This randomized clinical trial tests whether a virtual reality environment using a novel ceiling screen instead of a headset reduces the experience of pain during intravenous placement compared with standard care in a pediatric emergency department.
Key Points
Question
Can a virtual reality (VR) environment using a novel domed ceiling screen be effective in reducing distress among young children undergoing intravenous (IV) placement in pediatric emergency departments (PEDs)?
Findings
In this randomized clinical trial including 88 children, there was a significant reduction in overall pain scale scores over the course of IV placement in the VR intervention group compared with the control group.
Meaning
Virtual reality using a domed ceiling screen may be an effective distraction method to reduce distress in young children undergoing IV placement in PEDs.
Abstract
Importance
Distraction using virtual reality (VR) has been found to provide a clinically significant reduction in the experience of pain during various painful procedures. Commercially available VR systems usually require the user to wear a head-mounted display helmet, which can be challenging for young children, and whether VR can reduce pain during intravenous (IV) placement in young children is currently unknown.
Objective
To determine whether a VR environment using a novel domed ceiling screen reduces distress among children over the course of IV placement compared with standard care in a pediatric emergency department.
Design, Setting, and Participants
This randomized clinical trial was conducted from June 3, 2020, to February 8, 2021, at an urban tertiary academic children’s hospital. Included were children aged 6 months to 4 years undergoing IV placement in the pediatric emergency department.
Intervention
Children in the intervention group lay on a bed to experience a VR animation using a domed ceiling screen during the IV placement procedure, which was performed as usual. Children in the control group also lay on a bed during the procedure but did not view a VR animation.
Main Outcomes and Measures
The primary outcome was pain scores measured using the Face, Legs, Activity, Cry, and Consolability (FLACC) scale at 4 time points during IV placement: immediately after the child lay down on the bed (T1), the moment the tourniquet was applied (T2), the moment a sterile alcohol swab was applied (T3), and the moment the needle penetrated the skin (T4).
Results
Of the 88 children included in the final analysis, 44 received VR distraction (median [IQR] age, 24.0 [14.5-44.0] months; 27 boys [61.4%]), and 44 received standard care (median [IQR] age, 23.0 [15.0-40.0] months; 26 boys [59.1%]). The median [IQR] FLACC scores at T4 were 6.0 (1.8-7.5) in the intervention group and 7.0 (5.5-7.8) in the control group. The ordinal logistic regression model showed that children in the VR intervention group vs the control group had a lower probability of higher FLACC scores (odds ratio, 0.53; 95% CI, 0.28-0.99; P = .046).
Conclusions and Relevance
The findings of this trial indicate that displaying VR using a domed ceiling screen may be an effective distraction method that reduces distress in young children undergoing IV placement.
Trial Registration
isrctn.org Identifier: KCT0005122
Introduction
Visiting the pediatric emergency department (PED) is among the most stressful situations children can experience.1 In particular, intravenous (IV) placement is a common invasive procedure that invokes fear and pain in children.2 A lack of cooperation in children due to fear and anxiety about needles makes it more difficult for practitioners to perform IV placement.3 Frequent failure and retrial of the procedure negatively affects children, leading to maladaptive pain responses, needle phobia, long-term traumatic memories, and avoidance of medical centers.4
The pharmacological method of using topical anesthetics and systematic analgesics has less of an effect on pain relief than expected and cannot reduce anxiety before a procedure.5,6 In contrast, nonpharmacological distraction methods are not only effective in reducing distress but are also easily applicable to urgent procedures.7,8,9 In recent years, virtual reality (VR) has attracted attention as a novel digital distraction method that enables children to immerse themselves in an alternate world.10 In addition, distraction using VR has been found to provide clinically meaningful pain reduction during various procedures, such as burn wound care, lumbar puncture, and IV placement.11,12,13
Commercially available VR systems usually require the user to wear a head-mounted display helmet, which can be challenging for young children, who constitute the main age group visiting PEDs.14 Because wearing this helmet is difficult for young children, we developed a dome-shaped screen to deliver VR and conducted a pilot study prior to this randomized clinical trial (RCT).15 Considering some technical issues in implementing VR using the domed screen in clinical practice, the ceiling of our PED was renovated to install a dome-shaped screen, and we developed VR content suitable for children in the age group targeted in this study. The objective of this study was to gauge whether VR using a novel domed ceiling screen reduces distress in young children during IV placement compared with standard care in PEDs.
Methods
Study Design and Setting
This RCT was an experimental, parallel, 2-group, clinical study comparing the effect of VR using a domed ceiling screen vs no VR experience during IV placement in young children. This RCT was conducted according to the Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines in the PED of our hospital in Seoul, South Korea, from June 3, 2020, to February 8, 2021. Our hospital, Seoul National University Hospital, is a large, urban, academic tertiary care hospital with a 315-bed capacity, and its PED is visited by more than 20 000 children annually. This trial was approved by the institutional review board of the hospital and was registered in the Korean Clinical Trial Registry, with information provided in English at the International Clinical Trials Registry Platform (KCT0005122). Written informed consent was obtained from caregivers (ie, parents, guardians) of eligible children after researchers explained the trial.
Participants
Children visiting the PED were eligible if they were aged 6 months to 4 years and were undergoing IV placement for treatment or diagnosis. Children with developmental disabilities or facial anomalies that would make it difficult for physicians to apply the pain scale and children who were separated in a negative-pressure isolation room for suspected COVID-19 were excluded from the study. Children were also excluded if they needed urgent IV placement (eg, because of a hemodynamically unstable status or an altered level of consciousness) or if their caregivers had insufficient knowledge of Korean to understand the study protocol.
Randomization and Study Protocol
A researcher screened children visiting the PED during weekday daytime hours (9 am to 4 pm Monday through Friday). If the child was eligible, the researcher explained the trial and obtained written informed consent from his or her caregivers. There was no payment for participation. Then, the children were allocated to either the intervention or control group based on an encrypted random table. Simple randomization sequences were generated by a computerized random number generator. Allocation was concealed until consent was obtained.
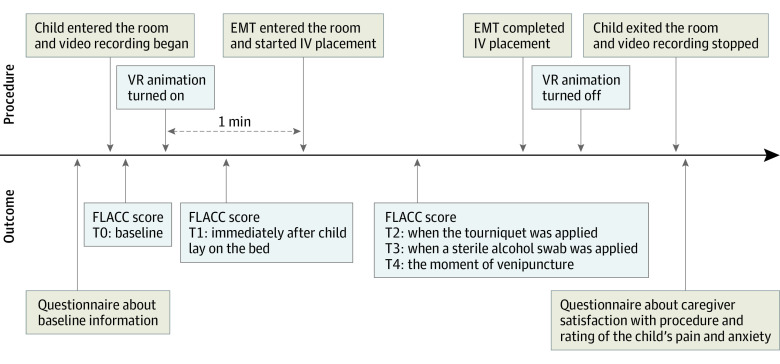
The study protocol is illustrated in Figure 1 and described in Supplement 1. Prior to the IV placement, caregivers completed a questionnaire to provide baseline information, such as whether the child had taken analgesics within 2 hours of visiting the PED and had previously experienced IV placement. When the researcher was ready for video recording, the child and caregivers entered the treatment room together. The video recording began shortly after the child and caregivers entered the treatment room and continued until they left the room after the procedure.
Figure 1. Study Protocol.
EMT indicates emergency medical technician; FLACC, Face, Legs, Activity, Cry, and Consolability; IV, intravenous; T0-T4, time points; VR, virtual reality.
For those children assigned to the intervention group, the VR animation was projected onto the domed ceiling screen, and the child was asked to lie on a bed to watch the VR animation. After the child watched the animation for approximately 1 minute, the emergency medical technician (EMT) entered the treatment room and started the IV placement procedure. In the control group, the child was asked to lie on a bed for IV placement, and EMTs were instructed to perform the IV placement procedure as they usually would. The IV placement procedure proceeded according to the following sequence: tourniquet application, venipuncture site cleansing, venipuncture, and taping of an indwelling IV cannula. To focus on the child’s facial expression, the researcher recorded videos using an action camera in addition to a camera installed on the ceiling throughout the procedure. After the IV placement was complete, the caregivers completed questionnaires about their satisfaction with the procedure and their child’s pain and anxiety.
Interventions
Our VR equipment consisted of a domed ceiling screen developed and installed by Gleam Systems and 2 projectors (EB-G7100, Epson) linked to a computer playing an animation. The domed ceiling screen had a hemispherical shape with a 123° angle of view. The diameter and height were 1700 and 520 mm, respectively (eFigure 1 in Supplement 2). An animation of approximately 3 minutes was created by Gleam Systems using a high-resolution, real-time, 3-dimensional game engine; the animation was displayed directly on the domed ceiling screen. The animation portrayed 4 animal characters (a bear, rabbit, cat, and squirrel) running through the forest, with the VR simulating that the child was running with animal friends (eFigure 2 in Supplement 2).
Outcomes
The primary outcome was the observed pain intensity during the IV placement procedure. This was measured using the Face, Legs, Activity, Cry, and Consolability (FLACC) scale16,17 at 4 time points during IV placement: immediately after the child lay down on the bed (T1), the moment the tourniquet was applied (T2), the moment a sterile alcohol swab was applied (T3), and the moment the needle penetrated the skin (T4). Baseline pain intensity was also measured immediately after the child entered the treatment room (T0). Pain intensity assessments were completed by 2 physicians who were blinded to the study protocol and purpose and who independently viewed the video recordings. The secondary outcomes included caregiver satisfaction on a 5-point Likert-type questionnaire and caregiver rating of the child’s pain and anxiety (visual analog scale, 0-10) after IV placement.
Sample Size
With a type I error rate of 0.05 and 80% power, a sample size of 37 per group was required to detect a 1.6-point reduction in the FLACC score with an SD of 2.4, based on the result of the previous pilot study. Assuming a 20% dropout rate, 44 children per group were required.
Statistical Methods
Our analysis was based on an intention-to-treat approach. Continuous variables were reported as medians and IQRs, and categorical variables were reported as frequencies and percentages. Comparisons were performed using the Mann-Whitney U test for continuous variables and the Fisher exact test or χ2 test for categorical variables as appropriate. Descriptive statistics were calculated, including the mean and median pain score at each time point (T0-T4), changes in pain score at each time point (T1-T4) compared with that at baseline (T0), mean pain score (T1-T4), and mean change in pain score from T0 to each time point (T1-T4).
Ordinal logistic regression was performed to provide odds ratios comparing the distribution of the primary outcome between the randomized groups. Odds ratios were estimated with a robust variance estimator in the generalized estimating equation to account for multiple measurements per patient. Additionally, a nonparametric repeated measures analysis of variance (RMANOVA) was conducted to evaluate the effect of VR distraction over the course of IV placement using pain scores measured after VR distraction was applied (ie, T1 to T4). When significant differences were found, the Mann-Whitney U test with Bonferroni correction for multiple comparisons was used.
Additional analyses were performed on the subgroup of children with a nonzero sum of pain scores for the entire procedure and on the subgroups by age. According to sex differences in pain perception and behavior,18,19 we conducted sex-stratified analyses to further understand sex differences in sensitivity to VR intervention. Per-protocol analysis was also conducted for the primary outcomes. A between-group comparison of caregiver responses to the postprocedural questionnaire was conducted using the Mann-Whitney U test. All statistical analyses were 2-tailed, and a P value less than .05 was considered significant. Statistical analyses were performed using R version 3.6.3 (R Foundation for Statistical Computing) and SAS version 9.4 (SAS Institute).
Results
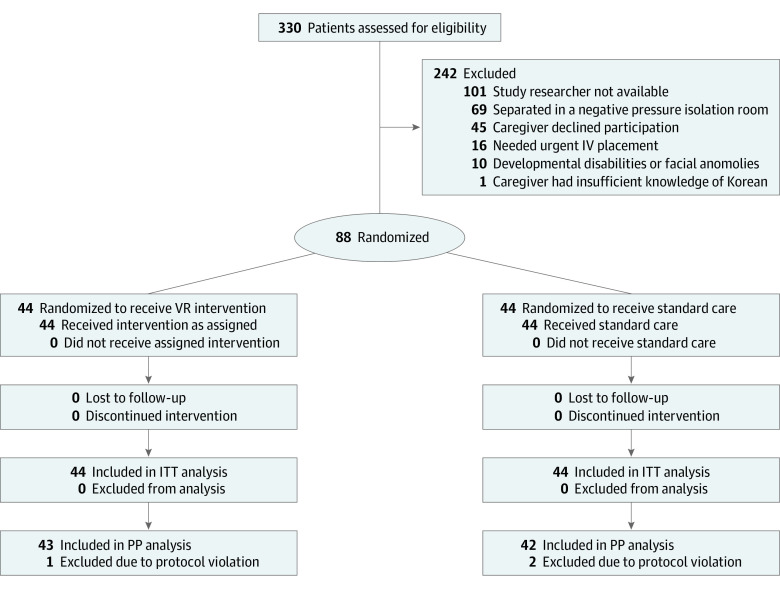
Figure 2 shows the flow diagram for the study, with 330 patients assessed for eligibility, with 88 randomized and 242 excluded. There were minor protocol violations with 3 children (1 in the intervention group and 2 in the control group). Table 1 shows the baseline characteristics of the 2 groups. The median (IQR) age of the children was 24.0 months (14.5-44.0) in the intervention group and 23.0 months (15.0-40.0) in the control group. There were 27 boys (61.4%) in the intervention group and 26 boys (59.1%) in the control group.
Figure 2. Flow Diagram for Study.
ITT indicates intention to treat; IV, intravenous; PP, per protocol; VR, virtual reality.
Table 1. Demographic Characteristics by Group.
| Characteristic | No. (%) | ||
|---|---|---|---|
| Intervention (n = 44) | Control (n = 44) | Total (N = 88) | |
| Children | |||
| Age, median (IQR), mo | 24.0 (14.5-44.0) | 23.0 (15.0-40.0) | 23 (15.0-42.5) |
| Sex | |||
| Male | 27 (61.4) | 26 (59.1) | 53 (60.2) |
| Female | 17 (38.6) | 18 (40.9) | 35 (39.8) |
| Reason for PED visit | |||
| Disease | 28 (63.6) | 26 (59.1) | 54 (61.4) |
| Trauma | 16 (36.4) | 18 (40.9) | 34 (38.6) |
| Previous venipuncture experience | |||
| Yes | 33 (75.0) | 31 (70.5) | 64 (72.7) |
| No | 11 (25.0) | 13 (29.5) | 24 (27.3) |
| Analgesics taken | |||
| Yes | 0 | 3 (6.8) | 3 (3.4) |
| No | 44 (100.0) | 41 (93.2) | 85 (96.6) |
| Fever | |||
| Yes | 3 (6.8) | 8 (18.2) | 11 (12.5) |
| No | 41 (93.2) | 36 (81.8) | 77 (87.5) |
| Caregivers | |||
| Age, median (IQR), y | 36.5 (34.5-40.0) | 37.0 (34.0-39.5) | 37.0 (34.0-40.0) |
| Relationship | |||
| Mother | 34 (77.3) | 34 (77.3) | 68 (77.3) |
| Father | 8 (18.2) | 8 (18.2) | 16 (18.2) |
| Grandmother | 2 (4.5) | 1 (2.3) | 3 (3.4) |
| Grandfather | 0 | 0 | 0 |
| Other | 0 | 1 (2.3) | 1 (1.1) |
| No. of children, median (IQR) | 1.0 (1.0-2.0) | 2.0 (1.0-2.0) | 2.0 (1.0-2.0) |
| Previous experience observing child’s venipuncture | |||
| Yes | 26 (59.1) | 29 (65.9) | 55 (62.5) |
| No | 18 (40.9) | 15 (34.1) | 33 (37.5) |
Abbreviation: PED, pediatric emergency department.
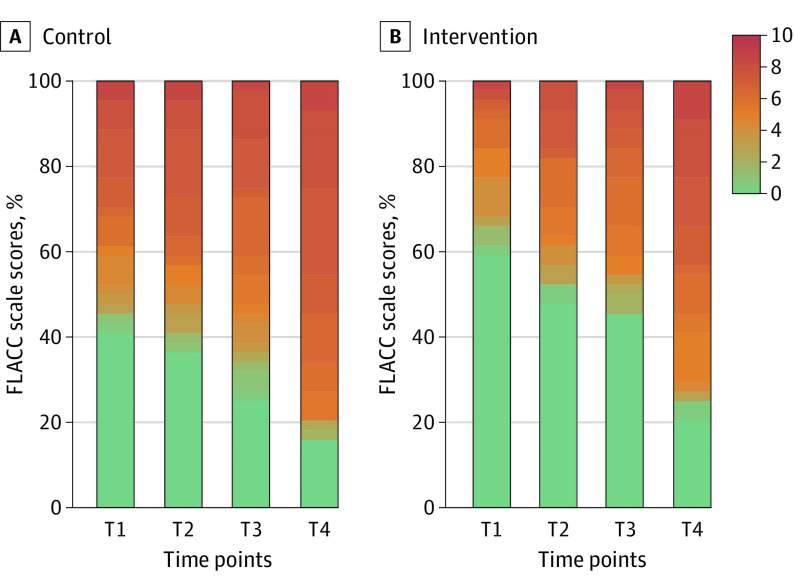
Descriptive statistics for FLACC scores at each time point by the group are shown in Figure 3 and eTable 1 in Supplement 2. The FLACC score tended to increase over the course of the IV placement in both groups, but the median pain scores at all time points were numerically lower in children exposed to the VR intervention compared with the control group. The median (IQR) FLACC scores at T4 were 6.0 (1.8-7.5) in the intervention group and 7.0 (5.5-7.8) in the control group.
Figure 3. Percentage Stacked Bar Chart of Face, Legs, Activity, Cry, and Consolability (FLACC) Scale Scores by Group and Time Point.
Responses in each of the 5 FLACC categories are scored between 0 (green) and 2, for a maximum total score of 10 (red), with higher scores indicating greater pain intensity. The time points during intravenous placement were as follows: T1, immediately after the child lay down on the bed; T2, the moment the tourniquet was applied; T3, the moment a sterile alcohol swab was applied; T4, the moment the needle penetrated the skin.
The generalized estimating equation analysis of the ordinal logistic regression model showed that the VR intervention group had a lower probability of having higher FLACC scores compared with the control group (odds ratio, 0.53; 95% CI, 0.28-0.99; P = .046) (Table 2). A nonparametric RMANOVA showed a significant main effect of both time point (F2.17,∞ = 40.39; P < .001) and group (F1.00,∞ = 4.05; P = .04). However, there was no significant interaction between time point and group (F2.17,∞ = 0.84; P = .44) (eTable 2 in Supplement 2). These results suggest that the overall FLACC scores of the intervention group were lower than those of the control group.
Table 2. Ordinal Regression Analysis of the Intervention Effects on the Primary Outcomes.
| Time | FLACC scale score, median (IQR) | OR (95% CI)a | |
|---|---|---|---|
| Intervention | Control | ||
| T1 | 0.0 (0.0-0.0) | 3.8 (0.0-7.0) | 0.39 (0.18-0.87) |
| T2 | 0.5 (0.0-6.0) | 4.5 (0.0-7.5) | 0.54 (0.25-1.18) |
| T3 | 2.2 (0.0-6.0) | 5.5 (0.2-7.2) | 0.51 (0.25-1.06) |
| T4 | 6.0 (1.8-7.5) | 7.0 (5.5-7.8) | 0.65 (0.31-1.34) |
| Overallb | 2.8 (1.1-5.4) | 4.9 (1.8-6.9) | 0.53 (0.28-0.99) |
Abbreviations: FLACC, Face, Legs, Activity, Cry, and Consolability; OR, odds ratio; T1, immediately after the child lay down on the bed; T2, the moment the tourniquet was applied; T3, the moment a sterile alcohol swab was applied; T4, the moment the needle penetrated the skin.
Odds of having higher FLACC scale score. Odds ratios were estimated with a robust variance estimator in the generalized estimating equation.
Overall group effect was considered statistically significant (P = .046). Group × time interaction was not considered statistically significant (P = .43).
In the analysis of only girls (intervention group, n = 17, vs control group, n = 18), there was a significant main effect of both group (F1.00,∞ = 6.56; P = .01) and time point (F2.26,∞ = 14.31; P < .001) and an interaction between time point and group (F2.26,∞ = 6.72; P < .001) (eTable 3 in Supplement 2). According to post hoc Mann-Whitney analyses, the pain score at T1 in the intervention group was lower than that in the control group (median [IQR], 0.0 [0.0-4.0] vs 7.2 [5.0-7.9]; Bonferroni-adjusted P < .001), with no significant differences between groups at other time points during IV placement (eTable 4 in Supplement 2). In contrast, there was only a significant effect of time point (F2.26,∞ = 28.54; P < .001) in the boy subgroup.
In the per-protocol analysis, we also found a significant main effect of group (F1.00,∞ = 4.84; P = .03) (eTable 5 in Supplement 2). A numerically lower mean pain score from T1 to T4 was observed in the intervention group than in the control group (median [IQR], 2.8 [1.1-5.2] vs 4.9 [1.9-6.9]) (eTable 6 in Supplement 2). After the children without observed distress throughout the procedure were removed from the analysis, there was also a significant main effect of group (F1,∞ = 5.34; P = .02) and numerically lower mean scores at the 4 time points during the procedure (median [IQR], 3.9 [1.9-5.8] vs 6.1 [3.0-6.9]) (eTables 7 and 8 in Supplement 2). We also performed analyses of each of the 5 subgroups based on age (6-11, 12-23, 24-35, 36-47, and 48-59 months). There was no significant main effect of group or interaction between groups in any of the subgroups (eTable 9 in Supplement 2).
The results of the caregiver responses to the postprocedural questionnaire are summarized in eTable 10 in Supplement 2. Caregiver ratings of the child’s pain (median [IQR], 5.0 [3.0-8.0] vs 7.0 [5.0-10.0]; P = .008) and anxiety (median [IQR], 5.0 [3.0-7.0] vs 8.0 [6.5-10.0]; P < .001) were lower in the intervention group than in the control group. However, there was no statistically significant difference between groups in caregiver satisfaction with the overall procedure (median [IQR], 4.0 [3.0-4.0] vs 4.0 [3.0-4.0]; P = .55).
Discussion
Based on the results of this RCT, VR using a domed ceiling screen may be an effective distraction method for IV placement. The ordinal logistic regression model showed that the VR intervention group had a lower probability of having higher FLACC scores than the control group. Additionally, there were overall differences in pain scores during the procedure between the 2 groups according to a significant main effect of group in nonparametric RMANOVA. Consistent with the results, the mean pain scores during the procedure were also numerically lower in the intervention group than in the control group.
Interestingly, there were significant findings in the girl subgroup only. Based on the post hoc analysis with Bonferroni correction, VR using a domed ceiling screen could be an effective distraction method for girls, especially in the preprocedural stage. Considering that there was no main effect of group in the boy subgroup, it seems that the difference in the effect of VR distraction with regard to sex contributed to the results in which no interaction effect was shown in the entire group. Given the high expectations for boys to have a high pain tolerance in Korea,20 distraction could have been more effective for girls, who are relatively more vulnerable to procedural distress.21 Even taking this into consideration, improving the content of the VR is required for future studies because the VR animation we prepared may not have been interesting enough for boys.
According to the subgroup analysis that excluded children who did not seem to be distressed during the whole procedure, the distraction aimed at reducing distress may be more effective in children who are actually experiencing distress. These results are consistent with those of a previous study that used music distraction.1 Virtual reality interventions are expected to effectively reduce procedural pain, especially in PED environments, which can be more frightening for children than other medical settings. However, when we performed subgroup analysis by age, there were no significant results related to the VR intervention. Because of the small sample size in each age group, it was impossible to show meaningful efficacy, and the VR animation may not be suitable to attract the attention of children of all ages.
Previous studies have reported that lower pain management ratings can decrease satisfaction significantly.22,23 Therefore, the caregiver’s perception that a child’s pain is being properly managed is clinically important in PEDs. The caregiver rating of child distress was significantly lower in the intervention group than in the control group in this trial. However, there was no difference in caregiver satisfaction with the overall procedure between the 2 groups. In fact, the satisfaction level was higher than moderate in both groups, making it difficult to compare the differences.
There were some issues that needed some improvement. The distance between the child and the screen was too far for the child to focus on it. For the VR to be immersive, the screen should be closer to the child, and it would be better to be able to adjust the screen height for each patient. In addition, it is challenging not to disrupt the child’s immersion in VR at the moment the EMT enters the treatment room or the moment the EMT comes in contact with the child’s body. When the children noticed that the EMT was preparing for the needle procedure, they could no longer focus on the animation. It may be more appropriate in future studies to change the screen shape to inhibit children from viewing the outside world.
The animation we used was not commercially produced but was created for the purpose of this study. However, as we mentioned earlier, our animation required interesting content to attract the attention of children of all ages and sexes. Virtual reality can be a more effective distraction tool when it involves devices that allow children to interact with virtual environments, such as by talking with virtual characters and playing simple games.24 It will be necessary to develop novel content so that children focus on the virtual environment and are more insensitive to external stimuli.
Limitations
This study has some limitations. It was impossible to blind the physicians who assessed the FLACC scale score because the room lights were turned off in the intervention group while the VR animation was on. Although physicians did not know the study protocol and purpose, there may have been an increased potential for bias. In addition, we used a portable light in the intervention group for IV placement after the animation started. Because the brightness and color of the portable light were different from those of the indoor light, it may have been more difficult than usual for EMTs to find a vein to puncture. In cases where the EMT was in contact with the child for a longer period of time to find a vein, the effectiveness of the intervention may have been reduced. In the next study, further adjustments to improve lighting in the treatment room will be needed.
Conclusions
The findings of this trial indicate that using a domed ceiling screen for VR may be an effective distraction method that reduces distress in young children undergoing IV placement. Future studies could evaluate more immersive VR systems, such as eye trackers, for young children in PED environments.
Trial protocol
eFigure 1. Virtual reality equipment consisted of a domed ceiling screen and two projectors
eFigure 2. Screenshot of the virtual reality animation
eTable 1. FLACC score by group
eTable 2. Nonparametric RMANOVA results
eTable 3. Nonparametric RMANOVA results stratified in sex
eTable 4. FLACC score by group stratified in sex
eTable 5. Nonparametric RMANOVA results in the per-protocol analysis
eTable 6. FLACC score by group in the per-protocol analysis
eTable 7. Nonparametric RMANOVA results except for children with a total score of 0
eTable 8. FLACC score by group except for children with a total score of 0
eTable 9. Nonparametric RMANOVA results stratified in age
eTable 10. Other outcomes by group
Data sharing statement
References
- 1.Hartling L, Newton AS, Liang Y, et al. Music to reduce pain and distress in the pediatric emergency department: a randomized clinical trial. JAMA Pediatr. 2013;167(9):826-835. doi: 10.1001/jamapediatrics.2013.200 [DOI] [PubMed] [Google Scholar]
- 2.Karakaya A, Gözen D. The effect of distraction on pain level felt by school-age children during venipuncture procedure: randomized controlled trial. Pain Manag Nurs. 2016;17(1):47-53. doi: 10.1016/j.pmn.2015.08.005 [DOI] [PubMed] [Google Scholar]
- 3.von Baeyer CL, Marche TA, Rocha EM, Salmon K. Children’s memory for pain: overview and implications for practice. J Pain. 2004;5(5):241-249. doi: 10.1016/j.jpain.2004.05.001 [DOI] [PubMed] [Google Scholar]
- 4.Buskila D, Neumann L, Zmora E, Feldman M, Bolotin A, Press J. Pain sensitivity in prematurely born adolescents. Arch Pediatr Adolesc Med. 2003;157(11):1079-1082. doi: 10.1001/archpedi.157.11.1079 [DOI] [PubMed] [Google Scholar]
- 5.Fanurik D, Koh JL, Schmitz ML. Distraction techniques combined with EMLA: effects on IV insertion pain and distress in children. Child Health Care. 2000;29(2):87-101. doi: 10.1207/S15326888CHC2902_2 [DOI] [Google Scholar]
- 6.Kamsvåg T, Hedén L, von Essen L, Ljungman G. Ibuprofen in needle procedures in children with cancer: a feasibility and pilot study. Acta Paediatr. 2021;110(2):704-710. doi: 10.1111/apa.15449 [DOI] [PubMed] [Google Scholar]
- 7.Gates M, Hartling L, Shulhan-Kilroy J, et al. Digital technology distraction for acute pain in children: a meta-analysis. Pediatrics. 2020;145(2):e20191139. doi: 10.1542/peds.2019-1139 [DOI] [PubMed] [Google Scholar]
- 8.Moadad N, Kozman K, Shahine R, Ohanian S, Badr LK. Distraction using the BUZZY for children during an IV insertion. J Pediatr Nurs. 2016;31(1):64-72. doi: 10.1016/j.pedn.2015.07.010 [DOI] [PubMed] [Google Scholar]
- 9.Sahiner NC, Bal MD. The effects of three different distraction methods on pain and anxiety in children. J Child Health Care. 2016;20(3):277-285. doi: 10.1177/1367493515587062 [DOI] [PubMed] [Google Scholar]
- 10.Malloy KM, Milling LS. The effectiveness of virtual reality distraction for pain reduction: a systematic review. Clin Psychol Rev. 2010;30(8):1011-1018. doi: 10.1016/j.cpr.2010.07.001 [DOI] [PubMed] [Google Scholar]
- 11.Hoffman HG, Chambers GT, Meyer WJ III, et al. Virtual reality as an adjunctive non-pharmacologic analgesic for acute burn pain during medical procedures. Ann Behav Med. 2011;41(2):183-191. doi: 10.1007/s12160-010-9248-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sander Wint S, Eshelman D, Steele J, Guzzetta CE. Effects of distraction using virtual reality glasses during lumbar punctures in adolescents with cancer. Oncol Nurs Forum. 2002;29(1):E8-E15. doi: 10.1188/02.ONF.E8-E15 [DOI] [PubMed] [Google Scholar]
- 13.Chen YJ, Cheng SF, Lee PC, Lai CH, Hou IC, Chen CW. Distraction using virtual reality for children during intravenous injections in an emergency department: a randomised trial. J Clin Nurs. 2020;29(3-4):503-510. doi: 10.1111/jocn.15088 [DOI] [PubMed] [Google Scholar]
- 14.Khadra C, Ballard A, Déry J, et al. Projector-based virtual reality dome environment for procedural pain and anxiety in young children with burn injuries: a pilot study. J Pain Res. 2018;11:343-353. doi: 10.2147/JPR.S151084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee HN, Bae W, Park JW, et al. Virtual reality environment using a dome screen for procedural pain in young children during intravenous placement: a pilot randomized controlled trial. PLoS One. 2021;16(8):e0256489. doi: 10.1371/journal.pone.0256489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Merkel S, Voepel-Lewis T, Malviya S. Pain assessment in infants and young children: the FLACC scale. Am J Nurs. 2002;102(10):55-58. doi: 10.1097/00000446-200210000-00024 [DOI] [PubMed] [Google Scholar]
- 17.Crellin DJ, Harrison D, Santamaria N, Babl FE. Systematic review of the Face, Legs, Activity, Cry and Consolability scale for assessing pain in infants and children: is it reliable, valid, and feasible for use? Pain. 2015;156(11):2132-2151. doi: 10.1097/j.pain.0000000000000305 [DOI] [PubMed] [Google Scholar]
- 18.Riley JL III, Robinson ME, Wise EA, Myers CD, Fillingim RB. Sex differences in the perception of noxious experimental stimuli: a meta-analysis. Pain. 1998;74(2-3):181-187. doi: 10.1016/S0304-3959(97)00199-1 [DOI] [PubMed] [Google Scholar]
- 19.Greenspan JD, Craft RM, LeResche L, et al. ; Consensus Working Group of the Sex, Gender, and Pain SIG of the IASP . Studying sex and gender differences in pain and analgesia: a consensus report. Pain. 2007;132(suppl 1):S26-S45. doi: 10.1016/j.pain.2007.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tüfekci FG, Celebioğlu A, Küçükoğlu S. Turkish children loved distraction: using kaleidoscope to reduce perceived pain during venipuncture. J Clin Nurs. 2009;18(15):2180-2186. doi: 10.1111/j.1365-2702.2008.02775.x [DOI] [PubMed] [Google Scholar]
- 21.Maani CV, Hoffman HG, Morrow M, et al. Virtual reality pain control during burn wound debridement of combat-related burn injuries using robot-like arm mounted VR goggles. J Trauma. 2011;71(1)(suppl):S125-S130. doi: 10.1097/TA.0b013e31822192e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Byczkowski TL, Fitzgerald M, Kennebeck S, et al. A comprehensive view of parental satisfaction with pediatric emergency department visits. Ann Emerg Med. 2013;62(4):340-350. doi: 10.1016/j.annemergmed.2013.04.025 [DOI] [PubMed] [Google Scholar]
- 23.Locke R, Stefano M, Koster A, Taylor B, Greenspan J. Optimizing patient/caregiver satisfaction through quality of communication in the pediatric emergency department. Pediatr Emerg Care. 2011;27(11):1016-1021. doi: 10.1097/PEC.0b013e318235be06 [DOI] [PubMed] [Google Scholar]
- 24.Hoffman HG. Interacting with virtual objects via embodied avatar hands reduces pain intensity and diverts attention. Sci Rep. 2021;11(1):10672. doi: 10.1038/s41598-021-89526-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eFigure 1. Virtual reality equipment consisted of a domed ceiling screen and two projectors
eFigure 2. Screenshot of the virtual reality animation
eTable 1. FLACC score by group
eTable 2. Nonparametric RMANOVA results
eTable 3. Nonparametric RMANOVA results stratified in sex
eTable 4. FLACC score by group stratified in sex
eTable 5. Nonparametric RMANOVA results in the per-protocol analysis
eTable 6. FLACC score by group in the per-protocol analysis
eTable 7. Nonparametric RMANOVA results except for children with a total score of 0
eTable 8. FLACC score by group except for children with a total score of 0
eTable 9. Nonparametric RMANOVA results stratified in age
eTable 10. Other outcomes by group
Data sharing statement