Abstract
The study is aimed to investigate the protective effect and potential mechanisms of sodium butyrate (NaBT) on soyasaponins (SA) induced intestinal epithelial cells (IECs) injury in vitro. The primary IECs of turbot were developed and treated with 0.4, 1 and 4 mM NaBT in the presence of 0.4 mg/mL SA for 6 h to explore the protective effects of NaBT. The results showed that the addition of NaBT significantly down-regulated gene expression of inflammatory cytokine TNF-α, IL-1β and IL-8, pro-apoptosis relevant gene BAX, caspase-3, caspase-7 and caspase-9 induced by SA, while up-regulated anti-apoptosis gene Bcl-2. SA stimulation did not induce reactive oxygen species production, but elevated gene expression of antioxidant enzyme heme oxygenase-1 and superoxide dismutase. Moreover, the gene expression of those antioxidant enzyme was further up-regulated in NaBT groups. Furthermore, NaBT supplementation decreased the acid phosphatase and alkaline phosphatase activities and suppressed phosphorylation of p38 and c-Jun N-terminal kinase (JNK). In conclusion, NaBT could mitigate SA-induced inflammation and apoptosis and elevate gene expression of antioxidant enzymes on IECs of turbot and p38 and JNK signaling pathway participated in those processes.
Keywords: Sodium butyrate, Soyasaponins, Inflammation, Apoptosis, MAPKs, Turbot
1. Introduction
Soyasaponins (SA) is one of the main thermostable antinutritional factors (ANFs) present in soybean meal (SBM), ranging from 0.43% to 0.67% [1]. Previous studies have demonstrated that the inclusion of SA in diet could increase the intestinal epithelial permeability and induce intestinal inflammation response in the distal intestine of Atlantic salmon in combination with other plant components present in legumes [2], [3], [4]. Krogdahl, Gajardo [5] revealed that SA caused increased severity of inflammation independent of basal diet in a dose-dependent manner. Moreover, study carried out on turbot showed that saponins could also increase intestinal epithelial cells (IECs) apoptosis and decrease antioxidant enzyme activity in vivo [6]. These studies manifested that SA could be the causal agent in SBM.
Sodium butyrate (NaBT), the sodium salt of butyric acid, has many beneficial effects and has been considered as a promising functional feed additive in aquafeeds. Previous in vivo studies have found that dietary supplementation of NaBT could decrease the overexpression of pro-inflammatory cytokines, inhibit cellular apoptosis and promote the antioxidative capacity of fish [7], [8], [9], [10], [11]. In terms of mechanism, studies on mammals have shown that NaBT can inhibit inflammation, apoptosis and oxidative damage by regulating mitogen-activated protein kinases (MAPKs) signaling pathway. MAPKs are a group of protein serine/threonine kinases that play an important role in response to extracellular stimuli, mainly consisting of p38, extracellular signal-regulated kinase (ERK), and c-Jun N-terminal kinase (JNK) [12], [13], [14], [15], [16]. Previously, studies on fish showed that NaBT supplementation protected turbot from SBM-induced intestinal impairment [17] and enhanced intestinal barrier function of young grass carp by MAPKs signaling pathway [9,11]. Whereas, these studies on fish are limited to in vivo, and researches on signal pathway only stay at the level of gene expression.
IECs are the most important cells in the intestinal tract in contact with the external environment and are also important multifunctional cells in the intestinal tract, which are essential for maintaining the normal function of the intestinal mucosal barrier. Accordingly, the present study aims to investigate the protective effect and potential mechanisms of NaBT on SA-caused IECs injury of turbot in vitro.
2. Materials and methods
2.1. Fish
Turbot (100 g ± 5 g) obtained from Yellow Sea Fishery Research Institute (Yantai, China) were fed with commercial feed to visual satiation twice daily at 8:00 and 18:00 in a flow-through system at Fisheries College of Ocean University of China. The water temperature ranged from 20 to 22°C, dissolved oxygen > 7.0 mg/mL, salinity 30 to 33‰ and pH 7.7–7.9. Animal care and handing procedures in present study were approved by the Animal Care Committee of Ocean University of China.
2.2. Isolation and culture of primary IECs
Fish were anesthetized with eugenol (1:10,000) (purity 99%, Shanghai Reagent Corp, China) after fasting for 24 h prior to sampling. The intestine was cut out longitudinally and rinsed with solution of hanks’ balanced salt solution/penicillin-streptomycin (Thermo Fisher Scientific, USA) repeatedly. Then minced tissue about 1 mm3 were obtained and digested with collagenase and dispase (Thermo Fisher Scientific, USA) for 15 to 20 min. Pipette the enzyme solution mildly after digestion, leave the contents to sediment, collect the supernatant and centrifuge for 5 min at 1000 rpm, repeat this procedure several times with L5520 medium (Sigma-Aldrich, USA). Collect the sediment and rinsed with culture medium for several times, then the cell were seeded into 6-well plates at a density of ∼1.0×106 cells/mL with L5520 medium (5 % fetal bovine serum (Biological Industries, China), 10 ng/mL epidermal growth factor (Sigma-Aldrich, USA), 0.2 % insulin-transferrin-selenium-sodium pyruvate (Thermo Fisher Scientific, USA), 100 IU/mL penicillin and 100 μg/mL streptomycin), and incubated in a 23℃ incubator in ambient air.
2.3. SA challenge and NaBT treatments
The cells were seeded into 6-well plates at a density of ∼1.0×106 cells/mL. After the cells adhered to the well for 24 h, fresh medium was introduced. Firstly, the cells were challenged with SA at 0 (control), 0.2, 0.4, 0.6, 0.8, and 1 mg/mL concentration for 6 h and the optimal SA concentration was determined according to the gene expression of inflammatory cytokines. Then, the cells were treated with 0, 0.4, 1 and 4 mM NaBT (Sigma-Aldrich, USA) together with 0.4 mg/mL SA for 6 h. All treatments were designed in triplicate wells.
2.4. Quantitative real-time PCR
Total RNA from the cells was isolated using RNAiso Plus (9108; Takara, Japan). The integrity of RNA was evaluated by running on a 1.2% agarose gel, and concentration determination was followed with NanoDrop®ND-1000 (Nano-Drop Technologies, USA). cDNA was obtained by following the manufacturer's instructions of PrimeScript® RT reagent Kit with gDNA Eraser (Takara, Japan).
Specific primers for the genes were generated by TSINGKE (China) and presented in Table 1. β-actin was used as the reference gene to normalize cDNA loading. The detailed methods were described in our previous work [18], with some modifications. In brief, the qPCR assays were carried out in a final volume of 20 μL containing 10 μL 2 × SYBR Green Real-time PCR Master Mix [SYBR® Premix Ex Taq™ (Tli RNaseH Plus)] (TaKaRa, Japan), 0.8 μL (10 μM) each forward and reverse primer, 1 μL 100 ng/μL complementary DNA template, and 7.4 μL dH2O.
Table 1.
Primers used for qRT-PCR analysis.
| Target genes | Forward primer | Reverse primer | Accession number |
|---|---|---|---|
| TNF-α | GGACAGGGCTGGTACAACAC | TTCAATTAGTGCCACGACAAAGAG | AJ276709.1 |
| IL-1β | CGCTTCCCCAACTGGTACAT | ACCTTCCACTTTGGGTCGTC | AJ295836.2 |
| IFN-γ | GCTTTCCCGATCATCTTCTG | GGTTTCCCAGATTCCCATTC | DQ400686.1 |
| IL-8 | GGCAGACCCCTTGAAGAATA | TGGTGAACCCTTCCCATTAT | [6] |
| TGF-β1 | TCAGCATTCCAGATGTAGGTG | GGAGAGTGGCTTCAGTTTTTC | KU238187.1 |
| Bcl-2 | TTCCTCAACTCTCAAAGCACAATTC | ATTACACTCGCTCGCCATTCC | MN782168 |
| BAX | AGCATCTTTGCTGACGGGAT | GCGCTCTCTGATGACCTGAA | MN782169 |
| Caspase-3 | TTCTGCCATTGTCTCTGTGC | GCCCTGCAACATAAAGCAAC | JU391554.1 |
| Caspase-7 | TCTGCAATGTCCTCAACGAG | TTGCGACCATGTAGTTGACC | JU373310.1 |
| Caspase-9 | CCCAGGACATGATCGACGAG | ACAATGGGAAGGCTCGACTG | KY979512.1 |
| Prdx-6 | ACTGCCCGCTGTGTGTTTGTG | CGGCGTGGCAACCTTCTTCTG | ADJ57694.1 |
| HO-1 | ACGAGGGTCTGTCGTTCTTTGCC | CCTGGAGCGTCTTTACTGGTTTA | JX453446.1 |
| SOD | AAACAATCTGCCAAACCTCTG | CAGGAGAACAGTAAAGCATGG | MG253620.1 |
| β-actin | CGTGCGTGACATCAAGGAG | AGGAAGGAAGGCTGGAAGAG | AY008305.1 |
Abbreviations: TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; IFN-γ, interferon-γ; IL-8, interleukin-8; TGF-β1, transforming growth factor-β1; Prdx-6, Peroxiredoxin-6; HO-1, heme oxygenase-1; SOD, superoxide dismutase;
2.5. Reactive oxygen species (ROS) production
The ROS level was detected by ROS kit (Beyotime Biotechnology Co. Ltd., cat. no. S0033S), which used fluorescent probe 2’ - 7’dichlorofluorescin diacetate (DCFH-DA). DCFH-DA itself has no fluorescence and can freely pass through the cell membrane, and can be hydrolyzed by esterase in the cell to produce fluorescent DCFH. Before the experiment, DCFH-DA was diluted with serum-free medium according to 1:1000. Remove the cell culture medium, add 1 mL diluted DCFH-DA to each well and incubate in the cell incubator for 30 min. Then, the cells were washed with L-15 medium for three times and observed under fluorescence microscope (Leica, Germany). The quantitative analysis were performed by Image J software.
2.6. Detection of acid phosphatase (ACP) and alkaline phosphatase (AKP) activity
ACP (cat. no. p0326) and AKP (cat. no. p0321s) kits were purchased from Beyotime Biotechnology Co. Ltd. (China). Cell lysates were obtained by cell lysis buffer without inhibitors (Beyotime Biotechnology Co. Ltd., cat. no. P0013J), the specific steps were performed according to the instructions, and the absorbance was measured at 405 nm by microplate reader. The protein concentration of the lysate was determined using the BCA kit (Beyotime Biotechnology Co. Ltd., cat. no. P0012).
2.7. Western blot analysis
Total protein extract of the cells was harvested in RIPA lysis buffer (Beijing Solarbio Science & Technology Co., Ltd., China) with inhibitors of protease and phosphatase. After centrifugation at 12,000 g for 15 min at 4°C, the supernatant was collected and the protein concentration was determined using the BCA kit (Beyotime Biotechnology Co. Ltd., cat. no. P0012). Equal amounts of proteins of each sample were separated on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, followed by transfer to polyvinylidene difluoride membranes (PVDF) (Millipore, USA). After blocking with 5% non-fat milk in tris-buffered saline with tween-20 (TBST) buffer for 2 h, the PVDF membrane was incubated with primary antibody at 4℃ for 12 h. Then, the membrane was cleaned three times with TBST for 5 min each time. The membranes were then incubated with the secondary antibodies with HRP-conjugated horseradish peroxidase for 1 h at room temperature. After washing, the immunoreactivity was visualized with BeyoECL Star (Beyotime Biotechnology Co. Ltd., cat. no. P0018AM). The total gray values of each protein band were digitized by Image J software. Mouse p-p38 (cat. no. sc-166182), JNK (cat. no. sc-7345), p-JNK (cat. no. sc-6254), ERK (cat. no. sc-514302), p-ERK (cat. no. sc-7383) were obtained from Santa Cruz Biotechnology Inc. (USA). p38 (cat. no. AM065) and secondary antibodies (goat anti-mouse IgG-HRP, cat. no. A0216; goat anti-rabbit IgG-HRP, cat. no. A0208) were purchased from Beyotime Biotechnology Co. Ltd. (China). GAPDH (cat. no. 309154) were obtained from Golden Bridge Biotechnology (China).
2.8. Statistical analysis
The experimental data were analyzed by T-Test or One-way analysis of variance ANOVA with SPSS 22.0. Difference among different groups were analyzed by Tukey's multiple comparisons test. P-value < 0.05 was considered statistically significant, data were expressed as means ± S.E. (standard error).
3. Results
3.1. Cell morphology of primary culture of IECs
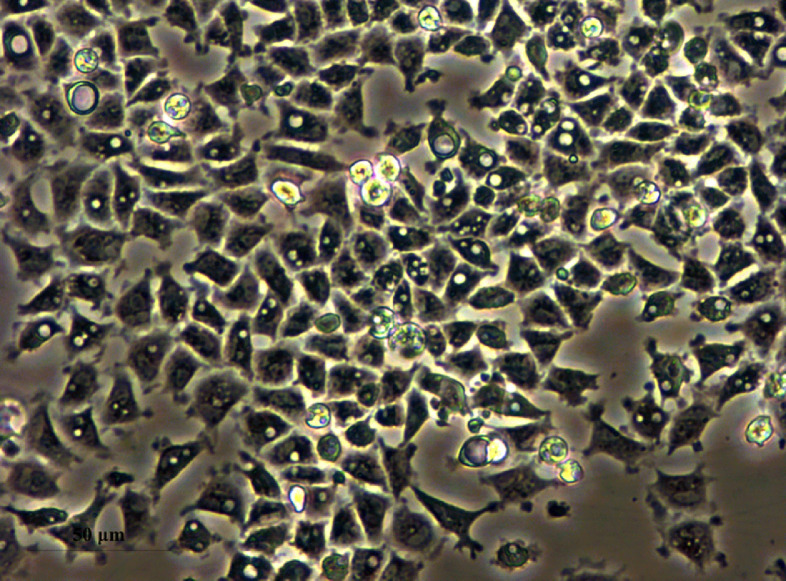
After seeding, the primary IECs could adhere well within 24 h, and showed flagstone-like appearance which is the typical features of epithelial-like cells (Fig. 1).
Fig. 1.
Morphology of primary culture intestinal epithelial cells of turbot.
3.2. Effects of NaBT on SA-induced inflammatory cytokine gene expression
SA at 0.4 mg/mL was selected for the following experiments as the gene expression of TNF-α, IL-1β and IL-8 was significantly up-regulated in response to 0.4 mg/mL SA treatment (Supplementary Fig. S1). Under this condition, NaBT was added to explore the anti-inflammatory effects. The results showed that the addition of 0.4 – 4 mM NaBT significantly down-regulated the gene expression of TNF-α, IL-1β and IL-8 (except 4 mM NaBT group) compared with SA group (Fig. 2).
Fig. 2.
The effect of NaBT on genes expression of inflammatory cytokine in SA-stimulated primary intestinal epithelial cells. The cells were treated with SA or co-treated with NaBT (0.4 mM, 1 mM, 4 mM) and SA for 6 h. Values are expressed as means ± SE (n=3). ## P<0.01, as compared with control; * P<0.05, ** P<0.01, *** P<0.001, as compared with SA. TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; IFN-γ, interferon-γ; IL-8, interleukin-8; TGF-β1, transforming growth factor-β1; SA, soyasaponins.
3.3. The effect of NaBT on apoptosis relevant gene expression
SA treatment significantly up-regulated the gene expression of BAX, caspase-3, caspase-7 and caspase-9, while down-regulated anti-apoptotic protein Bcl-2 gene expression (Fig. 3) compared with control group. Meanwhile, NaBT (0.4 - 4 mM) supplement significantly reversed the change of BAX, caspase-3, and Bcl-2 expression caused by SA. Moreover, the gene expression of caspase-7 was significantly down-regulated by 1 and 4 mM NaBT. Besides, the caspase-9 expression was also down-regulated in 0.4 mM NaBT treatment.
Fig. 3.
The effect of NaBT on apoptosis-relevant gene expression in SA-stimulated primary intestinal epithelial cells. The cells were treated with SA or co-treated with NaBT (0.4 mM, 1 mM, 4 mM) and SA for 6 h. Values are expressed as means ± SE (n=3). # P<0.05, ## P<0.01, as compared with control; * P<0.05, ** P<0.01, as compared with SA. SA, soyasaponins; NaBT, sodium butyrate.
3.4. Effects of NaBT on the level of ROS
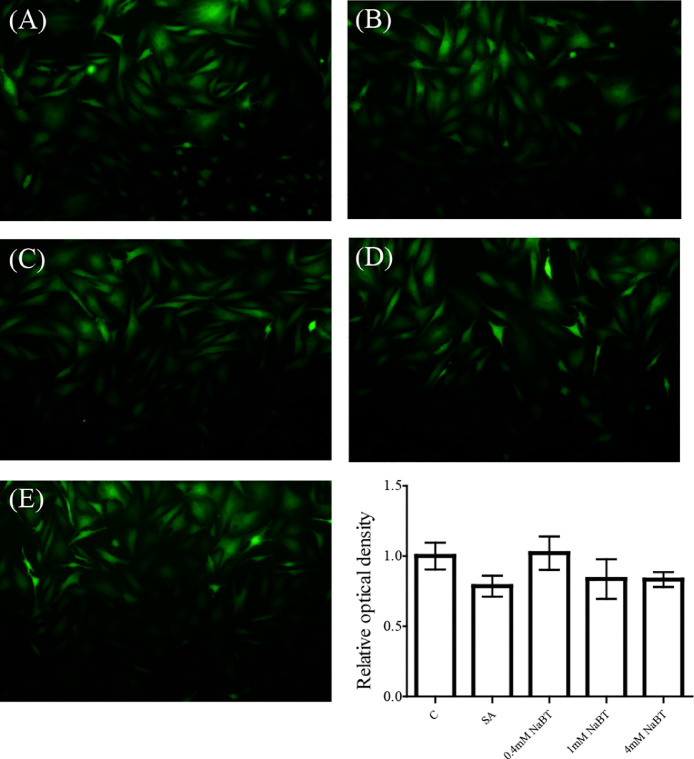
As showed in Fig. 4, SA treatment showed a slightly decreased ROS level, but there is no significant difference compared with control group. The addition of NaBT had no influence on ROS level compared to SA.
Fig. 4.
The effect of NaBT on ROS production. The cells were treated with SA or co-treated with NaBT (0.4 mM, 1 mM, 4 mM) and SA for 6 h. (A): C; (B): SA; (C): 0.4 mM NaBT; (D): 1 mM NaBT; (E): 4 mM NaBT.
3.5. Effects of NaBT on antioxidant enzyme relevant gene expression
The cells exposed to SA significantly up-regulated heme oxygenase-1 (HO-1) and superoxide dismutase (SOD) gene expression, and had no significant effect on the expression of peroxiredoxin-6 (Prdx-6) (Fig. 5). Compared with SA group, the addition of NaBT remarkable elevated Prdx-6, HO-1 and SOD (only in 4 mM NaBT group) expression. The mRNA levels were induced by 5–9-fold of NaBT treatments compared to controls.
Fig. 5.
The effect of NaBT on antioxidant-relevant gene expression in SA-stimulated primary intestinal epithelial cells. The cells were treated with SA or co-treated with NaBT (0.4 mM, 1 mM, 4 mM) and SA for 6 h. Values are expressed as means ± SE (n=3). # P<0.05, ## P<0.01, as compared with control; * P<0.05, ** P<0.01, *** P<0.01, as compared with SA. HO-1, Heme Oxygenase-1; SOD, superoxide dismutase; SA, soyasaponins; NaBT, sodium butyrate.
3.6. Effects of NaBT on ACP and AKP activities
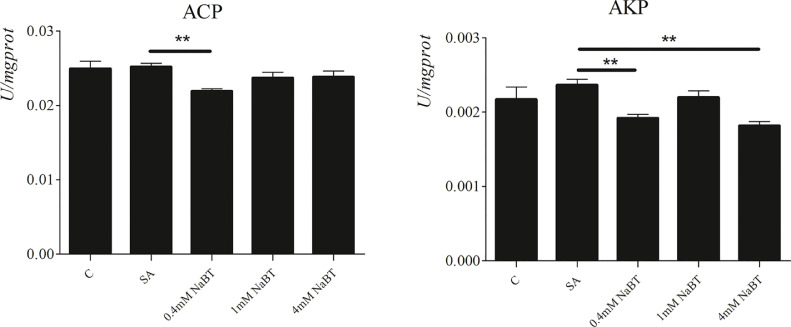
As shown in Fig. 6, compared with the control group, SA treatment had no significant effect on the activities of ACP and AKP. The addition of 0.4 mM NaBT significantly decreased ACP and AKP activities, and the decreased AKP activity also observed in 4 mM NaBT group compared with SA group.
Fig. 6.
The effect of NaBT on ACP and AKP production in SA-stimulated primary intestinal epithelial cells. The cells were treated with SA or co-treated with NaBT (0.4 mM, 1 mM, 4 mM) and SA for 6 h. Values are expressed as means ± SE (n=3). ** P<0.01, as compared with SA. ACP, acid phosphatase; AKP, alkaline phosphatase; SA, soyasaponins; NaBT, sodium butyrate.
3.7. Effects of NaBT on MAPKs signaling pathway
As shown in Fig 7, compared with the control group, the phosphorylation levels of p38, JNK and ERK did not show any difference in SA-stimulated cell. The addition of 1 and 4 mM NaBT significantly suppressed the phosphorylation of p38 and JNK, and 0.4 mM NaBT supplement decreased the phosphorylation of JNK compared with SA group.
Fig. 7.
The effect of NaBT on MAPK signaling in SA-stimulated primary intestinal epithelial cells. The cells were treated with SA or co-treated with NaBT (0.4 mM, 1 mM, 4 mM) and SA for 6 h. Values are expressed as means ± SE (n=3). * P<0.05, *** P<0.01, as compared with SA. SA, soyasaponins; NaBT, sodium butyrate.
4. Discussion
In vitro culture of IECs is of great significance for studying the absorption and metabolism of nutrients and intestinal pathological responses induced by various pathogenic factors. In this study, the IECs of the turbot were developed to explore the protective effects of NaBT in response to SA induced cell injure of turbot in vitro.
SA is a causal agent in SBM-induced enteritis. In this study, the IECs challenged with 0.4 mg/mL SA significantly up-regulated the gene expression of TNF-α and IL-1β, which were dramatically suppressed by the addition of NaBT. In fish, TNF-α and IL-1β are early pro-inflammatory cytokines at early stage of infection and play a key role in leading to inflammation by inducing a cascade of reaction and mediating the expression of other cytokines and chemokines [19,20]. Moreover, the expression of TNF-α and IL-1β is usually used to indicate the inflammation of fish in vivo and in vitro [21], [22], [23], [24], [25]. In present study, the significantly increased gene expression of TNF-α and IL-1β suggested SA successfully triggered inflammatory response on IECs of turbot. Generally speaking, acute inflammatory response is essential for tissue homoeostasis, however, if the inflammation cannot be resolved, it will develop chronic inflammation, which gives rise to many chronic inflammatory diseases [26], [27], [28]. NaBT, the sodium salt of butyrate, is a kind of potential anti-inflammatory substance. Previous studies on fish show that dietary NaBT could improve intestinal immunity and ameliorate SBM-induced intestinal inflammation [9,17]. In vitro research on RAW246.7 macrophages found that pre-treatment with NaBT (5 mM) could inhibit LPS-induced inflammation by down-regulating the expression of TNF-α and IL-6 [29]. Similarly, in present study, the gene expression of inflammatory cytokines TNF-α, IL-1β and IL-8 was significantly down-regulated following NaBT treatment under SA stimulated. These results confirmed the anti-inflammatory effects of NaBT on SA-stimulated IECs.
Intestinal epithelium is the key barrier to insulate the internal environment of the body from external harmful substances. The damage or excessive apoptosis of IECs can destroy barrier integrity and lead to inflammation [30]. Apoptosis usually inevitably happened in SBM-induced enteritis of turbot [6,31,32]. Previous in vivo study on turbot found that dietary saponin induced the IECs apoptosis in a dose-dependent manner by TdT-mediated dUTP nick end labeling [6]. Similarly, the cells apoptosis caused by SA was also observed in the current study, which was manifested as the increase of BAX, caspase-3, caspase-7 and caspase-9 and decrease of Bcl-2. Bcl-2 family, composed of anti-apoptotic and pro-apoptotic members, is the most characteristic protein family involved in the regulation of apoptotic cell death [33]. Bcl-2 and Bax are representative proteins of anti-apoptotic and pro-apoptotic protein families, respectively. The Bcl-2/Bax ratio determines the direction of apoptosis [34]. The caspase-cascade system plays an important role in the induction, transduction and amplification of intracellular apoptotic signals. At the beginning of apoptosis, initiator caspases (caspase-8 and -9) cleave and activate downstream effector caspases (caspase-3, -6, and -7), which directly cause apoptosis. It has been shown that NaBT could manipulate the expression of caspase-1, caspase-3, BAX, and Bcl-2 to ameliorate TNF-α induced apoptosis in SH-SY5Y cells [35]. Another experiment on juvenile grass carp showed that inclusion of NaBT in the diet down-regulated the gene expression of caspase-2, -3, -7, -8 -9 and BAX [11]. Here we found that administration with NaBT remarkably reversed those apoptosis-relevant gene expression in SA stimulated cells, exhibiting a potential apoptotic property.
Oxidative stress plays a pathogenic role in chronic inflammatory diseases. Previously, imbalance of redox homeostasis and impairment of intestinal antioxidant system were observed in SA induced intestinal inflammation of turbot [6]. Oxidative stress refers to the overproduced intracellular ROS and is caused by the imbalance between antioxidants and free radicals. However, in present study, SA stimulation did not cause oxidative stress in IECs of turbot as the level of ROS did not show any difference in all groups. Even so, the gene expression of antioxidant enzyme HO-1 and SOD were significantly up-regulated after SA treatment, and these gene expression were further increased after NaBT treatment. The gene expression and activity of antioxidant enzymes are regulated by multiple factors, such as oxidative status and inflammation [36]. In recent years, a large number of studies have found that antioxidant enzymes such as Prdx-6, HO-1 and SOD can not only scavenge free radicals and maintain redox balance, but also have immunomodulatory function [37], [38], [39]. As mentioned in the literature review of Sebastian, Salazar [40], HO-1 activation could reduce intestinal inflammation in several animal models of inflammatory bowel disease by inducing anti-inflammatory cytokine pathway. Overexpression of SOD3 suppressed the expression of inflammatory cytokines through modulating many signaling pathway [38]. Based on these studies, we speculated that the significantly up-regulated expression of antioxidant enzymes in this study might play an important anti-inflammatory role in NaBT group. A possible explanation for the up-regulated expression of HO-1 and SOD in SA group was that the increased expression of these enzyme was insufficient to counteract the inflammation. ACP and AKP are lysosomal enzymes, participate in the body's immune response and are regarded as a biomarker for toxicological evaluation of external toxicants. The increased ACP and AKP activity usually indicated a damage of lysosomal membranes and immunological toxicity [41]. In current study, although the stimulation of SA did not affect the activities of ACP and AKP, their activities were significantly suppressed after NaBT treatment, which indicated that the addition of NaBT had a cytoprotective effect on SA-treated cells.
MAPKs cascades are key signaling pathway mediating the transduction of extracellular signals to the intracellular environment. It has been reported that NaBT could mediate MAPKs signaling pathway. Wang, Wu [42] reported that dietary supplementation with NaBT could inhibit the phosphorylation of JNK in jejunal mucosa of weaned pigs accompanied with improved intestinal barrier and decreased inflammatory mediator production. Similarly, Wu, Tian [11] found that JNK signaling pathway involved in NaBT mediated inhibition of intestinal cell apoptosis, improvement of antioxidant level and intestinal structural integrity in grass carp. In present study, we found that the addition of NaBT significantly suppressed the relative protein levels of phosphorylated JNK and p38. The result was consistent with previous study, NaBT supplementation down-regulated p38 gene expression and improved intestinal immune function in young grass carp [9]. These data suggested that the addition of NaBT could protect IECs of turbot from SA-induced cell inflammation and apoptosis through mediating JNK and p38 signaling pathway.
In conclusion, the current study showed that NaBT treatment inhibited the gene expression of inflammatory cytokines and apoptosis mediators induced by SA, up-regulated the expression of prdx6, HO-1 and SOD and suppressed the activities of ACP and AKP. In addition, the JNK and p38 signaling pathways participated in the protective effects of NaBT on SA-stimulated IECs of turbot.
Funding
This work was supported by National Key R&D Program of China [2019YFD0900104]; the National Natural Science Foundation of China [No. 31872577]; and China Agriculture Researches System [Grant No. CARS 47-G10].
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We appreciate Zhichu Chen and Qiuchi Chen for their assistance in this study.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.fsirep.2021.100031.
Appendix. Supplementary materials
References
- 1.Ireland P.A., Dziedzic S.Z., Kearsley M.W. Saponin content of soya and some commercial soya products by means of high performance liquid chromatography of the sapogenins. J. Sci. Food Agric. 1986;37 694-89. [Google Scholar]
- 2.Couto A., Kortner T.M., Penn M., Østby G., Bakke A.M., Krogdahl Å., et al. Saponins and phytosterols in diets for European sea bass (Dicentrarchus labrax) juveniles: effects on growth, intestinal morphology and physiology. Aquacult. Nutr. 2015;21:180–193. [Google Scholar]
- 3.Knudsen D., Jutfelt F., Sundh H., Sundell K., Koppe W., Frokiaer H. Dietary soya saponins increase gut permeability and play a key role in the onset of soyabean-induced enteritis in Atlantic salmon (Salmo salar L.) Br. J. Nutr. 2008;100:120–129. doi: 10.1017/S0007114507886338. [DOI] [PubMed] [Google Scholar]
- 4.Chikwati E.M., Venold F.F., Penn M.H., Rohloff J., Refstie S., Guttvik A., et al. Interaction of soyasaponins with plant ingredients in diets for Atlantic salmon, Salmo salar L. Br. J. Nutr. 2011;107:1570–1590. doi: 10.1017/S0007114511004892. [DOI] [PubMed] [Google Scholar]
- 5.Krogdahl A., Gajardo K., Kortner T.M., Penn M., Gu M., Berge G.M., et al. Soya saponins induce enteritis in Atlantic Salmon (Salmo salar L.) J. Agric. Food Chem. 2015;63:3887–3902. doi: 10.1021/jf506242t. [DOI] [PubMed] [Google Scholar]
- 6.Gu M., Jia Q., Zhang Z., Bai N., Xu X., Xu B. Soya-saponins induce intestinal inflammation and barrier dysfunction in juvenile turbot (Scophthalmus maximus) Fish Shellfish Immunol. 2018;77:264–272. doi: 10.1016/j.fsi.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Ullah S., Zhang G., Zhang J., Tong S., Wang L., Kalhoro H., et al. Effects of microencapsulated sodium butyrate supplementation on growth performance, intestinal development and antioxidative capacity of juvenile black sea bream (Acanthopagrus schlegelii) Aquac. Res. 2020;51:4893–4904. [Google Scholar]
- 8.Liu W., Yang Y., Zhang J., Gatlin D.M., Ringo E., Zhou Z. Effects of dietary microencapsulated sodium butyrate on growth, intestinal mucosal morphology, immune response and adhesive bacteria in juvenile common carp (Cyprinus carpio) pre-fed with or without oxidised oil. Br. J. Nutr. 2014;112:15–29. doi: 10.1017/S0007114514000610. [DOI] [PubMed] [Google Scholar]
- 9.Tian L., Zhou X.-Q., Jiang W.-D., Liu Y., Wu P., Jiang J., et al. Sodium butyrate improved intestinal immune function associated with NF-κB and p38MAPK signalling pathways in young grass carp (Ctenopharyngodon idella) Fish Shellfish Immunol. 2017;66:548–563. doi: 10.1016/j.fsi.2017.05.049. [DOI] [PubMed] [Google Scholar]
- 10.Mirghaed A.T., Yarahmadi P., Soltani M., Paknejad H., Hoseini S.M. Dietary sodium butyrate (Butirex((R)) C4) supplementation modulates intestinal transcriptomic responses and augments disease resistance of rainbow trout (Oncorhynchus mykiss) Fish Shellfish Immunol. 2019;92:621–628. doi: 10.1016/j.fsi.2019.06.046. [DOI] [PubMed] [Google Scholar]
- 11.Wu P., Tian L., Zhou X.Q., Jiang W.D., Liu Y., Jiang J., et al. Sodium butyrate enhanced physical barrier function referring to Nrf2, JNK and MLCK signaling pathways in the intestine of young grass carp (Ctenopharyngodon idella) Fish Shellfish Immunol. 2018;73:121–132. doi: 10.1016/j.fsi.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 12.Kaminska B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy - from molecular mechanisms to therapeutic benefits. Biochim. Et Biophys. Acta-Proteins Proteom. 2005;1754:253–262. doi: 10.1016/j.bbapap.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 13.Sui X., Kong N., Ye L., Han W., Zhou J., Zhang Q., et al. p38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents. Cancer Lett. 2014;344:174–179. doi: 10.1016/j.canlet.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 14.Kyosseva S.V. Targeting MAPK signaling in age-related macular degeneration. Ophthalmol. Eye Dis. 2016;8:23–30. doi: 10.4137/OED.S32200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rezatabar S., Karimian A., Rameshknia V., Parsian H., Majidinia M., Kopi T.A., et al. RAS/MAPK signaling functions in oxidative stress, DNA damage response and cancer progression. J. Cell. Physiol. 2019;234:14951–14965. doi: 10.1002/jcp.28334. [DOI] [PubMed] [Google Scholar]
- 16.Yue J., Lopez J.M. Understanding MAPK signaling pathways in apoptosis. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21072346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y., Chen Z., Dai J., Yang P., Xu W., Ai Q., et al. Sodium butyrate supplementation in high-soybean meal diets for turbot (Scophthalmus maximus L.): Effects on inflammatory status, mucosal barriers and microbiota in the intestine. Fish Shellfish Immunol. 2019;88:65–75. doi: 10.1016/j.fsi.2019.02.064. [DOI] [PubMed] [Google Scholar]
- 18.Yu G., Ou W., Liao Z., Xu H., Liang M., Zhang Y., et al. Intestinal homeostasis of juvenile tiger puffer Takifugu rubripes was sensitive to dietary arachidonic acid in terms of mucosal barrier and microbiota. Aquaculture. 2019;502:97–106. [Google Scholar]
- 19.Reyes-Cerpa S., Maisey K., Reyes-Lpez F., Toro-Ascuy D., Mara A., Imarai M. Fish cytokines and immune response. New Adv. Contribut. Fish Biol. 2012 [Google Scholar]
- 20.Zou J., Secombes C.J. The function of fish cytokines. Biology. 2016;5:23. doi: 10.3390/biology5020023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng H., Guo Q., Duan X., Xu Z., Wang Q. l-arginine inhibited apoptosis of fish leukocytes via regulation of NF-κB-mediated inflammation, NO synthesis, and anti-oxidant capacity. Biochimie. 2019;158:62–72. doi: 10.1016/j.biochi.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 22.Jiang J., Yin L., Li J.-Y., Li Q., Shi D., Feng L., et al. Glutamate attenuates lipopolysaccharide-induced oxidative damage and mRNA expression changes of tight junction and defensin proteins, inflammatory and apoptosis response signaling molecules in the intestine of fish. Fish Shellfish Immunol. 2017;70:473–484. doi: 10.1016/j.fsi.2017.09.035. [DOI] [PubMed] [Google Scholar]
- 23.Jiang J., Shi D., Zhou X.-Q., Hu Y., Feng L., Liu Y., et al. In vitro and in vivo protective effect of arginine against lipopolysaccharide induced inflammatory response in the intestine of juvenile Jian carp (Cyprinus carpio var. Jian) Fish Shellfish Immunol. 2015;42:457–464. doi: 10.1016/j.fsi.2014.11.030. [DOI] [PubMed] [Google Scholar]
- 24.Zhao S., Chen Z., Zheng J., Dai J., Ou W., Xu W., et al. Citric acid mitigates soybean meal induced inflammatory response and tight junction disruption by altering TLR signal transduction in the intestine of turbot, Scophthalmus maximus L. Fish Shellfish Immunol. 2019;92:181–187. doi: 10.1016/j.fsi.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Li Y., Hu H., Liu J., Yang P., Zhang Y., Ai Q., et al. Dietary soya allergen β-conglycinin induces intestinal inflammatory reactions, serum-specific antibody response and growth reduction in a carnivorous fish species, turbot Scophthalmus maximus L. Aquac. Res. 2017;48:4022–4037. [Google Scholar]
- 26.Minihane A.M., Vinoy S., Russell W.R., Baka A., Roche H.M., Tuohy K.M., et al. Low-grade inflammation, diet composition and health: current research evidence and its translation. Br. J. Nutr. 2015;114:999–1012. doi: 10.1017/S0007114515002093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rogler G. Resolution of inflammation in inflammatory bowel disease. Lancet Gastroenterol. Hepatol. 2017;2:521–530. doi: 10.1016/S2468-1253(17)30031-6. [DOI] [PubMed] [Google Scholar]
- 28.Camba-Gomez M., Gualillo O., Conde-Aranda J. New perspectives in the study of intestinal inflammation: focus on the resolution of inflammation. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22052605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen G., Ran X., Li B., Li Y., He D., Huang B., et al. Sodium butyrate inhibits inflammation and maintains epithelium barrier integrity in a TNBS-induced inflammatory bowel disease mice model. EBioMedicine. 2018;30:317–325. doi: 10.1016/j.ebiom.2018.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blander J.M. On cell death in the intestinal epithelium and its impact on gut homeostasis. Curr. Opin. Gastroenterol. 2018;34:413–419. doi: 10.1097/MOG.0000000000000481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu G., Liu Y., Ou W., Dai J., Ai Q., Zhang W., et al. The protective role of daidzein in intestinal health of turbot (Scophthalmus maximus L.) fed soybean meal-based diets. Sci. Rep. 2021;11:3352. doi: 10.1038/s41598-021-82866-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Z.C., Zhao S.F., Liu Y., Yang P., Ai Q.H., Zhang W.Q., et al. Dietary citric acid supplementation alleviates soybean meal-induced intestinal oxidative damage and micro-ecological imbalance in juvenile turbot, Scophthalmus maximus L. Aquac. Res. 2018;49:3804–3816. [Google Scholar]
- 33.Tsujimoto Y. Role of Bcl-2 family proteins in apoptosis: apoptosomes or mitochondria? Genes Cells. 1998;3:697–707. doi: 10.1046/j.1365-2443.1998.00223.x. [DOI] [PubMed] [Google Scholar]
- 34.Korsmeyer S.J., Shutter J.R., Veis D.J., Merry D.E., Oltvai Z.N. Bcl-2/Bax - a rheostat that regulates an antioxidant pathway and cell-death. Semin. Cancer Biol. 1993;4:327–332. [PubMed] [Google Scholar]
- 35.Bayazid A.B., Jang Y.A., Kim Y.M., Kim J.G., Lim B.O. Neuroprotective effects of sodium butyrate through suppressing neuroinflammation and modulating antioxidant enzymes. Neurochem. Res. 2021;46:2348–2358. doi: 10.1007/s11064-021-03369-z. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez C., Mayo J.C., Sainz R.M., Antolin I., Herrera F., Martin V., et al. Regulation of antioxidant enzymes: a significant role for melatonin. J. Pineal Res. 2004;36:1–9. doi: 10.1046/j.1600-079x.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- 37.Campbell N.K., Fitzgerald H.K., Dunne A. Regulation of inflammation by the antioxidant haem oxygenase 1. Nat. Rev. Immunol. 2021;21:411–425. doi: 10.1038/s41577-020-00491-x. [DOI] [PubMed] [Google Scholar]
- 38.Nguyen Hoai N., Gia-Buu T., Cuong Thach N. Anti-oxidative effects of superoxide dismutase 3 on inflammatory diseases. J. Mol. Med. 2020;98:59–69. doi: 10.1007/s00109-019-01845-2. [DOI] [PubMed] [Google Scholar]
- 39.Knoops B., Argyropoulou V., Becker S., Ferte L., Kuznetsova O. Multiple roles of peroxiredoxins in inflammation. Mol. Cells. 2016;39:60–64. doi: 10.14348/molcells.2016.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sebastian V.P., Salazar G.A., Coronado-Arrazola I., Schultz B.M., Vallejos O.P., Berkowitz L., et al. Heme oxygenase-1 as a modulator of intestinal inflammation development and progression. Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.01956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou Y., Dahms H.-U., Dong F., Jing W., Wang L. Immune-associated parameters and antioxidative responses to cadmium in the freshwater crab Sinopotamon henanense. Ecotoxicol. Environ. Saf. 2016;129:235–241. doi: 10.1016/j.ecoenv.2016.03.040. [DOI] [PubMed] [Google Scholar]
- 42.Wang C.C., Wu H., Lin F.H., Gong R., Xie F., Peng Y., et al. Sodium butyrate enhances intestinal integrity, inhibits mast cell activation, inflammatory mediator production and JNK signaling pathway in weaned pigs. Innate Immun. 2018;24:40–46. doi: 10.1177/1753425917741970. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.