Key Points
Question
Does vitamin C supplementation for pregnant smokers increase offspring airway function at age 5 years and decrease wheeze?
Findings
In this follow-up study of a double-blind randomized clinical trial with 213 offspring of 251 pregnant smokers treated with vitamin C (500 mg/d) or placebo, forced expiratory flows measured at age 5 years indicated an improvement in offspring airway function with supplemental vitamin C vs placebo. Offspring of pregnant smokers allocated to vitamin C also had significantly decreased wheeze.
Meaning
These findings suggest that vitamin C supplementation for pregnant smokers may decrease the effects of smoking in pregnancy on childhood airway function and respiratory health.
This randomized clinical trial follows up an earlier study to assess the association of vitamin C supplementation (500 mg/d) vs placebo for pregnant smokers on offspring airway function at age 5 years and occurrence of wheeze.
Abstract
Importance
Vitamin C supplementation (500 mg/d) for pregnant smokers has been reported to increase offspring airway function as measured by forced expiratory flow (FEF) through age 12 months; however, its effects on airway function at age 5 years remain to be assessed.
Objective
To assess whether vitamin C supplementation in pregnant smokers is associated with increased and/or improved airway function in their offspring at age 5 years and whether vitamin C decreases the occurrence of wheeze.
Design, Setting, and Participants
This study followed up the Vitamin C to Decrease the Effects of Smoking in Pregnancy on Infant Lung Function (VCSIP) double-blind, placebo-controlled randomized clinical trial conducted at 3 centers in the US (in Oregon, Washington, and Indiana) between 2012 and 2016. Investigators and participants remain unaware of the treatment assignments. Forced expiratory flow measurements at age 5 years were completed from 2018 to 2021.
Interventions
Pregnant smokers were randomized to vitamin C (500 mg/d) or placebo treatment.
Main Outcomes and Measures
The primary outcome was the prespecified measurement of FEF between 25% and 75% expired volume (FEF25-75) by spirometry at age 5 years. Secondary outcomes included FEF measurements at 50% and 75% of expiration (FEF50 and FEF75), forced expiratory volume in 1 second (FEV1), and occurrence of wheeze.
Results
Of the 251 pregnant smokers included in this study, 125 (49.8%) were randomized to vitamin C and 126 (50.2%) were randomized to placebo. Of 213 children from the VCSIP trial who were reconsented into this follow-up study, 192 (90.1%) had successful FEF measurements at age 5 years; 212 (99.5%) were included in the analysis of wheeze. Analysis of covariance demonstrated that offspring of pregnant smokers allocated to vitamin C compared with placebo had 17.2% significantly higher mean (SE) measurements of FEF25-75 at age 5 years (1.45 [0.04] vs 1.24 [0.04] L/s; adjusted mean difference, 0.21 [95% CI, 0.13-0.30]; P < .001). Mean (SE) measurements were also significantly increased by 14.1% for FEF50 (1.59 [0.04] vs 1.39 [0.04] L/s; adjusted mean difference, 0.20 [95% CI, 0.11-0.30]; P < .001), 25.9% for FEF75 (0.79 [0.02] vs 0.63 [0.02] L/s; 0.16 [95% CI, 0.11-0.22]; P < .001), and 4.4% for FEV1 (1.13 [0.02] vs 1.09 [0.02] L; 0.05 [95% CI, 0.01-0.09]; P = .02). In addition, offspring of pregnant smokers randomized to vitamin C had significantly decreased wheeze (28.3% vs 47.2%; estimated odds ratio, 0.41 [95% CI, 0.23-0.74]; P = .003).
Conclusions and Relevance
In this follow-up study of offspring of pregnant smokers randomized to vitamin C vs placebo, vitamin C supplementation during pregnancy resulted in significantly increased airway function of offspring at age 5 years and significantly decreased the occurrence of wheeze. These findings suggest that vitamin C supplementation for pregnant smokers may decrease the effects of smoking in pregnancy on childhood airway function and respiratory health.
Trial Registration
ClinicalTrials.gov Identifier: NCT03203603
Introduction
In utero smoke exposure from maternal smoking during pregnancy is a well-established risk factor for impaired fetal lung development, decreased airway function, and increased risk for wheeze and asthma in offspring,1,2 with health care costs of more than $1 billion annually in the US.3 Despite antismoking efforts, more than 10.0% of US individuals continue to smoke during pregnancy,4 resulting in more than 400 000 infants exposed per year. In utero smoke exposure remains an important determinant of lifelong reduced airway function and greater respiratory morbidity.5,6,7 Longitudinal studies demonstrate that the trajectories for airway function are established early in life,8 and children in the lower percentiles tend to have persistently lower airway function into adulthood.9,10 Importantly, individuals with decreased airway growth relative to lung parenchyma growth (dysanaptic growth)11,12 early in life have increased respiratory morbidity and may be at increased risk for chronic obstructive pulmonary disease (COPD) in adulthood as airway function declines with age.8,10,13
In 2 controlled randomized clinical trials (RCTs),14,15,16 we demonstrated that vitamin C supplementation (500 mg/d) for women unable to quit smoking during pregnancy significantly improved their offspring’s lung and/or airway function; however, some of the results were mixed. These RCTs were based on preclinical evidence in primates showing that the major effects of in utero smoke are caused by nicotine crossing the placenta to interact with nicotinic receptors in the developing lung airways. This interaction leads to altered patterns of lung growth and development, including the presence of dysanapsis confirmed by stereology, and hence altered airway function at birth.17 These earlier studies demonstrated that supplemental vitamin C blocked the effects of in utero nicotine exposure on newborn monkey lung function.18 In our first clinical study,16 offspring born to pregnant smokers randomized to vitamin C had significant improvements in newborn pulmonary function test (PFT) results and a 47.5% decrease in the occurrence of wheeze through age 12 months. However, there was no difference in PFT results at age 12 months. Our second RCT 14,15 showed that compared with placebo, vitamin C supplementation for pregnant smokers resulted in their offspring having increased forced expiratory flow at 75% of expiration (FEF75). Their offspring also had significantly increased FEF between 25% and 75% expired volume (FEF25-75) and FEF at 50% of expiration (FEF50) at age 3 months. At age 12 months, all 3 FEF measurements significantly increased; however, there was no significant difference in the occurrence of wheeze.14,15
Although previous cohort studies8,10 have provided insight into trajectories of airway function, no studies to date have demonstrated whether airway function can be improved very early in life and then maintained through preschool age. To begin to address this issue, we continued to follow up the children in our Vitamin C to Decrease the Effects of Smoking in Pregnancy on Infant Lung Function (VCSIP) trial cohort. The primary objective of this follow-up study was to compare FEF25-75 measurements at age 5 years for the offspring of pregnant smokers randomized to supplemental vitamin C (500 mg/d) vs placebo treatment. We hypothesized that vitamin C supplementation for pregnant smokers would continue to increase their offspring’s FEF25-75 measurements compared with placebo. We also hypothesized that vitamin C administration would decrease the occurrence of wheeze, a secondary outcome of the study.
Methods
Participants
For this double-blind, placebo-controlled RCT, we recruited pregnant individuals from 3 sites: Oregon Health & Science University (OHSU) in Portland, PeaceHealth Southwest Washington Medical Center in Vancouver, and Indiana University in Indianapolis. The data coordinating center was based at OHSU. Eligible participants were individuals aged 15 years or older who self-reported as female and had a singleton gestation between 13 and 22 weeks confirmed by ultrasonography and were current cigarette smokers (≥1 cigarette in the last week), English speaking, and receiving prenatal care at surrounding clinics to the recruitment sites.
Each site’s institutional review board approved the protocols for the VCSIP trial and this follow-up study (Supplement 1 and Supplement 2, respectively). All individuals provided written informed consent for participation in the RCT as well as the follow-up of their child. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Trial Design and Study Protocol
Details of the trial design and study protocol through age 12 months were published previously.14,15 VCSIP was a double-blind RCT of vitamin C (500 mg/d) vs placebo treatment for pregnant smokers. After participants successfully completed a medication adherence period, they were randomized to vitamin C (500 mg/d) or a similar placebo, stratified by gestational age (GA; ≤18 vs >18 weeks) and study site. Research staff met with participants at each prenatal visit to collect interval smoking histories, assess medication adherence, provide brief smoking cessation counseling, and collect study biomarkers. Participants were randomized to the intervention between December 2012 and June 2015. Prenatal interval visits, labor and delivery room data, and postnatal data collected through age 12 months were published previously.14,15 The National Heart, Lung, and Blood Institute (NHLBI) funded the original research grant that lasted 5 years, with outcomes in the offspring collected through age 12 months.14,15 The NHLBI grant was renewed to enable continued follow-up of the children to assess the potential persistence of the beneficial effects of in utero vitamin C administered to pregnant smokers on their offspring’s airway function and occurrence of wheeze.
All offspring of randomized VCSIP participants were eligible for reconsent into this follow-up study. Follow-up of the last child to their sixth birthday was completed in December 2021. The investigators and participants remain unaware of the treatment group allocation. The data coordinating center at OHSU continues to maintain the blinding of the study.
Measurements and Outcomes
Spirometry at Age 5 Years
The primary outcome for this VCSIP follow-up study was an extension of the original RCT—that is, FEF25-75 measurements at age 5 years (defined as occurring between the child’s fifth and sixth birthday) in children born to pregnant smokers randomized to vitamin C vs placebo treatment. Prespecified secondary spirometry outcomes were FEF50, FEF75, and forced expiratory volume in 1 second (FEV1) measurements. Experienced respiratory technicians used a model 6800 spirometer (Vitalograph) to perform spirometry measurements at each site. We adhered to American Thoracic Society (ATS)/European Respiratory Society (ERS) acceptance guidelines,19 including a test free from artifacts, a preferred minimum expiratory time of 1 second, and 2 or more maneuvers with forced vital capacity (FVC) and FEV1 within 90.0%. All bronchodilator inhalers, syrups, or pills were held for more than 24 hours before testing. All results were overread by a reviewer unaware of treatment assignment.
Respiratory Questionnaires and Occurrence of Wheeze
An additional secondary outcome (primary clinical outcome) of this follow-up trial was the prespecified occurrence of wheeze between the child’s fourth and sixth birthday. A standardized respiratory questionnaire (RQ)20 was administered quarterly to the child’s parent or primary caretaker. Wheeze was defined as a positive response to any of the following: parental report of wheeze, health care professional diagnosis of wheeze or asthma, or any bronchodilator or steroid use (eTable 1 in Supplement 3). Only children with 1 or more RQs completed after their fourth birthday were included in the analysis.
Statistical Analysis
Statistical analyses evaluated the effects of vitamin C supplementation for pregnant smokers on their offspring’s airway function test results at age 5 years and on the occurrence of wheeze (Supplement 2). FEF25-75 measured at age 5 years was the primary outcome. Analysis was based on intention to treat.
A general linear model analysis of covariance was used to compare FEF25-75 measurements at the 5-year-old time point between treatment groups.21 The model was fit with 6 experimental factors plus 2-way interactions. These design factors included treatment arm, clinical site, and GA at randomization (≤18 vs >18 weeks), and the covariates of infant sex, maternal race and ethnicity, and child height at testing were included in the final model regardless of statistical significance. A stepwise approach was used for selecting significant 2-way interactions.
With regard to race and ethnicity, previous data have shown that airway function in childhood differs by these constructs. In addition, separate normative standards are published by race. Thus, this difference may be associated with predictors of race and ethnicity (eg, exposure to air pollution) or other social determinants of health (eg, health care). In this study, participants self-reported maternal race (American Indian or Alaska Native, Asian, Black or African American, Native Hawaiian or other Pacific Islander, White, or other) and ethnicity (Hispanic or non-Hispanic origin).
Using similar statistical modeling as for the primary outcome of FEF25-75 measurements, we assessed differences between the 2 allocation groups for the additional airway function outcomes of FEF50, FEF75, FEV1, FVC, and FEV1/FVC at the 5-year spirometry test.
Multiple logistic regression was used to jointly examine the effects of vitamin C supplementation for pregnant smokers, clinical site, and GA at randomization (≤18 vs >18 weeks); the covariates of child sex and maternal race and ethnicity; and significant 2-way interactions of all variables on the occurrence of wheeze.22
Other adjusted odd ratios (ORs) and corresponding 95% CIs and P values were obtained according to reference groups for each variable. Statistical analyses were performed with SAS, version 9.4 (SAS Institute Inc).
Results
Characteristics of the Trial Participants at Follow-Up
In this follow-up study of the VCSIP trial, we reconsented 213 children of 251 pregnant smokers at a mean (SD) age of 3.1 (0.8) and 3.2 (0.8) years in the vitamin C and placebo treatment groups, respectively. All 213 children were included in this analysis; selected baseline and follow-up characteristics of the pregnant smokers and their offspring were comparable between the 2 allocated groups (Table 1 and eTable 2 in Supplement 3). There were no differences in birth or postnatal characteristics between the vitamin C vs placebo groups followed up through age 5 years, including median (IQR) maternal urine cotinine levels during pregnancy (5201 [1885.4-7655.0] vs 5586 [1966.8-8528.0] ng/mL) and maternal self-reported number of cigarettes smoked daily 1 week before randomization (7.5 [4-10] vs 8 [4-10]) and at offspring age 12 months (10 [6-10] vs 10 [6-13]). As reported previously, mean (SD) fasting plasma ascorbic acid levels after randomization at mid- and late gestation were higher in pregnant smokers randomized to vitamin C (48.8 [19.7] vs 47.5 [19.9] μmol/L), as evidence of adherence to the intervention. There was no difference between allocated groups in other variables important in childhood respiratory health (Table 1).
Table 1. Maternal, Birth, and Postnatal Characteristics of Participantsa.
| Characteristic | Value | P value | |
|---|---|---|---|
| Vitamin C group (n = 106) | Placebo group (n = 107) | ||
| Baseline maternal | |||
| Age at enrollment, mean (SD), y | 26.5 (5.3) | 26.7 (5.9) | NA |
| History of asthma, No. (%) | 35 (33.0) | 33 (30.8) | NA |
| <High school education, No. (%) | 17 (16.0) | 26 (24.3) | NA |
| Public health insurance, No. (%) | 94 (88.7) | 90 (84.1) | NA |
| Gestational age at randomization, mean (SD), wk | 18.4 (3.0) | 18.1 (2.8) | NA |
| No. of cigarettes/d in the week before randomization, median (IQR) | 7.5 (4-10) | 8 (4-10) | NA |
| Urine cotinine at randomization, median (IQR), ng/mL | 5201 (1885.4-7655.0) | 5586 (1966.8-8528.0) | NA |
| Plasma ascorbic acid at randomization, mean (SD), μmol/L | 48.8 (19.7) | 47.5 (19.9) | NA |
| Birth and postnatal | |||
| Birth weight, mean (SD), g | 3137.2 (519.0) | 3069.1 (555.9) | .36 |
| Gestational age, mean (SD), wk | 38.7 (1.9) | 38.6 (1.7) | .59 |
| Female sex of child, No. (%) | 54 (50.9) | 53 (49.5) | .78 |
| Race and ethnicity of child (based on mother), No. (%) | |||
| Black, Hispanic or non-Hispanic | 16 (15.1) | 12 (11.2) | .45 |
| White, Hispanic | 1 (0.9) | 4 (3.7) | |
| White, non-Hispanic | 82 (77.4) | 82 (76.6) | |
| Otherb | 7 (6.6) | 9 (8.4) | |
| No. of cigarettes/d, median (IQR)c | |||
| On 3-mo RQ | 8 (5-10) | 10 (5-10) | .46 |
| On 12-mo RQ | 10 (6-10) | 10 (6-13) | .26 |
| Between child’s fourth and sixth birthdayc,d | 7.5 (3.5-10.9) | 8.4 (2.0-12.1) | .87 |
| Caregiver reported 0 cigarettes smoked between child’s fourth and sixth birthday, No. (%)c,e | 16 (15.1) | 16 (15.1) | >.99 |
| Child attended daycare (or preschool) between fourth and sixth birthday, No. (%)f | 31 (29.8) | 27 (25.7) | .51 |
| Pets in home between child’s fourth and sixth birthday, No. (%)f | 78 (75.0) | 87 (82.9) | .16 |
| Diagnosis between child’s fourth and sixth birthday, No. (%)f | |||
| Eczema | 24 (23.1) | 33 (31.4) | .18 |
| Food allergy | 18 (17.3) | 12 (11.4) | .23 |
Abbreviations: NA, not applicable; RQ, respiratory questionnaire; VCSIP, Vitamin C to Decrease the Effects of Smoking in Pregnancy on Infant Lung Function.
Children reconsented into the VCSIP follow-up. Analyses of the number of cigarettes per day included children who had at least 1 RQ completed after their fourth birthday; the median number of RQs completed was 8 (range, 1-8) in each group.
Includes American Indian or Alaska Native, Asian, Native Hawaiian or other Pacific Islander, or other race or ethnicity.
Number of cigarettes per day reported by mother or primary caretaker on quarterly RQs.
Based on 104 vs 106 children in the vitamin C vs placebo groups.
Based on 106 vs 106 children in the vitamin C vs placebo groups.
Based on 104 vs 105 children in the vitamin C vs placebo groups.
Spirometry at Age 5 Years
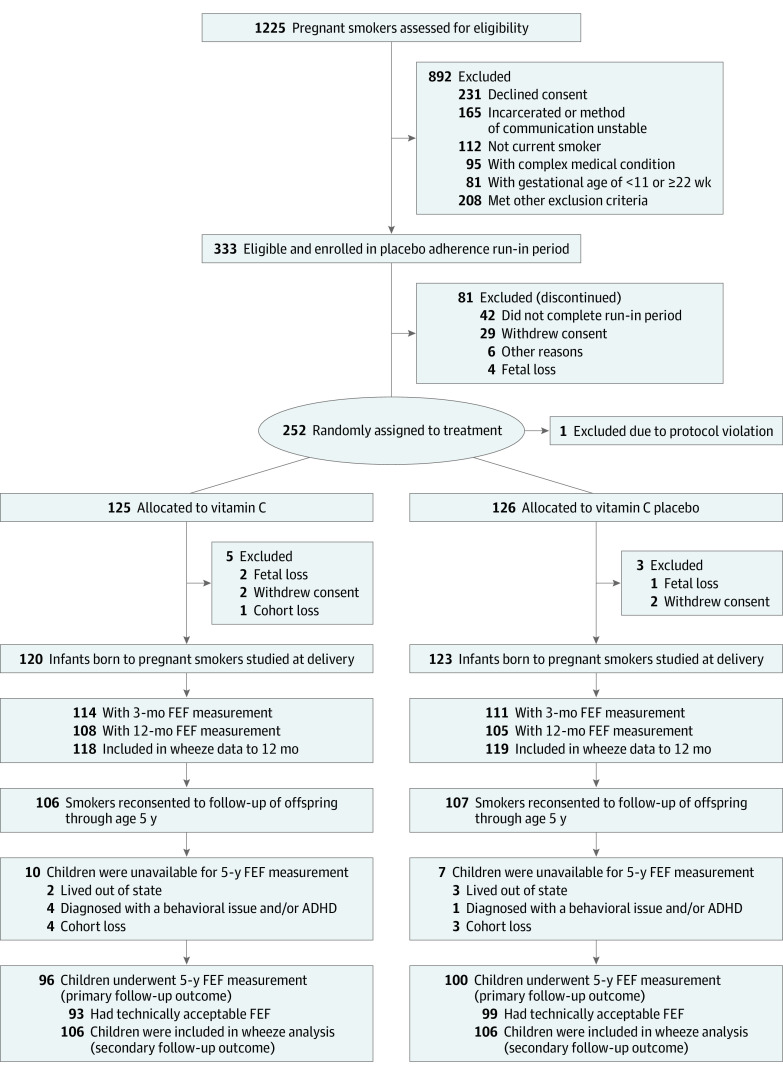
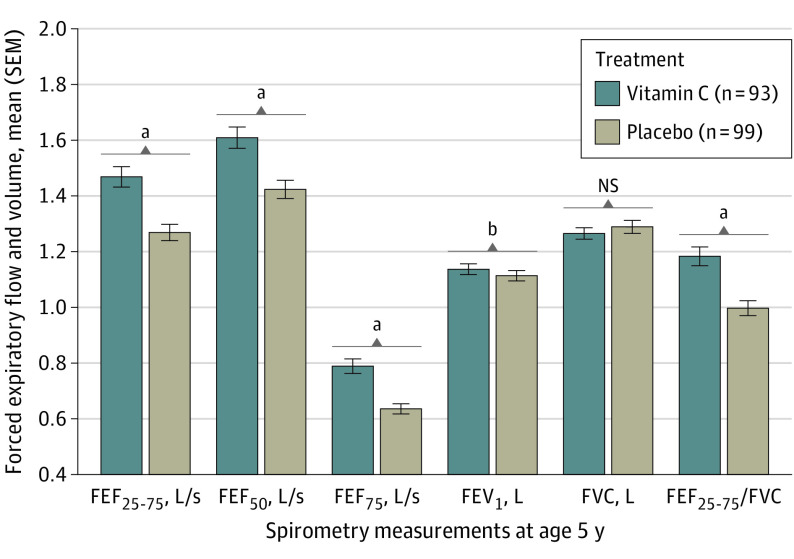
Of the 213 children reconsented into this follow-up study, 196 (92.0%) were available for spirometry measurements at age 5 years (Figure 1); of these, results were technically acceptable for 192 (98.0%) offspring (93 and 99 in the vitamin C and placebo groups, respectively). Height at the time of spirometry testing was comparable between treatment groups (Table 2). Of the 213 children who were reconsented, 192 (90.1%) had successful FEF measurements at age 5 years. Children born to pregnant smokers randomized to vitamin C vs placebo had significantly increased FEF25-75 measurements, the primary spirometric outcome for the study. Mean (SE) measurements of FEF25-75 were 17.2% greater in the vitamin C group (1.45 [0.04] vs 1.24 [0.04] L/s; adjusted mean difference, 0.21 [95% CI, 0.13-0.30]; P < .001). Mean (SE) measurements were also significantly increased by 14.1% for FEF50 (1.59 [0.04] vs 1.39 [0.04] L/s; adjusted mean difference, 0.20 [95% CI, 0.11-0.30]; P < .001), 25.9% for FEF75 (0.79 [0.02] vs 0.63 [0.02] L/s; 0.16 [95% CI, 0.11-0.22]; P < .001), and 4.4% for FEV1 (1.13 [0.02] vs 1.09 [0.02] L; 0.05 [95% CI, 0.01-0.09]; P = .02) (Figure 2 and Table 2). FVC did not differ between treatment groups (0.2% change; mean [SE], 1.26 [0.02] vs 1.25 [0.02]; adjusted mean difference, 0.002 [95% CI, −0.05 to 0.05]; P = .92). The FEF25-75/FVC ratio, an index of dysanaptic growth between the airway and lung parenchyma, was greater for the vitamin C group compared with placebo (18.4% change; mean [SE], 1.19 [0.03] vs 1.00 [0.03]; adjusted mean difference, 0.18 [95% CI, 0.10-0.26]; P < .001 (Figure 2 and Table 2). Forced expiratory flow measurements at age 5 years were completed from 2018 to 2021.
Figure 1. Study Flow Diagram.
Enrollment, randomization, and follow-up of pregnant smokers randomized to supplemental vitamin C (500 mg/d) or placebo and their offspring at age 5 years. ADHD indicates attention-deficit/hyperactivity disorder; FEF, forced expiratory flow.
Table 2. Airway Function Test Results Obtained by Spirometry at Age 5 Years in Children Born to Pregnant Smokers Randomized to Vitamin C Supplementation vs Placeboa.
| Measurement | Mean (SE) | Vitamin C − Placebo | Adjusted P valueb | ||
|---|---|---|---|---|---|
| Vitamin C group (n = 93) | Placebo group (n = 99) | Adjusted mean difference (95% CI)b | % Change | ||
| FEF25-75, L/s | 1.45 (0.04) | 1.24 (0.04) | 0.21 (0.13 to 0.30) | 17.2 | <.001 |
| FEF50, L/s | 1.59 (0.04) | 1.39 (0.04) | 0.20 (0.11 to 0.30) | 14.1 | <.001 |
| FEF75, L/s | 0.79 (0.02) | 0.63 (0.02) | 0.16 (0.11 to 0.22) | 25.9 | <.001 |
| FEV1, L | 1.13 (0.02) | 1.09 (0.02) | 0.05 (0.01 to 0.09) | 4.4 | .02 |
| FVC, L | 1.26 (0.02) | 1.254 (0.02) | 0.002 (−0.05 to 0.05) | 0.2 | .92 |
| FEF25-75/FVC | 1.19 (0.03) | 1.001 (0.03) | 0.18 (0.10 to 0.26) | 18.4 | <.001 |
Abbreviations: FEF, forced expiratory flow (subscript values 25-75, 50, and 75 indicate between 25% and 75%, at 50%, and at 75% of the expired volume, respectively); FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity.
Children in the 2 treatment groups did not differ in terms of age or height (both groups were 112 cm) at 5-year spirometry measurement.
P values and mean differences (95% CIs) were adjusted for design factors of site, gestational age at randomization, and covariates of height, race and ethnicity, and sex as well as significant 2-way interactions.
Figure 2. Airway Function Test Results Obtained by Spirometry Measurements at Age 5 Years in Children Born to Pregnant Smokers Randomized to Vitamin C Supplementation (500 mg/d) vs Placebo.
Spirometry measurements are shown for offspring of 93 and 99 pregnant smokers in the vitamin C and placebo treatment groups, respectively. P values were adjusted for trial stratification variables of study site and gestational age at randomization, sex, race and ethnicity, and height at testing. All forced expiratory flow (FEF) and forced expiratory volume in 1 second (FEV1) measurements increased significantly in offspring of vitamin C–treated pregnant smokers. Of note, the ratio of FEF between 25% and 75% expired volume to forced vital capacity (FEF25-75/FVC), an indicator of increased airway growth relative to lung volume (dysanapsis), was also significantly increased in the offspring of vitamin C–treated smokers. FEF50 indicates FEF at 50% of expiration; FEF75, FEF at 75% of expiration; and NS, not significant.
aP < .01.
bP < .05.
Clinical Respiratory Outcome (Wheeze)
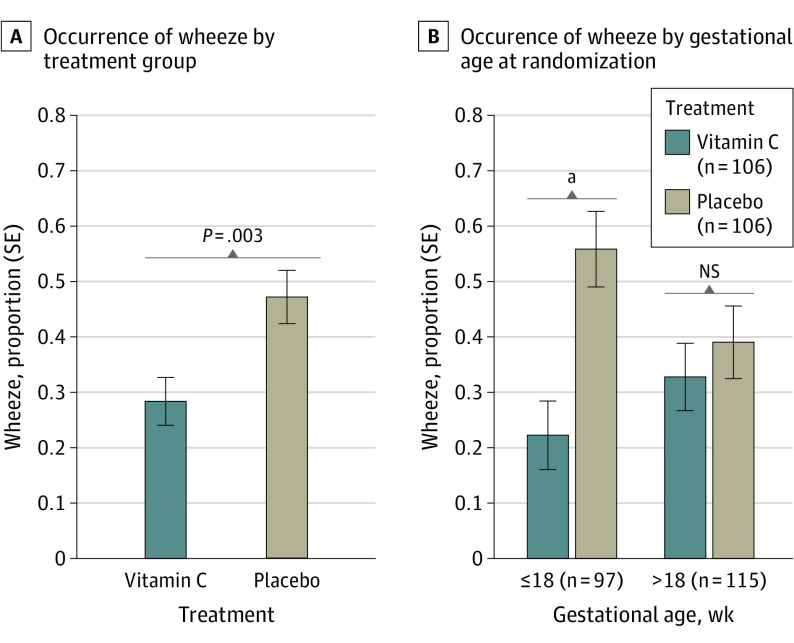
Follow-up data were available for clinical outcomes assessed with the RQ for 212 of 213 (99.5%) eligible offspring, including 106 children each in the vitamin C and placebo groups, respectively. The median number of completed RQs was 8 (range, 1-8) in both treatment groups. Children born to vitamin C–treated pregnant smokers had a significantly lower occurrence of wheeze between their fourth and sixth birthday (28.3% vs 47.2%; OR, 0.41 [95% CI, 0.23-0.74]; P = .003). The interaction for treatment and GA at randomization was significant (P = .04), with children whose mothers were randomized to and began the intervention at 18 weeks GA or earlier demonstrating the largest benefit of vitamin C treatment (OR, 0.22 [95% CI, 0.09-0.54]; P = .001]) (Figure 3).
Figure 3. Any Occurrence of Wheeze Between the Fourth and Sixth Birthday in Offspring of Pregnant Smokers Randomized to Vitamin C Supplementation (500 mg/d) vs Placebo During Pregnancy, Overall, and by Gestational Age at Randomization.
A, Multiple logistic regression was used to compare the occurrence of wheeze between offspring in the vitamin C and placebo treatment groups, adjusting for trial design factors of study site and gestational age at randomization and covariates of race and ethnicity and sex, and significant 2-way interactions of all of these variables. The analysis included 212 children (106 each in the vitamin C and placebo treatment groups). Children born to vitamin C–treated pregnant smokers had a significant decrease in current wheeze (28.3% vs 47.2%; estimated odds ratio, 0.41 [95% CI, 0.23-0.74]; P = .003). B, There was a significant interaction between treatment group and gestational age at randomization (P = .04), with children born to pregnant smokers randomized at 18 weeks of gestational age or earlier demonstrating the largest benefit from vitamin C treatment (odds ratio, 0.22 [95% CI, 0.09-0.54]). NS indicates not significant.
aP = .001.
Discussion
In this double-blind, placebo-controlled RCT with longitudinal follow-up, supplemental vitamin C administered to individuals during pregnancy contributed to significantly better airway function (FEF25-75) and lower occurrence of wheeze in their offspring. Moreover, initiation of the vitamin C intervention before 18 weeks of gestation may have had a greater effect on the occurrence of wheeze compared with treatment initiated after 18 weeks of gestation. Airway function at age 5 years suggests that (1) an in utero intervention targeted for a specific toxin can provide lasting improvements in offspring respiratory health at least until preschool age and (2) the timing of the intervention during fetal lung development may be important.
The effect of vitamin C vs placebo supplementation for pregnant smokers before 23 weeks of gestation consistently resulted in significantly better airway function, as seen by the higher FEF measurements observed for all flow parameters evaluated (Table 2). The height, sex, and race and ethnicity of the children did not differ between treatment groups and were adjusted for, as is standard for lung function measurements; these covariates do not account for observed spirometric differences between the 2 groups. These findings are consistent with and extend our early findings from this cohort during the first year of life.14,15 We a priori selected FEF25-75 as the primary spirometric outcome at age 5 years, based on our previous studies demonstrating that FEF25-75 was more sensitive than timed expiratory volumes (eg, FEV0.5 and FEV1) in young children.23 In children with asthma, FEF25-75 was previously reported to be better than FEV1 in assessing treatment effects.24 We demonstrated that FEF25-75 in the offspring of vitamin C–supplemented pregnant smokers was improved by 17.2% over the placebo group (mean [SE] 1.45 [0.04] vs 1.24 [0.04] L/s; adjusted mean difference, 0.21 [95% CI, 0.13-0.30]) and, importantly, was associated with a significant 40.0% decrease (P = .003) in the occurrence of wheeze.
Both groups had a high occurrence of wheeze (28.3% vs 47.2% in the vitamin C vs placebo group; 37.7% overall), which highlights the high respiratory morbidity associated with in utero smoke exposure. For reference, in a nonselected prospective population-based birth cohort, 22.0% of 5-year-old children were reported to have current wheeze, and maternal smoking during pregnancy significantly increased the risk of wheeze (relative risk, 2.15 [95% CI, 1.26-3.66]).25 Burke et al7 demonstrated previously that children born to individuals who smoked prenatally had an increased OR of 1.52 (95% CI, 1.23-1.87) for the occurrence of wheeze at age 5 years. In our study, prenatal nicotine exposure (as measured by maternal urine cotinine levels and self-reported number of cigarettes per day) and postnatal nicotine exposure (as obtained by maternal self-reported cigarettes per day) did not differ between treatment groups (Table 1), although we do not have measures of current maternal urine cotinine levels. Our results highlight the important clinical significance of this in utero intervention on long-term respiratory morbidity. These findings are also consistent with our previous reports of lower wheeze during infancy in vitamin C treatment groups compared with placebo for maternal cigarette smoking during pregnancy.15,16 This study demonstrated a significant interaction between treatment group (vitamin C vs placebo) and the GA at which the intervention was initiated (≤18 vs >18 weeks). This finding suggests that the timing of the intervention during fetal lung development may be important; however, this result should be interpreted with caution and verified in continued follow-up and in future studies, given the potential for confounding as suggested by differences in response between the early and late randomized placebo groups. In addition, there was no significant interaction of spirometric outcomes and GA at randomization.
FEF improvement with vitamin C vs placebo occurred in the absence of a significant difference in FVC (Table 2). This observation suggests that the improvement was primarily related to increased airway size rather than differences in lung volume. Vitamin C treatment significantly improved FEV1 measurements at age 5 years, which is consistent with the significant improvement in FEV0.5 we observed in this cohort during infancy.14,15 The relative ineffectiveness of FEV1 to detect lung disease in young children is most evident in those with cystic fibrosis and asthma, who may have FEV1 values well within normal limits even though high-resolution computerized tomography (HRCT) imaging demonstrates significant lung disease.26 The 4.0% increase in FEV1 in our vitamin C–treated group is modest but may underestimate the differences in airway size between our vitamin C and placebo groups.
The trajectory of airway function from early in life and continuing through adulthood may be an important risk factor for the development of COPD in adulthood. In a longitudinal study of adults, half of the COPD cases resulted from attaining a lower maximal value of airway function in early adulthood, rather than from an accelerated decline of airway function during adulthood.27 In addition, HRCT imaging in adults indicated that a smaller airway size relative to the size of the lung volume was associated with greater risk for the subsequent development of COPD.12 In our cohort of young children, we found a higher ratio of FEF25-75/FVC in the vitamin C vs placebo treatment group (Figure 2 and Table 2). This physiologic finding also suggests that vitamin C treatment minimized the adverse effects of in utero smoking on airway development relative to the development of lung volume. We previously reported that nicotine seems to impair the normal differential growth patterns between the airways and lung parenchyma (dysanapsis),28 which begins in utero, and vitamin C supplementation during pregnancy may minimize this altered airway development.
Strengths and Limitations
A major strength of our study is the continued blinded follow-up of our targeted in utero intervention through age 5 years. Our assessment of FEF measurements is also the gold standard for assessing airway function. In addition to the a priori selected primary outcome of FEF25-75, all FEFs assessed from the flow volume curve by spirometry were significantly improved with the vitamin C intervention. Furthermore, increased FEF measurements in the vitamin C–treated group were consistent across all 3 sites (data not shown), supporting the generalizability of our findings.14,15 Additionally, major strengths of our study include the excellent follow-up of our cohort, the high completion rate of attempted spirometry at age 5 years (98%) following ATS/ERS criteria, and the use of 2 different respiratory outcomes, both of which demonstrated improvement with supplemental vitamin C compared with placebo.
As in any longitudinal follow-up, cohort loss can potentially bias outcomes. However, there were no differences in the demographics of the children and their mothers and in early airway function measured at age 3 and age 12 months but not studied at age 5 years (eTable 2 in Supplement 3). We conclude that there was not a differential loss of children in the cohort based on any known factor relevant to outcome. In addition, wheeze is a complex clinical respiratory outcome. Although we used a standardized RQ, this outcome was assessed using responses from a parent or the child’s primary caretaker. In addition, nonatopic and atopic mechanisms can contribute to airway obstruction and wheeze,23,29 and vitamin C supplementation may not modify both mechanisms. We did not have an objective measure of atopy for the majority of our participants.
Conclusions
In this double-blind, placebo-controlled RCT, vitamin C supplementation during pregnancy of mothers who continue to smoke significantly improved airway function and respiratory outcomes of wheeze in their offspring at age 5 years. Of the phases of airway function growth into adulthood, the childhood growth phase is the least well investigated yet is potentially the most important in defining whether early life factors determine airway function trajectories, maximal airway function, and subsequent risk for COPD. Future follow-up studies of our cohort using spirometry as well as HRCT imaging may more directly demonstrate the effect of vitamin C treatment on airway size relative to lung volume. Further follow-up of this cohort is required to determine whether the improved respiratory outcomes in the offspring of pregnant smokers randomized to vitamin C vs placebo persist as well as to increase understanding of the mechanisms for the improvement.
Trial Protocol for VCSIP Study
Trial Protocol and Statistical Analysis Plan for VCSIP Follow-up
eTable 1. Wheeze Outcome Variables
eReferences
eTable 2. Characteristics of Children With Completed 12-Month and 5-Year Pulmonary and/or Airway Function Test Results
Data Sharing Statement
References
- 1.Hayatbakhsh MR, Sadasivam S, Mamun AA, Najman JM, Williams GM, O’Callaghan MJ. Maternal smoking during and after pregnancy and lung function in early adulthood: a prospective study. Thorax. 2009;64(9):810-814. doi: 10.1136/thx.2009.116301 [DOI] [PubMed] [Google Scholar]
- 2.Stocks J, Hislop A, Sonnappa S. Early lung development: lifelong effect on respiratory health and disease. Lancet Respir Med. 2013;1(9):728-742. doi: 10.1016/S2213-2600(13)70118-8 [DOI] [PubMed] [Google Scholar]
- 3.Stoddard JJ, Gray B. Maternal smoking and medical expenditures for childhood respiratory illness. Am J Public Health. 1997;87(2):205-209. doi: 10.2105/AJPH.87.2.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nighbor TD, Coleman SRM, Bunn JY, et al. Smoking prevalence among U.S. national samples of pregnant women. Prev Med. 2020;132:105994. doi: 10.1016/j.ypmed.2020.105994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neuman Å, Hohmann C, Orsini N, et al. ; ENRIECO Consortium . Maternal smoking in pregnancy and asthma in preschool children: a pooled analysis of eight birth cohorts. Am J Respir Crit Care Med. 2012;186(10):1037-1043. doi: 10.1164/rccm.201203-0501OC [DOI] [PubMed] [Google Scholar]
- 6.Best D; Committee on Environmental Health; Committee on Native American Child Health; Committee on Adolescence . From the American Academy of Pediatrics: technical report—secondhand and prenatal tobacco smoke exposure. Pediatrics. 2009;124(5):e1017-e1044. doi: 10.1542/peds.2009-2120 [DOI] [PubMed] [Google Scholar]
- 7.Burke H, Leonardi-Bee J, Hashim A, et al. Prenatal and passive smoke exposure and incidence of asthma and wheeze: systematic review and meta-analysis. Pediatrics. 2012;129(4):735-744. doi: 10.1542/peds.2011-2196 [DOI] [PubMed] [Google Scholar]
- 8.Agusti A, Faner R. Lung function trajectories in health and disease. Lancet Respir Med. 2019;7(4):358-364. doi: 10.1016/S2213-2600(18)30529-0 [DOI] [PubMed] [Google Scholar]
- 9.Stern DA, Morgan WJ, Wright AL, Guerra S, Martinez FD. Poor airway function in early infancy and lung function by age 22 years: a non-selective longitudinal cohort study. Lancet. 2007;370(9589):758-764. doi: 10.1016/S0140-6736(07)61379-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guerra S, Lombardi E, Stern DA, et al. Fetal origins of asthma: a longitudinal study from birth to age 36 years. Am J Respir Crit Care Med. 2020;202(12):1646-1655. doi: 10.1164/rccm.202001-0194OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allen JL. Airway function throughout the lifespan: pediatric origins of adult respiratory disease. Pediatr Investig. 2019;3(4):236-244. doi: 10.1002/ped4.12165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith BM, Kirby M, Hoffman EA, et al. ; MESA Lung, CanCOLD, and SPIROMICS Investigators . Association of dysanapsis with chronic obstructive pulmonary disease among older adults. JAMA. 2020;323(22):2268-2280. doi: 10.1001/jama.2020.6918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Svanes C, Sunyer J, Plana E, et al. Early life origins of chronic obstructive pulmonary disease. Thorax. 2010;65(1):14-20. doi: 10.1136/thx.2008.112136 [DOI] [PubMed] [Google Scholar]
- 14.McEvoy CT, Shorey-Kendrick LE, Milner K, et al. Oral vitamin C (500 mg/d) to pregnant smokers improves infant airway function at 3 months (VCSIP): a randomized trial. Am J Respir Crit Care Med. 2019;199(9):1139-1147. doi: 10.1164/rccm.201805-1011OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McEvoy CT, Shorey-Kendrick LE, Milner K, et al. Vitamin C to pregnant smokers persistently improves infant airway function to 12 months of age: a randomised trial. Eur Respir J. 2020;56:1902208. doi: 10.1183/13993003.02208-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McEvoy CT, Schilling D, Clay N, et al. Vitamin C supplementation for pregnant smoking women and pulmonary function in their newborn infants: a randomized clinical trial. JAMA. 2014;311(20):2074-2082. doi: 10.1001/jama.2014.5217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sekhon HS, Keller JA, Benowitz NL, Spindel ER. Prenatal nicotine exposure alters pulmonary function in newborn rhesus monkeys. Am J Respir Crit Care Med. 2001;164(6):989-994. doi: 10.1164/ajrccm.164.6.2011097 [DOI] [PubMed] [Google Scholar]
- 18.Proskocil BJ, Sekhon HS, Clark JA, et al. Vitamin C prevents the effects of prenatal nicotine on pulmonary function in newborn monkeys. Am J Respir Crit Care Med. 2005;171(9):1032-1039. doi: 10.1164/rccm.200408-1029OC [DOI] [PubMed] [Google Scholar]
- 19.Beydon N, Davis SD, Lombardi E, et al. ; American Thoracic Society/European Respiratory Society Working Group on Infant and Young Children Pulmonary Function Testing . An official American Thoracic Society/European Respiratory Society statement: pulmonary function testing in preschool children. Am J Respir Crit Care Med. 2007;175(12):1304-1345. doi: 10.1164/rccm.200605-642ST [DOI] [PubMed] [Google Scholar]
- 20.Ferris BG. Epidemiology standardization project (American Thoracic Society). Am Rev Respir Dis. 1978;118(6, pt 2):1-120. [PubMed] [Google Scholar]
- 21.Kutner M, Nachtsheim C, Neter J, Li W. Applied Linear Statistical Models. 5th ed. McGraw-Hill; 2004. [Google Scholar]
- 22.McCullagh P, Nelder JA. Generalized Linear Models. 2nd ed. Chapman & Hall/CRC; 1989. doi: 10.1007/978-1-4899-3242-6 [DOI] [Google Scholar]
- 23.Sarria EE, Mattiello R, Yao W, et al. Atopy, cytokine production, and airway reactivity as predictors of pre-school asthma and airway responsiveness. Pediatr Pulmonol. 2014;49(2):132-139. doi: 10.1002/ppul.22784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szefler SJ, Goldstein S, Vogelberg C, et al. Forced expiratory flow (FEF25-75%) as a clinical endpoint in children and adolescents with symptomatic asthma receiving tiotropium: a post hoc analysis. Pulm Ther. 2020;6(2):151-158. doi: 10.1007/s41030-020-00117-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicolaou NC, Simpson A, Lowe LA, Murray CS, Woodcock A, Custovic A. Day-care attendance, position in sibship, and early childhood wheezing: a population-based birth cohort study. J Allergy Clin Immunol. 2008;122(3):500-506.e5. doi: 10.1016/j.jaci.2008.06.033 [DOI] [PubMed] [Google Scholar]
- 26.Martínez TM, Llapur CJ, Williams TH, et al. High-resolution computed tomography imaging of airway disease in infants with cystic fibrosis. Am J Respir Crit Care Med. 2005;172(9):1133-1138. doi: 10.1164/rccm.200412-1665OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lange P, Celli B, Agustí A, et al. Lung-function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med. 2015;373(2):111-122. doi: 10.1056/NEJMoa1411532 [DOI] [PubMed] [Google Scholar]
- 28.Sarria EE, Mattiello R, Rao L, et al. Quantitative assessment of chronic lung disease of infancy using computed tomography. Eur Respir J. 2012;39(4):992-999. doi: 10.1183/09031936.00064811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ly NP, Gold DR, Weiss ST, Celedón JC. Recurrent wheeze in early childhood and asthma among children at risk for atopy. Pediatrics. 2006;117(6):e1132-e1138. doi: 10.1542/peds.2005-2271 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol for VCSIP Study
Trial Protocol and Statistical Analysis Plan for VCSIP Follow-up
eTable 1. Wheeze Outcome Variables
eReferences
eTable 2. Characteristics of Children With Completed 12-Month and 5-Year Pulmonary and/or Airway Function Test Results
Data Sharing Statement