Abstract
Herein, we report the first metal-free and molecular halogen reagent-free dihomohalogenation methodology by using Selectfluor as an oxidant and tetrabutylammonium bromide/chloride salts as a halogen source. This effective strategy provides various fluorine-free halogenated products easily in quantitative yields from alkenes, alkynes, and natural products.
Halogenation reactions have gained great importance since their discovery, and halides are important in organic synthesis as they are used in the synthesis of various valuable compounds.1,2 Among the halides, chlorides and bromides attract more attention than iodides and fluorides owing to their great commercial importance.3 On account of chlorine gas or bromine being extremely toxic, researchers are seeking to develop new halogenation strategies.4,5 In addition, conducting chemistry without using metals is also crucial in terms of medicinal chemistry6 and late-stage functionalization (LSF) of natural products and pharmaceuticals.7
Selectfluor is not only a well-known, safe, easily soluble, easy-to-handle, stable solid, and reactive electrophilic fluorination reagent8,9 but is also a robust and efficient “fluorine-free” green oxidant in organic synthesis.10−12 Selectfluor has been used effectively as an oxidant in transition metal-catalyzed cross-coupling reactions,13 selective oxidations,14 regioselective ring openings,15 Diels–Alder cycloadditions,16,17 carbene transfer reactions,18 and other organic transformations19,20 hitherto. Moreover, oxidative monochlorination of some reactive heterocycles, such as aminopyridines and aminodiazines, was efficiently performed using metal salts with the help of Selectfluor.21
Interestingly, tetrabutylammonium bromide and chloride salts have not yet been discovered in oxidative dihomohalogenation reactions until now. To our delight, in this work, we report Selectfluor-mediated metal-free dihomobromination and dihomochlorination methodologies using tetrabuthylammonium bromide/chloride salts under mild reaction conditions with short reaction times.
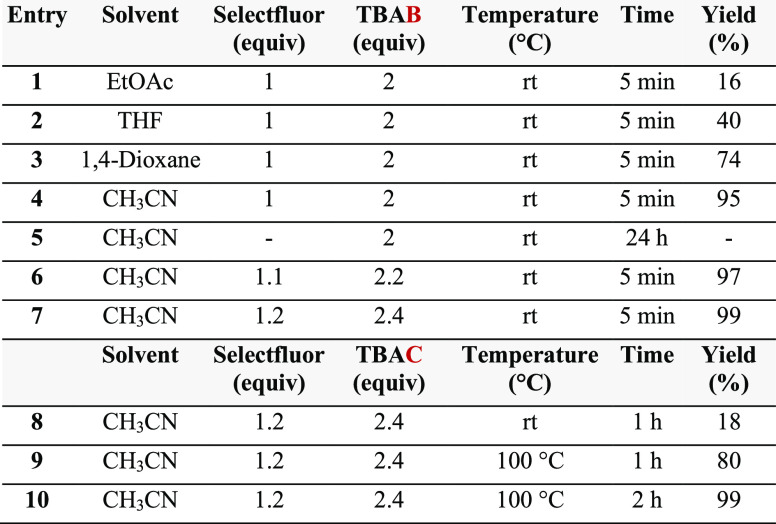
First, we used benzonorbornadiene 1k as a test molecule to optimize the halogenation conditions (Table 1). Studies to determine the solvent of the reaction (Table 1, entries 1–4) revealed that acetonitrile was the best choice for bromination (Table 1, entry 4) within 5 min. Next, it should be noted that oxidative bromination does not occur even with stirring for 24 h without Selectfluor (Table 1, entry 5). The amounts of reagents were gradually increased in order to obtain a quantitative yield (Table 1, entries 6 and 7), and the best result was achieved using 1.2 equiv of Selectfluor and 2.4 equiv of tetrabutylammonium bromide salt (Table 1, entry 7). From the oxidative chlorination point of view, an 18% yield was obtained at room temperature in a 1 h reaction (Table 1, entry 8). In an effort to increase the yield, the reaction temperature was set to 100 °C, and 80% yield was obtained as a result of the 1 h reaction (Table 1, entry 9), while a 2 h reaction led to the synthesis of dicholoro benzonorbornadiene quantitatively (Table 1, entry 10). As a result of detailed optimization experiments, the presented oxidative bromination/chlorination protocol was performed when 1.2 equiv of Selectfluor and 2.4 equiv of TBAB in 2 mL of CH3CN was used in 5 min at room temperature for bromination (Table 1, entry 7) and 2 h at 100 °C for chlorination (Table 1, entry 10).
Table 1. Optimizing the Conditions for the Bromination and Chlorination of Benzonorbornadienea.
Reaction conditions: Benzonorbornadiene (1k) (0.5 mmol), Selectfluor (209 mg, 0.6 mmol), and TBAC (333 mg, 1.2 mmol) or TBAB (386 mg, 1.2 mmol), 2 mL of CH3CN, 5 min and room temperature for bromination, 2 h and 100 °C for chlorination. Conversions were calculated by 1H NMR with 1,3-dinitrobenzene as an internal standard.
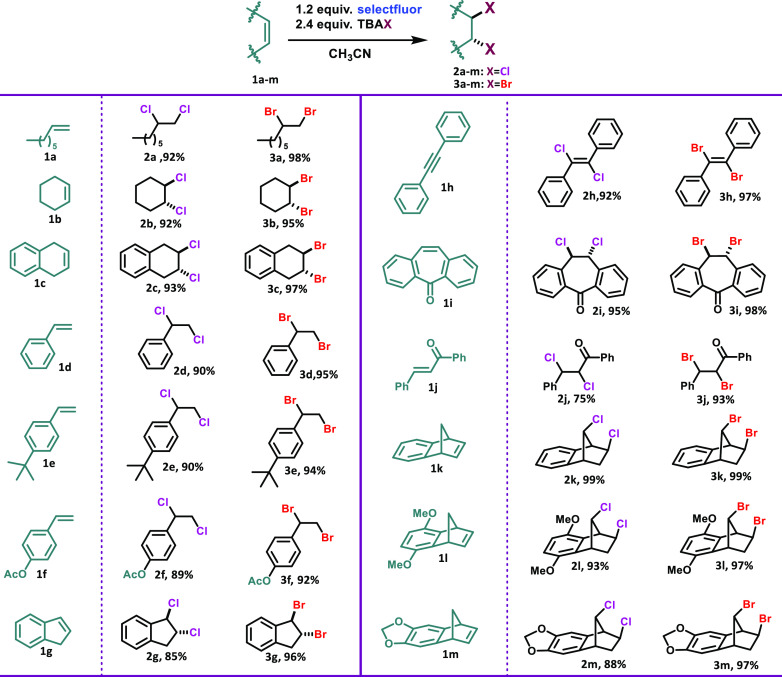
After obtaining the optimum oxidative halogenation conditions from bicyclic 1k, reactions of different unsaturated analogues (26 examples) were examined to extend the scope of the developed methodology (Scheme 1). Under these circumstances, many dihalogenated structures were synthesized in good to excellent yields (75%–99%). Oxidative halogenation of the aliphatic alkene 1a enabled the synthesis of 2a and 3a in yields of 92% and 98%, respectively. The corresponding halogenation products were obtained in high yields similarly from the halogenation of the cyclic alkenes 1b and 1c. Styrene 1d and its derivatives with an activated (1e) and a deactivated double bond (1f) also worked efficiently under this oxidative system. Oxidative chlorination of polycyclic hydrocarbon 1g resulted in 85% yield, while the bromination occurred with 96%. In the case of alkynes (1h), the corresponding dihalogens were obtained (2h, 3h) selectively. Dibenzosuberenone provided the related dichloro (2i) and dibromo (3i) compounds quantitatively in 24 h. As expected, the oxidative chlorination of a deactivated double bond is especially difficult. Halogenation of chalcone 1j enabled the synthesis of the dichloro compound (2j) in 75% yield and the brominated one (3j) in 93% yield in 24 h. Oxidation of 1l resulted in the rearrangement products 2l (93%) and 3l (97%). Finally, another bicyclic structure, 1m, was investigated and the chlorination product was obtained in 88% yield and the bromination product in 97% yield. In general, when the reaction times were extended, elimination products were also formed.
Scheme 1. Oxidative Chlorination and Bromination of Olefins with Selectfluor and TBAX (Cl, Br).
All reactions were carried out using 0.5 mmol of olefin, 0.6 mmol of Selectfluor, and 1.2 mmol of TBAX (Cl, Br) in 2 mL of CH3CN. For chlorination: 100 °C, 2 h (1i and 1j: 24 h). For bromination: rt, 5 min (1i and 1j: 24 h). Conversions were calculated by 1H NMR with 1,3-dinitrobenzene as internal standard.
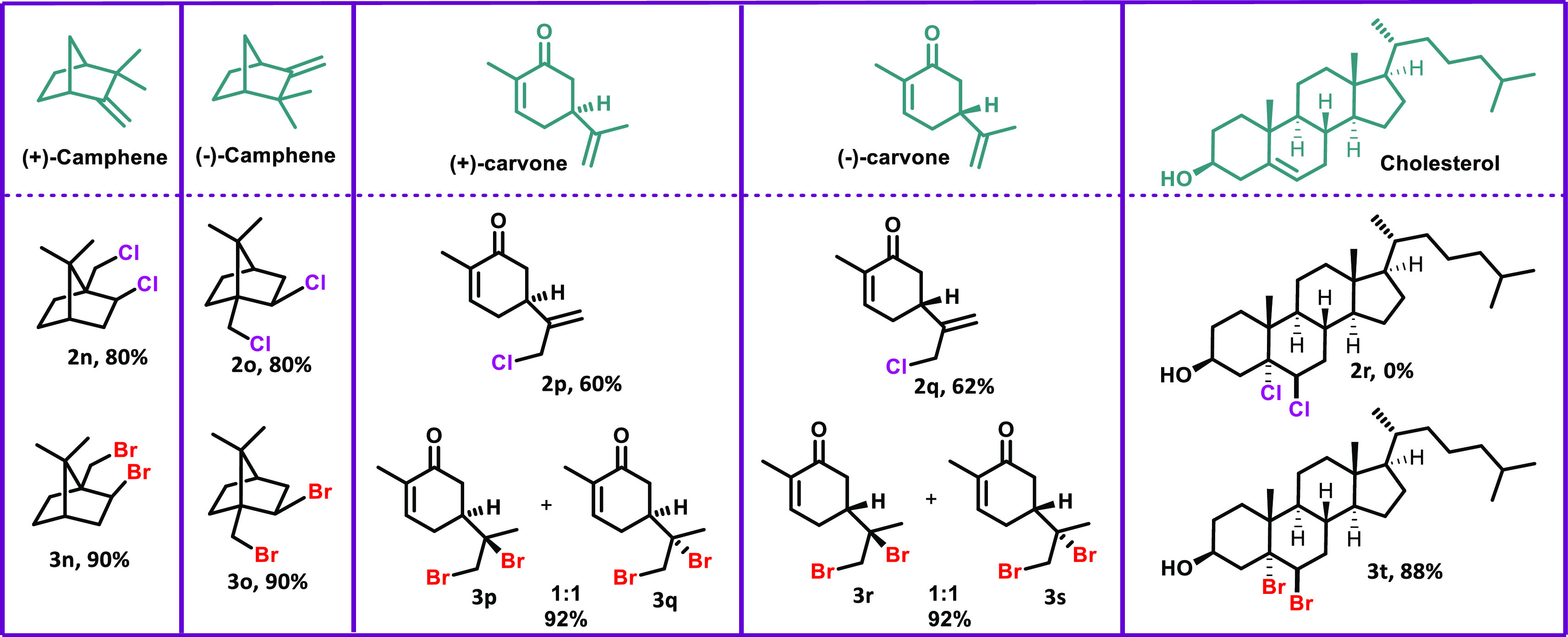
LSF strategies are currently receiving great interest in both the drug discovery and chemical biology fields because they enable the efficient derivatization of natural products.7,22 Oxidative bromination and chlorination studies were carried out on 5 different natural products to show that the present method allows derivatization of natural products efficiently in high yields (Scheme 2). When both (+) and (−) enantiomers of camphene were halogenated separately, Wagner–Meerwein rearrangement products dichloro 2n and 2o (80%) and dibromo 3n and 3o (90%) were obtained. By bromination of carvone enantiomers, dibromo compounds 3p–3q and 3r–3s were obtained in a 1:1 diastereomeric mixture (92%), as expected. However, when the carvone enantiomers were chlorinated, 2p (60%) and 2q (62%) were formed by elimination following the chlorination. From the halogenation of cholesterol under general reaction conditions, dibromo 3t was obtained in 88% yield, but dichloro 2r could not be isolated (Table 2). The successful transformation of cholesterol to the dibromide 3t showed that the developed method could be used for a late-stage functionalization reaction. All of the natural products used in the present study were chiral compounds. Because halogenation and Wagner–Meerwein rearrangement do not cause racemization and the initial enantiomeric excess is preserved in the resulting products, no extra analysis was required for the determination of enantiomeric excess.
Scheme 2. Scale-Up Experiments.
Reaction conditions: Benzonorbornadiene (1g, 7.03 mmol), Selectfluor (2.94 g, 8.44 mmol), TBAX (Cl, Br) (16.87 mmol), CH3CN (20 mL). For chlorination: 100 °C, 2 h. For bromination: rt, 5 min.
Table 2. Oxidative Halogenation of Natural Productsa.
All reactions were carried out using 0.5 mmol of natural product, 0.6 mmol of Selectfluor, and 1.2 mmol of TBAX (Cl, Br) in 2 mL of CH3CN. For chlorination: 100 °C, 2 h. For bromination: rt, 5 min. Conversions were calculated by 1H NMR with 1,3-dinitrobenzene as internal standard.
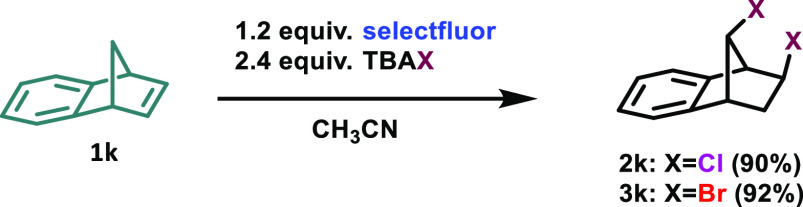
Scale-up experiments were performed in order to put forth the practicality of the oxidative halogenation methodology by using benzonorbornadiene (1 g, 7.03 mmol, 14-fold increase) as a test molecule under the optimized reaction conditions. As can be clearly seen from Scheme 2, the isolated yields of 2k (1.35 g, 90% yield) and 3k (1.95 g, 92%) are quite satisfactory.
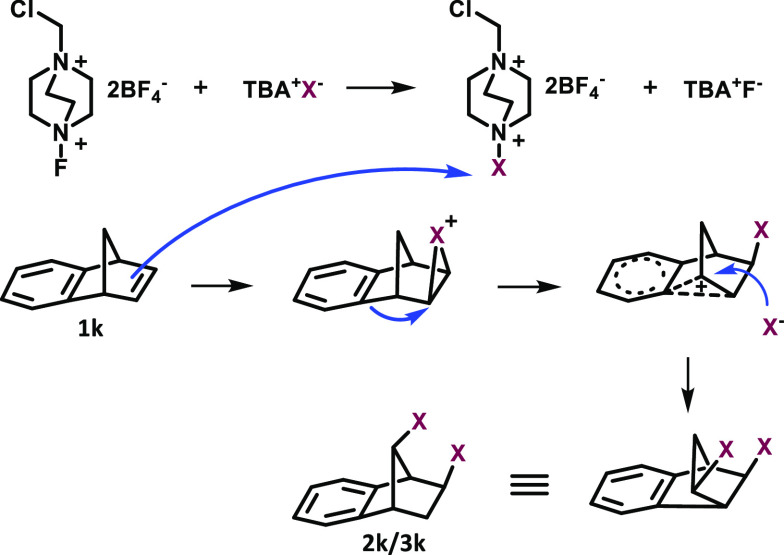
For the oxidative halogenation, we propose the mechanism given in Scheme 3. The Wagner Meerwein rearrangement during the bromination of benzonorbornadiene and its derivatives strongly showed that the reaction proceed via cationic intermediates. In this mechanism, first Selectfluor oxidizes halogen anion X– to halogen cation X+. Then the alkene attacks X+. Classical halogenation occurs in alkenes, cyclic alkenes, acetylenes, and chalcones by attack of X– on the positive charge center. On the other hand, in bicyclic alkenes, halogenation occurs by attack of X– via Wagner–Meerwein rearrangement.23
Scheme 3. Proposed Mechanism for Halogenation of Bicyclic Alkenes by Wagner–Meerwein Rearrangement.
In summary, we succeeded in developing the first metal- and molecular halogen-free dihalogenation of several olefins by using TBAX (Br, Cl) as a halogen source and Selectfluor as an oxidant. Both the olefin functionalization (26 examples) and the LSF experiments (11 examples) of natural products worked perfectly with the yields mostly reaching over 95%. The major advantages of the presented methodology over the existing ones are that (i) there is no need for the molecular chlorine gas and bromine reagent, which are extremely toxic, (ii) it does not require the presence of any metal, (iii) it enables the synthesis of corresponding halogenated products with high yields and selectivity, (iv) it allows derivatization of natural chiral molecules, and (v) it uses a safe solvent in mild conditions. Therefore, we think that this oxidative halogenation protocol will create a new perspective for the dihomohalogenation of olefins and natural products.
Acknowledgments
This study is funded by the Research Fund of Atatürk University, Turkey (Project number: FAD-2019-7025). We would like to thank Atatürk University for its support.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.2c02627.
Experimental procedures, NMR, and HR-MS spectra of products (PDF)
Author Contributions
Ziya Dağalan: Methodology, Optimization, Investigation, Writing—original draft, Visualization. Ramazan Koçak: Conceptualization, Writing review and editing, Supervision, Project administration, Validation. Arif Daştan: Supervision, Funding acquisition, Writing review and editing, Bilal Nişancı: Conceptualization, Methodology, Investigation, Writing—original draft, Supervision, Funding acquisition, Project administration, Validation, Provision.
The authors declare no competing financial interest.
Supplementary Material
References
- Badetti E.; Romano F.; Marchiò L.; Taşkesenlioğlu S.; Daştan A.; Zonta C.; Licini G. Effective bromo and chloro peroxidation catalysed by tungsten (VI) amino triphenolate complexes. Dalton Trans. 2016, 45, 14603–14608. 10.1039/C6DT01780K. [DOI] [PubMed] [Google Scholar]
- Chen Z. Recent development of biomimetic halogenation inspired by vanadium dependent haloperoxidase. Coord. Chem. Rev. 2022, 457, 214404. 10.1016/j.ccr.2021.214404. [DOI] [Google Scholar]
- Gribble G. W. Recently discovered naturally occurring heterocyclic organohalogen compounds. Heterocycles 2012, 84, 157–207. 10.3987/REV-11-SR(P)5. [DOI] [Google Scholar]
- Van Kerrebroeck R.; Horsten T.; Stevens C. V. Bromide oxidation: a safe strategy for electrophilic brominations. Eur. J. Org. Chem. 2022, e202200310. 10.1002/ejoc.202200310. [DOI] [Google Scholar]
- Gu L.; Lu T.; Zhang M.; Tou L.; Zhang Y. Efficient oxidative chlorination of aromatics on saturated sodium chloride solution. Adv. Synth. Catal. 2013, 355, 1077–1082. 10.1002/adsc.201300062. [DOI] [Google Scholar]
- Lasso J. D.; Castillo-Pazos D. J.; Li C.-J. Green chemistry meets medicinal chemistry: a perspective on modern metal-free late-stage functionalization reactions. Chem. Soc. Rev. 2021, 50, 10955. 10.1039/D1CS00380A. [DOI] [PubMed] [Google Scholar]
- Guillemard L.; Kaplaneris N.; Ackermann L.; Johansson M. J. Late-stage C–H functionalization offers new opportunities in drug discovery. Nat. Rev. Chem. 2021, 5, 522–545. 10.1038/s41570-021-00300-6. [DOI] [PubMed] [Google Scholar]
- Nyffeler P. T.; Durón S. G.; Burkart M. D.; Vincent S. P.; Wong C. H. Selectfluor: mechanistic insight and applications. Angew. Chem., Int. Ed. 2005, 44, 192–212. 10.1002/anie.200400648. [DOI] [PubMed] [Google Scholar]
- Wang W.; Huo T.; Zhao X.; Qin Q.; Liang Y.; Song S.; Liu G.; Jiao N. Nitromethane-enabled fluorination of styrenes and arenes. CCS Chem. 2020, 2, 566–575. 10.31635/ccschem.020.202000172. [DOI] [Google Scholar]
- Yang K.; Song M.; Ali A. I.; Mudassir S. M.; Ge H. Recent Advances in the Application of Selectfluor as a “Fluorine-free” Functional Reagent in Organic Synthesis. Chem. Asian J. 2020, 15, 729–741. 10.1002/asia.202000011. [DOI] [PubMed] [Google Scholar]
- Capilato J. N.; Lectka T. Arene Amination Instead of Fluorination: Substitution Pattern Governs the Reactivity of Dialkoxybenzenes with Selectfluor. J. Org. Chem. 2021, 86, 5771–5777. 10.1021/acs.joc.1c00231. [DOI] [PubMed] [Google Scholar]
- Laali K. K.; Nandi G. C.; Bunge S. D. Selectfluor-mediated mild oxidative halogenation and thiocyanation of 1-aryl-allenes with TMSX (X= Cl, Br, I, NCS) and NH4SCN. Tetrahedron Lett. 2014, 55, 2401–2405. 10.1016/j.tetlet.2014.02.110. [DOI] [Google Scholar]
- Yang Y.; Lin Y.; Rao Y. Ruthenium (II)-catalyzed synthesis of hydroxylated arenes with ester as an effective directing group. Org. Lett. 2012, 14, 2874–2877. 10.1021/ol301104n. [DOI] [PubMed] [Google Scholar]
- Gao D.-W.; Vinogradova E. V.; Nimmagadda S. K.; Medina J. M.; Xiao Y.; Suciu R. M.; Cravatt B. F.; Engle K. M. Direct access to versatile electrophiles via catalytic oxidative cyanation of alkenes. J. Am. Chem. Soc. 2018, 140, 8069–8073. 10.1021/jacs.8b03704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.; Ren S.; Zhang J.; Zhang W.; Liu Y. Selectfluor-mediated simultaneous cleavage of C–O and C–C Bonds in α, β-epoxy ketones under transition-metal-free conditions: a route to 1, 2-diketones. J. Org. Chem. 2015, 80, 6856–6863. 10.1021/acs.joc.5b00857. [DOI] [PubMed] [Google Scholar]
- Chen K.; Zhu S. NHC–AuCl/Selectfluor: An Efficient Catalytic System for π-Bond Activation. Synlett 2017, 28, 640–653. 10.1055/s-0036-1588693. [DOI] [Google Scholar]
- Zhu S.; Hu L.; Jiang H. Gold-catalyzed tandem Diels–Alder reactions of enynals/enynones with alkenes: generation and trapping of cyclic o-QDMs. Org. Biomol. Chem. 2014, 12, 4104–4111. 10.1039/C4OB00321G. [DOI] [PubMed] [Google Scholar]
- Ma J.; Jiang H.; Zhu S. NHC–AuCl/Selectfluor: a highly efficient catalytic system for carbene-transfer reactions. Org. Lett. 2014, 16, 4472–4475. 10.1021/ol502018d. [DOI] [PubMed] [Google Scholar]
- Yan M.; Zhou D.; Gao Y.; Ma Y. Metal-Free Synthesis of Methylene-Bridged Bisamide via Selectfluor-Mediated Oxidative Methylenation. ChemistrySelect 2018, 3, 13006–13009. 10.1002/slct.201802450. [DOI] [Google Scholar]
- Xie L.-Y.; Qu J.; Peng S.; Liu K.-J.; Wang Z.; Ding M.-H.; Wang Y.; Cao Z.; He W.-M. Selectfluor-mediated regioselective nucleophilic functionalization of N-heterocycles under metal-and base-free conditions. Green Chem. 2018, 20, 760–764. 10.1039/C7GC03106H. [DOI] [Google Scholar]
- Hu J.; Zhou G.; Tian Y.; Zhao X. Selectfluor-promoted regioselective chlorination/bromination of 2-aminopyridines and 2-aminodiazines using LiCl/LiBr. Org. Biomol. Chem. 2019, 17, 6342–6345. 10.1039/C9OB00972H. [DOI] [PubMed] [Google Scholar]
- Hong B.; Luo T.; Lei X. Late-stage diversification of natural products. ACS Cent. Sci. 2020, 6, 622–635. 10.1021/acscentsci.9b00916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daştan A.; Balci M. High temperature bromination. Part 18: Bromination of benzonorbornadiene derivatives: Polybrominated benzonorbornenes and benzonorbornadienes. Tetrahedron 2005, 61, 5481–5488. 10.1016/j.tet.2005.03.132. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.