Abstract
Patient: Male, 66-year-old
Final Diagnosis: Metformin associated lactic acidosis
Symptoms: Fatigue • nausea • vomiting • abdominal pain • watery diarrhea
Medication: —
Clinical Procedure: —
Specialty: Critical Care Medicine • Endocrinology and Metabolic • Nephrology
Objective:
Unusual clinical course
Background:
Metformin is recommended as the first-line therapy for type 2 diabetes mellitus, according to the American Diabetes Association. It is considered a safe medication with minimal adverse effects, with the most common being gastrointestinal. Metformin-associated lactic acidosis (MALA) is a rare but life-threatening complication. MALA usually occurs in patients with kidney dysfunction. However, it can still occur with preserved kidney function with the ingestion of a large dose of metformin.
Case Report:
A 66-year-old man with a significant medical history of type 2 diabetes mellitus presented after an intentional ingestion of a high dose of metformin (3000 mg/day). He was admitted to our hospital with symptoms of fatigue, nausea, vomiting, abdominal pain, and watery diarrhea lasting for 3 days. His initial laboratory findings were remarkable, with a serum creatinine level of 819 µmol/L. Arterial blood gas revealed severe lactic acidosis, with a pH of 6.94, HCO3– of 3 mEq/L, anion gap of 48 mmol/L, and lactate level of 15 mmol/L. Emergent continuous renal replacement therapy was done. Two days later, his condition improved considerably, and the lactic acidosis was resolved entirely. He was discharged on day 11 of hospitalization.
Conclusions:
MALA is rare but life-threatening complication of treatment with metformin. MALA should be considered when there is evidence of metformin ingestion and renal insufficiency in patients with lactic acidosis. The curative treatment of MALA is based on hemodialysis, but the main remedy is prevention, which requires patient compliance with taking metformin as prescribed.
Keywords: Acute Kidney Injury; Diabetes Mellitus, Insulin-Dependent, 10; Metformin
Background
Biguanide compounds were first extracted from the French lilac in the 17th century and were used for the treatment of diabetes in France beginning in the 1950s. Metformin, a biguanide compound, was approved for use in the United States in 1994 [1]. Because of the improvement in morbidity and mortality in type 2 diabetes mellitus with metformin use, which is explained in the United Kingdom Prospective Diabetes Study, metformin has been recommended as the first-line oral therapy for type 2 diabetes by the American Diabetes Association and European Association for the Study of Diabetes since 2009. Thus, not surprisingly, it is a commonly prescribed medication by most healthcare providers in most medical institutions because of its many benefits and general safety. However, ingested metformin can lead to metformin-associated lactic acidosis (MALA), a rare but fatal adverse effect. According to several reports, the estimated incidence of MALA is 0.03 to 0.06 per 1000 patient-years [2]. The etiology of lactic acidosis relates to many factors and includes several problems, such as inhibited gluconeogenesis by decreased lactate metabolism in the liver, dysregulation of pyruvate dehydrogenase complex activity, and decreased transport to mitochondria, resulting in the elevated conversion of pyruvate into lactate [3,4]. Moreover, the reduction of hepatic lactate clearance due to inhibiting the mitochondrial respiratory chain can lead to high lactate levels [5]. MALA often happens in patients with reduced kidney function. However, it can occur with normal kidney function if a patient ingests a large dose of metformin [4,6]. Hemodialysis plays an essential role, along with supportive care, in managing MALA [4].
Case Report
A 66-year-old man with a medical history of type 2 diabetes mellitus and hypertension was admitted to the Emergency Department with symptoms of fatigue, abdominal pain, nausea, vomiting, and severe diarrhea lasting for 3 days. The diabetes was managed with metformin 1000 mg twice daily and sitagliptin 100 mg once daily, with reasonable glycemic control. His last visit to the doctor had been 2 months ago, and he had normal kidney function at that time (serum creatinine level: 79 µmol/L). However, his blood glucose level had gone up to 9 mmol/L for the last 5 days, and he intentionally increased his metformin dose to 1000 mg 3 times a day without being recommended to do so by his physicians. On clinical examination, he was alert and afebrile (36.6°C), with a blood pressure of 160/100 mmHg, heart rate of 110 beats/min, respiratory rate of 20 breaths/min, and SpO2 of 99% on room air. There were signs of dehydration, oliguria, diminished skin turgor, and dry mucous membranes. The rest of the clinical symptoms were unremarkable. The initial laboratory parameters are summarized in Table 1. An initial arterial blood gas revealed a pH of 6.94; PO2 of 151 mmHg; PCO2 of 14 mmHg; bicarbonate of 3 mmol/L, anion gap 48 mmol/L, and lactate level of 15 mmol/L.
Table 1.
Blood investigation results in the Emergency Department.
| Test | Value | Reference range |
|---|---|---|
| Sodium (mmol/L) | 123 | 135–145 |
| Potassium (mmol/L) | 4.7 | 3.3–8.3 |
| Chloride (mmol/L) | 72 | 98–106 |
| Glucose (mmol/L) | 24.96 | 4.1–5.9 |
| Urea (mmol/L) | 28.98 | 3.3–8.3 |
| Creatinine (µmol/L) | 819 | 62–106 |
| Aspartate transaminase (U/L) | 36 | <40 |
| Alanine transaminase (U/L) | 24 | <40 |
| White blood cells (G/L) | 17.41 | 4–10 |
| Red blood cells (T/L) | 4.55 | 4.2–6 |
| Hemoglobin (g/L) | 131 | 130–170 |
| Hematocrit (L/L) | 0.406 | 0.38–0.49 |
| Platelet (G/L) | 314 | 140–350 |
With these symptoms and laboratory parameters, MALA with acute renal failure was diagnosed. Sodium bicarbonate 4.2% and glucose control with insulin infusion were administrated. After that, his blood pressure dropped to 70/50 mmHg; therefore, a norepinephrine infusion was initiated. After 2 h in the Emergency Department, there was no improvement, so he was transferred to the Intensive Care Unit, where the decision to proceed with emergent continuous renal replacement therapy was made.
Over the next 8 h, there was a noticeable improvement in lactic acidosis. Also, his clinical condition stabilized, and norepinephrine was stopped. A total amount of intravenous crystal-loid fluid was 2350 mL, given over 12 h. After hemodiafiltration, lasting 2 days, his kidney function was gradually recovered, with the restoration of urine output, and the lactic acidosis was resolved completely. All alterations in indexes of arterial blood gases and lactate measurements are shown in Table 2.
Table 2.
Arterial blood gas and lactate levels by time of test.
| Initiation | h 2 | h 4 | h 8 | h 12 | h 14 | h 20 | h 48 | |
|---|---|---|---|---|---|---|---|---|
| pH | 6.94 | 7.15 | 7.18 | 7.27 | 7.43 | 7.46 | 7.42 | 7.4 |
| pCO2 (mmHg) | 14 | 19 | 23 | 22 | 29 | 37 | 41 | 52 |
| pO2 (mmHg) | 151 | 170 | 139 | 149 | 143 | 116 | 143 | 145 |
| HCO3− (mmol/L) | 3 | 9.5 | 11 | 12.9 | 20.5 | 26.3 | 26.6 | 32.2 |
| Lactate (mmol/L) | 15 | 15.5 | 13.9 | 10.2 | 7.6 | 5.4 | 5.7 | 2.5 |
He was then transferred to the Endocrinology Department to manage type 2 diabetes mellitus and acute renal failure. On day 11 of hospitalization, he was discharged home, with good health status, blood glucose levels effectively controlled, and renal function fully recovered. All changes in serum creatinine levels are shown in Figure 1.
Figure 1.
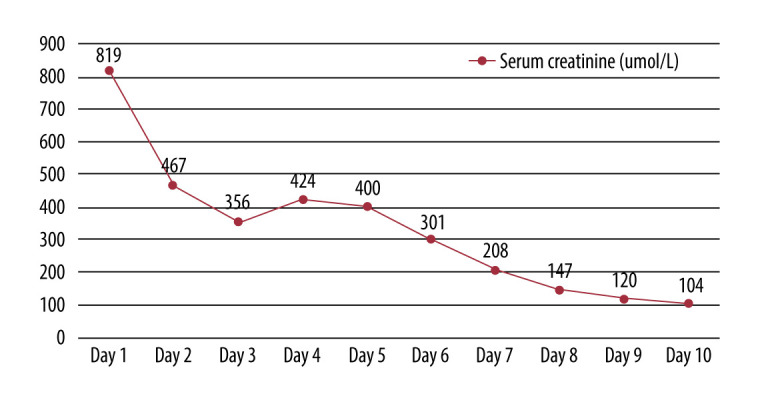
All changes in the patient’s serum creatinine levels.
As a part of our multidisciplinary discharge planning, this patient was provided with follow-up appointments within 1 month in the Endocrinology and Nephrology Departments. Two appointments after discharge, his glycemic control and renal function remained stable.
Discussion
Metformin is the most commonly prescribed treatment for newly diagnosed type 2 diabetes mellitus [7] because of its effectiveness. It is generally considered a safe drug, with minimal gastrointestinal adverse effects: diarrhea, nausea, vomiting, and abdominal bloating [2,8]. Despite its safety, metformin can lead to MALA, a rare but severe complication, particularly in patients with renal failure. Metformin is safely indicated for patients with an estimated glomerular filtration rate (eGFR) greater than 45 mL/min/1.73 m2 and is contraindicated in patients with an eGFR less than 30 mL/min/1.73 m2 [9]. For patients with eGFR between 30 and 45 mL/min/1.73 m2, the FDA recommends not initially treating with metformin [9]. If the patient is on metformin treatment and the eGFR later falls below 45 mL/min/1.73 m2, the recommendation is to consider the benefits and risks of maintaining medication. The FDA also recommends that all patients taking metformin have eGFR monitored annually. Patients with a high risk for developing kidney injury, including the elderly and patients with infection, inflammation, and autoimmune diseases, should be assessed more frequently [9].
Our patient had a serum creatinine level of 79 µmol/L, corresponding to an approximate eGFR of 80 mL/min/1.73 m2 at 2 months before his presentation to the hospital. He had a typical tolerance of 2000 mg metformin per day and had reasonable glycemic control. Nevertheless, the intentional increase of his dose of metformin to 3000 mg per day led to gastrointestinal reactions, including nausea, vomiting, abdominal pain, and diarrhea, which caused severe dehydration, followed by the development of acute kidney injury, one of the results of MALA.
Symptoms of MALA are nonspecific and can resemble sepsis, with gastrointestinal symptoms including nausea, vomiting, abdominal pain, and leukocytosis. These gastrointestinal symptoms associated with metabolic acidosis can lead doctors to make a misdiagnosis and can lead to a patient’s death due to doctor’s not recognizing it as MALA.
MALA is not easy to distinguish from sepsis, especially in severe cases. Therefore, measuring serum metformin levels can be helpful for diagnosis. However, serum metformin is not routinely measured in most hospitals. MALA should be suspected in patients with lactic metabolic acidosis and in patients who have the following conditions: evidence of metformin ingestion; severe metabolic acidosis with pH ≤7.1; remarkably increased lactate concentration ≥15 mmol/L with anion gap >20 mmol/L; a deficient serum bicarbonate level <10 mmol/L; and kidney failure.
There is no antidote for metformin toxicity. Supportive care and hemodialysis are the mainstays of curative therapy for MALA. Hemodialysis could adjust acid-base metabolic disturbances, elevate metabolism, and release metformin. Hemodialysis can be discontinued when the serum lactate level is under 3 mmol/L and the pH >7.35 [10].
Conclusions
MALA is a rare but life-threatening complication of treatment with metformin. It is essential that all medical professionals educate patients regarding the adverse effects of metformin therapy. Kidney function needs to be monitored during metformin therapy. MALA should be considered in patients with lactic metabolic acidosis, evidence of metformin ingestion, and renal insufficiency. There is no antidote for metformin toxicity, with supportive care and hemodialysis being the mainstays of curative therapy. However, the primary remedy is prevention, which requires patient compliance with taking metformin as prescribed.
Footnotes
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Thomas I, Gregg B. Metformin; A review of its history and future: From lilac to longevity. Pediatr Diabetes. 2017;18(1):10–16. doi: 10.1111/pedi.12473. [DOI] [PubMed] [Google Scholar]
- 2.DeFronzo R, Fleming GA, Chen K, et al. Metformin-associated lactic acidosis: Current perspectives on causes and risk. Metabolism. 2016;65(2):20–29. doi: 10.1016/j.metabol.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 3.Omar A, Ellen R, Sorisky A. Metformin-associated lactic acidosis in a patient with normal renal function. Can J Diabetes. 2016;40(4):280–81. doi: 10.1016/j.jcjd.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Keller G, Cour M, Hernu R, et al. Management of metformin-associated lactic acidosis by continuous renal replacement therapy. PLoS One. 2011;6(8):e23200. doi: 10.1371/journal.pone.0023200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agius L, Ford BE, Chachra SS. The metformin mechanism on gluconeo-genesis and AMPK activation: The metabolite perspective. Int J Mol Sci. 2020;21(9):3240. doi: 10.3390/ijms21093240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lalau JD, Race JM. Metformin and lactic acidosis in diabetic humans. Diabetes Obes Metab. 2000;2(3):131–17. doi: 10.1046/j.1463-1326.2000.00053.x. [DOI] [PubMed] [Google Scholar]
- 7.Gong L, Goswami S, Giacomini KM, et al. Metformin pathways: Pharmacokinetics and pharmacodynamics. Pharmacogenet Genomics. 2012;22(11):820–27. doi: 10.1097/FPC.0b013e3283559b22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: A patient-centered approach: Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35(6):1364–79. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.US Food Drug Administration FDA Drug Safety Communication: FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function. 2017.
- 10.Calello DP, Liu KD, Wiegand TJ, et al. Systematic review and recommendations from the Extracorporeal Treatments in Poisoning Workgroup. Crit Care Med. 2015;43(8):1716–30. doi: 10.1097/CCM.0000000000001002. [DOI] [PubMed] [Google Scholar]


