Abstract
Background:
Atrial fibrosis is a hallmark of structural remodeling in atrial fibrillation (AF). Plasma Procollagen Type III N-Terminal Propeptide (PIIINP) reflects collagen synthesis and degradation while Collagen Type I Carboxy-Terminal Telopeptide (ICTP) reflects collagen degradation. We aimed to study baseline plasma PIIINP and ICTP and their associations with incident AF in participants initially free of overt cardiovascular disease.
Methods:
In a stratified sample of the Multi-Ethnic Study of Atherosclerosis (MESA), initially aged 45–84 years, 3071 participants had both PIIINP and ICTP measured at baseline. Incident AF in 10 year follow-up was based on a hospital ICD code for AF or atrial flutter, in- or out-patient Medicare claims through 2011 (primarily in those aged 65–84 years), or ECG 10 years after baseline (n=357). The associations of PIIINP and ICTP with incident AF were estimated using Poisson regression with follow-up time offset.
Results:
Baseline PIIINP (5.50±1.55 μg/L) and ICTP (mean ± SD, 3.41±1.37 μg/L) were positively related (both p<0.0001) to incident AF in a model adjusting for age, race/ethnicity, and sex, with an apparent threshold (relative incidence density 2.81 (1.94–4.08) for PIIINP ≥8.5 μg/L (3.5% of the sample) and 3.46 (2.36–5.07) for ICTP ≥7 μg/L (1.7% of the sample). Findings were attenuated but remained statistically significant after further adjustment for systolic blood pressure, height, body mass index, smoking, and renal function. Additional adjustment for other risk factors and biomarkers of inflammation did not alter conclusions.
Conclusions:
Plasma collagen biomarkers, particularly at elevated levels, were associated with excess risk for atrial fibrillation.
Keywords: cohort study, atrial fibrillation, incidence, collagen, PIIINP, ICTP
Graphical Abstract
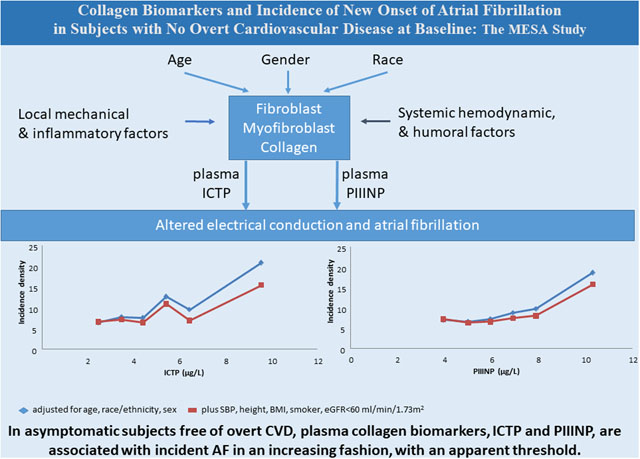
In 2010 the US prevalence of AF was 6.1 million and is expected to rise to at least 12.1 million by 2050.1,2 AF is associated with significant morbidity and mortality.3,4,5,6 Cardiac fibrosis is thought to play a central role in the pathogenesis of AF.7 Development and progression of atrial fibrosis are hallmarks of structural remodeling in AF and considered the substrate of AF perpetuation.8 Type I and III collagens are the major collagens in both normal and diseased tissue. Two major biomarkers of collagen metabolism, Procollagen Type III N-Terminal Propeptide (PIIINP) and Collagen Type I Carboxy-Terminal Telopeptide (ICTP), reflect collagen synthesis and degradation and degradation only, respectively.9 AF is common in subjects with symptomatic and clinically recognizable inflammation conditions. Low-grade, asymptomatic inflammation may also contribute to the pathogenesis of AF.10,11,12,13 Processes of inflammation and pathologic fibrosis are often intertwined.14
Retrospective studies have suggested that circulating fibrosis biomarkers are associated with AF, but prospective studies are limited. In the Cardiovascular Health Study, a prospective cohort study of elderly men and women in which levels of plasma PIIINP were assessed in 2,935 participants, 767 developed AF during 9 years follow-up. Risk of incident AF generally increased with PIIINP, with a non-significant tendency to flatten at higher PIIINP levels.15 ICTP was not studied in CHS. In the Atherosclerosis Risk in Communities study the relationships between matrix metalloproteinases 1, 2 and 9, tissue inhibitor matrix metalloproteinase 1, 2 and C-terminal propeptide of collagen type-I with incident AF were examined after adjusting for confounders.16 In models adjusted for age, sex and race, all biomarkers were associated with AF, but only the relationship between plasma matrix metalloproteinase-9 remained significant in the fully-adjusted model.
The predictive value of collagen biomarkers for AF is largely unknown in subjects free of clinically recognized cardiovascular disease (CVD). Studying such persons affords the opportunity to examine the contribution of fibrosis to AF before the fibrosis can be the result of clinically overt CVD. It also offers advantages for determining the directionality of the fibrosisAF relationship, because people free of CVD would be less likely to have clinically unrecognized AF at baseline, which could itself drive atrial mechanical remodeling and consequent fibrosis. Therefore our hypothesis was that PIIINP and ICTP are positively associated with incidence of AF in middle-aged to older adults free of prevalent, clinically apparent CVD. Understanding associations between these collagen biomarkers and incident AF will suggest potential mechanisms of disease and may help in development of prevention strategies.
Methods
Study Population
The Multi-Ethnic Study of Atherosclerosis (MESA) was initiated to investigate the prevalence, correlates, and progression of subclinical CVD in people initially free of overt clinical CVD, radiation or chemotherapy-treated cancer, other serious major illness, or cognitive impairment in the judgment of the screening interviewer prior to baseline.17 Between 2000 and 2002, 6,814 men and women of white, black, Hispanic, or Chinese race/ethnicity, aged 45–84 years were enrolled. The institutional review boards at all participating centers approved the study, and all participants signed informed consent. Following standard MESA procedures for data availability, the data, analytic methods, and study materials will be made available to other researchers for purposes of reproducing the results or replicating the procedure after submission of a request to the MESA Publications and Presentations Committee (https://mesa-nhlbi.org/Publications.aspx) and obtaining that committee’s approval.
In the present stratified design, we excluded 1000 randomly selected MESA participants whose blood sample inventory had been depleted in earlier studies, as well as those who were missing baseline continuous blood pressure waveform measurement (the latter based on the original purpose of the design). We included 3071 participants, namely, all with any adjudicated CVD event (coronary heart disease, stroke, peripheral vascular disease or heart failure) prior to 2011 plus a 56% random sample of all remaining participants (who had not had a CVD event through 2011). With random sampling and case weighting, our data analyses are representative of all of MESA, although with somewhat expanded standard errors of our estimates. All included participants had assessment of both PIIINP and ICTP at baseline.
Height and weight were measured in the MESA clinic and body mass index (BMI) was defined as weight/height2 (kg/m2). Questionnaires were administered to ascertain sex, race/ethnicity, medical history, medication use, and smoking status. Sitting rest blood pressure was determined oscillometrically; the average of the second and third measurements was used. Diabetes was defined as fasting plasma glucose (determined from a venous blood sample) ≥126 mg/dL or taking antidiabetic medication. Blood lipids were measured at the Collaborative Studies Clinical Laboratory (Fairview-University Medical Center, Minneapolis, MN) using CDC/NHLBI standards. High density lipoprotein cholesterol (HDL-C) was measured (Roche Diagnostics, Indianapolis, IN) after precipitation of non-HDL-C with magnesium/dextran. Low density lipoprotein cholesterol was calculated using the Friedewald equation.
Collagen Biomarkers
Blood was drawn following a 12 hour fast, and EDTA plasma was stored at −70°C. EDTA plasma PIIINP (μg/L) and ICTP (μg/L) were selected as measures reflecting type III and type I collagen turnover, respectively. Assays were performed in the Molecular Epidemiology and Biomarker Research Laboratory (University of Minnesota, under direction of Myron Gross, PhD) using competitive radioimmunoassay kits (UNIQ #06099, ICTP and UniQ #06098 PIIINP, Orion Diagnostica, Espoo, Finland)18. In the PIIINP assay, a known amount of labelled PIIINP and an unknown amount of unlabeled PIIINP in the sample compete for the limited number of high affinity binding sites of the antibody. After separating the free antigen, the amount of labeled PIIINP in the sample tube is inversely proportional to the amount of PIIINP in the sample. The description of the ICTP assay is parallel to that for PIIINP, using an ICTP specific antibody. The concentrations in unknown samples are obtained from a calibration curve. Coefficients of variation for internal quality control samples during the main runs were 9.3% for PIIINP high control, 16.5% for PIIINP low control, 6.3% for ICTP high control, and 8.8% for ICTP low control.
Inflammatory Markers
High Sensitivity C-reactive protein (hs-CRP) was measured using the BNII nephelometer (Dade Behring Inc, Deerfield, IL). Intra-assay and interassay analytic coefficient of variance (CV) ranged from 2.3 to 4.4% and 2.1 to 5.7%, respectively. Interleukin 6 (IL-6) was measured by ultrasensitive ELISA (Quantikine HS Human IL-6 Immunoassay, R&D Systems, Minneapolis, MN). The laboratory analytic CV for this assay was 6.3%. D-dimer was measured using an immunoturbidimetric assay on a Sta-R analyzer (Liatest D-DI, Diagnostica Stago, Parsippany, NJ). The lower limit of detection was 0.01μg/mL. Intra-assay CV for D-dimer was 12.2% and inter-assay CV range, 5–14%. The NMR measurement of GlycA and the lipoprotein particles in the MESA study have been described in our previous publication.19,20 NMR spectra used were those acquired for NMR LipoProfile® testing at the CLIA-certified LipoScience (now LabCorp) clinical laboratory in Raleigh, NC. GlycA is a composite of alpha glycoprotein inflammatory markers; its chemical origins, and its analytic and biological variability have been described in detail.19 Reported coefficients of variation for intra- and interassay precision were 1.9% and 2.6%, respectively. The sum of small plus medium high-density lipoprotein particles (HSM-P), which we have shown to have anti-inflammatory properties, had coefficients of variation of 9% (small HDL particles) and 14% (medium HDL particles).19
Outcome Events: Atrial Fibrillation
AF was identified based on assignment of an ICD-9 diagnosis code for AF (427.31) or atrial flutter (427.32) in any position at hospital discharge or (for those enrolled in fee-for-service Medicare) in inpatient or outpatient Medicare claims through 2011; or in a 12-lead electrocardiogram at Exam 5 (10 years after baseline). It is recognized both that age is a strong risk factor for AF and that inclusion of Medicare outpatient claims provides more complete AF case-finding in ages ≥65 years than in younger people; baseline age-specific rates were 4.4% (72 AF cases) in ages 45–64, vs 19.68% (285 AF cases) in ages 65–84 years.
Statistical analysis
All analyses were weighted inversely to the sampling weight (1 or 0.56, in effect treating each participant who did not have a CVD event before 2011 as representing1/0.56 = 1.79 participants). Mean and standard deviation (SD) or counts and percentages were calculated for description. We initially performed Poisson regression with follow-up time offset predicting new onset of AF from PIIINP and ICTP, both as continuous variables. We also examined the data across equal concentration interval categories of PIIINP and ICTP (categories specified in the tables, lowest and highest categories are open-ended) as a way to display the shapes of the associations with the outcomes. Because the analyses of the equal interval categories strongly suggested threshold associations of the collagen biomarkers with incident AF, we report the associations in categories, with the lowest collagen biomarker levels as the reference, plus the p-value for linear trend from the continuous models. The cutpoints were selected based on boundaries of the equal interval biomarker category variable. We also examined spline plots (data not shown), which agree with our visual judgments. In this way we regard the continuous models only as general tests of upward trend and do not report linear slopes. The minimal model included age, race/ethnicity, and sex as covariates. The risk factor model added covariates height, heart rate, systolic and diastolic blood pressure (BP), BP lowering medications, body mass index (BMI), former and current smoking, diabetes, total cholesterol, HDL-C, triglycerides, cholesterol-lowering medication, clinic, estimated glomerular filtration rate (eGFR) and low eGFR (< 60 mL/min/1.73m2), hsCRP, IL-6, D-dimer, GlycA, and small plus medium HDL particles. (The propriety of inclusion of height and BMI in the same model is discussed in the Supplemental Methodological Note.) After backwards elimination, several variables were not associated with AF and the risk factor model shown in the tables included only systolic blood pressure, height, BMI, current smoking, and low eGFR. We recognize that age, renal status, and some risk variables may be causally linked to fibrosis as indicated by collagen biomarkers. To the extent that they are causally linked to fibrosis, we may be adjusting for mediating factors, and therefore underestimating the strength of association. In sensitivity analyses, we employed the random sample of cohort participants without weighting and/or using Cox proportional hazards regression. These analyses yielded similar conclusions, with the estimated associations in the Cox models slightly stronger than in the Poisson models (data not shown). The advantage of the Poisson models is that they give direct estimates of the absolute incidence densities. At the suggestion of a reviewer, we examined AF with or without incident CVD during followup, according to whether the incident CVD occurred before, with (30 days before or after), or after AF diagnosis. In this exploratory analysis, we identified AF without incident CVD, at least until 30 days after AF diagnosis and compared that to AF that was accompanied by CVD before or with AF diagnosis. For the AF without incident CVD, we computed continuous net reclassification improvement (NRI) to compare models with covariates only to models that included a collagen biomarker. We considered P<0.05 to be noteworthy in general screening of findings. Analyses were performed using PC-SAS version 9.4 (SAS institute, Cary, North Carolina).
Results
Table 1 and Supplemental Table 1 summarize the baseline characteristics according to PIIINP and to ICTP equal concentration interval categories. The mean age increased monotonically across all categories of ICTP and from the 1st category of PIIINP through the 7 μg/L category. There were small differences in ethnic distribution among the PIIINP categories, while Chinese were less likely to have high ICTP. While there was no significant difference for systolic and diastolic BP among the categories, there was an increase in height, heart rate, BMI, and antihypertensive medication use with higher PIIINP and ICTP levels. Total cholesterol and HDL-C were lower in the higher PIIINP and ICTP levels. There was no difference in diabetes. Renal function was lower with higher PIIINP and ICTP levels. Hs-CRP, IL-6 and D-dimer were higher with higher PIIINP and ICTP levels. Small and medium HDL particles were lower with higher PIIINP and ICTP. Both current smoking and GlycA were inversely associated with PIIINP, although smoking was unrelated to ICTP and GlycA had a nomonotonic, but generally positive association with ICTP. ICTP and PIIINP had a correlation coefficient of 0.4.
Table 1:
Baseline characteristics according to PIIINP and to ICTP 1 μg/L interval categories
| Procollagen Type III N- Terminal Propeptide (PIIINP) category | p trend | ||||||
|---|---|---|---|---|---|---|---|
| Minimum - Maximum | 1.32–4.49 | 4.5–5.49 | 5.5–6.49 | 6.5–7.49 | 7.5–8.49 | 8.5–27.71 | |
| Sample size | N=735 | N=1050 | N=721 | N=332 | N=126 | N=107 | |
| Mean (μg/L) | 3.93 | 5.00 | 5.94 | 6.92 | 7.89 | 10.3 | |
| ICTP (μg/L) | 2.8±1.11 | 3.18±1.11 | 3.5±1.47 | 4.03±1.95 | 4.13±2.53 | 5.25±3.8 | <0.0001 |
| Age (years) | 61.7±12.7 | 62.1±13.3 | 62.9±13.3 | 63.9±13.8 | 62.0±12.8 | 63.0±13.2 | 0.006 |
| Male (%) | 44.6 | 50.5 | 49.7 | 49.1 | 46.2 | 52 | 0.53 |
| Height (cm) | 165±12.9 | 166.5±12.9 | 167.1±12.9 | 167.2±13.2 | 166.3±13.6 | 167.1±13.1 | 0.02 |
| Heart rate (beats/min) | 62.6±11.1 | 62.9±12.1 | 63±12.2 | 62.8±12.8 | 64.8±13.7 | 64.6±15.2 | 0.03 |
| Systolic pressure (mmHg) | 125.8±25.6 | 125.7±27.5 | 126.7±27.3 | 129.4±29.3 | 127.8±29.1 | 126±27 | 0.32 |
| Diastolic pressure (mmHg) | 71.8±12.5 | 71.9±12.8 | 72.1±13.1 | 72.7±13 | 73.6±13.9 | 70.8±14.1 | 0.79 |
| Antihypertensive Rx (%) | 29.9 | 31.5 | 33.8 | 39.4 | 46.1 | 38.6 | 0.01 |
| Body Mass Index (kg/m2) | 26.9±6 | 28±6.7 | 28.7±6.9 | 29.7±7.3 | 30.4±7.3 | 30.3±8.8 | <0.0001 |
| Former smoker (%) | 35 | 37 | 38.2 | 36.2 | 39.8 | 38.7 | 0.79 |
| Current smoker (%) | 15.4 | 12.5 | 9.4 | 9 | 9.9 | 12.5 | 0.003 |
| eGFR* <60 ml/min/1.73 m2 (%) | 2.6 | 4 | 5.2 | 8.1 | 8.2 | 10.5 | <0.0001 |
| Collagen Type I Carboxy-Terminal Telopeptide (ICTP) categories | P trend | ||||||
| Minimum - Maximum | 1.12–2.99 | 3–3.99 | 4–4.99 | 5–5.99 | 6–6.98 | 7.01–23.45 | |
| Sample size | N=1329 | N=1080 | N=430 | N=132 | N=45 | N=55 | |
| Mean (μg/L) | 2.44 | 3.45 | 4.4 | 5.41 | 6.43 | 9.55 | |
| PIIINP (μg/L) | 4.95±1.46 | 5.52±1.55 | 6.21±2.55 | 6.57±2.21 | 7.27±2.65 | 7.75±3.18 | <0.0001 |
| Age (years) | 59.9±12.1 | 63.2±13.3 | 65.7±13.4 | 65.9±13.6 | 68.8±12.2 | 70.6±12.6 | <0.0001 |
| Male (%) | 46.1 | 50.4 | 50.7 | 50.8 | 50.7 | 54.3 | 0.07 |
| Height (cm) | 166±13 | 166.6±13 | 166.6±13.4 | 167±12.5 | 168.1±10.9 | 167.4±12.9 | <0.0001 |
| Heart rate (beats/min) | 62.4±11.4 | 63.2±12.3 | 63.5±13 | 64.5±14.3 | 64.1±14.7 | 64.3±13.9 | 0.0002 |
| Systolic pressure (mmHg) | 124.2±26.3 | 127.3±27 | 129.4±27.6 | 125.2±27.2 | 134.8±37.4 | 137.6±32.9 | 0.06 |
| Diastolic pressure (mmHg) | 72.1±13.2 | 72.3±12.5 | 71.7±12.2 | 70.2±13.1 | 71.1±14.1 | 73.4±15.4 | 0.86 |
| Antihypertensive Rx (%) | 27.9 | 32.6 | 42.8 | 50.1 | 46.4 | 60.8 | <0.0001 |
| Body Mass Index (kg/m2) | 27.4±6.4 | 28.5±6.8 | 29.4±7.4 | 30.6±7.8 | 31.4±7.4 | 29.3±7.3 | <0.0001 |
| Former smoker (%) | 36.7 | 36.5 | 37.5 | 37.1 | 41.7 | 38.3 | 0.36 |
| Current smoker (%) | 12.4 | 11.6 | 12.7 | 10 | 11.1 | 11.6 | 0.29 |
| eGFR* <60 ml/min/1.73 m2 (%) | 1.3 | 3 | 10.8 | 10.4 | 19 | 56.6 | <0.0001 |
eGFR: Estimated glomerular filtration rate ml/min/1.73 m2 based on CKD-EPI formula using serum creatinine.
Results are given either in percentage (%) or as mean ± standard deviation. Equal concentration interval categories of PIIINP and ICTP were used (note that lowest and highest categories are open-ended).
Table 2 shows the mean ICTP and PIIINP concentrations about to AF without or without accompanying CVD. The incident disease subgroups with consistently high collagen markers were the 166 people with incident AF without any incident CVD event and the 61 people with incident AF who did subsequently have an incident CVD event, but not until at least 30 days after the diagnosis of AF.
Table 2.
Collagen biomarker mean values according to presence or absence of atrial fibrillation (AF) with or without CVD
| PIIINP (μg/L) | ICTP (μg/L) | ||
|---|---|---|---|
| N | Mean±SE | Mean±SE | |
| No AF | |||
| Neither incident CVD nor incident AF | 2217 | 5.41±0.03 | 3.28±0.03 |
| Incident CVD, no incident AF | 497 | 5.58±0.07 | 3.44±0.06 |
| AF without other CVD | |||
| No incident CVD, any incident AF | 166 | 5.89±0.13 | 3.88±0.14 |
| Incident CVD after incident AF | 61 | 5.90±0.26 | 4.48±0.23 |
| AF accompanied by other CVD | |||
| Incident CVD within 30 days of incident AF | 63 | 5.52±0.15 | 3.63±0.14 |
| Incident CVD before incident AF | 67 | 5.39±0.18 | 3.97±0.33 |
Incident AF during the 10 year follow-up was identified in 357 participants. Figure 1 summarizes the AF incidence density for each category of each collagen biomarker. Positive linear trend was significant in each model (Table 3). Threshold relationships were suggested visually in Figure 1, with small gradual increase across the lower categories, particularly in the demographic adjusted model. The apparent threshold for PIIINP was at 8.5 μg/L (3.5% of the sample), with relative incidence density (RID) 2.81 (1.94–4.08) in the demographic adjusted model and 2.29 (1.57–3.34) in the more adjusted model. Correspondingly for ICTP the apparent threshold was at 7 μg/L (1.7% of the sample), with RID 3.46 (2.36–5.07) in the demographic adjusted model and 2.44 (1.60–3.72) in the more adjusted model. Associations of ICTP and PIIINP with incident AF not accompanied by incident CVD event (N=227) were stronger than those for total AF. NRI is about 0.14 (p<0.003) for both markers. We also note that the adjusted incidence density rates in those for whom the addition of the collagen markers suggested a higher risk vs a lower risk were 7.8 vs 5.8 per 1000 person years (p-0.003) for ICTP and 8.2 vs 5.6 per hundred person years (p<0.0001) for PIIINP. The collagen markers were not related to incident stroke (data not shown).
Figure 1:
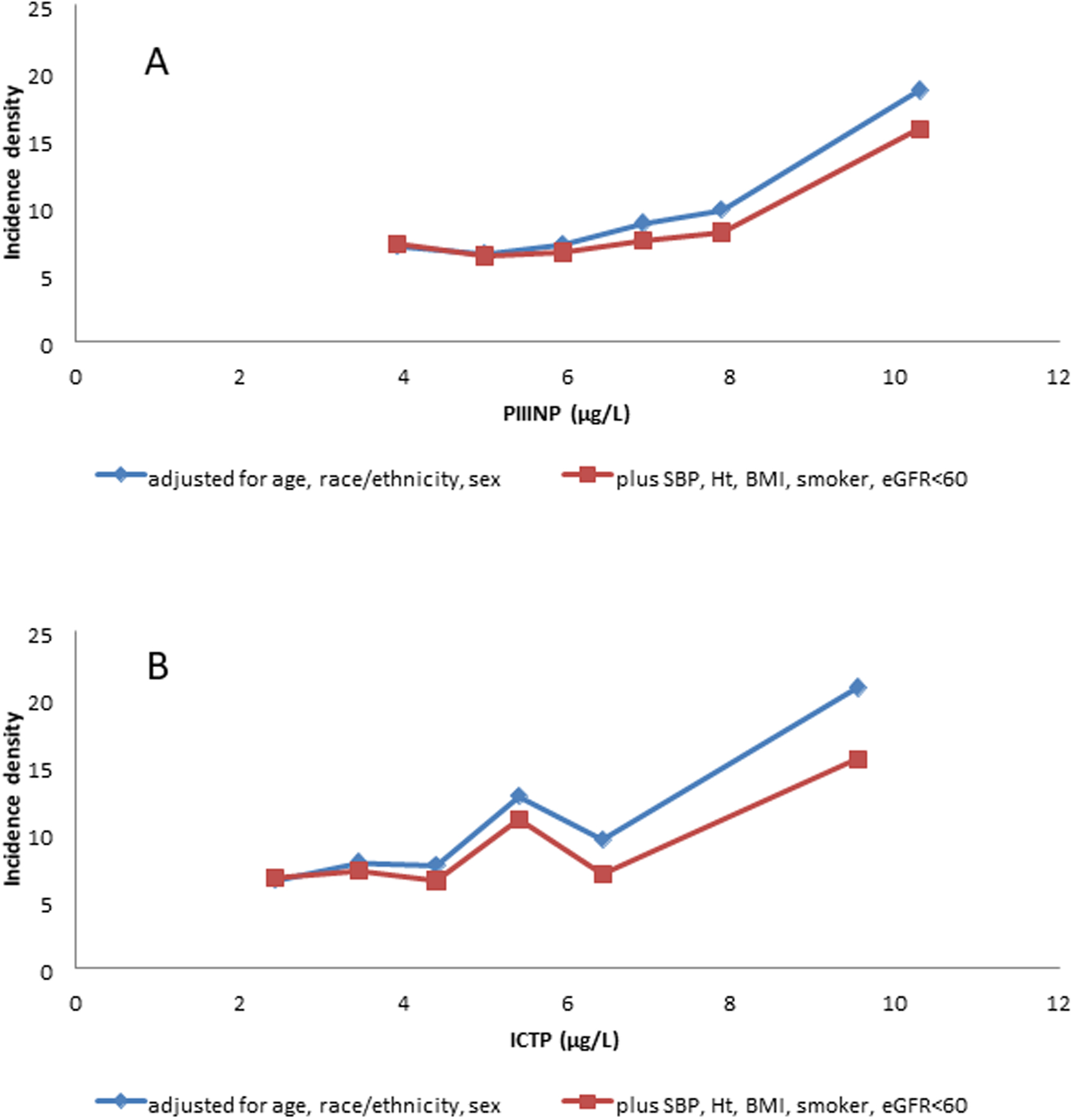
Incidence densities per 1000 person years for atrial fibrillation according to 1 μg/L interval categories, with open-ended extreme categories, of Plasma Procollagen Type III N-Terminal Propeptide (PIIINP, A) and Plasma Collagen Type I CarboxyTerminal Telopeptide (ICTP, B).
Blue line: adjusted for demographics (age, race/ethnicity, and sex)
Red line: adjusted for demographics and for systolic blood pressure (SBP), height (Ht), body mass index (BMI), current smoker, and estimated glomerular filtration rate (eGFR)<60 ml/min/1.73 m2. Covariates are set to their overall means in the plotted points.
Further adjustment for heart rate, diastolic blood pressure, blood pressure lowering medications, former smoking, diabetes, total cholesterol, HDL-cholesterol, triglycerides, cholesterol-lowering medication, hsCRP, IL6, D-dimer, GlycA, and small plus medium HDL particles), did not substantially alter the estimates and is not shown.
Table 3:
Incident Atrial Fibrillation and Collagen Biomarkers (PIIINP and ICTP) in adjusted Poisson models Relative risks across 1 μg/L interval categories of each collagen biomarker
| PIIINP category | p trend | ||||||
|---|---|---|---|---|---|---|---|
| Mean (μg/L) | 3.93 | 5.00 | 5.94 | 6.92 | 7.89 | 10.3 | |
| Sample size | N=735 | N=1050 | N=721 | N=332 | N=126 | N=107 | |
| All incident atrial fibrillation (N=357) | |||||||
| Model: adjusted for age, race/ethnicity, and sex | |||||||
| Relative Risk (CI) | 1 (ref) | 0.92(0.71–1.18) | 1.02(0.78–1.33) | 1.25(0.92–1.69) | 1.4(0.89–2.18) | 2.81(1.94–4.08) | <0.0001 |
| Model: further adjusted for SBP, Ht, BMI, smoker, eGFR<60 | |||||||
| Relative Risk (CI) | 1 (ref) | 0.87(0.68–1.12) | 0.91(0.7–1.18) | 1.03(0.76–1.41) | 1.12(0.71–1.76) | 2.29(1.57–3.34) | 0.003 |
| Incident atrial fibrillation without other CVD (N=227) | |||||||
| Model: adjusted for age, race/ethnicity, and sex | |||||||
| Relative Risk (CI) | 1 (ref) | 0.82(0.61–1.11) | 1.02(0.75–1.38) | 1.28(0.9–1.82) | 1.39(0.83–2.33) | 3.48(2.33–5.19) | <0.0001 |
| Model: further adjusted for SBP, Ht, BMI, smoker, eGFR<60 | |||||||
| Relative Risk (CI) | 1 (ref) | 0.78(0.58–1.06) | 0.91(0.67–1.24) | 1.07(0.74–1.53) | 1.10(0.65–1.86) | 2.75(1.83–4.15) | 0.006 |
| Incident atrial fibrillation accompanied by other CVD (N=130, 59 of which involved heart failure) | |||||||
| Model: adjusted for age, race/ethnicity, and sex | |||||||
| Relative Risk (CI) | 1 (ref) | 1.2(0.74–1.93) | 1.03(0.61–1.74) | 1.14(0.62–2.12) | 1.39(0.57–3.38) | 0.85(0.26–2.82) | 0.36 |
| Model: further adjusted for SBP, Ht, BMI, smoker, eGFR<60 | |||||||
| Relative Risk (CI) | 1 (ref) | 1.14(0.7–1.84) | 0.92(0.54–1.57) | 0.95(0.5–1.79) | 1.17(0.47–2.88) | 0.75(0.22–2.51) | 0.09 |
| ICTP category | p trend | ||||||
| Mean (μg/L) | 2.44 | 3.45 | 4.4 | 5.41 | 6.43 | 9.55 | |
| Sample size | N=1329 | N=1080 | N=430 | N=132 | N=45 | N=55 | |
| All incident atrial fibrillation (N=357) | |||||||
| Model: adjusted for age, race/ethnicity, and sex | |||||||
| Relative Risk (CI) | 1 (ref) | 1.21(0.97–1.5) | 1.18(0.89–1.55) | 2.02(1.41–2.9) | 1.49(0.85–2.61) | 3.46(2.36–5.07) | <0.0001 |
| Model: further adjusted for SBP, Ht, BMI, smoker, eGFR<60 | |||||||
| Relative Risk (CI) | 1 (ref) | 1.08(0.86–1.34) | 0.97(0.73–1.28) | 1.69(1.17–2.45) | 1.04(0.59–1.84) | 2.44(1.60–3.72) | 0.0005 |
| Incident atrial fibrillation without other CVD (N=227) | |||||||
| Model: adjusted for age, race/ethnicity, and sex | |||||||
| Relative Risk (CI) | 1 (ref) | 1.38(1.07–1.79) | 1.22(0.88–1.7) | 2.08(1.34–3.23) | 1.92(1.04–3.54) | 4.80(3.17–7.25) | <0.0001 |
| Model: further adjusted for SBP, Ht, BMI, smoker, eGFR<60 | |||||||
| Relative Risk (CI) | 1 (ref) | 1.24(0.95–1.61) | 1.02(0.73–1.44) | 1.77(1.12–2.78) | 1.39(0.74–2.61) | 3.67(2.31–5.83) | 0.001 |
| Incident atrial fibrillation accompanied by other CVD (N=130, 59 of which involved heart failure) | |||||||
| Model: adjusted for age, race/ethnicity, and sex | |||||||
| Relative Risk (CI) | 1 (ref) | 0.83(0.54–1.27) | 1.07(0.65–1.75) | 1.85(0.97–3.52) | 0.63(0.15–2.62) | 0.84(0.26–2.74) | 0.36 |
| Model: further adjusted for SBP, Ht, BMI, smoker, eGFR<60 | |||||||
| Relative Risk (CI) | 1 (ref) | 0.75(0.49–1.15) | 0.88(0.53–1.49) | 1.58(0.8–3.1) | 0.42(0.1–1.78) | 0.49(0.14–1.73) | 0.89 |
BMI: body mass index; eGFR: estimated glomerular filtration rate; ICTP: Collagen Type I Carboxy-Terminal Telopeptide; Ht: height; PIIINP: Plasma Procollagen Type III N-Terminal Propeptide; SBP: systolic blood pressure
Other demographic variables and risk factors in the more adjusted model were associated with incident AF, including age (RID per yr 1.10, 95% CI 1.08–1.11), black and Hispanic race/ethnicity (lower risk than whites or Chinese), height (RID per cm 1.04, 1.02–1.05), systolic BP (RID per 10 mmHg 1.08, 1.04–1.13), BMI (RID per kg/m2 1.05, 1.03–1.07), and current smoking (RID vs never smoker 1.46, 1.07–1.98). Low eGFR<60 ml/min/1.73 m2 was correlated with incident AF when adjusted for PIIINP (RID vs higher eGFR 1.48, 1.13–1.94), but was not significant when adjusted for ICTP. Other risk factor and inflammatory variables were at most weakly associated with future AF.
Discussion
Our main and novel findings were that in a large multi-ethnic and middle-aged sample, initially free of clinically recognized CVD, the collagen biomarkers PIIINP and ICTP predicted new onset of AF during a median follow-up of approximately 10 years. The shape of relationship was generally increasing, with apparent thresholds above 8.5 μg/L for PIIINP and above 7 μg/L for ICTP. The strong effect sizes in the highest categories of PIIINP and ICTP fit well with two earlier reports. Collagen biomarkers were strongly related to total death and chronic inflammatory-related severe hospitalization and death.18 For the less frequently occurring outcome heart failure with preserved ejection fraction, a threshold association was found in the relationship of PIIINP,21 as we report here for AF. Circulating collagen biomarkers should be interpreted with consideration of their meaning in relation to collagen molecules systemically. Collagen biomarkers that are found in the circulation are fragments of collagen molecules that arise from collagen activity throughout the body, including both normal tissue maintenance and tissue repair. Therefore circulating collagen biomarkers are an imperfect measure of tissue repair. At any one time, several organs may be undergoing tissue repair with attendant collagen molecule production. In this case, epidemiologic disease associations would be consistent over broad ranges of PIIINP and ICTP. If only one organ were undergoing tissue repair at the time of plasma collagen biomarker measurement, only a small portion of the variation in plasma collagen biomarker would be expected from that organ repair. Then, epidemiologically, a threshold would be expected.
We explored subsets of AF. We found that the subset of AF which was not associated with incident CVD at all during MESA followup or until at least 30 days after AF diagnosis was strongly associated with the collagen biomarkers, in contrast with AF which was associated with CVD either diagnosed before or concurrent with AF. These findings are suggestive that fibrosis is a cause of AF when CVD is not present, but AF may also be responsive to other features of CVD in those who have AF accompanied by CVD.
There is very limited information in the literature regarding the predictive value of PIIINP and ICTP for AF in study participants without clinically recognized CVD at baseline. In the Cardiovascular Health Study, PIIINP was measured in 2935 participants and the risk of incident AF was studied over a median follow-up of 8.8 years.15 AF was identified based on an annual resting EKG through the 9th year of follow-up and discharge diagnoses for all hospitalizations based on the ICD codes. The Cardiovascular Health Study sample was older (mean age range 77–78 years) than the MESA study sample. More than 50% of the participants were treated for hypertension. In contrast to our study some participants in the Cardiovascular Health Study had a history of heart failure, myocardial infarction or stroke. In that study, they found a linear relationship between the risk of AF and PIIINP approximately up to the median value both before and after adjustment for demographics, CVD risk factors and history of heart failure and stroke. Above the median value of PIIINP the association between PIIINP and AF risk was not significant, while in our MESA study only the highest category of PIINP and ICTP levels were predictive for AF.
Fibrosis is a process of normal repair that is activated in response to injury to maintain the original tissue architecture and functional integrity. Prolonged chronic injurious stimuli may cause deregulation of normal processes and result in an excess deposition of extracellular matrix and fibrosis. Fibrosis involves complex multistage inflammatory processes with inflammatory cell and extracellular matrix expansion where numerous factors including cytokines/chemokines, growth factors, adhesion molecules, and signaling processes play a role.10,14,22,23
Several hypotheses for our findings may be formulated.22,23,24 In the presence of risk factors like obesity, obstructive sleep apnea, and diabetes, inflammation and an increased sympathetic tone may play a pathological role for AF. Moreover, an important concept is that inflammation contributes to AF before there is consolidation of the inflammatory process leading to structural changes and fibrosis25. However, we reported here that five inflammatory markers did not predict future AF; perhaps such inflammatory factors become important more proximally to the emergence of AF. The molecular basis for the heritability of AF has been explored in depth over the past decade. Rare variants have been identified in ion channels, transcription factors and a wide range of other genes.26
As with other epidemiological studies, the ascertainment and classification of AF is a potential limitation of the present study. Because we had access to hospital discharge diagnoses through MESA cardiovascular events surveillance, plus Medicare outpatient and inpatient claims, our ascertainment may be relatively good.27 However, we are aware that paroxysmal AF may go unrecognized by the patient and not occur at a clinic visit. Therefore, the true AF prevalence is likely to be higher. The type of AF, whether symptomatic or asymptomatic (paroxysmal, persistent, or chronic), cannot be discriminated in this study. A cause-effect relationship cannot be firmly established. It is possible that residual confounding might explain the prediction of new cases of AF by low level collagen biomarkers. Statistically, detecting and describing nonlinear relationships is inherently much more challenging than finding linear associations.
In conclusion, our results add further insight regarding collagen biomarkers and AF. Collagen biomarkers were associated with incident AF in an increasing fashion, with an apparent threshold in asymptomatic subjects free of overt CVD.
Supplementary Material
What is Known?
Collagen biomarkers in the circulation are fragments of collagen molecules that arise from collagen activity throughout the body, including both normal tissue maintenance and repair.
Some atrial fibrillation is known to be associated with fibrosis in the atria.
What the Study Adds?
In a large sample of men and women free of clinical cardiovascular disease at baseline, atrial fibrillation that was not associated with cardiovascular disease was strongly associated with circulating Type I and Type III collagen during 10 years followup.
Atrial fibrillation with concurrent or prior cardiovascular disease was not associated with either circulating Type I or Type III collagen.
The associations with collagen markers showed a pronounced threshold.
Sources of Funding:
The Multi-Ethnic Study of Atherosclerosis was supported by contracts N01-HC-95159, N01HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute and by grants UL1-TR-000040 and UL1-TR-001079 from NCRR. Collagen turnover markers were funded by R01 HL098382 to Drs. Jacobs and Duprez. Identification of atrial fibrillation was facilitated by R01 HL127659 to Dr. Heckbert. Nuclear magnetic resonance-derived GlycA and lipoprotein particle values were provided at no cost by Liposcience Inc (now LabCorp, Inc).
Footnotes
Disclosures: None
References:
- 1.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 2006;114:119–125. [DOI] [PubMed] [Google Scholar]
- 2.Colilla S, Crow A, Petkun W, Singer DE, Simon T, Liu X. Estimates of current and future incidence and prevalence of atrial fibrillation in the US adult population. Am J Cardiol. 2013;112:1142–1147. [DOI] [PubMed] [Google Scholar]
- 3.Wallace ER, Siscovick DS, Sitlani CM, Dublin S, Mitchell PH, Odden MC, Hirsch CH, Thielke S, Heckbert SR.. Incident Atrial Fibrillation and Disability-Free Survival in the Cardiovascular Health Study. J Am Geriatr Soc. 2016;64:838–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piccini JP, Hammill BG, Sinner MF, Hernandez AF, Walkey AJ, Benjamin EJ, Curtis LH, Heckbert SR. Clinical course of atrial fibrillation in older adults: the importance of cardiovascular events beyond stroke. Eur Heart J. 2014;35:250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thacker EL, McKnight B, Psaty BM, Longstreth WT Jr, Sitlani CM, Dublin S, Arnold AM, Fitzpatrick AL, Gottesman RF, Heckbert SR. Atrial fibrillation and cognitive decline: a longitudinal cohort study. Neurology 2013;81:119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen LY, Sotoodehnia N, Bůžková P, Lopez FL, Yee LM, Heckbert SR, Prineas R, Soliman EZ, Adabag S, Konety S, Folsom AR, Siscovick D, Alonso A. Atrial fibrillation and the risk of sudden cardiac death: the Atherosclerosis Risk in Communities study and Cardiovascular Health Study. JAMA Intern Med. 2013;173:29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernstein B, Nattel S. Atrial fibrosis: Mechanisms and Clinical Relevance in Atrial Fibrillation. J Am Coll Cardiol 2008;51:802–809. [DOI] [PubMed] [Google Scholar]
- 8.Dzeshka MS, Lip GY, Snezhitskiy V, Shantsila E. Cardiac Fibrosis in Patients With Atrial Fibrillation: Mechanisms and Clinical Implications. J Am Coll Cardiol. 2015;66:943–959. [DOI] [PubMed] [Google Scholar]
- 9.Lopez B, Gonzales A, Diez J. Circulation biomarkers of collagen metabolism in cardiac diseases. Circulation 2010;121:1645–1654. [DOI] [PubMed] [Google Scholar]
- 10.Hu YF, Chen YJ, Lin YJ, Chen SA. Inflammation and the pathogenesis of atrial fibrillation. Nat Rev Cardiol. 2015;12:230–243. [DOI] [PubMed] [Google Scholar]
- 11.Klein Klouwenberg PM, Frencken JF, Kuipers S, Ong DS, Peelen LM, van Vught LA, Schultz MJ, van der Poll T, Bonten MJ, Cremer OL,MARS Consortium. Incidence, Predictors, and Outcomes of New-Onset Atrial Fibrillation in Critically Ill Patients with Sepsis. A Cohort Study. Am J Respir Crit Care Med. 2017;195:205211. [DOI] [PubMed] [Google Scholar]
- 12.Kuipers S, Klein Klouwenberg PM, Cremer OL. Incidence, risk factors and outcomes of new-onset atrial fibrillation in patients with sepsis: a systematic review. Crit Care. 2014;18(6):688. doi: 10.1186/s13054-014-0688-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walkey AL, Greiner MA, Heckbert SR, Jensen PN, Piccini JP, Sinner MF, Curtis LH, Benjamin EJ. Atrial Fibrillation Among Medicare Beneficiaries Hospitalized With Sepsis: Incidence and Risk Factors. Am Heart J. 2013;165:949–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18:1028–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenberg MA, Maziarz M, Tan AY, Glazer NL, Zieman SJ, Kizer JR, Ix JH, Djousse L, Siscovick DS, Heckbert SR, Mukamal KJ. Circulating fibrosis biomarkers and risk of atrial fibrillation: The Cardiovascular Health Study (CHS). Am Heart J. 2014;167:723–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huxley RR, Lopez FL, MacLehose RF, Eckfeldt JH, Couper D, Leiendecker-Foster C, Hoogeveen RC, Chen LY, Soliman EZ, Agarwal SK, Alonso A. Novel Association between Plasma Matrix Metalloproteinase-9 and Risk of Incident Atrial Fibrillation in a Case-Cohort Study: The Atherosclerosis Risk in Communities Study. PLoS One. 2013; 8:e59052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 18.Duprez DA, Gross MD, Sanchez OA, Kizer JR, Ix JH, Lima J, Tracy RP, Jacobs DR Jr. Collagen Turnover Markers in Relation to Future Cardiovascular and Noncardiovascular Disease: The Multi-Ethnic Study of Atherosclerosis. Clin Chem. 2017;63:1237–1247. [DOI] [PubMed] [Google Scholar]
- 19.Duprez DA, Otvos J, Tracy RP, Feingold KR, Greenland P, Gross MD, Lima JA, Mackey RH, Neaton JD, Sanchez OA, Jacobs DR. High-Density Lipoprotein Subclasses and Noncardiovascular, Noncancer Chronic Inflammatory-Related Events Versus Cardiovascular Events: The Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2015;4:e002295. doi: 10.1161/JAHA.115.002295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duprez DA, Otvos J, Sanchez OA, Mackey RH, Tracy R, Jacobs DR. Comparison of the Predictive Value of GlycA and Other Biomarkers for Total Death, Incident Cardiovascular Events, Noncardiovascular and Noncancer Inflammatory-Related Events. Clin. Chem 2016;62:1020–1031. [DOI] [PubMed] [Google Scholar]
- 21.Duprez DA, Gross M, Kizer JR, Ix JH, Hundley WG, Jacobs DR Jr. Predictive Value of Markers of Collagen Turnover for Systolic and Diastolic Heart Failure: The MESA Study. J Am Heart Assoc. 2018;7(5). pii: e007885. doi: 10.1161/JAHA.117.007885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andrade J, Khairy P, Dobrev D, Nattel S. The Clinical Profile and Pathophysiology of Atrial Fibrillation. Relationships Among Clinical Features, Epidemiology, and Mechanisms. Circ. Res 2014;114:1453–1468. [DOI] [PubMed] [Google Scholar]
- 23.Nattel S. Molecular and Cellular Mechanisms of Atrial Fibrosis in Atrial Fibrillation. JACC Clinical Electrophysiol. 2017;3:425–435. [DOI] [PubMed] [Google Scholar]
- 24.Patel P, Dokainish H, Tsai P, Lakkis N. Update on the association of inflammation and atrial fibrillation. J Cardiovasc Electrophysiol. 2010;21:1064–1070. [DOI] [PubMed] [Google Scholar]
- 25.Aviles RJ, Martin DO, Apperson-Hansen C, Tracy RP, Van Wagoner DR, Psaty BM, Lauer MS, Chung MK. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108:3006–3010. [DOI] [PubMed] [Google Scholar]
- 26.Christophersen IE, Ellinor PT. Genetics of atrial fibrillation: from families to genomes. J Hum Genet. 2016;61:61–70. [DOI] [PubMed] [Google Scholar]
- 27.Jensen PN, Johnson K, Floyd J, Heckbert SR, Carnahan R, Dublin S. A systematic review of validated methods for identifying atrial fibrillation using administrative data. Pharmacoepidemiol Drug Saf. 2012;21 Suppl 1:141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


