (version 4.0.3; R Foundation for Statistical Computing). Categorical data were summarized as the frequency and percentage. Numerical data were summarized as the mean and SD (or median and IQR or range if normally distributed). A linear mixed model analysis was used to determine the longitudinal association between KD clusters and clinical outcomes, so as to take into account clustering of repeated measurements within subjects [18]. Separate intercepts were estimated for each subject, over which a normal distribution was drawn. Then, the variance was estimated from that normal distribution and added to the model as a random intercept (ε), to adjust for repeated measurements within subjects, as follows: Y = β0 + β1X + ε. For upper limb function, the dependent variable was the NHPT score, the independent variable was FMSC, and the covariates were age and sex. For information processing speed, the dependent variable was the SDMT score, the independent variable was CSC, and the covariates were age, sex, and level of education. Since there was a significant relationship between time and SDMT performance, most likely due to practice effects, an additional random intercept for time (in days) was added to the cognition model. This allowed varying intercepts based on time in order to account for practice effects and imbalances in time intervals between clinical visits across subjects [19].
Importantly, given that the effect estimates of a linear mixed model analysis in a cohort with repeated measures are overall effects (ie, effect estimates entangle both differences across subjects and changes within subjects over time), a “hybrid” linear mixed model analysis was performed to disentangle the between-subject and within-subject effects of the longitudinal association [20]. This was done by centering around the mean and calculating deviation scores at each clinical visit for each subject. The mixed model analysis was then performed with both the centered values and the deviation score of this centered value for each individual, as follows: 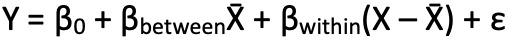 , where βbetween is the between-subject effect and βwithin is the within-subject effect [18].
, where βbetween is the between-subject effect and βwithin is the within-subject effect [18].
The output of all linear mixed models included effect estimates, 95% CIs, P values, and percentage explained variance. Covariates were considered relevant if the effect estimate between the dependent and independent variables changed by 10% or more after including the covariates into the model [21].
Results
A total of 102 patients with MS were included, of whom 91 completed the follow-up at M12; 6 patients dropped out at M3, 1 at M6, 1 at M9, and 3 at M12. The demographic and clinical characteristics at baseline are summarized in Table 1. The patients had a mean age of 46.4 years, most were female (75/102, 73.5%), and most had the relapsing-remitting MS subtype (61/102, 59.8%). The median disease duration since diagnosis was 5.7 years and the median EDSS score was 3.5. The mean follow-up duration was 376.9 (SD 109.4) days. At M12, the retention rate of patients with active keyboard use was 83.3% (85/102). Figure 2 shows the monthly retention rate and the average number of keystroke events per day. The clinical outcomes per visit and keystroke cluster data corresponding with each clinical visit are summarized in Table 2 and Multimedia Appendices 4 and 5. Part of the study follow-up coincided with the COVID-19 pandemic, which resulted in missing clinical visits, most prominently at M6 and M9.
Table 1.
Baseline patient demographic and clinical characteristics.
| Characteristics | Patients with multiple sclerosis (N=102) | ||
| Age (years), mean (SD) | 46.4 (10.4) | ||
| Sex, n (%) | |||
|
|
Female | 75 (73.5) | |
|
|
Male | 27 (26.5) | |
| Education levela, n (%) | |||
|
|
Low | 3 (2.9) | |
|
|
Middle | 34 (33.3) | |
|
|
High | 65 (63.7) | |
| Multiple sclerosis type, n (%) | |||
|
|
Primary progressive | 11 (10.8) | |
|
|
Secondary progressive | 30 (29.4) | |
|
|
Relapsing remitting | 61 (59.8) | |
| Disease duration since diagnosis (years), median (IQR) | 5.7 (3.0-13.1) | ||
| Expanded Disability Status Scale score, median (range) | 3.5 (1.5-7.0) | ||
aEducation levels were defined according to Rijnen et al [22].
Figure 2.
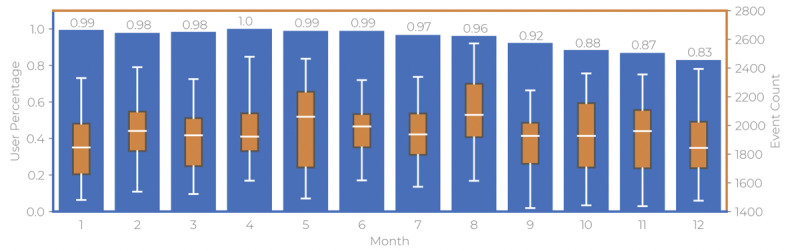
Bar graph depicting the retention rate (left y-axis, “user percentage”) of patients per month with superimposed box plots of the number of daily keystroke events (right y-axis, “event count”). The values above the bars show the retention rates as percentages.
Table 2.
Clinical outcomes and keystroke dynamics clusters for each clinical visit.
| Outcomes | M0 | M3 | M6 | M9 | M12 | |
| Nine-Hole Peg Testa | ||||||
|
|
Subjects, n | 102 | 93 | 76 | 58 | 89 |
|
|
Time (seconds), median (IQR) | 21.2 (19.4-25.0) | 21.0 (18.7-24.0) | 20.5 (18.8-22.5) | 20.2 (18.5-22.0) | 20.3 (18.7-23.0) |
| Symbol Digit Modalities Test | ||||||
|
|
Subjects, n | 102 | 93 | 76 | 58 | 90 |
|
|
Mean score (SD) | 54.4 (10.3) | 56.8 (10.4) | 57.9 (12.0) | 61.3 (12.8) | 60.3 (12.9) |
| Fine motor score cluster | ||||||
|
|
Subjects, n | 96 | 88 | 72 | 55 | 71 |
|
|
Time (seconds), mean (SD) | 0.45 (0.16) | 0.44 (0.16) | 0.44 (0.17) | 0.39 (0.15) | 0.42 (0.17) |
|
|
Daysb (n), mean (SD) | 14.3 (2.4) | 26.4 (4.8) | 26.2 (4.8) | 25.5 (5.4) | 15.5 (5.5) |
| Cognition score cluster | ||||||
|
|
Subjects, n | 101 | 89 | 70 | 55 | 72 |
|
|
Time (seconds), mean (SD) | 1.01 (0.42) | 0.95 (0.40) | 0.92 (0.40) | 0.86 (0.38) | 0.90 (0.44) |
|
|
Daysb (n), mean (SD) | 7.8 (1.0) | 13.7 (1.0) | 13.3 (2.2) | 13.1 (2.1) | 8.4 (2.7) |
aFor the Nine-Hole Peg Test, an average outlier threshold of 40 seconds was implemented, excluding 14 of 387 samples (3.6%).
bOnly days with ≥50 keystroke events.
Upper Limb Function
For the association between the NHPT and FMSC, 98 patients with MS were included in the mixed model analysis with an average of 3.9 observations per patient. Overall, the mean (SD) for FMSC was 0.43 (0.16) seconds and the median (IQR) for the NHPT was 20.6 (18.8-23.3) seconds. The results of the mixed model analysis are shown in Table 3 and depicted visually in Figure 3. In the overall model, FMSC was significantly associated with the NHPT and explained 42% of the variance in the NHPT results. Age and sex were not found to be relevant confounders in this association. In the hybrid model, a one-SD (0.16-second) increase in FMSC was significantly associated with an increase in NHPT of 2.5 seconds between patients and 1.1 seconds within patients.
Table 3.
Results of linear mixed model analyses of Nine-Hole Peg Test results over time with a random intercept on subject level.
|
|
β (95% CI) | P value | Random effect variance, % | Explained variance, % | |||
| Intercept only | 13.7 | N/Aa | |||||
| Fine motor score cluster | 12.62 (9.61-15.63) | <.001 | 8 | 42 | |||
| Fine motor score cluster and covariatesb | 12.56 (8.96-16.16) | <.001 | 7.7 | 43.9 | |||
| Hybrid model | 7.7 | 43.7 | |||||
|
|
Between subjects | 15.91 (12.18-19.63) | <.001 | N/A | N/A | ||
|
|
Within subjects | 6.94 (2.00-11.87) | <.001 | N/A | N/A | ||
aN/A: not applicable.
bCovariates included age and sex.
Figure 3.
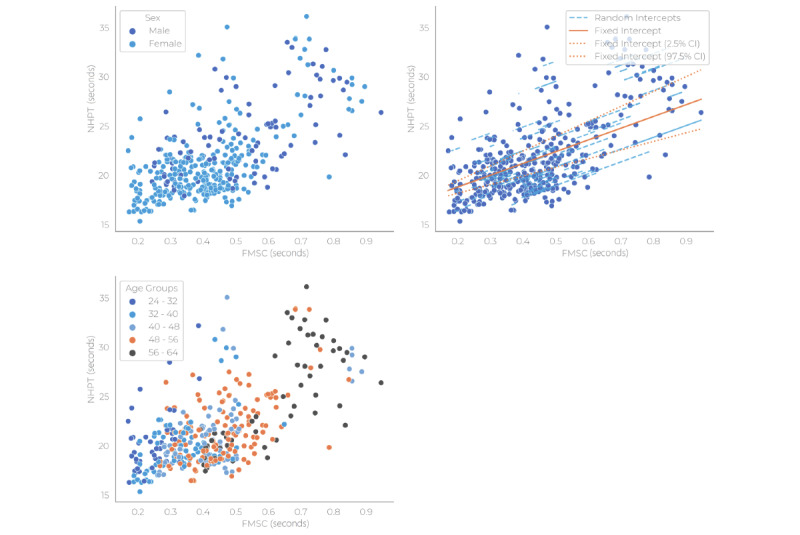
Scatter plots and linear mixed model fit for the Nine-Hole Peg Test and fine motor score cluster by covariates (sex and age), with random intercepts on subject level, and the number of days that constituted the keystroke cluster data points. FMSC: fine motor score cluster; NHPT: Nine-Hole Peg Test.
Information Processing Speed
All 102 patients with MS were included in the analysis of the association between information processing speed and the cognition keystroke cluster. The patients had an average of 3.8 repeated observations. The overall mean (SD) was 0.94 (0.41) seconds for CSC and 58.9 (12.1) points for SDMT. The output of the mixed model analyses is summarized in Table 4 and shown visually in Figure 4. In the overall model, CSC was significantly associated with SDMT and, together with age, sex, and level of education, explained 30.4% of the variance in SDMT. In the hybrid model, an increase of 1 SD (0.41 seconds) in CSC was significantly associated with a decrease of –4.6 in SDMT between patients. The within-subject association between CSC and SDMT, however, was not statistically significant.
Table 4.
Results of linear mixed model analyses of the Symbol Digit Modalities Test results over time with random intercepts on subject level and time (in days).
|
|
β (95% CI) | P value | Random effect variance, % | Explained variance, % | |||||||
| Intercept only | 110.9 | N/Aa | |||||||||
| Cognition score cluster | –8.57 (–12.02 to –5.12) | <.001 | 82.7 | 25.4 | |||||||
| Cognition score cluster and covariatesb | –5.02 (–9.02 to –1.02) | .02 | 77.1 | 30.4 | |||||||
| Hybrid model (including covariatesa) | 74.4 | 32.9 | |||||||||
|
|
Between subjects | –11.25 (–17.28 to –5.21) | <.001 | N/A | N/A | ||||||
|
|
Within subjects | –0.35 (–5.60 to 4.89) | .9 | N/A | N/A | ||||||
aN/A: not applicable.
bCovariates included age, sex, and level of education.
Figure 4.
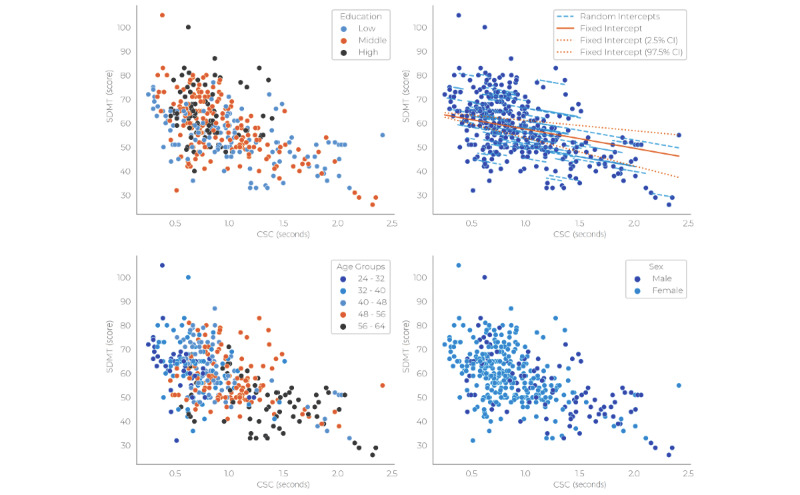
Scatter plots and linear mixed model fit for Symbol Digit Modalities Test and cognition score cluster by covariates (level of education, age, and sex) and random intercepts on subject level. SDMT: symbol digit modalities test; CSC: cognition score cluster.
Discussion
Principal Findings
This study investigated the longitudinal association between smartphone KD and commonly used clinical measures for upper limb function and information processing speed in patients with MS. In the overall model, the fine motor keystroke cluster was significantly associated with the NHPT (β=12.6, 95% CI 9.6-15.6); higher latency for presses and releases of alphanumeric keys during typing was related to a worse performance on the NHPT. When splitting the model for between-subject and within-subject effects, the association remained significant for both (β=15.9, 95% CI 12.2-19.6, and β=6.9, 95% CI 2.0-11.9, respectively). For the association between the cognitive keystroke cluster and SDMT, the time in days was included to account for practice effects on the SDMT and the imbalance in intervals between visits across subjects. CSC was found to be significantly negatively associated with SDMT; higher latency for backspace and punctuation mark keypresses was related to a worse SDMT score. This association had a β of –5.0 (95% CI –9.0 to –1.0) after adjusting for age, sex, and level of education. In the hybrid model for the cognitive keystroke cluster, the between-subject effect increased to β=–11.2 (95% CI –17.3 to –5.2), whereas the within-subject effect decreased to β=–0.4 (95% CI –5.6 to 4.9). To improve the interpretability of these associations, rather than considering 1-unit changes in keystroke clusters, the effect sizes can be recalculated to represent a change of 1 SD in keystroke clusters. In this distribution-based approach, a 1-SD change in FMSC corresponded with a change in NHPT of 1.1 seconds within patients or a 2.5-second difference between patients. Likewise, a 1-SD change in CSC corresponded with a change in SDMT of –0.14 points (although this was not significant) within patients or a –4.6-point difference between patients. Therefore, in our current cohort, a 2-SD change in FMSC and a 1-SD change in CSC would correspond to clinically relevant changes, as a 20% change in NHPT and a 4-point change in SDMT are considered clinically relevant based on group studies [14,16].
Comparison With Prior Work
Measurements of task or activity performance are an integral part of assessing and monitoring chronic neurological disorders such as MS. Typing on a smartphone is a near-daily activity from which biometric information pertaining to physical or mental functions can be derived. Despite this, the use of KD in the assessment of diseases is relatively underutilized, especially considering that touchscreen typing has existed for over a decade. Hence, our objective was to validate the use of passive KD, measured with the Neurokeys app, to improve disease management in MS. To this end, earlier investigations by our research group reported on the clinimetric properties of reliability, validity, and 3-month responsiveness of KD in MS [10,11]. To the best of our knowledge, other applications of KD analysis in diseases are limited to the detection of early-stage Parkinson disease [23-28], upper limb dysfunction in amyotrophic lateral sclerosis [29], and severity in mood disorders [30,31]. The objective of the studies of Parkinson disease was to differentiate subjects with disease or early disease from those without, making the study endpoints not directly comparable to ours. Nevertheless, the study on amyotrophic lateral sclerosis found worse typing to be associated with progression of the disease, which is similar to our current findings. Last, the 2 studies on mood disorders found significant regression effects between severity of depressive symptoms and smartphone keyboard activity. This is in line with our findings, in which worse typing parameters corresponded to worse performance on the clinical tests. In addition, concurrent to our findings, these studies showed that KD can be utilized and can even outperform clinical standards in the detection and assessment of disease status through capitalizing on motor anomalies and, to some extent, cognitive dynamics that affect typing behavior.
Of note is that, besides our 3-month responsiveness interim analysis, there are currently no studies investigating KD in a longitudinal setting in patients with MS. While research investigating differences between subjects is of great importance, especially in early validation research, differences across subjects cannot directly be extrapolated to changes that occur within individuals over time. Therefore, analyzing change over time within patients is essential for monitoring or predictive modeling in MS. Splitting our model to separately determine between-subject and within-subject effects showed that the latter were stronger than the former. This suggests that in our sample, differences in upper limb function and information processing speed tended to be greater across patients than within patients, as shown by the SD of the outcomes across patients being much larger than the average change over time. This is not surprising, given that research in outcome measures in MS often struggles to achieve adequate sensitivity to change over time compared to correlations across patients [32]. For upper limb function, our model that separated the between-subject and within-subject effects still showed a strong, significant within-subject effect estimate, indicating that the fine motor keystroke cluster is sensitive to change within individuals.
For information processing speed, prior to adjustment for time, we also found a significant within-subject effect estimate for the cognitive keystroke cluster (data not shown). However, accounting for practice effects by adding the time point as a random effect to the model resulted in a lower, statistically nonsignificant within-subject effect. This suggests that the association between SDMT and the cognitive keystroke cluster in our current cohort was affected more strongly by the effect of learning than by changes in the keystroke cluster within patients. This explanation is supported by the results of modeling the effect of time as a fixed term instead of a random effect. In this model, the effect of time was stronger than the within-subject effect of the cognition keystroke cluster. In addition, when time as a fixed term was modeled categorically (such as M0, M3, or M6), instead of being linear, the effect of time on the association between SDMT and cognition keystroke cluster was larger at later time points than earlier time points. As the amount of learning differs between patients, patients who are less severely affected by MS tend to have stronger practice effects than patients with more severe disability [33], and the larger positive slopes at later time points can be explained by practice effects causing a larger spread in SDMT data over time while the cognition keystroke cluster stays more or less stable.
Despite practice effects most likely diluting our findings on the within-subject association, the strong between-subject effect demonstrates the promise of the use of KD as a biomarker of information processing speed. Therefore, monitoring of cognition using KD needs further investigation with clinical cognitive outcomes that are more sensitive or less affected by practice effects and with a study population that allows a closer focus on cognitive function (ie, by including the presence of cognitive deficits as a selection criterion or using a longer follow-up duration) to demonstrate effects larger than measurement variability or learning effects. Similarly, a smartphone-based cognition test in the same cohort was found to be valid on the cross-sectional level, but lacked responsiveness when looking at change longitudinally, as changes within subjects are subtler than differences across subjects and measurements can be variable [34]. Additionally, more advanced analysis methods, such as nonlinear models, may increase sensitivity and allow higher frequency keystroke data and further investigation [12,35].
Limitations
A few limitations should be considered. First, despite modeling time in the analyses to take into account score changes over time, practice effects were not adjusted for, such by having healthy controls throughout the study. In addition, practice effects may have been exacerbated in the current cohort by their weekly performance of a smartphone variant of the SDMT concurrently with the digital biomarkers. Second, we investigated 2 commonly affected domains in MS that are directly involved with typing on the smartphone: upper limb function and information processing speed. In reality, MS entails a much broader array of functional spheres and relevant treatment outcomes. We collected a broad scope of clinical outcomes in our current cohort, and these data should be examined in future work in order to incorporate KD as a complete tool for monitoring MS. Lastly, a significant number of patients had missing clinical data, most prominently at M6 and M9, due to the COVID-19 pandemic. This also created a bias, as patients with secondary or primary progressive MS missed their clinical visits more often than patients with relapsing-remitting MS.
Conclusions
Keystroke clusters constructed from passively acquired smartphone KD data were shown to reflect function in patients with MS in a longitudinal setting, as measured with commonly used clinical outcome measures of upper limb function and cognitive functioning. In the longitudinal repeated measures analysis, the fine motor keystroke cluster was found to be associated with upper limb function across and within patients. This attests to the use of KD for monitoring and predictive purposes in MS. Monitoring cognitive function with KD needs further investigation, as a significant association was found in the overall model, but this relied mostly on differences between patients rather than changes within patients, likely exacerbated by practice effects related to the clinical measures. Altogether, KD during typing provided detailed data on the temporal and granular level on everyday upper limb and cognitive function. Our current findings are the first to demonstrate associations between clinical outcomes in MS and smartphone typing performance. With the ongoing expansion of therapeutic interventions, KD as a remote passive biomarker may improve clinical assessment and patient-centered disease management in MS. Important steps for future research are investigating other highly relevant MS outcomes, such as disease activity, and the external validity of the current results by monitoring function in clinical practice on the individual-patient level.
Acknowledgments
The authors would like to thank all the patients for their participation in this study. The authors also disclose receipt of the following financial support for the research, authorship, and publication of this article: funding from the Public Private Partnership Allowance, made available by Health-Holland, Top Sector Life Sciences and Health (grant number LSHM16060-SGF), and Stichting Multiple Scleroris Research (grant number 16-946 MS) to stimulate public-private partnerships; unrestricted funding was also received from Biogen.
Abbreviations
- CSC
cognition score cluster
- EDSS
Expanded Disability Status Scale
- FMSC
fine motor score cluster
- KD
keystroke dynamics
- MRI
magnetic resonance imaging
- MS
multiple sclerosis
- MSFC
Multiple Sclerosis Functional Composite
- NHPT
Nine-Hole Peg Test
- SDMT
Symbol Digit Modalities Test
Supplementary table 1. Principal component analysis of the timing-related and error-related/paralinguistic keystroke features.
Supplementary table 2. Correlation matrix of the timing-related keystroke features.
Supplementary table 3. Correlation matrix of the error-related/paralinguistic keystroke features.
Supplementary figure 1. Line graph of individual patients’ NHPT scores during the study.
Supplementary figure 2. Line graph of individual patients’ SDMT scores during the study.
Data Availability
Anonymized data not published within the article are available upon request from a qualified investigator. Such requests must be submitted in writing and will be reviewed for researcher qualifications and the legitimacy of the research purpose.
Footnotes
Authors' Contributions: KHL contributed to designing and conceptualizing the study, had a major role in the acquisition, analysis, and interpretation of the data, and drafted and revised the manuscript for intellectual content. JT had a major role in the acquisition, analysis, and interpretation of the data and contributed to revising the manuscript for intellectual content. BLW contributed to analysis and interpretation of the data and revising the manuscript for intellectual content. GL contributed to analysis and interpretation of the data and revising the manuscript for intellectual content. KM contributed to revising the manuscript for intellectual content. BU contributed to designing and conceptualizing the study and revising the manuscript for intellectual content. VDG contributed to designing and conceptualizing the study, analyzing and interpreting the data, and revising the manuscript for intellectual content. JK contributed to designing and conceptualizing the study, analyzing and interpreting the data, and revising the manuscript for intellectual content.
Conflicts of Interest: KHL, BLW, and VDG declare no conflicts of interest. JT, GL, and KM are employees of Neurocast BV (an industry partner). BU received consultancy fees from Biogen Idec, Genzyme, Merck Serono, Novartis, Roche, and Teva. JK has accepted speaker and consultancy fees from Merck, Biogen, Teva, Genzyme, Roche, and Novartis.
References
- 1.Hauser SL, Cree BA. Treatment of multiple sclerosis: a review. Am J Med. 2020 Dec;133(12):1380–1390.e2. doi: 10.1016/j.amjmed.2020.05.049. https://europepmc.org/abstract/MED/32682869 .S0002-9343(20)30602-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faissner S, Plemel JR, Gold R, Yong VW. Progressive multiple sclerosis: from pathophysiology to therapeutic strategies. Nat Rev Drug Discov. 2019 Dec 09;18(12):905–922. doi: 10.1038/s41573-019-0035-2.10.1038/s41573-019-0035-2 [DOI] [PubMed] [Google Scholar]
- 3.Lubetzki C, Zalc B, Williams A, Stadelmann C, Stankoff B. Remyelination in multiple sclerosis: from basic science to clinical translation. Lancet Neurol. 2020 Aug;19(8):678–688. doi: 10.1016/S1474-4422(20)30140-X.S1474-4422(20)30140-X [DOI] [PubMed] [Google Scholar]
- 4.Motl RW, Sandroff BM, Kwakkel G, Dalgas U, Feinstein A, Heesen C, Feys P, Thompson AJ. Exercise in patients with multiple sclerosis. Lancet Neurol. 2017 Oct;16(10):848–856. doi: 10.1016/S1474-4422(17)30281-8.S1474-4422(17)30281-8 [DOI] [PubMed] [Google Scholar]
- 5.Goverover Y, Chiaravalloti ND, O'Brien AR, DeLuca J. Evidenced-based cognitive rehabilitation for persons with multiple sclerosis: an updated review of the literature from 2007 to 2016. Arch Phys Med Rehabil. 2018 Feb;99(2):390–407. doi: 10.1016/j.apmr.2017.07.021.S0003-9993(17)31117-6 [DOI] [PubMed] [Google Scholar]
- 6.Lublin Fd, Reingold Sc, Cohen Ja, Cutter Gr, Sørensen Per Soelberg, Thompson Aj, Wolinsky Jerry S, Balcer Laura J, Banwell Brenda, Barkhof Frederik, Bebo Bruce, Calabresi Peter A, Clanet Michel, Comi Giancarlo, Fox Robert J, Freedman Mark S, Goodman Andrew D, Inglese Matilde, Kappos Ludwig, Kieseier Bernd C, Lincoln John A, Lubetzki Catherine, Miller Aaron E, Montalban Xavier, O'Connor Paul W, Petkau John, Pozzilli Carlo, Rudick Richard A, Sormani Maria Pia, Stüve Olaf, Waubant Emmanuelle, Polman Chris H. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014 Jul 15;83(3):278–86. doi: 10.1212/WNL.0000000000000560. https://europepmc.org/abstract/MED/24871874 .WNL.0000000000000560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983 Nov 01;33(11):1444–52. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 8.Cutter GB, Baier ML, Rudick RA, Cookfair D, Fischer J, Petkau J, Syndulko K, Weinshenker B G, Antel J P, Confavreux C, Ellison G W, Lublin F, Miller A E, Rao S M, Reingold S, Thompson A, Willoughby E. Development of a multiple sclerosis functional composite as a clinical trial outcome measure. Brain. 1999 May;122 ( Pt 5):871–82. doi: 10.1093/brain/122.5.871. [DOI] [PubMed] [Google Scholar]
- 9.Goldman MD, LaRocca NG, Rudick RA, Hudson LD, Chin PS, Francis GS, Jacobs A, Kapoor R, Matthews PM, Mowry EM, Balcer LJ, Panzara M, Phillips G, Uitdehaag BM, Cohen JA, Multiple Sclerosis Outcome Assessments Consortium Evaluation of multiple sclerosis disability outcome measures using pooled clinical trial data. Neurology. 2019 Nov 19;93(21):e1921–e1931. doi: 10.1212/WNL.0000000000008519. https://europepmc.org/abstract/MED/31641014 .WNL.0000000000008519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lam K, Meijer K, Loonstra F, Coerver E, Twose J, Redeman E, Moraal B, Barkhof F, de Groot V, Uitdehaag B, Killestein J. Real-world keystroke dynamics are a potentially valid biomarker for clinical disability in multiple sclerosis. Mult Scler. 2021 Aug 05;27(9):1421–1431. doi: 10.1177/1352458520968797. https://journals.sagepub.com/doi/abs/10.1177/1352458520968797?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lam K, Twose J, McConchie H, Licitra G, Meijer K, de Ruiter L, van Lierop Z, Moraal B, Barkhof F, Uitdehaag B, de Groot V, Killestein J. Smartphone-derived keystroke dynamics are sensitive to relevant changes in multiple sclerosis. Eur J Neurol. 2022 Feb 14;29(2):522–534. doi: 10.1111/ene.15162. https://europepmc.org/abstract/MED/34719076 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Twose J, Licitra G, McConchie H, Lam KH, Killestein J. Early-warning signals for disease activity in patients diagnosed with multiple sclerosis based on keystroke dynamics. Chaos. 2020 Nov;30(11):113133. doi: 10.1063/5.0022031. [DOI] [PubMed] [Google Scholar]
- 13.Neurokeys. Neurocast BV. [2022-10-13]. https://neurokeys.app/
- 14.Feys P, Lamers I, Francis G, Benedict R, Phillips G, LaRocca N, Hudson LD, Rudick R, Multiple Sclerosis Outcome Assessments Consortium The Nine-Hole Peg Test as a manual dexterity performance measure for multiple sclerosis. Mult Scler. 2017 Apr 16;23(5):711–720. doi: 10.1177/1352458517690824. https://journals.sagepub.com/doi/abs/10.1177/1352458517690824?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denney DR, Lynch SG, Parmenter BA. A 3-year longitudinal study of cognitive impairment in patients with primary progressive multiple sclerosis: speed matters. J Neurol Sci. 2008 Apr 15;267(1-2):129–36. doi: 10.1016/j.jns.2007.10.007.S0022-510X(07)00710-1 [DOI] [PubMed] [Google Scholar]
- 16.Benedict RH, DeLuca J, Phillips G, LaRocca N, Hudson LD, Rudick R, Multiple Sclerosis Outcome Assessments Consortium Validity of the Symbol Digit Modalities Test as a cognition performance outcome measure for multiple sclerosis. Mult Scler. 2017 Apr 16;23(5):721–733. doi: 10.1177/1352458517690821. https://journals.sagepub.com/doi/abs/10.1177/1352458517690821?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen J. A power primer. Psychol Bull. 1992 Jul;112(1):155–9. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 18.Twisk JWR. Applied Mixed Model Analysis: A Practical Guide, 2nd Ed. Cambridge, UK: Cambridge University Press; 2019. [Google Scholar]
- 19.Curran PJ, Bauer DJ. The disaggregation of within-person and between-person effects in longitudinal models of change. Annu Rev Psychol. 2011 Jan 10;62(1):583–619. doi: 10.1146/annurev.psych.093008.100356. https://europepmc.org/abstract/MED/19575624 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Twisk JW, de Vente W. Hybrid models were found to be very elegant to disentangle longitudinal within- and between-subject relationships. J Clin Epidemiol. 2019 Mar;107:66–70. doi: 10.1016/j.jclinepi.2018.11.021.S0895-4356(18)30783-2 [DOI] [PubMed] [Google Scholar]
- 21.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993 Dec 01;138(11):923–36. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 22.Rijnen SJM, Meskal I, Emons WHM, Campman CAM, van der Linden SD, Gehring K, Sitskoorn MM. Evaluation of normative data of a widely used computerized neuropsychological battery: applicability and effects of sociodemographic variables in a Dutch sample. Assessment. 2020 Mar 12;27(2):373–383. doi: 10.1177/1073191117727346. https://journals.sagepub.com/doi/abs/10.1177/1073191117727346?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arroyo-Gallego T, Ledesma-Carbayo MJ, Sanchez-Ferro A, Butterworth I, Mendoza CS, Matarazzo M, Montero P, Lopez-Blanco R, Puertas-Martin V, Trincado R, Giancardo L. Detection of motor impairment in Parkinson's Disease via mobile touchscreen typing. IEEE Trans Biomed Eng. 2017 Sep;64(9):1994–2002. doi: 10.1109/TBME.2017.2664802.7859354 [DOI] [PubMed] [Google Scholar]
- 24.Adams WR. High-accuracy detection of early Parkinson's Disease using multiple characteristics of finger movement while typing. PLoS One. 2017 Nov 30;12(11):e0188226. doi: 10.1371/journal.pone.0188226. https://dx.plos.org/10.1371/journal.pone.0188226 .PONE-D-17-31940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iakovakis D, Hadjidimitriou S, Charisis V, Bostantzopoulou S, Katsarou Z, Hadjileontiadis LJ. Touchscreen typing-pattern analysis for detecting fine motor skills decline in early-stage Parkinson's disease. Sci Rep. 2018 May 16;8(1):7663–13. doi: 10.1038/s41598-018-25999-0. doi: 10.1038/s41598-018-25999-0.10.1038/s41598-018-25999-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iakovakis D, Chaudhuri KR, Klingelhoefer L, Bostantjopoulou S, Katsarou Z, Trivedi D, Reichmann H, Hadjidimitriou S, Charisis V, Hadjileontiadis LJ. Screening of Parkinsonian subtle fine-motor impairment from touchscreen typing via deep learning. Sci Rep. 2020 Jul 28;10(1):12623–13. doi: 10.1038/s41598-020-69369-1. doi: 10.1038/s41598-020-69369-1.10.1038/s41598-020-69369-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papadopoulos A, Iakovakis D, Klingelhoefer L, Bostantjopoulou S, Chaudhuri KR, Kyritsis K, Hadjidimitriou S, Charisis V, Hadjileontiadis LJ, Delopoulos A. Unobtrusive detection of Parkinson's disease from multi-modal and in-the-wild sensor data using deep learning techniques. Sci Rep. 2020 Dec 07;10(1):21370–10. doi: 10.1038/s41598-020-78418-8. doi: 10.1038/s41598-020-78418-8.10.1038/s41598-020-78418-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pham TD. Pattern analysis of computer keystroke time series in healthy control and early-stage Parkinson's disease subjects using fuzzy recurrence and scalable recurrence network features. J Neurosci Methods. 2018 Sep 01;307:194–202. doi: 10.1016/j.jneumeth.2018.05.019.S0165-0270(18)30157-2 [DOI] [PubMed] [Google Scholar]
- 29.Londral A, Pinto S, de Carvalho M. Markers for upper limb dysfunction in Amyotrophic Lateral Sclerosis using analysis of typing activity. Clin Neurophysiol. 2016 Jan;127(1):925–931. doi: 10.1016/j.clinph.2015.06.017.S1388-2457(15)00671-9 [DOI] [PubMed] [Google Scholar]
- 30.Zulueta J, Piscitello A, Rasic M, Easter R, Babu P, Langenecker SA, McInnis M, Ajilore O, Nelson PC, Ryan K, Leow A. Predicting mood disturbance severity with mobile phone keystroke metadata: a BiAffect digital phenotyping study. J Med Internet Res. 2018 Jul 20;20(7):e241. doi: 10.2196/jmir.9775. https://www.jmir.org/2018/7/e241/ v20i7e241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vesel C, Rashidisabet H, Zulueta J, Stange J, Duffecy J, Hussain F, Piscitello Andrea, Bark John, Langenecker Scott A, Young Shannon, Mounts Erin, Omberg Larsson, Nelson Peter C, Moore Raeanne C, Koziol Dave, Bourne Keith, Bennett Casey C, Ajilore Olusola, Demos Alexander P, Leow Alex. Effects of mood and aging on keystroke dynamics metadata and their diurnal patterns in a large open-science sample: A BiAffect iOS study. J Am Med Inform Assoc. 2020 Jul 01;27(7):1007–1018. doi: 10.1093/jamia/ocaa057. https://europepmc.org/abstract/MED/32467973 .5848291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Groot V, Beckerman H, Uitdehaag B M J, de Vet H C W, Lankhorst G J, Polman C H, Bouter L M. The usefulness of evaluative outcome measures in patients with multiple sclerosis. Brain. 2006 Oct 15;129(Pt 10):2648–59. doi: 10.1093/brain/awl223.awl223 [DOI] [PubMed] [Google Scholar]
- 33.Roar M, Illes Z, Sejbaek T. Practice effect in Symbol Digit Modalities Test in multiple sclerosis patients treated with natalizumab. Mult Scler Relat Disord. 2016 Nov;10:116–122. doi: 10.1016/j.msard.2016.09.009.S2211-0348(16)30172-9 [DOI] [PubMed] [Google Scholar]
- 34.Lam K, van Oirschot P, den Teuling B, Hulst H, de Jong B, Uitdehaag B, de Groot V, Killestein J. Reliability, construct and concurrent validity of a smartphone-based cognition test in multiple sclerosis. Mult Scler. 2022 Feb 26;28(2):300–308. doi: 10.1177/13524585211018103. https://journals.sagepub.com/doi/abs/10.1177/13524585211018103?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lam K, Bucur IG, Van Oirschot P, De Graaf F, Weda H, Strijbis E, Uitdehaag B, Heskes T, Killestein J, De Groot V. Towards individualized monitoring of cognition in multiple sclerosis in the digital era: A one-year cohort study. Mult Scler Relat Disord. 2022 Apr;60:103692. doi: 10.1016/j.msard.2022.103692. https://linkinghub.elsevier.com/retrieve/pii/S2211-0348(22)00207-3 .S2211-0348(22)00207-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary table 1. Principal component analysis of the timing-related and error-related/paralinguistic keystroke features.
Supplementary table 2. Correlation matrix of the timing-related keystroke features.
Supplementary table 3. Correlation matrix of the error-related/paralinguistic keystroke features.
Supplementary figure 1. Line graph of individual patients’ NHPT scores during the study.
Supplementary figure 2. Line graph of individual patients’ SDMT scores during the study.
Data Availability Statement
Anonymized data not published within the article are available upon request from a qualified investigator. Such requests must be submitted in writing and will be reviewed for researcher qualifications and the legitimacy of the research purpose.


