Highlights
-
•
Sequence features and tissue distribution of CD83 molecule were analyzed in flounder.
-
•
The specific antibodies against flounder CD83 molecule were developed.
-
•
Dendritic cells of flounder were isolated and identified in vitro.
-
•
Dendritic cells and CD83 were respond to LPS in vitro and in vivo.
Keywords: Paralichthys olivaceus, Dendritic cells, CD83, Tissue distribution, Immunological characteristic
Abstract
Dendritic cells (DCs) are the professional antigen presenting cells, which play important roles in regulating the immune response and tolerance. In mammals, CD83 is considered as a maturation marker for DCs, which is a type I membrane glycoprotein. In this research, the gene sequence of the CD83 in Paralichthys olivaceus was obtained on NCBI, the sequence characteristics were studied and CD83 was detected in the head kidney, spleen, gut, gill, liver and muscle, with lower expression in the liver and muscle and higher expression in the head kidney and spleen. And then the CD83 recombinant protein was expressed and the specific antibodies (Abs) were prepared, transiently expressed CD83 eukaryotic proteins could be identified by western blot and indirect immunofluorescence. Then single cell suspensions of head kidney were obtained and DCs were collected by density gradient centrifugation after 7 days of cultivation. Giemsa staining revealed that the nuclei were kidney and dumbbell shaped, and the cell surfaces had dendritic or pseudopod-like protrusions. In addition, the DCs could phagocytose fluorescent microspheres, and the phagocytosis rate was 79.27±1.01%. After incubation with allogeneic lymphocytes, it was found that the lymphocytes clustered and proliferated significantly with the ratio ranging from 39.0% to 59.8% in a dose-dependent manner, which proved that the DCs could elicit mixed lymphocyte responses. After LPS immunization in vivo, the relative expression of CD83 in the head kidney and spleen showed a trend of increasing and then decreasing, peaking at 6 h. Meanwhile, the expression of CD83, MHC II, CD80/86 and CD40 were significantly up-regulated in DCs after stimulated with LPS for 24 h in vitro. And then IIF revealed that CD83+ cells could be detected in the LPS-stimulated group using CD83 Abs, while no positive signal was detected in the control group, which suggested that CD83 protein may be considered as a specific marker for the maturation of DCs in teleost.
1. Introduction
Dendritic cells (DCs), named for their distinctive morphology, are the most potent antigen-presenting cells (APCs) of the immune system [3]. Their superior abilities to activate naive T lymphocytes and initiate adaptive immune responses distinguishes them from other APCs [6,13]. In the presence of inflammatory and immune responses, immature DCs constitutively patrol through peripheral tissues and take up antigens [20]. Then they migrate to the peripheral lymph nodes, during which the internalized antigens are processed into proteolytic peptides and loaded onto MHC class I and II molecules [15,23]. At the same time, pathogen signals induce DCs to enter a developmental program, called maturation. As mature DCs, they express additional costimulatory molecules that can induce T lymphocytes [16,22]. CD83, expressed on mature DCs, is considered as a cell surface marker of mature DCs in mammals. The correlated increased expression of CD83 with co-stimulatory molecules CD80 and CD86 during the maturation of DC suggests its role in immune responses [18].
Teleost fish constitute the first animal group in which all the main elements of a complete adaptive immune system are present [24]. However, studies on the identification and the functional characteristics of DCs in teleost fish remain limited due to the absence of specific identification tools such as molecular markers, and the significant differences between the immune systems of teleost and mammals [14,18]. For example, fish lack lymph nodes, and the question of where antigen presentation takes place is of particular interest [4]. In spite of these challenges, dendritic-like cells have been identified recently in fish. In zebrafish (Danio rerio), a population of DC-like cells has been identified on the basis of peanut agglutinin (PNA) lectin-binding affinity, dendritic morphology, and stimulatory effects on T cell activation [14]. And then a method of culturing from hematopoietic tissue was established in rainbow trout (Oncorhynchus mykiss) to obtain enriched cells that shared many DC characteristic features [4]. Later on, DC-SCRIPT, a well-conserved zinc finger protein that is preferentially expressed in all sub-types of human DCs, was identified as a potential molecular marker for DCs in Barramundi (Lates calcarifer) [26]. Meanwhile, Shao revealed that the surface phenotypic hallmarks of mammalian DCs, including MHC-II, CD80/86, CD83, and CD209, were distributed on the surfaces of zebrafish (D. rerio) DCs [18].
The gene of CD83 has been annotated on NCBI in flounder (Paralichthys olivaceus). In the present research, with the aim of identifying dendritic cells and the maturation marker CD83 in flounder, the gene sequence of the CD83 was obtained on NCBI, the sequence characteristics and tissue distribution characteristics were studied; the CD83 recombinant protein was expressed and the specific antibodies of CD83 were prepared; DCs were obtained in vitro and their functions of phagocytosis and eliciting mixed lymphocyte responses were investigated; the changes of CD83 molecule expression and dendritic cells after lipopolysaccharide (LPS) stimulation were studied in vitro and in vivo experiments.
2. Material and method
2.1. Experimental animals and cells
Healthy flounders (Paralichthys olivaceus, 35–100 g) were purchased from a farm (Rizhao, Shandong, China), and kept in a tank at 20 °C for a week. The air pump was used for continuous inflation and oxygenation. Healthy individuals were selected for the experiments.
Healthy New Zealand white rabbits weighting of 2 kg were purchased from Qingdao Drug Inspection Institute (Shandong, China) and kept in the laboratory for two weeks, and then were used for antibodies production.
HINAE cells, generously provided by Dr. Ikuo Hirono of Tokyo University of Marine Scienceand Technology [19], were inoculated in 6-well plates, cultured in Leibovitz's l-15 medium containing 20% FBS, 100 IU/ml penicillin, and 100 µg/ml streptomycin.
This study was carried out in strict accordance with the recommendations of the Guide for the Use of Experimental Animals of the Ocean University of China, in agreement with the International Guiding Principles for Biomedical Research Involving Animals (EU84 2010/63). All experimental methods were approved by the Instructional Animal Care and Use Committee of the Ocean University of China (permit number: 20180101).
2.2. Bioinformatic analysis
To analyze the flounder CD83 gene, the sequence of CD83 (accession No.KR998303.1) was retrieved from the NCBI database (https://www.ncbi.nlm.nih.gov). The conserved protein domain of CD83 was analyzed using online SMART tool (http://smart.embl-heidelberg.de/). Three-dimensional structure of CD83 was established by iTASSER (https://zhanggroup.org/I-TASSER/). N-terminal glycosylation sites and phosphorylation sites were respectively predicted using NetNGlyc 1.0 Server (http://www.cbs.dtu.dk/services/NetNGlyc/) and NetPhos 3.1 Server (http://www.cbs.dtu.dk/services/NetPhos/).
2.3. RNA extraction and quantitative real-time PCR (qPCR) for tissue distribution
For mRNA preparation, the gill, spleen, kidney, liver, gut and muscle were collected aseptically from six flounders, respectively. The total RNA was extracted by the TRIzol method according to the manufacturer's instructions, the RNA is treated with the DNase to remove trace amounts of DNA after elution from the filter. The quantity and concentration of the RNA were detected by NanoDrop 8000 spectrophotometer (Thermo Scientific, USA), and the cDNA was synthesized by using Reverse Transcriptase M-MLV kit (TaKaRa, China) according to manufacturer's instructions.
The expression levels of flounder CD83 in various tissues were determined with the LightCycler 480 PCR system (Roche) using the SYBR Green Realtime PCR Master Mix (TaKaRa, China). CD83 RT-F/R (Table 1) were used as gene-specific primers in real-time PCR. β-actin primers (β-actin F/β-actin R) were introduced as the reference genes. The cycling protocol was: 95 °C, 30 s, (95 °C, 15 s→60 °C, 5 s) x 40 cycles, 95 °C, 5 s, 65 °C,60 s. Melting curve analysis and sequencing were used to detect the specificity of PCR products. All samples were analyzed in triplicate. Expression of the target gene was normalized to the housekeeping gene β-actin, and calculated with the 2−△△Ct method.
Table 1.
Primers used in the present study.
| Primer name | Primer sequence (5′−3′) | The serial number |
|---|---|---|
| CD83-F (extracellular region) | CGGGATCCGCAGTGATGGGCACTGTGATG | |
| CD83-R (extracellular region) | CGCTCGAGGTCATCGATGGTCAGTTTTTCTT | |
| CD83-Full-F | CCGCTCGAGATGACTCCACAGCGCCTG | |
| CD83-Full-R | CCGGAATTCCACAGACGTGCTTCATCGAGT | |
| CD83-F(RT) | GAGTGCAACCATCAGCACGAC | KR998303.1 |
| CD83-R(RT) | CCCAACGGCACGACGACATAC | |
| MHCII-F | TCCCAATAGCGATCTTGTTTC | 109643252 |
| MHCII-R | CAGCGTCTTTGACTTCTACCC | |
| CD80/86-F | CGCAGTGGGTTTGCTGTAGGG | 109634414 |
| CD80/86-R | GGTTGCTGGTTCTGGTTTGGA | |
| CD40-F | CTCCCTTTGGTCCTCGTGGTT | AB081752.1 |
| CD40-R | AGTTGGCAGTTTGGCTGTAGTTG | |
| β-actin-F | GAGGGAAATCGTGCGTGACTA | 109639577 |
| β-actin-R | ATTGCCGATGGTGATGACCTG |
2.4. Production of CD83 protein and antibodies
RNA was extracted from the spleen and reversely transcribed into cDNA as a template for the extracellular domain of CD83 and it was amplified using specific primers listed in Table 1. The PCR product was purified and inserted into the pET-32a prokaryotic expression vector to construct the pET-32a-CD83 recombinant expression vector. The constructed recombinant plasmid was then transformed into E. coli competent cells (Merck millipore), and induced with isopropy-β-D-thiogalactoside (IPTG) for 6 h at 37 °C during exponential growth. The recombinant proteins CD83 (rCD83) were affinity-purified using His Trap™ HP Ni-Agarose (GE healthcare, China) according to the manufacturer's instructions. The purified rCD83 were detected by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and stained with Coomassie brilliant blue R-250.
The concentrations of purified proteins were determined by the Bradford method. The polyclonal antibody against rCD83 were produced as previously described. After three boosters, the serum samples were collected and purified by protein G agarose affinity chromatography (Pierce/Thermo Scientific), and then the rabbit anti-flounder CD83 polyclonal Abs were obtained and the specificity of the Abs were analyzed using Western blotting and indirect immunofluorescence assay (IIFA).
2.5. Eukaryotic transfection, western-blot and immunofluorescence
Using cDNA of spleen as template, the full-length CD83 was cloned, and the PCR product was inserted into pTag-RFP eukaryotic expression vector to construct pTag-RFP-CD83 recombinant plasmid. Subsequently, using Lipofectamine™ 3000 Reagent (Thermo Fisher Scientific), the plasmid pTag-RFP and pTag-RFP-CD83 were respectively transfected into the HINAE cells cultured in minimum essential medium (DMEM) and observed by fluorescence microscope (Olympus, Japan) after 48 h.
The purified rCD83 and pTag-RFP-CD83 transfected HINAE cells lysates were applied on SDS-PAGE and transferred onto PVDF membranes (Merck Millipore, Germany). And then the membrane was blocked with PBS containing 5% BSA for 1 h at 37 °C, and incubated with Abs for 1.5 h at 37 °C, then washed three times with PBST. Antibody binding was detected with goat-antirabbit IgG-alkaline phosphatase conjugate (diluted 1:4000 in PBS) (Merck Millipore) for 1 h at 37 °C, and washed three times with PBST. Finally, the bands were stained with freshly prepared substrate solution (100 mM NaCl, 100 mM Tris and 5 mM MgCl2, pH 9.5) containing NBT (Sigma) and BCIP (Sigma) for 5 min and stopped by washing with distilled water. Serum from no immunized rabbit was used as control.
The transfected HINAE cells were collected and fixed using 4% paraformaldehyde fixative for 15 min at room temperature. The cells were subsequently blocked with 5% BSA for 1 h at 37 °C, and incubated with Abs for 1.5 h at 37 °C, then washed three times with PBST. In parallel experiments, the unrelated antibodies rabbit IgG were used as negative controls. And then the cells were incubated with secondary Alexa Fluor 647-conjugated anti-rabbit IgG at 37 °C for 1 h. Further staining with DAPI was performed before photomicrography was conducted. Fluorescence images of the samples were obtained using the fluorescence microscope (Olympus, Japan).
2.6. Dendritic cells culture and morphology identification
The fish was bled from the caudal vein, and then the spleen and HK were aseptically removed and squeezed with 65% RPMI-1640 solution (containing 20 IU ml−1 heparin, 0.1% w/v NaN3 and 1% w/v BSA) through a nylon gauze filter. Subsequently, cell suspensions were centrifuged and the supernatants were laid over a 1.020–1.070 g/cm3 discontinuous Percoll density gradient and centrifuged for 30 min at 840 x g. Subsequently, the cells layer from the Percoll interface were collected and adjusted to a concentration of 5 × 106 cells/mL in complete l-15 medium: l-15 Leibovitz medium supplemented 10% foetal bovine serum (FBS) and 1% penicillin-streptomycin (PS; 10,000 U/mL stock). Cells were plated in 25 cm2 or 75 cm2 cell culture flasks (Corning, NY) and cultured at room temperature for about 7 days, and the cells were counted with blood counting plates. Prior to harvest, culture flasks were agitated to suspend non-adherent cells and the media was collected and pooled. The cell suspension was layered over a Percoll gradient at 1.058 g/ cm3 and 1.020 g/cm3 and then centrifuged for 30 min at 840× g. The cells at the surface between the Percoll layers were collected and washed in fresh medium (5 min, 680× g). Then the morphology was determined by Wright–Giemsa staining and the images were taken on the microscope (Olympus, Japan).
2.7. Phagocytosis assay
The isolated DCs from head kidney in l-15 medium were counted and diluted to 1 × 107 cells/ml. The 1.0 ml cell suspensions were mixed with 1.0 μl fluoresbrite carboxylate YG 1.0 μm microspheres (Polysciences) and incubated for 3 h in 24 well culture plates at 22 °C. The medium instead of microspheres was used as negative control. Following incubation, cells were collected and non-ingested beads were removed by placing the cell-bead suspensions on top of a glucose cushion (3 ml PBS, pH 7.3, with 3% (W/V) BSA and 4.5% (W/V) d-glucose) and centrifuged at 100 g for 10 min at 4 °C. After ingestion, the isolated cells were analyzed using BD Accuri C6 Flow Cytometer. Meanwhile, the cell suspensions were settled onto glass slides, fixed with 4% paraformaldehyde for 20 min at 22 °C, and observed under the fluorescence microscope (Zeiss, Germany).
2.8. Mixed leukocyte reaction assay
For lymphocytes of peripheral blood, the isolated protocol according to a method described previously. In brief, the peripheral blood was drawn from the caudal vein and diluted in solution (65% RPMI-1640 containing 20 IU ml−1 heparin, 0.1% w/v NaN3 and 1% w/v BSA). Then the peripheral blood cell suspensions were centrifuged and the supernatants were laid over a 1.020–1.070 g/cm3 discontinuous Percoll density gradient. After centrifuged, the leukocytes layers from the Percoll interface were collected to a 25 cm2 cell culture flask and cultured.
Allogeneic lymphocytes and DCs were counted and resuspended in l-15 medium supplemented with 5% flounder serum, 1% PS at a concentration of 5 × 106 cells/mL. Allogeneic lymphocytes were stained using 5 μM/mL carboxy fluorescein succinimidyl ester (CFSE; Sigma) for 20 min at 37 °C. Cells were then washed twice in PBS by centrifugation. Lymphocytes and DCs were mixed at ratios of 1:1, 1:2, 1:4 and 1:8 and then incubated at 22 °C protecting from light. Negative controls were composed of responder cells alone. After 3 days, cells were analyzed by flow cytometry using decrease in CFSE fluorescence as an indicator of proliferation.
2.9. LPS treatment assay in vivo and in vitro
A total of 30 healthy flounders were selected. The 2 mg/ml LPS and PBS were emulsified and mixed with Freund's incomplete adjuvant, and immunized Japanese flounder by intraperitoneal injection. Each flounder was injected with 100μl LPS mixture. After 0 h, 6 h, 12 h, 24 h, 2d, 3d, 5d and 7d, three fishes were randomly selected, and the spleens and head kidneys were collected. The total RNA was extracted by Trizol method and reverse transcribed into cDNA according to the manufacturer's instructions. The relative expression of CD83 in the head kidney and spleen at different time points were measured by qPCR.
For vitro experiment, primary DCs were cultured from head kidney cell suspensions and adjusted to 5 × 106/ml. These cells were stimulated with 10 μg of LPS in vitro. In parallel experiments, PBS was added as negative control. After 24 h of incubation at room temperature, these cells were harvested, and the expression levels of CD83, MHCII, CD80/86 and CD40 were detected by qPCR. And then IIFA for CD83 of the collected DCs was detected. The cells of the two groups were fixed with 4% paraformaldehyde, blocked with 5% BSA for 1 h at 37 °C, and incubated with Abs for 1.5 h at 37 °C, then washed three times with PBST. And then the cells were incubated with secondary Cy3-conjugated anti-rabbit IgG at 37 °C for 1 h. Further staining with DAPI was performed before photomicrography was conducted. Fluorescence images of the samples were obtained using fluorescence microscope (Olympus, Japan).
2.10. Statistics
All data were analyzed using SPSS 19.0 and expressed as mean ±SD. Significant differences were determined using the t-test.
3.Results
3.1. Bioinformatic analysis
According to the sequence published on NCBI, the coding region of the CD83 gene sequence in flounder is 669 bp, which encodes a total of 222 amino acids (aa). Analysis of the conserved domains of protein showed that flounder CD83 is a transmembrane protein containing an immunoglobulin-like domain (aa29-aa136). The aa1-aa24 are signal peptides, aa25-aa151 are extracellular regions, aa152-aa174 are transmembrane regions, and aa175-aa222 are intracellular regions (Fig. 1A). At the same time, using the iTASSER online analysis platform, a three-dimensional model of the flounder CD83 was constructed (Fig. 1B). The online analysis of N-glycosylation and phosphorylation was showed in Fig. 1C.
Fig. 1.
Conserved domains, tertiary structure model, N-glycosylation sites and phosphorylation sites are analysed for the protein sequence of flounder CD83. A: SMART conserved domain analysis; B: CD83 molecular three-dimensional model was constructed by iTASSER; C: CD83 molecular N-glycosylation site and phosphorylation site were analysed .
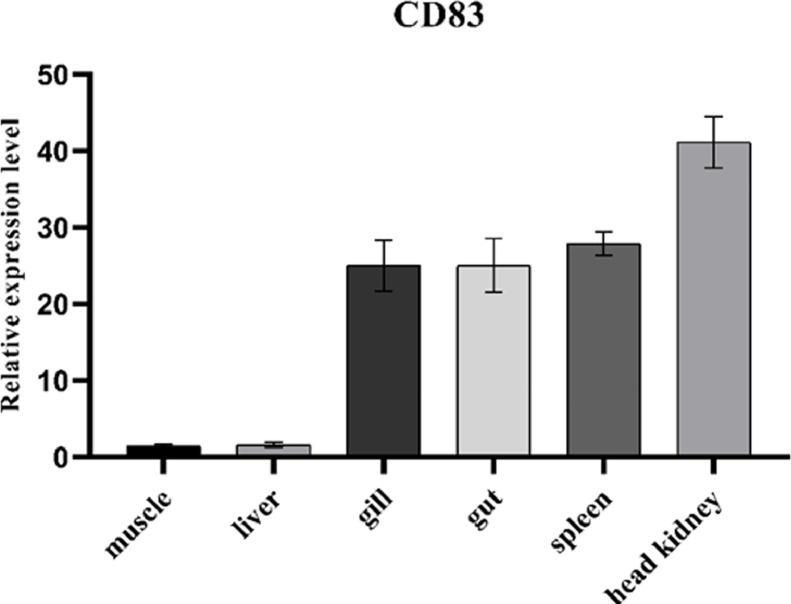
3.2. Tissue distribution of CD83
Using β-actin as an internal reference gene, the tissue distribution characteristics of CD83 were detected. The results showed that CD83 was distributed in the head kidney, spleen, hind gut, gills, liver, and muscle, with low expression in liver and muscle, and high expression in head kidney and spleen (Fig. 2).
Fig. 2.
The relative expression of CD83 gene in different tissues of flounder. The results are presented as the means ± SD.
3.3. Production of rCD83 and eukaryotic transfection
The SDS-PAGE analysis showed that the rCD83 of flounder was successfully expressed in E. coli competent cells (BL21) with the pET-32a vector after IPTG induction. The molecular weight of the expressed proteins was approximately 33 kDa, which was in accordance with predicted molecular mass. The recombinant proteins with high purity were obtained and used for immunization.
The recombinant plasmid pTag-RFP-CD83 was transfected into the HINAE cell line and it was observed under an inverted fluorescence microscope after 48 h. Obvious red fluorescence could be seen in the transfected group, but no red fluorescence was seen in the negative control of the un-transfected group (Fig. 3), which proves that the plasmid is successfully transfected.
Fig. 3.
The results of eukaryotic transfection. Observation of HINAE-RFP-CD83 and HINAE-Control cell lines with an inverted fluorescence microscope. The arrow shows the cells transfected with the vector pTag-RFP-CD83. Bar=100 μm.
3.4. Western blot and Indirect immunofluorescence
Western blot experiments were performed using purified recombinant protein and lysate of HINAE cell line transfected with pTag-RFP-CD83 eukaryotic plasmid as substrates, respectively. The results showed that the CD83 Abs could show a single band at 33 kDa (Fig. 4A) and 52 kDa (Fig. 4B) were shown, they are consistent with the molecular weight recombinant prokaryotic and eukaryotic proteins, respectively, while the negative rabbit serum control group did not show any band.
Fig. 4.
Western blot results of CD83 Abs and Indirect immunofluorescence results. A. Lane M: Marker; Lane 1: CD83Abs to rCD83; Lane 2: negative control. B. Lane M: Marker; Lane 1: CD83 Abs to pTag-RFP-CD83 eukaryotic transfected HINAE cells lysates; Lane 2: negative control. C. Indirect immunofluorescence results of antibody and HINAE cell lines transfected with eukaryotic plasmid. Bar=200 μm.
IIFA results showed that the CD83 Abs could bind cells transfected with pTag-RFP-CD83 eukaryotic plasmid and could not bind cells transfected with pTag-RFP null plasmid, while the negative serum could not bind cells transfected with pTag-RFP-CD83 eukaryotic plasmid (Fig. 4C). The results indicated that the prepared CD83 Abs has good specificity and could be used for subsequent experiments.
3.5. Dendritic cells culture and morphology identification
Single cells isolated from the head kidney appeared smooth and round at 1 d (Fig. 5A), some of the cells showed protrusions on the surface at 3 d (Fig. 5B), while obvious dendritic like protrusions were visible at 5–7 d (Fig. 5C, D). Single cells were also isolated from the spleen (Fig. 5E), allowed to fully adhere at the first 6 h (Fig. 5F), some of the cells were seen to become suspended overnight (Fig. 5G), and then these cells were collected (Fig. 5H), and the purity of the primary DC cultures is 81.0 ± 0.7%. DCs from head kidney and spleen cultures were stained with Wright-Giemsa, and the results showed that the nuclei were reniform and dumbbell-shaped, with dendritic or pseudopod-like protrusions on the cell surface (Fig. 6).
Fig. 5.
Dendritic cells culture and morphological observation. A-D: Head kidney cells culture process;A:1d; B:3d; C:5d; D:7d;E-F: Spleen cells culture process;E: Initial cells; F:6 h; G:12 h; H: enriched suspension cells. Bar=10 μm.
Fig. 6.
Giemsa staining results of dendritic cells. A-D: DCs from head kidney; E-F: DCs from spleen. Bar=10 μm.
3.6. Phagocytosis analysis
After incubation of the enriched cells with fluorescent microspheres, microscopic observation showed that green fluorescent microspheres were engulfed within the cells (Fig. 7A), indicating that the cultured dendritic cells had phagocytic function. The results of flow cytometry showed that the phagocytosis rate of DCs in the LPS-stimulated group was 78.95 ± 0.64% and that of dendritic cells in the control group was 79.27 ± 1.01% (Fig. 7B), and the results of significance analysis showed that there was no significant difference in the phagocytosis rate of dendritic cells between the two groups (Fig. 7C).
Fig. 7.
Identification of phagocytic function of cultured cells. A: Microscope observation, Bar=10 μm; B: Flow cytometry results; C: Statistical analysis of the phagocytosis rate of LPS activation group and control group. The results are presented as the means ± SD.
3.7. Mixed leukocyte reaction analysis
CFSE-labeled allogeneic lymphocytes were cultured for 3 days, and flow cytometry results showed positive proliferation peaks in all groups of lymphocytes with the addition of DCs, while there were no positive proliferation peaks in the negative control group. The proliferation ratios of lymphocytes were 39.0%, 44.9%, 53.5%, and 59.8%, respectively (Fig. 8A), and there was a dose dependence. Meanwhile, the aggregation of lymphocytes could be observed by microscopic observation (Fig. 8B).
Fig. 8.
Detecting the proliferation of lymphocytes by flow cytometry (A) and microscopic observation (B) . (A) flow cytometry: a, Cells scatter diagram; b, Negative control; c, DCs 1:8; d, DCs 1:4; e, DCs 1:2; f, DCs 1:1. (B) microscopic observation: a, Lymphocytes added with DCs; b, Control. Bar=20 μm.
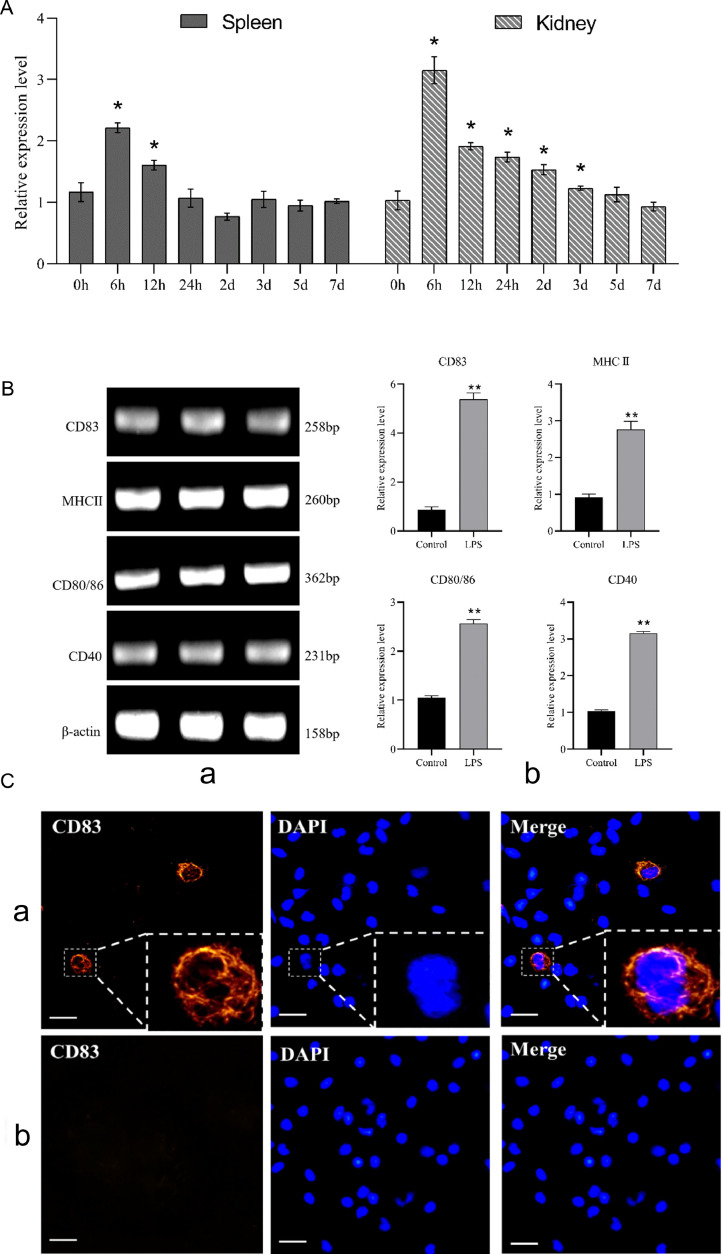
3.8. LPS treatment assay in vivo and in vitro
The relative expressions of CD83 gene in the spleen and head kidney were measured by qPCR at different time points after immunization. The experimental results showed that the expression of CD83 molecule in both spleen and head kidney showed significant changes, with an overall trend of increasing and then decreasing, and both reached the peak at 6 h (Fig. 9A).
Fig. 9.
LPS treatment assay in vivo and vitro by qPCR(A,B) and IIFA(C). (A) qPCR: The relative expression of CD83 in spleen and kidney of flounder after LPS injection. The results are presented as the means ± SD. * represent the statistical significance (p<0.05). (B) qPCR: Gene expression characteristics of CD83, MHC II, CD80/86, CD40 after LPS stimulated dendritic cells in vitro. a, RT-PCR results of surface molecules on dendritic cells; b, The expression level of surface molecules related to dendritic cells after activation of LPS. **represent the statistical significance (p<0.01). (C) IIFA: Fluorescence microscope observation of the expression of CD83 molecules after LPS stimulated dendritic cells in vitro. a, DCs in the LPS stimulation group; b, DCs in the control group. Bar=10 μm.
DCs cultured in vitro were stimulated with LPS and the relative expression of membrane surface marker molecules was measured after 24 h. qPCR revealed that CD83, MHC II, CD80/86, and CD40 genes were significantly up-regulated in expression (Fig. 9B). IIFA results showed that a small number of CD83+ cells could be detected in the DCs of the LPS-stimulated group using the prepared antibody, while no positive signal was detected in the control group (Fig. 9C).
4. Discussion
In this study, the CD83 sequence of flounder was analyzed for structural domainsand active sites , and a three-dimensional model of the protein was constructed. Meanwhile, the distribution characteristics of CD83 molecule in different tissues of flounder were explored. The CD83 molecule is a transmembrane protein containing an immunoglobulin-like structural domain, which is similar to human CD83 [5]. CD83 is composed of an extracellular IgV-like domain, a transmembrane domain and a cytoplasmic tail [1,2,17]. The results showed that CD83 contained 5 N-glycosylation sites and 34 phosphorylation sites, which may be related to its biological function. Glycosylation is an important post-transcriptional modification of CD83, and the human CD83 contains three N-glycosylation sites, which are necessary for the function [17]. The tissue distribution of CD83 shows that its expression is highest in the cephalic kidney and spleen, presumably because the cephalic kidney and spleen are important immune organs in teleost.
In mammals, DCs are usually obtained by culturing precursor cells from bone marrow, but teleost lack bone marrow [4]. In the present study, DCs were cultured in vitro from the head kidney and spleen of flounder. The kidney contains precursor cells, which are gradually cultured to produce dendritic cells in the presence of molecules such as endogenous growth factors of precursor cells and cytokines contained in the serum, and then isolated and enriched using Percoll gradients, taking advantage of their low buoyancy density. In the later stage of primitive dendritic cell culture, we observed that the cells had dendritic protrusion, which was consistent with the morphology and differentiation characteristics of dendritic cells. The spleen contains intrinsic dendritic cells, and splenic dendritic cells were obtained by taking advantage of this property of their transient apposition to the wall. Dendritic cells are transient adherent, suspended cells will be collected for culture every time in the cell culture process, which will gradually dilute and discard the other adherent cells. Microscopic observation and Giemsa staining revealed that the cultured dendritic cells were surrounded by dendritic-like protrusions and the nuclei showed irregular morphology such as kidney-shaped and dumbbell-shaped, which were consistent with the morphological features of mammalian DCs. In capture at the end of the antigen, immature DCs in chemotactic factor, under the action of migration to lymphatic area near the place in the body, which are transported to the surface of the cell for recognition by T lymphocytes, thus completing antigen presentation and activating primary T lymphocytes [11], dendritic cells also changed from immature state to mature state during cell culture. The low content of DCs isolated from the spleen, it was not enough to complete the function test, so the subsequent function identification was carried out with DCs cultured from the head kidney. In terms of phagocytosis, flounder DCs were found to be phagocytic in this study and could engulf fluorescent microspheres. In mammals, it is generally accepted that immature DCs are usually more phagocytic than those activated by antigen stimulation [12]. To investigate whether DCs of flounder possess similar properties, LPS was used to stimulate DCs and to examine the changes in phagocytosis rate before and after stimulation. The results of the significance analysis showed that there was no significant difference in the phagocytosis rate between the LPS stimulated and control groups. Similar results were also observed in rainbow trout, where there was no significant change in the phagocytosis rate of cells before and after stimulation of DCs using poly I: C [4]. Eliciting a mixed lymphocyte response is also an important function of dendritic cells, and the CFSE dye labeling of lymphocytes was used to detect cell proliferation in this study. As the cells divide, the CFSE dye also divides equally into two daughter cells, thus halving the fluorescence intensity of the cells [21]. The experimental results showed that after the addition of DCs to the culture, lymphocytes produce a significant proliferation peak and the percentage of cell proliferation showed a certain dose-dependent effect.
In terms of membrane surface molecules, the DCs expressed CD83, MHC II, CD80/86, and CD40 marker molecules, and all showed significantly upregulated expression after LPS stimulation. In zebrafish, CD83, MHC II, and CD80/86 also showed up-regulated expression after KLH and LPS stimulation of isolated DCs [18]; in rainbow trout, the expression of CD83 and MHC II also showed up-regulated expression after poly I: C stimulation of isolated dendritic cells [4]. These results in teleost are similar to those in mammals, where CD83, MHC II, CD80/86, and CD40 showed upregulated expression after stimulation with LPS in mouse dendritic cells [25]. MHC II, CD80/86, and CD40 are key membrane surface molecules in the antigen presentation pathway of mammalian dendritic cells and are involved in the formation of immune [7], [8], [9], therefore, the experimental results also suggest that similar antigen delivery pathways may exist between flounder and mammalian dendritic cells. And in capture at the end of the antigen, immature DC streated with chemotactic factor, It binds to it and forms some complexes, which are transported to the surface of the cell for recognition by T lymphocytes, thus completing antigen presentation and activating primary T lymphocytes [10], dendritic cells also changed from immature state to mature state during cell culture. We also demonstrated that DCs in LPS-stimulated group expressed CD83 protein at the protein level, while no positive signals were found in the control, suggesting that CD83 protein in flounder may be expressed mainly in mature DCs after stimulation activation, as in mammals, and could be used as a specific marker for dendritic cell maturation. In human dendritic cells, CD83 can also be detected at the genetic level, but the protein form of CD83 can only be detected on the surface of mature dendritic cells. And Dendritic cells are activated by antigen stimulation, promoting their maturation [11]. In this study, CD83 positive cell were low which might because there are fewer mature dendritic cells, or the primary cells cultured in vitro are less activated by antigen. It has been reported that in mammalian dendritic cells, CD83 is already expressed intracellularly before localizing to the dendritic cell surface, but is present in the Golgi complex and early endosomes, where it is released from the Golgi apparatus and endosomes and expressed on the cell surface only after TLR signaling stimulation and regulation by TNF-α. LPS is TLR ligand that activates TLR signaling mechanism, so it is speculated that the mechanism of CD83 protein membrane surface expression in dental flounder may be similar [4]. However, CD83 can be either transmembrane or soluble form in the mammalian system, it should be the difference between teleost and mammals. So far there are few references on the CD83 expression in cells of fish, the transmembrane or soluble forms of CD83 need further exploration.
In conclusion, this study analyzed the molecular properties and tissue distribution characteristics of CD83, obtained DCs and prepared CD83 polyclonal antibody for the first time in flounder, and investigated the expression characteristics of CD83 and changes in DCs after LPS stimulation, revealing that CD83 may be a marker molecule in the maturation period of DCs in flounder.
Declaration of Competing Interest
The authors state that the study was conducted without any commercial and financial relationships, which can be interpreted as potential conflicts of interest.
Acknowledgements
This study was supported by the National Key Research and Development Program of China (2018YFD0900503), the National Natural Science Foundation of China (32173005; 31730101; 31672684; 31672685), Shandong Provincial Natural Science Foundation (ZR2020KC025), the Fundamental Research Funds for the Central Universities (201822015), the Director Foundation of Functional Laboratory for Marine Fisheries Science and Food Production Processes, the Qingdao National Laboratory for Marine Science and Technology (2018MFSD-01), NBRPC (2012CB114406), the Key Research and Development Program of Shandong Province (2016GNC115001), and the OUC-AU joint projects (861901153077).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.fsirep.2021.100030.
Appendix. Supplementary materials
References
- 1.Aerts-toegaert C., Heirman C., Tuyaerts S., Corthals J., Aerts J.L., Bonehill A., Thielemans K., Breckpot K. CD83 expression on dendritic cells and T cells : correlation with effective immune responses. Eur. J. Immunol. 2007;37:686–695. doi: 10.1002/eji.200636535. [DOI] [PubMed] [Google Scholar]
- 2.Lechmann M., Berchtold S., Hauber J., Steinkasserer A. CD83 on dendritic cells : more than just a marker for maturation. Trends Immunol. 2002;23(6):273–275. doi: 10.1016/S1471-4906(02)02214-7. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau J., Briere F., Caux C., Davoust J., Lebecque S., Liu Y., Pulendran B., Palucka K. Immunobiology of dendritic cells. Annu. Rev. Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 4.Bassity E., Clark T.G. Functional identification of dendritic cells in the Teleost Model, Rainbow Trout (Oncorhynchus mykiss) PLoS One. 2012;7:e33196. doi: 10.1371/journal.pone.0033196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breloer M., Fleischer B. CD83 regulates lymphocyte maturation, activation and homeostasis. Trends Immunol. 2008;29:186–194. doi: 10.1016/j.it.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Geissmann F., Manz M.G., Jung S., Sieweke M.H., Merad M., Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Courtney A.H., Lo W.-L., Weiss A. TCR signaling: mechanisms of initiation and propagation. Trends Biochem. Sci. 2018;43(2):108–123. doi: 10.1016/j.tibs.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gizinski A.M., Fox D.A., Sarkar S. Co-stimulation and T cells as therapeutic targets. Best Pract. Res. Clin. Rheumatol. 2010;24(4):463–477. doi: 10.1016/j.berh.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang T., Cheng X., Truong B., Sun L., Yang X., Wang H. Molecular basis and therapeutic implications of CD40/CD40L immune checkpoint. Pharmacol. Ther. 2020 doi: 10.1016/j.pharmthera.2020.107709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Münz C. Autophagy proteins influence endocytosis for MHC restricted antigen presentation. Semin. Cancer Biol. 2020;66:110–115. doi: 10.1016/j.semcancer.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Farrokhi S., Shabani M., Aryan Z., Zoghi S., Krolo A., Boztug K., Rezaei N. MHC class II deficiency: report of a novel mutation and special review. Allergol. Immunopathol. (Madr.) 2018;46(3):263–275. doi: 10.1016/j.aller.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Guermonprez P., Valladeau J., Zitvogel L., Amigorena S., Théry C., Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu. Rev. Immunol. 2002;20:621–667. doi: 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- 13.Liu K., Victora G.D., Schwickert T.A., Guermonprez P., Meredith M.M., Yao K., Chu F.-.F., Randolph G.J., Rudensky A.Y., Nussenzweig M. In vivo analysis of dendritic cell development and homeostasis. Science. 2009;324:392–397. doi: 10.1126/science.1170540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lugo-villarino G., Balla K.M., Stachura D.L., Bañuelos K., Werneck M.B.F. Identification of dendritic antigen-presenting cells in the zebrafish. Proc. Natl Acad. Sci. 2010;107:15850–15855. doi: 10.1073/pnas.1000494107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nussenzweig M.C., Steinman R.M. Contribution of dendritic cells to stimulation of the murine syngeneic mixed leukocyte reaction. J. Exp. Med. 1980;151:1196–1212. doi: 10.1084/jem.151.5.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radwan J., Babik W., Kaufman J., Lenz T.L., Winternitz J. Advances in the evolutionary understanding of MHC polymorphism. Trends Genet. 2020;36:298–311. doi: 10.1016/j.tig.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 17.Scholler N., Hayden-ledbetter M., Hellström I., Hellström K.-.E., Ledbetter J.A. CD83 is a sialic acid-binding Ig-like lectin (Siglec) adhesion receptor that binds monocytes and a subset of activated CD8 + T cells. J. Immunol. 2001;166:3865–3872. doi: 10.4049/jimmunol.166.6.3865. [DOI] [PubMed] [Google Scholar]
- 18.Shao T., Zhu L.Y., Nie L., Shi W., Dong W.R., Xiang L.X., Shao J.Z. Characterization of surface phenotypic molecules of teleost dendritic cells. Dev. Comp. Immunol. 2015;49:38–43. doi: 10.1016/j.dci.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 19.Liu F., Tang x, Sheng X., Xing J., Zhan W. DNA vaccine encoding molecular chaperone GroEL of Edwardsiella tarda confers protective efficacy against edwardsiellosis. Mol. Immunol. 2016;79:55–65. doi: 10.1016/j.molimm.2016.09.024. [DOI] [PubMed] [Google Scholar]
- 20.Shortman K., Naik S.H. Steady-state and inflammatory dendritic-cell development. Nat. Rev. Immunol. 2007;7:19–30. doi: 10.1038/nri1996. [DOI] [PubMed] [Google Scholar]
- 21.Terrén, I., Orrantia, A., Vitallé, J., Zenarruzabeitia, O., Borrego, F., 2020. Chapter Twelve - CFSE dilution to study human T and NK cell proliferation in vitro, in: GalluzziL., RudqvistN.-P.B.T.-M. in E. (Eds.), Tumor Immunology and Immunotherapy – Cellular Methods Part A. Academic Press, pp. 239–255. 10.1016/bs.mie.2019.05.020. [DOI] [PubMed]
- 22.Thibodeau J., Moulefera M.A., Balthazard R. On the structure–function of MHC class II molecules and how single amino acid polymorphisms could alter intracellular trafficking. Hum. Immunol. 2019;80:15–31. doi: 10.1016/j.humimm.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Valečka J., Almeida C.R., Su B., Pierre P., Gatti E. Autophagy and MHC-restricted antigen presentation. Mol. Immunol. 2018;99:163–170. doi: 10.1016/j.molimm.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 24.Vremec D., Pooley J., Hochrein H., Wu L., Shortman K. CD4 and CD8 expression by dendritic cell subtypes in mouse thymus and spleen. J. Immunol. 2000;164:2978–2986. doi: 10.4049/jimmunol.164.6.2978. [DOI] [PubMed] [Google Scholar]
- 25.Xu L., Kwak M., Zhang W., Lee P.C., Jin J. Time-dependent effect of E . coli LPS in spleen DC activation in vivo : alteration of numbers, expression of co-stimulatory molecules, production of pro-inflammatory cytokines, and presentation of antigens. Mol. Immunol. 2017;85:205–213. doi: 10.1016/j.molimm.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 26.Zoccola E., Delamare-deboutteville J., Barnes A.C. Identification of barramundi (Lates calcarifer) DC-SCRIPT, a specific molecular marker for dendritic cells in fish. PLoS One. 2015;10:1–20. doi: 10.1371/journal.pone.0132687. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.