Highlights
-
•
Stinging catfish Heteropneustes fossilis were found to resist endotoxin up to 0.1mg.
-
•
Endotoxin at 0.05mg per fish enhanced some of the non-specific immune responses while at low dose (0.01mg per fish) failed to stimulate immune responses.
-
•
Endotoxin at 0.05mg immunostimulatory while at 0.1 mg immunosuppressive for stinging catfish Heteropneustes fossilis.
-
•
Endotoxin at 0.05mg per fish was found to protect stinging catfish from Aeromonas hydrophila challenge.
Keywords: Endotoxin, Lipopolysachharide, Heteropneustes fossilis, Immunity, Haematology
Abstract
Endotoxin, the outer cell wall membrane lipopolysaccharide component of the Gram-negative bacteria is a factor responsible for a number of complications/disorders and plays important role in the associated with pathophysiological complications and pathogenesis of many diseases in animals. Unlike higher animals which are extremely sensitive to endotoxin, fish are found to be resistant to endotoxic shock and earlier studies though limited have demonstrated the patho-physiological, immuno-endocrinological and immuno-neurological effects of LPS/endotoxin in aquatic animals including fish. Herein in the present investigation, the effect of pure endotoxin on immuno-haematological parameters of stinging catfish, Heteropneustes fossilis ranging from 50–60 g was studied by intraperitoneally injecting 0.1, 0.05 and 0.01 mg endotoxin per fish. H. fossilis yearlings were found to resist the endotoxin concentration up to 0.1 mg without any mortality. While, no change in immune parameters was recorded in stinging catfish injected with low dose of endotoxin (0.01 mg), most of the immune parameters were found to be significantly elevated in catfish injected with 0.05 mg endotoxin. Different serum and immune parameters like protein, globulin, lysozyme, respiratory burst activity, myeloperoxidase activity, natural agglutination titre were found to be significantly high (p < 0.01) at a dose of 0.05 mg endotoxin per fish. On the contrary, most of these parameters were decreased at high dose i.e., 0.1 mg endotoxin per fish, thereby indicating the immuno-suppressive effect of the endotoxin. The findings of the modulation of innate immunity also corroborated with the results of Aeromonas hydrophila pathogen challenge study with highest percent of mortality in group injected with 0.1 mg endotoxin per fish and least percentage in group injected with 0.05 mg endotoxin per fish.
1. Introduction
Endotoxin is the highly conserved outer cell wall membrane lipopolysachharide (LPS) component of Gram -negative bacteria which is involved in pathogenesis of many diseases. Endotoxin (synonym ‘LPS’) being an amphiphilic cell surface antigen and common to all Gram -negative bacteria comprised of components that confer both immunogenic and endotoxic properties to the molecule [[1], [2], [3]]. While, higher vertebrates often experience endotoxic shock during Gram negative bacterial infections, various patho-physiological complications including cytotoxic, hypotension, thrombocytopenia, hypoferremia, sepsis, liver disease, vascular disease, etc are often induced by endotoxin in sensitive hosts [4], [5], [6], [7], [8], [9], [10], [11], [12]. Among various bacterial cell surface structures (fimbriae, flagella, capsule or LPS/endotoxin), endotoxin/LPS is believed to one of the most potent stimulators of innate immunity [13]. It can execute immuno-stimulatory or suppressive effect on host [14] and its profound effect on immune components like T cells, B cells, macrophages etc and production of antibody, cytokines etc are well demonstrated [15], [16].
Higher animals are extremely sensitive to endotoxin even at a very low concentration but lower vertebrates like frog and fish are found to be resistant to endotoxic shock [17]. Although fish are often reported to be resistant to endotoxin, the constant exposure to Gram-negative bacteria and their endotoxin can affect the health status of fish. It is already demonstrated that the LPS is responsible for the pathogenicity of several Gram-ve bacterial pathogens in several aquatic animals including fish [18]. Further, endotoxin/LPS in pure or crude form could induce stress leading to a wide variety of metabolic effects, patho-physiological, immunological, immuno-endocrinological and neuro-immunological effects in aquatic vertebrates including fish [[7], [8], [9],19,20]. However, literatures in this regard are very limited in different fish species and many times experimental data are conflicting and divergent [6,9,[19], [20], [21], [22]]. Therefore, the present study investigates the effects of endotoxin at varying concentration on the hematology, innate immunity and protection of stinging catfish, Heteropneustes fossilis a catfish species of considerable economical importance.
2. Materials and methods
2.1. Fish
Stinging catfish, (Heteropneustes fossilis) of weight ranging from 50-60 g were used in the present investigation.
2.2. Endotoxin
Pure E. coli endotoxin (E. coli 055:B5 endotoxin) procured from Cambrex, (Cambrex Bioscience Walkersville Inc., Walksville, USA), was used in the present investigation.
2.3. Experimental design
H. fossilis yearlings were acclimatized under laboratory conditions 15 days prior to the start of the experiment. Ten yearlings were maintained per tank (1000 L) and fed with artificial carp diet with daily two – third water exchange. Ten catfish per group in duplicate set were intraperetoneally injected with 0.1ml of endotoxin in such a manner that individual fish in a particular group received 0.1 mg endotoxin, 0.05 mg endotoxin and 0.01 mg endotoxin while another group injected with same amount of phosphate buffered saline (PBS, pH 7.2) was kept as control group. Blood was collected from H. fossilis from all groups at 7th and 15th days post injection (dpi) to assess various immuno-haematological parameters. During the entire study period the alkalinity (75–80 mg ml−1), pH (7.4 - 7.8) and temperature (270–290 C) of the rearing tanks were found to be within the permissible limit.
2.4. Haematological parameters
2.4.1. Collection of blood
Blood from H. fossilis in different groups was collected aseptically by caudal fin after 7th and 15th dpi. One part of the blood was collected and mixed with anticoagulant (heparin solution) to evaluate different haematological parameters like total erythrocytes count, total leucocytes count and haemoglobin content were done as per the method of Blaxhall and Daisley [23]. Similarly another part was allowed to clot at room temperature for 45 min. After that it was centrifuged at 2000 × g for 5 min. The supernatant was collected for evaluating different serum parameters.
2.4.2. Differential leucocytes count (DLC)
The slides were stained with combination of Wright's and Giemsa stains. At first, modified Wright's stain was applied into the smears and kept for 10 min. it was followed by dilution (1:10 with distilled water, pH 7–7.2) and kept for another 20 min. the smear was then stained with diluted Giemsa (1:10 in distilled water) for 90 min. finally, the slides were washed, air dried and observed under the microscope (× 1000). Different types of leucocytes were counted on randomly selected fields from the smear.
2.4.3. Total erythrocytes count (TEC)
Blood was drawn up to 0.5 marks in RBC diluting pipette of haemocytometer (Hi media, India). The pipette was immediately filled up to the 101 mark with RBC diluting fluid. The pipette was shaken for 30 s and from it few drops of diluted blood were expelled. Then tip of the pipette was touched to Neubauer's slide and cover slip junction. Diluted blood was drawn inside automatically by capillary action and counting was done under 10 × objective lens of microscope. The Neubauer's slide was divided into ruled areas of 1 sq. mm with the center 1 sq. mm divided into 25 groups of 16 small squares each. The cells within the boundaries of five of these small squares (80 smallest squares) were counted. The total erythrocyte count was done using the following formula
TEC = total number of cells in five small squares × 10,000 / cu. mm of blood
2.4.4. Total leucocytes count (TLC)
The blood was sucked up to 0.5 mark and diluted up to 11 mark with diluting fluid (0.1 N HCl containing 1% Giemsa) in the pipette meant for WBC counting. The counting was done in four big squares at the four corners of the Neubauer's slide each of which contain 16 small squares.
2.4.5. Hemoglobin content
Total haemoglobin percentage was calculated as per the method of Blaxhall and Daisley [23]. blood was drawn up to 20 mm mark of pipette of haemoglobinometer and then put inside the calibrated test tube prefilled up to 10 mm mark with 0.1N HCl. the samples were gently mixed with a glass rod for 3–5 min for lysis. To it, 0.1 N HCl was gradually added and diluted till the colour matched with the side tubes and the results as obtained from the graduated tube directly were expressed in gram percentage of blood.
2.5. Serum parameters study
2.5.1. Total serum protein, albumin and globulin content
The serum protein content of various groups was estimated as per the standard method of Bradford [24]. Briefly, 0.2 ml of serum from individual fish of various experimental groups was separately added to 2.0 ml commissa blue-g-reagent (1 % blue g. 5 % ethanol and 10 % phosphoric acid) and the absorbance was read at 550 nm. The protein content was calculated by regression analysis with bovine serum albumin as standard. The albumin content was estimated spectrophotometrically using standard kits (Glaxo, India). The globulin content was estimated as per the following method. 50 µl saturated ammonium sulphate solution was added drop wise to 50 µl serum followed by vortexing. Centrifugation was done at 10,000 × g for 5 min. Then, 20 µl of this sample was dissolved with 80 µl carbonate-bicarbonate buffer (pH 9.3) and the globulin content was determined by estimating the protein content as done above. Finally the albumin: globulin (A:G) ratio was calculated.
2.5.2. Serum enzymatic activities
Different serum enzymatic activities such as Alkaline phosphatase, Serum glutamate pyruvate transaminase (SGPT) and Serum glutamate oxaloacetate transaminase (SGOT) were estimated as per standard enzyme estimation method by using commercial kit [25].
2.5.3. Myeloperoxidase activity
The myeloperoxidase activity was studies as per the method of Quade and Roth [26]. Briefly, 15 µl of serum was diluted in 135 µl of Hank's balanced salt solution (Ca++ and Mg++ free) and to it 50 µl of 20 mM 3, 3’, 5, 5’ tetra methyl benzidine and 5 mM H2O2 were added. The reaction was stopped after 2 min by adding 50 µl of 4 M sulphuric acid and the optical density (OD) was read at 450 nm in a spectrophotometer.
2.5.4. Lysozyme activity
A turbidimetric assay utilizing lyophilized Micrococcus lysodeikticus (Sigma, USA) was done to determine lysozyme activity in serum as described by Shankaran and Shanto [27] and Studinicka et al. [28] with slight modifications. M. lysodeikticus at a concentration of 0.2 mg ml-1 (in 0.02 M sodium citrate buffer) was added to serum samples at 10:1 ratio. Immediately after adding M. lysodeikticus, initial OD was taken at 450 nm. After incubating for 1 h at 240C, OD was taken. Lysozyme activity was expressed as units/ml where one unit is defined as the decrease in absorbance of 0.001 min-1.
2.5.5. Respiratory burst assay
The respiratory burst activity was measured by the reduction of NBT by intra cellular super oxide radicals [29]. Briefly, 100 µl of heparinished blood from fish of each group was mixed in 100 µl of 0.2% NBT (Sigma, USA) solution for 30 min at 250C. After incubation, 50 µl from the above mixture was added with 1ml of N.N., Diethylmethyl formamide (Qualigens, India) and then centrifuged at 3 000 × g for 5 min. The optical density of the supernatant was measured at 540 nm in a spectrophotometer.
2.5.6. Bacterial agglutination activity
The natural bacterial agglutinating activity of the sera samples of all the groups was studied in ‘U’ shaped microtitre plates. Two-fold serial dilution of 50 µl serum of fish was made with equal volume of PBS (pH 7.2) in each well, to plates were incubated overnight at room temperature. The titre was calculated as the reciprocal of the highest dilution of serum complete agglutination of the bacterial cells.
2.5.7. Hemagglutination activity
The hemagglutination activity of serum samples from various endotoxin injected and control groups was carried out using a standardized method [30]. This assay was done in 'U' shaped microtitre plates by serial two-fold dilution of 50 µl serum (inactivated at 450C for 30 min) with PBC (pH 7.2).Then 50 µl of freshly prepared 1 % New Zealand white rabbit RBC suspension was added to each well. The plates were kept at room temperature (28–300C) for 2 h or over night at 40C, in case agglutination was not visible within 2 h. The titre was calculated as the reciprocal of the highest dilution of serum showing complete agglutination of RBC.
2.6. Challenge study
After one week of final sampling, fishes from all the experimental groups were challenged with virulent Aeromonas hydrophila pathogen to find out the mortality pattern. Approximately, 0.1 ml of overnight grown A. hydrophila @ 106 CFU ml−1 (LD50 dose) was intra-peritoneally injected to all the endotoxin injected and control fishes. After challenge the mortality was recorded up to 10 days post challenge to find out the percentage of mortality.
2.7. Statistical analysis
The statistical analysis system (SAS) software (version 6.12) was used to analyse all the data. One-way analysis of variance (ANOVA) followed by DMRT was done to compare the variations in various serum and immune parameters at significance level of difference at 0.01 % level in different endotoxin injected groups [31].
3. Results
No mortality or abnormality could be recorded in any of the endotoxin injected groups before the pathogen challenge study. H. fossilis yearlings were found to resist the endotoxin up to the 0.1 mg endotoxin per fish. Fish were found to intake feed actively during feeding and no sign and symptoms or abnormalities of disease/injury during the experiment.
3.1. Haematological parameters
The RBC content (× 106 cell ml−1) was lowest in group injected with 0.1 mg endotoxin at 7th dpi. However, the RBC counts didn't vary significantly in any of the groups at both the sampling period. On the other hand, the WBC content (× 103 cell ml−1) was significantly lowered (p < 0.01) in group injected with 0.1 mg endotoxin at both 7th and 15th dpi while the count was highest in group injected with 0.05 mg endotoxin. No variation in the count could be recorded in 0.01 mg endotoxin injected and control groups. Similarly, no significant variation (p > 0.01) in the haemoglobin content could be recorded in any of the experimental groups and the content varied from 9.3 to 10.0 gm percentage in all groups (Table 1).
Table 1.
The mean (±SD) of total erythrocyte count (TEC), total leucocytes count (TLC) and hemoglobin content in H. fossilis injected with various concentrations of endotoxin at 7th and 15th days post injection (dpi).
| Parameters | 0.1 mg | 0.05 mg | 0.01 mg | Control | ||||
|---|---|---|---|---|---|---|---|---|
| 7th dpi | 15th dpi | 7thdpi | 15th dpi | 7th dpi | 15thdpi | 7th dpi | 15th dpi | |
| TEC (× 106 cells/ ml) |
1.65 ± 0.37 |
1.95 ± 0.37 |
1.84 ± 0.52 |
2.01 ± 0. 26 |
1.79 ± 0.38 |
1.88 ± 0.49 |
1.92 ± 0.46 |
2.16 ± 0.37 |
| TLC (× 103 cells/ ml) |
13.07 ± 0.33c |
16.3 ± 0.2.4 c |
21.1 ± 0.31a |
23.8 ± 0.03a |
17.8 ± 0.1b |
20.1 ± 0.2b |
18.4 ± 0.4 b |
19.0 ± 0.51b |
| Haemoglobin (gm %) | 9.5 | 9.5 | 9.5 | 10 | 8.9 | 9.3 | 9.3 | 9.6 |
*Superscripts indicate significance difference among different endotoxin injected groups with respect to specific sampling period.
Similarly, the differential leucocytes count also varied among the groups. The lymphocytes percentage was significantly lowest (p < 0.01) in group injected with 0.1 mg endotoxin irrespective of sampling period. Conversely, the percentage of lymphocytes, monocytes and neutrophils were significantly higher (p < 0.01) in 0.05 mg endotoxin injected group as compared to other groups (Table 2). The percentage of monocytes and neutrophils did not vary significantly (p > 0.01) among the 0.01 mg endotoxin injected and control groups. Similarly, the percentage of neutrophils was least (p < 0.01) in group injected with 0.1 mg endotoxin irrespective of sampling periods from the other groups.
Table 2.
The mean (± SD) of differential leucocyte counts of H. fossilis injected with different concentrations of endotoxin at 7th and 15th days post injection (dpi).
| Types of cells | 0.1 mg | 0.05 mg | 0.01 mg | Control | ||||
|---|---|---|---|---|---|---|---|---|
| 7th dpi | 15thdpi | 7th dpi | 15th dpi | 7th dpi | 15thdpi | 7th dpi | 15th dpi | |
| Lymphocytes (%) |
61 ± 1.2b |
65 ± 1.6 b |
79 ± 1.51a |
81 ± 4.91a |
73 ± 2.41a |
80 ± 3.78a |
40 ± 2.9c |
40 ± 1.17c |
| Monocyte (%) | 8 .0 ± 2.6 b |
7.0 ± 3.1 b |
9.1 ± 0.67a |
9 .0 ± 0.8a |
7 .0 ± 0.83b |
7.5 ± 0.8b |
8.1 ± 0.23b |
8.7 ± 0.06b |
| Neutrophil (%) | 6 .0 ± 3.8c |
6.1 ± 3.1 c |
9 .0 ± 0.92a |
9 .0 ± 1.51a |
7.8 ± 1.51a |
8.2 ± 1.51a |
7.4 ± 1.51a |
8.3 ± 1.51a |
*Superscripts indicate significance difference among different endotoxin injected groups with respect to specific sampling period.
3.2. Serum and Immunological parameters
3.2.1. Protein, Albumin and Globulin
The effect of endotoxin on different blood serum and immune parameters of H. fossilis injected with endotoxin is presented in Table 3. Among different serum parameters, serum protein content was least recorded in control group at both the sampling period. No significant difference (p > 0.01) among the serum protein content could be recorded in control, 0.1 mg and 0.01 mg endotoxin injected groups at 7th dpi. On the other hand, serum protein content was significantly high (p < 0.01) in group injected with 0.05 mg endotoxin as compared to that of other groups. The mean (± SD) serum protein content of fishes of this group was 3.46 (± 0.2) mg ml−1 and 4.29 (±0.19) mg ml−1 at 7th and 15th dpi, respectively.
Table 3.
Effect of different endotoxin concentrations on various blood serum parameters of H. fossilis at 7th and 15th days post injection (dpi).
| Groups | Protein (mg/ml) | Albumin (mg/ml) | Globulin (mg/ml) | A:G ratio | ||||
|---|---|---|---|---|---|---|---|---|
| 7th dpi | 15th dpi | 7th dpi | 15th dpi | 7th dpi | 15th dpi | 7th dpi | 15th dpi | |
| 0.1 mg | 3.07± 0.41 b |
3.87± 0.68 b |
1.62± 0.15 |
1.78± 0.24 |
1.27± 0.46c |
2.07± 0.14b |
0.97± 0.13 |
0.95± 0.13 |
| 0.05 mg |
3.46± 0.39 a |
4.29± 0.44 a |
1.72± 0.18 |
1.82± 0.15 |
1.75± 0.23 a |
2.47± 0.46 a |
1.25± 0.44 |
0.86 ± 0.28 |
| 0.01 mg |
3.22± 0.27 b |
3.91± 0.24 b |
1.64± 0.19 |
1.94± 0.16 |
1.62± 0.11b |
1.96± 0.20 b |
0.98± 0.06 |
0.98± 0.09 |
| Control | 2.93± 0.29 b |
3.09± 0.29 c |
1.32± 0.18 |
1.58± 0.43 |
1.65± 0.15 b |
1.51± 0.13 b |
0.81± 0.09 |
1.04± 0.11 |
*Superscripts indicate significance difference in the mean (± SD) values of individual serum parameters among different endotoxin injected groups with respect to specific sampling period.
The albumin content was least (1.32 ± 0.18 mg ml−1) in control group while it was highest (1.72 ± 0.18 mg ml−1) in 0.05 mg endotoxin injected group at 7th dpi. However, the albumin content did not vary significantly (p > 0.01) in all endotoxin injected groups irrespective of its concentration at both 7th and 15th dpi.
The globulin content was significantly low (1.27 ± 0.03 mg ml−1) in 0.1 mg endotoxin injected group at 7th dpi but found to be increased (2.07 ± 0.08 mg ml−1) at 15th dpi. Significantly high (p < 0.01) globulin level was recorded in 0.05 mg endotoxin injected group at both sampling periods. The mean (±SD) globulin content of this group was 1.75 (± 0.19) mg ml−1 and 2.47 (± 0.19) mg ml−1 at 7th and 15th dpi, respectively. No significant difference (p > 0.01) among the globulin level in 0.01 mg endotoxin injected and control groups could be recorded. Similarly, no significant difference (p > 0.01) among the A:G ratio could be recorded among all groups except 0.05 mg endotoxin injected group (Table 3).
3.2.2. ALP level
The ALP level was found to increase in all the endotoxin injected groups but significant increased (p < 0.01) in 0.1 mg endotoxin injected group at 7th dpi and maintained at a high level up to 15th dpi. The ALP content of fishes in this group was 0.49 (± 0.04) U ml−1 and 0.38 (± 0.06) U ml−1 serum at 7th and 15th dpi, respectively. However, the ALP level did not vary significantly (p > 0.01) in other groups (Fig. 1a).
Fig. 1.
(a–c). Effect of different endotoxin concentrations on various serum enzyme parameters of Heteropneustes fossilis at 7th and 15th days post injection (dpi) a) Alkaline phosphatase activity; b) SGOT level; c) SGPT level
*: superscripts indicate significant difference in the mean (± SD) values of individual enzyme content at P < 0.01 among different endotoxin injected and control groups at specific sampling period.
3.2.3. SGOT and SGPT level
The endotoxin didn't affect the SGOT level in any of the injected groups and no significant difference (p > 0.01) in the SGOT level was found to in any of the groups irrespective of sampling periods. Similarly, no difference in the SGPT level irrespective of sampling periods was found among the experimental groups except in 0.1 mg endotoxin injected group (Fig. 1b). The SGPT level was significantly high (p < 0.01) with 0.18 (± 0.006) U ml−1 and 0.19 (± 0.005) U ml−1 serum in this group at 7th and 15th dpi, respectively (Fig. 1c).
3.2.4. Myeloperoxidase activity
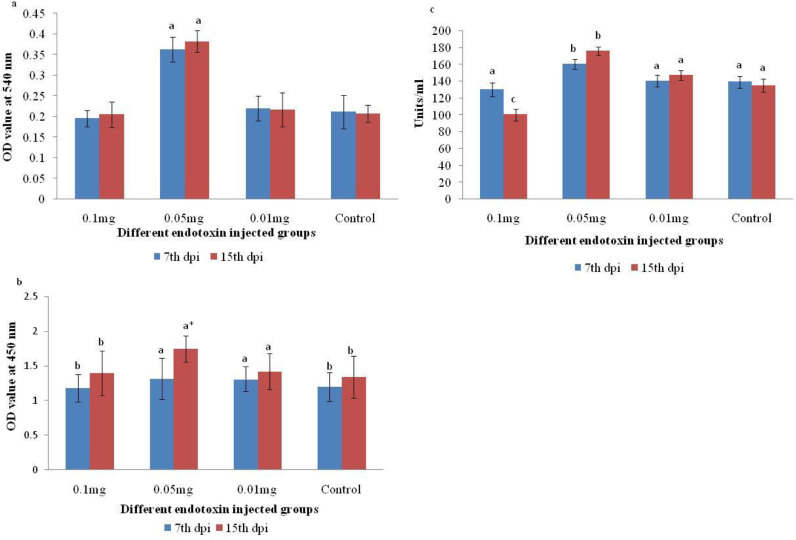
The myeloperoxidase activity was significantly high (p < 0.01) in 0.05 mg endotoxin injected group at both sampling period. The mean (±SD) OD value of 1.32 (± 0.3) and 1.75 (± 0.19) was recorded at 7th and 15th dpi, respectively. On the contrary, no significant difference in the mean (±SD) OD value could be recorded in other groups at 15th dpi (Fig. 2a).
Fig. 2.
(a–c). Effect of different endotoxin concentrations on various non-specific immune parameters of Heteropneustes fossilis at 7th and 15th days post injection (dpi); (a) Respiratory burst activity; (b) Myeloperoxidase activity; (c) Lysozyme content
*: superscripts indicate significant difference in the mean (± SD) values of individual non-specific immune parameters at P < 0.01 among the different endotoxin injected and control groups at specific sampling period.
3.2.5. Lysozyme level
No significant difference (p > 0.01) in the serum lysozyme level in control, 0.1 mg and 0.01 mg endotoxin injected groups could be recorded at 7th and 15th dpi except in 0.1 mg endotoxin, the content was significantly low at 15th dpi. However in group injected with 0.05 mg endotoxin, the serum lysozyme level with mean (± SD) value of 160 (± 6.0) and 176 (± 5.0) units per ml serum was found to be significantly high (p < 0.01) from that of other groups at both 7th and 15th dpi, respectively (Fig. 2b).
3.2.6. Respiratory burst activity
The respiratory burst activity as measured by NBT reduction assay was significantly high (p < 0.01) in group injected with 0.05 mg endotoxin with mean (±SD) OD values of 0.362 (± 0.03) and 0.382 (± 0.026) at 7th and 15th dpi, respectively. On the other hand, the mean (±SD) OD value was least (0.195 ± 0.02) in group injected with 0.1 mg endotoxin at 7th dpi but the level was increased at 15th dpi. No significant difference (p > 0.01) in the mean (± SD) OD value could be recorded in 0.01 mg endotoxin injected and control groups irrespective of sampling periods (Fig. 2c).
3.2.7. Bacterial agglutination titre
No significant difference (p > 0.01) in the bacterial agglutination titre could be recorded among groups injected with 0.1 mg, 0.01 mg endotoxin as well as control group at 7th and 15th dpi. On the contrary, the agglutination titre of the 0.05 mg endotoxin injected group was significantly high (p < 0.01) at both the sampling periods (Table 4).
Table 4.
Agglutinating and haemagglutinating activity of serum of H. fossilis injected with different concentrations of endotoxin at 7th and 15th days post injection (dpi).
| Groups | Agglutination titre (Log2) | Haemagglutination titre (Log2) | ||||
|---|---|---|---|---|---|---|
| 7th dpi | 15th dpi | 7th dpi | 15th dpi | |||
| 0.1 mg | 3.0 ±1.1 | 2.33±1.0 | 3.33±0.75 | 2.0±0.0 | ||
| 0.05 mg | 3.6±0.75a | 4.0±0.5a | 3.33±0.75 | 4.3±0.57a | ||
| 0.01 mg | 2.66±0.57 | 2.66±0.57 | 2.0±0.0 | 2.0±0.0 | ||
| Control | 2.66±0.57 | 2.0±0.0 | 1.66±0.5 | 1.66±0.5 | ||
*Superscripts indicate significance difference in the mean (± SD) titre values (Log2) among the different endotoxin injected groups with respect to specific sampling period.
3.2.8. Haemaglutination titre
The haemaglutination titre was higher in all endotoxin injected groups but not at significant level (p > 0.01) except in group injected with 0.05 mg endotoxin. Significantly high haemagglutinating activity with a titre value (Log2) of 4.3 (± 0.57) was recorded in group injected with 0.05 mg endotoxin at 15th dpi (Table 4).
3.3. Challenge study
On challenging with virulent A. hydrophila after one week of 2nd sampling, highest percentage of mortality (70 %) was recorded in 0.1 mg endotoxin injected group followed by 60 % mortality in both 0.01 mg endotoxin injected and control groups. However, significantly least percentage of mortality (40%) was recorded in 0.05 mg endotoxin injected group (Fig. 3).
Fig. 3.
The mortality percentage of Heteropneustes fossilis injected with various doses of endotoxin challenged after 15th days post injection with virulent Aeromonas hydrophila @ 106 CFU ml−1
*: Superscript indicates significance difference (p ≤ 0.01) in the mortality percentage from other groups.
4. Discussion
Endotoxin, the major virulence factor of many Gram –ve bacteria, is responsible for the clinical manifestations of diseases in host. It can induce innumerable biological effects ranging from simple response to intense reactions which may even lead to death in several animals including humans. Higher vertebrates are highly sensitive to endodtoxin even at very low concentration. Unlike higher vertebrates, lower vertebrates like fish and frogs are found to resist endotoxic shock [17]. Herein the effect of varying doses of endotoxin on certain blood and serum biochemical and immunological responses of stinging catfish H. fossilis was studied.
Herein in this study, H. fossilis yearlings were found to resist the toxicity of endotoxin upto the dose of 0.1 mg endotoxin per fish. Earlier, researchers have demonstrated the resistance pattern of different fish species towards LPS/endotoxin [7,21]. While, Wedemeyer et al. [7] demonstrated the resistivity of coho salmon, Oncorhynchus kisutch and rainbow trout, Oncorhynchus mykiss towards Escherichia coli and Aeromonas salmonicida endotoxin up to 80 mg per ml, Nayak et al. [21] found Indian major carp, rohu, Labeo rohita to be resistant to bacterial endotoxin upto 20EU per fish. The resistance of H. fossilis to endotoxin could be attributed to its preference for living conditions in very dirty and muddy areas leading to resistance either due to routine exposure to aquatic environment where they are intimately associated with Gram -ve bacteria or serum factors that conferred the sensitivity to toxin in mammals [32].
The patho-physiological effects in a host in response to endotoxins/LPS are predominantly dose-dependent. It is believed that with increased dose, the peak effect becomes more pronounced, and the duration of the effects prolonged [33]. In this present investigation, the lower dose of endotoxin (i.e., 0.01 mg) failed to modulate serum biochemical and immunological parameters of H. fossilis. The level of various immune parameters were not changed significantly and were either at par with control group or after an initial enhancement at 7th dpi, decreased to normal level as that of control at 15th dpi. However, at 0.05 mg of endotoxin concentration, the responses enhanced significantly but at higher dose (0.1 mg endotoxin) detrimental effects were recorded in catfish.
Literatures on the effects of LPS/endotoxin on fish serum biochemical parameters are limited and often contradictory. While, Selvaraj et al. [34] recorded decreased protein and globulin level by injecting crude A. hydrophila LPS to common carp, Cyprinus carpio, Nayak et al. [21] demonstrated enhanced protein and globulin level by injecting pure E. coli endotoxin to L. rohita. On the contrary, no significant difference among serum parameters like total protein, albumin and globulin content in any of the endotoxin injected groups could be recorded. Such differences could be attributed to the fish species used as well as dose and source of LPS since different bacteria produce different morphologically heterogeneous LPS molecules.
The effect of toxicant on serum enzymatic activity is one of the most important biochemical parameters, which is affected under stress [35]. Like higher vertebrates, the blood chemistry parameters varied in the response to stress with anemia, polycythemia, leucopenia or leucocytosis depending upon the stress nature [36]. In this study endotoxin especially at high dose act as a potential stress or with significant reduction in TLC, TEC, DLC (lymphocytes, neutrophils) levels especially at 7th dpi. Similarly, it was demonstrated that various serum enzymes increase in the fish in response to stress [[37], [38], [39], [40], [41], [42]]. Herein in this study, various serum enzymatic parameters like transamines and ALP values enhanced significantly in response to high dose of endotoxin (0.1 mg endotoxin per fish) in H. fossilis. But these parameters didn't vary significantly at lower dose (0.01 mg endotoxin per fish). However, serum glutamate oxaloacetate transaminase (SGOT) and serum glutamate pyruvate transaminase (SGPT) which are involved in transamination reactions in living system, didn't vary significantly in endotoxin injected catfish, H. fossilis.
Earlier, several researchers have demonstrated the modulation of piscine immune system by endotoxin/LPS [10,43] but high concentrations is often required for inducing immune responses through PRRs such as β -2 integrins mediated recognition [44,45]. The ability of LPS as a potent mediator of phagocytic, lysozyme, anti-protease and oxidative burst activities has been demonstrated in fish [46]. In this study, endotoxin was found to increase of various immune parameters of H. fossilis. The serum protein and globulin content was significantly high (P ≤ 0.01) in 0.05 mg of endotoxin per fish injected group. Similarly, LPS has been demonstrated to increase the respiratory burst activity of rainbow trout, Oncorhynchus mykiss [46.47], Indian major carp, rohu, L. rohita [21].
Similarly, myeloperoxidase, a hemoprotein stored in primary azurophilic granules of neutrophils and secreted during activation of neutrophils, plays an important role in the defense of the organism. In this study, the significant rise in myeloperoxidase activity at 0.05 mg endotoxin per fish injected group showed the activation of neutrophils by endotoxin. Earlier Nayak et al. [21] also demonstrated that the respiratory burst activity and myeloperoxidase activity in L. rohita injected endotoxin to increase significantly at dose dependent manner.
Among other non-specific immune parameters, endotoxin/LPS are also reported to induce lysozyme expression leading to increase its concentration in the serum and mucus of animals [48]. Lysozyme which plays an important role in piscine protective mechanism [49], was found to be unchanged at lower dose followed by increase and then decreased at higher dose. Significantly high lysozyme level was recorded in fish injected with 0.05 mg endotoxin. Earlier findings also indicated the stimulation of plasma lysozyme activity by LPS in fish like Atlantic salmon (Salmo salar), Indian major carp, L. rohita [21,48].
Likewise, the agglutination titre in irrespective of endotoxin injected groups indicated a possible enhancement of natural agglutinin level in serum of the treated fish due to leucocyte proliferation and differentiation. Furthermore, hemagglutination activity is believed to be an important biological function of LPS [50] and this is also apparent from the findings of the present study i.e., irrespective of doses agglutinating activity of H. fossilis serum increased in responses to endotoxin.
Endotoxin/LPS can activate the immune system in different eukaryotic species [51] and also responsible for production of cytokines, Pro-inflammatory cytokines and inflammatory effector substances such as nitric oxide [9,52,53]. In fish, it often acts a potent stimulator of the cellular immunity and protective antigen to several fish pathogens [54]. LPS have shown a tendency towards high distribution in haematopoetic organs like head-kidney and spleen [55] and dietary supplementation or bath immersion of LPS/endotoxin can effectively stimulate piscine immunity [10,21,43].
Nonetheless, the disease protective capacity of endotoxin/LPS alone and/or in combination with other agents was also earlier reported in several fish species [21,34,[56], [57], [58], [59], [60], [61], [62]]. In this study, the variations /changes of non-specific immune parameters among various endotoxin injected groups were also reciprocated in the challenge study. On challenge with virulent A. hydrophila, significantly lowest mortality (40%) was recorded in the group injected with 0.05 mg endotoxin per fish but it was 70% in 0.1 mg endotoxin injected group. The survivability of fish appears to be dependent upon the concentration of endotoxin/LPS. The low concentration of endotoxin/LPS is effective in inducing higher protection [46,54,62,63]. Earlier, Nya and Austin [46] demonstrated high relative percent survivability of rainbow trout fed with low dose of LPS and less survivability in high LPS fed fish against A. hydrophila. Similar type of trend was also observed in Labeo bata fed with 50,100 and 150 mg E. coli LPS kg−1 feed against Edwardsiella tarda [63].
5. Conclusions
The research on bacterial endotoxin mostly focused to understand in detail the cellular and molecular mechanism(s) by which this unique highly conserved microbial component contributes to the changes in the immune response to Gram-negative bacterial infections. Herein we have demonstrated the dose dependent variation among several immune parameters. While immunomodulating effect of endotoxin at 0.05 mg per fish was recorded, higher dose i.e., 0.1 mg per fish was found to suppress the immune status of the host.
Compliance with ethical standards
All the animal experiments conducted in this present study were in accordance with the ethical guidelines and approval of the university.
Declaration of Competing Interest
The authors declare that they have no known competing interests
Acknowledgement
The authors duly acknowledge the Head of the Department, for his support.
References
- 1.Nakahla A.N., Szalai A.J., Banoub J.H., Keough K.M.W. Serum anti-LPS antibody production by rainbow trout (Oncorhynchus mykiss) in response to the administration of free and liposomally incorporated LPS from Aeromonas salmonicida. Fish Shellfish Immunol. 1997;7:387–401. [Google Scholar]
- 2.Raetz C.R., Whitfield C. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bishop R.E. Fundamentals of endotoxin structure and function. Contrib. Microbiol. 2005;12:1–27. doi: 10.1159/000081687. [DOI] [PubMed] [Google Scholar]
- 4.Morrison D.C., Duncan R.L., Goodman S.A. In vivo biological activities of endotoxin. Prog. Clin. Biol. Res. 1985;189:81–99. [PubMed] [Google Scholar]
- 5.Nowotny A. Molecular aspects of endotoxin reactions. Bacteriol. Rev. 1969;33:72–98. doi: 10.1128/br.33.1.72-98.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fletcher T.C., White A. Metabolic and immunological effects of endotoxin in the plaice, Pleuronectes platessa L. J. Fish Biol. 1987;31:81. [Google Scholar]
- 7.Wedemeyer G., Ross A.J., Smith L. Some metabolic effects of bacterial endotoxin in salmonid fishes. J. Fish Res. Board Canada. 1968;26:115–122. [Google Scholar]
- 8.Wedemeyer G. Pituitary activation by bacterial endotoxin in the rainbow trout (Salmo gairdneri) J. Bacteriol. 1969;100:542–543. doi: 10.1128/jb.100.1.542-543.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balm P.H., van Lieshout E., Lokate J., Bonga S.E.W. Bacterial lipopolysaccharide (LPS) and interleukin 1 (IL-1) exert multiple physiological effects in the tilapia Oreochromis mossambicus (Teleostei) J. Comp. Physiol. B. 1995;165:85–92. doi: 10.1007/BF00301472. [DOI] [PubMed] [Google Scholar]
- 10.Guttvik A., Paulsen B., Dalmo R.A., Espelid S., Lund V., Bogwald J. Oral administration of lipopolysaccharide to Atlantic salmon (Salmo salar l.) fry. Uptake, distribution, influence on growth and immune stimulation. Aquaculture. 2002;214:35–53. [Google Scholar]
- 11.Erridge C., Bennett-Guerrero E., Poxton I.R. Structure and function of lipopolysaccharide. Microbes Infect. 2002;4(8):837–841. doi: 10.1016/s1286-4579(02)01604-0. [DOI] [PubMed] [Google Scholar]
- 12.MacKenzie S., Montserrat N., Mas M., Acerete L., Tort L., Krasnov A., et al. Bacterial lipopolysaccharide induces apoptosis in the trout ovary. Reprod. Biol. Endocrinol. 2006;4:46–58. doi: 10.1186/1477-7827-4-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Futoma-Koloch B. Immune response against bacterial lipopolysaccharide. J. Mol. Immunol. 2016;2:105. doi: 10.4172/jmi.1000e105. [DOI] [Google Scholar]
- 14.Uchiyama T., Kamagata Y., Yoshioka M. Mechanism of lipopolysaccharide induced immunosupression, immunological activity of B cells subset responding to T dependent and T independent antigens in lipopolysaccharide pre-injected mice. Infect. Immun. 1984;45(2):367–371. doi: 10.1128/iai.45.2.367-371.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uchiyama T. Modulation of immune response by bacterial lipopolysaccharide (LPS): roles of macrophages and T cells in in vitro adjuvant effects of LPS on antibody response to T cell dependent and T cell independent antigens. Microbiol. Immunol. 1982;26:213–225. doi: 10.1111/j.1348-0421.1982.tb00173.x. [DOI] [PubMed] [Google Scholar]
- 16.Doñate C., Roher N., Balasch J.C., Ribas L., Goetz F.W., Planas J.V., Tort L., MacKenzie S. CD83 expression in sea bream macrophages is a marker for the LPS-induced inflammatory response. Fish Shellfish Immunol. 2007;23:877–885. doi: 10.1016/j.fsi.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 17.Berczi I., Bertok L., Bereznai T. Comparative studies on the toxicity of Escherichia coli lipopolysaccharide endotoxin in various animal species. Can. J. Microbiol. 1966;12(5):1070–1071. doi: 10.1139/m66-143. [DOI] [PubMed] [Google Scholar]
- 18.Baba T., Imamura J., Izawa K. Immune protection in carp, Cyprinus carpio L., after immunization with Aeromonas hydrophila crude lipopolysaccharide. J. Fish Dis. 1988;11:237–244. [Google Scholar]
- 19.Balm P.H.M., Pepels P., van Lieshout E., Bonga S.E.W. Neuroimmunological regulation of a-MSH release in tilapia (Oreochromis mossambicus) Fish Physiol. Biochem. 1993;11:125–130. doi: 10.1007/BF00004558. [DOI] [PubMed] [Google Scholar]
- 20.Pepels P.P.L.M., van Helvoort H., Bonga S.E.W., Balm P.H.M. Corticotropin-releasing hormone in the teleost stress response: rapid appearance of the peptide in plasma of tilapia (Oreochromis mossambicus) J. Endocrinol. 2004;180:425–438. doi: 10.1677/joe.0.1800425. [DOI] [PubMed] [Google Scholar]
- 21.Nayak S.K., Swain P., Nanda P.K., Dash S., Shukla S., Meher P.K., Maiti N.K. Effect of endotoxin on the immunity of Indian major carp, Labeo rohita. Fish Shellfish Immunol. 2007;24(3):394–399. doi: 10.1016/j.fsi.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Swain P., Nayak S.K., Nanda P.K., Dash S. Biological effects of bacterial lipopolysaccharide (endotoxin) in fish: a review. Fish Shellfish Immunol. 2008;25(3):191–201. doi: 10.1016/j.fsi.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 23.Blaxhall P.C., Daisley K.W. Routine haematological methods for use with fish blood. J. Fish Biol. 1973;5:771–778. [Google Scholar]
- 24.Bradford M.M. A rapid and sensitive method for the quantification of microgram quantities of protein. Ann. Biochem. 1976;72:248. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 25.International federation of clinical chemistry, physicochemical quantities and units in clinical chemistry. J. Clin. Chem. Clin. Biochem. 1980;18:829–854. [PubMed] [Google Scholar]
- 26.Quade M.J., Roth J.A. A rapid, direct assay to measure degranulation of bovine neutrophil primary granules. Vet. Immunol. Immunopathol. 1997;58:239–248. doi: 10.1016/s0165-2427(97)00048-2. [DOI] [PubMed] [Google Scholar]
- 27.Sankaran K., Shanto G. On the variation in catalytic activity of lysozyme in fishes. Ind. J. Biochem. Biophys. 1972;91:162–165. [PubMed] [Google Scholar]
- 28.Studnicka M., Siwicki A., Ryka B. Lysozyme level in carp (Cyprinus carpio L.) Bamidgeh. 1986;38(1):22–25. [Google Scholar]
- 29.Anderson D.P., Siwicki A.K. Duration of protection against Aeromonas salmonicida in brook trout immunostimulated with glucan or chitosan by injection and immersion. Prog. Fish Cult. 1994;56:258–261. [Google Scholar]
- 30.Blazer V.S., Wolke R.E. The effects of α-tocopherol on the immune response and non-specific resistance factors of rainbow trout (Salmo gairdneri Richardson) Aquaculture. 1984;37:1–9. [Google Scholar]
- 31.SAS Institute Inc . 2nd Ed. SAS Institute Inc; Cary, NC: 1991. SASR system for Regression; p. 210. [Google Scholar]
- 32.Iliev D.B., Roach J.C., Mackenzie S., Planas J.V., Goetz F.W. Endotoxin recognition: in fish or not in fish? FEBS Lett. 2005;579:6519–6528. doi: 10.1016/j.febslet.2005.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lohuis J.A.C.M., Verheijden J.H.M., Burvenich C., van Miert A.S.J.P.A.M. Pathophysiological effects of endotoxins in ruminant. Vet. Quart. 1988;10:109–116. doi: 10.1080/01652176.1988.9694157. [DOI] [PubMed] [Google Scholar]
- 34.Selvaraj V., Sampath K., Sekar V. Extraction and characterization of lipopolysaccharide from Aeromonas hydrophila and its effects on survival and hematology of the carp, Cyprinus carpio. Asian Fish Sci. 2004;17:163–173. [Google Scholar]
- 35.K.Das B., Mukherjee S.C. Toxicity of cyper methrinin Labeo rohita fingerlings: Biochemical, enzymatic and haematological consequences. Comp. Biochem. Physiol. C. 2003;124:109–121. doi: 10.1016/s1532-0456(02)00219-3. [DOI] [PubMed] [Google Scholar]
- 36.Wedemeyer G.A., McLeay D.J. In: Stress and Fish. Pickering A.D., editor. Academic press; London: 1981. Methods for determining the tolerance of fishes to environmental stressors; pp. 247–275. Edited by. Edited by. [Google Scholar]
- 37.A.M.Hilmy N.El-Domaity, Daabees M.Y. The use of the chelating agent EDTA in the treatment of acute cadmium toxicity, tissue distribution and some blood parameters in the Egyptian toad Bufo regularis. Comp. Biochem. Physiol. 1986;85:67–74. doi: 10.1016/0742-8413(86)90053-8. [DOI] [PubMed] [Google Scholar]
- 38.Benson W.H., Baer K.N., Stackhouse R.A, Watson C.F. Influence of cadmium exposure on selected hematological parameters in fresh water teleost, Notemigous chrysoleucas. Ecotoxicol. Environ. Saf. 1987;13:92–96. doi: 10.1016/0147-6513(87)90046-7. [DOI] [PubMed] [Google Scholar]
- 39.Hilmy A.M., Domaity N.El. Some physiological and biochemical indices of zinc toxicity in two freshwater fishes, Clarias lazera and Tilapia zillii. Comp. Biochem. Physiol. 1987;87:297–301. doi: 10.1016/0742-8413(87)90011-9. [DOI] [PubMed] [Google Scholar]
- 40.Singh H.S., Reddy T.V. Effect of copper sulfate on hematology, blood chemistry and hepato-somatic index of an Indian catfish, Heteropneustes fossilis (Bloch) and its recovery. Ecotoxicol. Environ. Saf. 1990;20:30–35. doi: 10.1016/0147-6513(90)90043-5. [DOI] [PubMed] [Google Scholar]
- 41.Karan V., Vitorovic S., et al. Functional enzyme activity and gill histology of carp after copper sulfate exposure and recovery. Ecotoxicol. Environ. Saf. 1998;40:49–55. doi: 10.1006/eesa.1998.1641. [DOI] [PubMed] [Google Scholar]
- 42.Al-Attar A.M. Biochemical effects of short term cadmium exposure on the freshwater fish, Oreochromis niloticus. J. Biol. Sci. 2005;5:260–265. [Google Scholar]
- 43.Dalmo R.A., Kjerstad A.A., Arnesen S.M., Tobias P.S., Bogwald J. Bath exposure of Atlantic halibut (Hippoglossus hippoglossus L.) yolk sac larvae to bacterial lipopolysaccharide (LPS): absorption and distribution of the LPS and effect on fish survival. Fish Shellfish Immunol. 2000;10:107–128. doi: 10.1006/fsim.1999.0231. [DOI] [PubMed] [Google Scholar]
- 44.MacKenzie S., Planas J.V., Goetz F.W. LPS-stimulated expression of a tumor necrosis factor-a mRNA in primary trout monocytes and in vitro differentiated macrophages. Dev. Comp. Immunol. 2003;27:393–400. doi: 10.1016/s0145-305x(02)00135-0. [DOI] [PubMed] [Google Scholar]
- 45.Iliev D.B., Liarte C.Q., Mackenzie S., Goetz F.W. Activation of rainbow trout (Oncorhynchus mykiss) mononuclear phagocytes by different pathogen associated molecular pattern (PAMP) bearing agents. Mol. Immunol. 2005;42:1215–1223. doi: 10.1016/j.molimm.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 46.Nya E.J., Austin B. Use of bacterial lipopolysaccharide (LPS) as an immunostimulant for the control of Aeromonas hydrophila infections in rainbow trout Oncorhynchus mykiss (Walbaum) J. Appl. Microbiol. 2010;108:686–694. doi: 10.1111/j.1365-2672.2009.04464.x. [DOI] [PubMed] [Google Scholar]
- 47.Solem S.T., Jorgensen J.B., Robertsen B. Stimulation of respiratory burst and phagocytic activity in Atlantic salmon Salmo salar L. macrophages by LPS. Fish Shellfish Immunol. 1995;5:475–491. [Google Scholar]
- 48.Paulsen S.M., Lunde H., Engstad R.E., Robertsen B. In vivo effects of beta-glucan and LPS on regulation of lysozyme activity and mRNA expression in Atlantic salmon (Salmo salar L.) Fish Shellfish Immunol. 2003;14:39–54. doi: 10.1006/fsim.2002.0416. [DOI] [PubMed] [Google Scholar]
- 49.Ingram G.A., Alexander J.B. The immune response of the brown trout Salmo trutta to lipopolysaccharide. J. Fish Biol. 1980;16:181–197. [Google Scholar]
- 50.Alam M., Miyoshi S., Tomochika K., Shinodo S. Haemagglutination is a novel biological function of lipopolysaccharide (LPS) as seen with the Vibrio cholerae 0139 LPS. Clin. Diagn. Lab. Immunol. 1997;4(5):604–606. doi: 10.1128/cdli.4.5.604-606.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alexander C., Rietschel E.T. Bacterial lipopolysaccharides and innate immunity. J. Endotoxin Res. 2001;7:167–202. [PubMed] [Google Scholar]
- 52.McCann S.M., Kimura M., Karanth S., Yu W.H., Mastronardi C.A., Rettori V. The mechanism of action of cytokines to control the release of hypothalamic and pituitary hormones in infection. Ann. New York Acad.Sci. 2000;917:4–18. doi: 10.1111/j.1749-6632.2000.tb05368.x. [DOI] [PubMed] [Google Scholar]
- 53.Akira S., Hemmi H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol. Lett. 2003;85:85–95. doi: 10.1016/s0165-2478(02)00228-6. [DOI] [PubMed] [Google Scholar]
- 54.Mac Arthur J.I., Fletcher T.C., Pirie B.J., Davidson R.J., Thomson A.W. Peritoneal inflammatory cells in plaice, Pleuronectes platessa L.: effect of stress and endotoxin. J. Fish Biol. 1984;25:69–81. [Google Scholar]
- 55.Dalmo R.A., Bogwald J. Distribution of intravenously and perorally administered Aeromonas salmonicida lipopolysaccharide in Atlantic salmon, Salmo salar L. Fish Shellfish Immunol. 1996;6:427–441. [Google Scholar]
- 56.Saeed M.O., Plumb J.A. Immune response of channel catfish to lipopolysaccharide and whole cell Edwardsiella ictaluri vaccines. Dis. Aquat. Org. 1986;2:21–25. [Google Scholar]
- 57.Velji M.I., Albright L.J., Evelyn T.P. Protective immunity in juvenile Coho salmon Oncorhynchus kisutch following immunization with Vibrio ordalii lipopolysaccharide or from exposure to live V. ordalii cells. Dis. Aquat. Org. 1990;9:25–29. [Google Scholar]
- 58.AL-Harbi A.H., Austin B. The immune response of turbot, Scophthalmus maximus (L.), to lipopolysaccharide from a fish-pathogenic cytophaga-like bacterium. J. Fish Dis. 1992;15:449–452. [Google Scholar]
- 59.Steine N.O., Melingen G.O., Wergeland H.I. Antibodies against Vibrio salmonicida lipopolysaccharide (LPS) and whole bacteria in sera from Atlantic salmon (Salmo salar L.) vaccinated during the smolting and early post-smolt period. Fish Shellfish Immunol. 2001;11(1):39–52. doi: 10.1006/fsim.2000.0292. [DOI] [PubMed] [Google Scholar]
- 60.Selvaraj V., Samapath K., Sekar V. Adjuvant and immunostimulatory effect of beta glucan administration in combination with lipopolysaccharide enhances survival and some immune parameters in carp challenged with Aeromonas hydrophila. Vet. Immunol. Immunopathol. 2006;114(1-2):15–24. doi: 10.1016/j.vetimm.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 61.Selvaraj V., Sampath K., Sekar V. Administration of lipopolysaccharide increases specific and non-specific immune parameters and survival in carp (Cyprinus carpio) infected with Aeromonas hydrophila. Aquaculture. 2009;286:176–183. [Google Scholar]
- 62.Salati F., Hamaguchi M., Kusuda R. Immune response of red sea bream to Edwardsiella tarda antigens. Fish Pathol. 1987;22:93–98. [Google Scholar]
- 63.Sahoo L., Parhi J., Debnath C., Prasad K.P. Effect of feeding lipopolysaccharide as an immunostimulant on immune response and immune gene expression of Labeo bata. Vet. Immunol. Immunopathol. 2017;188:48–58. doi: 10.1016/j.vetimm.2017.04.012. [DOI] [PubMed] [Google Scholar]