Abstract
Striped catfish, Pangasianodon hypophthalmus was immunized with Biofilm (BF) and Free cell (FC) of Aeromonas hydrophila vaccine at 1010 CFU g−1 for 20 days and monitored for growth parameters, immune responses and disease resistance up to 60 day post vaccination (dpv). Pangasius catfish in the BF vaccinated group had considerably higher growth and feed utilization than the FC vaccinated and unvaccinated groups (p < 0.05). Biofilm vaccinated group showed a significant increase (p < 0.05) in the mean weight gain (46.91 ± 0.59) than the FC (35.94 ± 0.21) and unvaccinated group (34.92 ± 0.35). The vaccinated fishes were challenged with A. hydrophila at 107 CFU/ml. Significant higher relative percentage survival (RPS) was recorded with BF (84.21 ± 1.49%) compared to that with FC (33.33 ± 1.21%). Polyclonal antibody-based ELISA was used to quantify the antibody titre. BF vaccinated group showed significantly higher antibody titer compared to other treatments (p < 0.05). Moreover, higher haematological parameters recorded in the present study were differentially stimulated by the oral administration of A. hydrophila biofilm vaccine. The mean total protein, albumin, and globulin levels of the BF vaccine groups were significantly higher (p < 0.05) than the mean total protein, albumin, and globulin contents of the unvaccinated group. Furthermore, biochemical stress parameters (SGPT, SGOT) in the vaccinated groups showed an incremental trend in the early days of the experimental period. However, the values were significantly lower (p < 0.05) in the biofilm group on 20 dpv onwards indicating improved health condition. Vaccinated BF fishes showed gut associated lymphoid tissues (GALT) within the laminar propria of mid gut. But in FC group fishes showed less aggregation of lymphoid cells. The unvaccinated control fish had no lymphoid cell aggregation in their intestines. The findings of the current research suggested that biofilm vaccine has the capability to be one of the potential oral vaccines in striped catfish against A. hydrophila infection.
Keywords: Biofilm based oral vaccine, Polyclonal antibody, Haemato-biochemical indices, Striped catfish, Gut associated lymphoid tissues, Growth performance
1. Introduction
During the last few decades aquaculture has played a vital and steadily rising role in food safety and monetary stability to the world. However, since 2000, global aquaculture now not enjoys the high annual growth and fallen to moderate 5.8% by the period of 2001–2016 [1]. Diseases have been a continuous concern for numerous significant Asian aquaculture producers, notably India. Striped catfish, Pangasianodon hypophthalmus (known as pangas/pangasius) an exotic fish species cultured across the country for its faster growth often compromised with bacterial infection. The motile aeromonas septicemia (MAS) and bacillary necrosis in pangasius (BNP) are two main bacterial diseases causing huge economic losses in pangasius farming [2], [3], [4], [5]. Prophylaxis through antibiotic had been an effective strategy in the beginning [6] but antibiotic-resistant pathogenic bacteria are becoming increasingly common, posing a severe threat to human and animal health around the world [7]. Prevention of epidemics is consequently necessary to avoid major economic losses and the development of an appropriate vaccine against the virulent pathogen is therefore imperative [8]. Disease prevention through vaccination and immunostimulation have been shown to be highly beneficial, since the 1980s, when the Norwegian salmon industry proficiently validated the concept of mass vaccination [9]. Vaccination strategy has been established for more than 40 countries against 22 bacterial diseases for more than 17 aquaculture species [10]. The administration of monovalent and polyvalent vaccines in Norwegian salmon industry has not only paved the way for potential solution for prevention of infectious diseases and higher yield but also reduced the burden of antibiotic usage in aquatic environment [11].
The immune protection provided by injection vaccination is significantly superior to that offered by the immersion strategy and the oral approach [12]. However, antigens that persist at the injection site can act as inflammatory reactions, possibly leading to negative side effect [13], growth penalty [14,15] and malfunction of the reproductive organs [16]. Oral vaccines are an attractive alternative to overcome such constraints. Feed based oral immunization is one of the most popular technique among the administered types because of its low cost, easy to handle and can immunize fishes of all size at a time [17].
However, conventional free cell bacterial vaccine provide lower and inharmonious immune response and disease resistance in fish [18] due to acid destruction of the antigen in the stomach / foregut prior to the entering the hind gut where immune induction initiated [19]. Several techniques have been developed and tried to protect the antigens from destruction viz., encapsulated antigen microspheres [20,21], liposome vaccine [22] enteric coated vaccine [23] and artemia [24] for protecting the antigens from enzyme destruction, which many are costly and complex. Against this background, biofilm based oral vaccines look promising because of its cost-effective preparation, non-stressful and mass immunization.
Biofilm based oral vaccine has been conceptualized in our laboratory. In vitro biofilm of A. hydrophila developed on a substrate (chitin flakes) later inactivated and mixed with fish feed. The biofilm vaccine feed has given significant encouraging results in different models of fish experimented in herbivore [25], omnivore [26], carnivore [27], Nile tilapia [28], Asian seabass [29] and penaeid shrimp [30] in terms of antibody titre and protection upon challenge. However, vaccine efficiency should not rely on protection alone [31] rather its ability on growth performance, blood profile as well as biochemical response to vaccine [16] and those parameters can provide necessary facts to select an ideal antigen for vaccination [32]. So far, there has been no published information on growth performance and gut morphology [33] of fishes fed on biofilm A. hydrophila as oral vaccine. Therefore, the present study was designed for the evaluation of A. hydrophila biofilm oral vaccine on growth, haemato-biochemical profile and gut histology of striped catfish, P. hypophthalmus.
2. Materials and methods
2.1. Isolation, identification and maintenance of pure culture
A virulent A. hydrophila isolated from diseased gold fish was injected and re-isolated from common carp (Cyprinus carpio) as described by Mamun et al. [34]. Following confirmation, the bacteria were grown in Trypton Soya Broth (1.5w/v). Cultured broth were transferred to the 50 ml centrifuge tube later harvested as pellet by centrifugation at 10,000 rpm for 10 min. Bacterial sample were preserved with 15% glycerol and 1.5% TSB (w/v). The bacterial suspension was aliquoted into 2 ml micro-centrifuge tubes and preserved at -40 °C for further use.
2.2. In vitro preparation of A. hydrophila biofilm cells
Production of A. hydrophila biofilm cells were prepared according to Azad et al.[35]. Media were prepared in 250 ml conical flask bearing TSB (0.225 w/v) and chitin flakes (0.3%) were sterilized by autoclaving. One milliliter (ml) fresh bacterial broth inoculated into the prepared conical flask and agitated for 6 h daily in refrigerated shaker incubator with 120 strokes/min at 37 °C. The biofilm cells were harvested on day four by washing thrice in the same flask with sterile phosphate buffer saline (PBS, pH 7.2) to remove free cells. Before mixing with the feed biofilm cells were heat inactivated (100 °C for 50 min).
2.3. In vitro preparation of A. hydrophila free cells
Planktonic A. hydrophila cells were produced according to Azad et al. [35]. Hundred (100) ml media (TSB, 1.5%) autoclaved at 121 0C for 15 min and inoculated with A. hydrophila, incubated for overnight at 37 °C. Free cells antigen collected by centrifugation at 10,000 rpm for 10 min and further washed thrice using sterile PBS (pH 7.2). At the end, the bacterial pellets were re-suspended in PBS to achieve higher CFU/ml (1010 cells/ml). Free cells or planktonic cells were heat inactivated before incorporating in the feed.
2.4. Characterization of biofilms through light microscopy and field emission scanning electron microscope (FE-SEM)
Four-day old biofilm of A. hydrophila dislodged from the substrates by vortexing for 5 min and mounted fresh on to the slide, was observed under the phase contrast microscope (Olympus, BX3-25ND25, Japan) and microphotographed.
Biofilm cells were characterized in FE-SEM according to the manufacturer's instruction. BF cells were separated from chitin after vortexing for 3 min, the supernatant, with the cluster of biofilm was centrifuged at 200 rpm for 5 min to remove chitin particles, biofilm in the supernatant was pelletized at 1000 rpm for 10 min and the pellet was resuspended in sterile PBS. A small quantity of prepared biofilm cells were dispersed on a SEM specimen mount. Enough argon was maintained in the chamber so that the vacuum reads 0.08 mbar. Sample was coated with gold (Eiko 1B-3 at 0.15 torr) as appropriate for a specified period of time and current to obtain an acceptable coating. Finally sample was transferred back to the specimen box after coating for analyzing at Carl Zeiss Sigma VP Field Emission Scanning Electron Microscope in the Central Laboratory of DST-PURSE PROGRAMME, Mangalore University.
2.5. Incorporation of biofilm and free cells in feed
Biofilm vaccine (BF) as well as free cells (FC) were mixed into the feed as stated by Azad et al. (1999). Different feed ingredients such as fish meal (24%), groundnut oil cake (24%), rice bran (17%), wheat flour (17%) tapioca flour (14%) were collected from local fish market. The ingredients were well sieved, mixed and cooked and cool to ambient temperature. Cod liver oil (3%) vitamins and mineral premix (1%) were added to cooked ingredients followed by incorporation of BF and FC vaccine separately. A basal diet (C) with above components without vaccine antigen was prepared with sterile PBS (pH 7.2). Both BF and FC vaccine were quantified and incorporated in feed at 1010 cells g−1 of feed at the end. The feed dough was pelletized by hand pelletizer later dried and stored at 4 °C in a refrigerator.
2.6. Rearing of pangasius fish stock
Healthy fry of P. hypophthalmus were procured from Zonal Agricultural and Horticultural Research Station, Mudigere (13.1378°N 75.6060°E.), Chikkamagaluru, India. Further, the fish were reared for one month to grow into juvenile stage. The fish were fed with dry small pellets, prepared with feed ingredients such as fish meal 24%, groundnut oil cake 24%, rice bran 17%, wheat flour 17%, tapioca 14%, cod liver oil 3%, minerals and vitamins 1%.
2.7. Oral vaccination of pangasius catfish
Juvenile P. hypophthalmus (12.42 ± 0.14 g) were maintained in cemented rectangular tanks of containing 950 L water. Treatments were assigned into two groups’ biofilm (BF) and free cell (FC) and one control (C) with triplicate. Thirty fishes (juveniles) were released in each replicate. Optimum water quality were maintained by removing of wastes by siphoning (20% water) from the bottom of the tank at every alternate day. Both the vaccinated group (BF and FC) fishes fed vaccine at the dose of 1010 g−1 of feed. Pangasius juveniles in the control group (C) fed basal diet prepared with PBS. The vaccinated and basal feed was given at 3% of the body weight at 9.00 and 17.00 h for 20 days and the complete acceptance of feed was monitored each day. Wastes were siphoned from the bottom of the tanks once in day and subsequently added 20% water. The water quality parameters such as water temperature, pH, DO and ammonia were 22.5–27.4 °C, 6.9–7.8, 6.5–7 ppm and 0.04–0.06 ppm, respectively throughout the experimental period. After 20 days of trial, vaccinated feed were completely withdrawn and control feed was given till the experimental period.
Upon completion of 20 days of oral vaccination, three fish from each treatment and control were anesthetized (1 ppm, Clove Oil) and bled from their caudal vein of fish using disposable syringes (2 ml) flushed with heparin (Sigma, UK). Blood sample were collected on 0, 10, 20, 30, 40, 50 and 60 dpv. Harvested blood sample separated into two aliquots, one for CBC (Complete Blood Count), and the other sample for serum collection. Blood was centrifuged at 10,000 rpm for 10 min and sera collected from 3 fish per treatment were accumulated and stored at -40 °C for further record.
2.8. Estimation of growth parameters
The fishes were sampled at every 15 days to analyze the growth parameters. The parameters such as weight gain, % weight gain, specific growth rate (SGR), feed conversion ratio (FCR), protein efficiency ratio (FCR), average daily gain (ADG), feeding rate (FR) and survival rate were determined according to Rathore et al. [36].
2.9. Protection upon challenge with A. hydrophila
2.9.1. Estimation of lethal dose50 (LD50) of A. hydrophila
A. hydrophila isolate was tested for pathogenicity in fingerlings of P. hypophthalmus (15 ± 5.50 g) were maintained in seven glass aquaria (180 l capacity), in triplicate, with 10 fish in each aquarium. Fish were injected (intramuscular) each with 0.1 ml of A. hydrophila containing 103, 104, 105, 106, 107, 108 and 109 and CFU/ml (the practice were performed by the permission of the Animal Ethics Committee, College of Fisheries, Mangalore). Control fish in tank eight received 0.1 ml PBS. Mortality was noted at different hours interval for 10 days. The pathogenicity (degree of virulence) were estimated as reported by Reed and Muench [37]. LD50 of A. hydrophila in pangasius was found to be 4.5 × 107 CFU/ml (Table 1). At higher concentration (109 CFU/ml) 90 % mortality was observed and there was no mortality within 10 d post injection at 103 CFU/ml. No mortality was observed in fish injected with phosphate buffered saline. Therefore, 4.5 × 107 CFU/ml, was considered for challenge studies.
Table 1.
Details of LD50 of Aeromonas hydrophila in pangasius catfish.
| No. of Bacterial cell (CFU/ml) | Initial number | No. of fish dead | No. of fish survived | Death ratio | Survival ratio | Mortality rate | Cumulative mortality (%) |
|---|---|---|---|---|---|---|---|
| 109 | 30 | 30 | 0 | 96 | 0 | 96/96 | 100 |
| 108 | 30 | 27 | 3 | 66 | 3 | 66/69 | 95.65 |
| 107 | 30 | 20 | 10 | 39 | 13 | 39/52 | 75 |
| 106 | 30 | 11 | 19 | 19 | 32 | 19/51 | 37.19 |
| 105 | 30 | 7 | 23 | 8 | 55 | 8/63 | 12.70 |
| 104 | 30 | 1 | 29 | 1 | 84 | 1/85 | 1.18 |
| 103 | 30 | 0 | 30 | 0 | 114 | 0/114 | 0.00 |
Proportionate distance =
Log LD50 = Dilution above 50% - (Proportionate distance × Dilution factor)
LD50 = 4.5 × 107 CFU/ml
2.9.2. Vaccine efficacy determination by challenge study
Twenty six orally vaccinated P. hypophthalmus from each tank of vaccinated (BF and FC) and unvaccinated fishes were injected (IM) with 0.2 ml of 18 h old culture of A. hydrophila at 4.5 × 107 CFU/ml. Manifestation of clinical signs, morbidity and mortality were viewed for 12 days. Fresh blood from moribund fish and streaking were done from kidney on RS media (Specific for A. hydrophila isolation) for the confirmation of fish death. Vaccine efficacy were measured by relative percent survival was recorded as described by Amend [38].
2.10. Serum antibody response of P. hypophthalmus
2.10.1. Purification of immunoglobulin
Pangasius IgM purification process in the present study were done according to Babu et al. [39] using a 5 ml BSA-CL agarose column. Extracted samples were merged and dialysis were done at 4 °C using a membrane. The membrane were transferred to the tray to condense and concentrated IgM was preserved at -40 °C for further analysis.
2.10.2. Development of polyclonal antibody (PAb) against P. hypophthalmus IgM
Two healthy rabbits were reared with proper house for the development of polyclonal antibody. Serum were taken before injecting the purified IgM, considered as control. Rabbits were injected intramuscularly (IM) with 400 µl pure IgM (100 µgml−1) emulsified in Freund's complete Adjuvant (1:1). On days 14, 28, and 35, the remaining three booster doses were given with incomplete Freund's adjuvant. Anti-IgM rabbit antiserum that had been highly immunized was filtered through a 0.45 m syringe filter and purified on a Protein-A Sepharose column. The antiserum was separated into 0.5 ml aliquots and kept at -20 °C until it was used. During the rabbits' rearing, the College's Animal Ethics Committee's codal formalities were observed.
2.10.3. Characterization of polyclonal antibodies
Pangasius immunoglobulins were characterized by 15% SDS-PAGE Laemmli [40]. Briefly, samples were mixed 1:1 with sample buffer, boiled for 5 min at 95 °C, and then placed onto the gel with the molecular weight markers. The dye front was allowed to reach the bottom of the gel by running it at a continuous voltage (80 V). The polyvinyldine difluoride (PVDF) membrane (0.2 µm, Amersham) was activated by soaking it in cooled methanol for 15 min, then washing it and equilibrating it separately in a Western blot buffer. Using a semi-dry-transblot device, proteins were transferred to PVDF membrane for 1 h at a steady 250 milliampere (mA) current and 25 volts (Chromous Biotech, Bangalore). PVDF protein lanes were cut into strips and reacted overnight with blocking solution (3% BSA-PBS). After that, the paper lanes were incubated with immune serum for 2 ½ h. Anti-rabbit IgG-HRP (1:2000) was produced in blocking buffer and incubated for 45 min at room temperature (RT) on a rocker shaker after three washes with PBS –Tween 20 (0.05%). Strips were washed three times with wash buffer before being incubated for 5 min with the substrate-chromogen complex (H2O2 – 3, 3′- Diaminobenzidine tetrahydrochloride hydrate, Sigma, USA) in a rocker shaker. The reaction was stopped by using wash buffer to clean the PVDF membrane strips. After the strips were properly dried, a brown band appeared, which was compared to a standard protein molecular weight marker (wide range, Sigma USA).
2.10.4. Evaluation of serum antibody response by polyclonal antibody (PAb) based ELISA
The ELISA was performed as stated by Furuta et al. [41] with some modifications. A. hydrophila antigen (0.1 ml) was coated to Elisa plate wells (109 cells). Free sites were block by coating the plate with 300 µl of PBS (0.05% tween and 1% milk powder) for 1 h. Poured off the caesin, plate were wash (3 × 5 min) with PBS tween 20. Serum samples were added (starting dilution 1:4) and incubated for 1 h at 37 °C. Plate were washed (3 × 5 min) with PBS tween 20 and one time with PBS. After that, 0.1 ml of anti- P. hypophthalmus IgM polyclonal antibody was added, which was incubated for 1 h at room temperature before being washed. The bound antibodies were washed after a 45 min reaction at room temperature with HRP conjugated DAKO goat anti mouse IgG (Sigma, USA) diluted 1:4000. The substrate (TMB) was added and incubated for 5 min before the reaction was halted by adding 50 µl/2N H2SO4 to each well. In an ELISA reader, the color developed was read at 450 nm (Bioteck, USA).
2.11. Analysis of haemato-biochemical parameters
Hematological parameters such as hemoglobin, hematocrit, erythrocyte count, mean corpuscular volume (MCV), mean cell hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), total leucocyte count (TLC), platelets were determined using automatic hematology analyzer (PCE-210) and biochemical parameters such as serum glutamate pyruvate transaminase (SGOPT), serum glutamate oxaloacetate transaminase (SGOT), alkaline phosphate (ALP), cholesterol, triglycerides, serum glucose, total protein, albumin, globulin, A:G ratio and total immunoglobulin were analyzed using kits (AGD Biomedicals Pvt. Ltd., Mumbai) by clinical chemistry analyzer (AGD-2020) at Animal Disease Diagnostic Laboratory and Information Centre (Institute of Animal Health and Veterinary Biologicals, Mangalore, Karnataka).
2.12. Histology of gut
For histological study, healthy fishes were euthanized and mid gut were separated, washed in normal saline and preserved in 10% neutral buffered formalin for 72 h for fixation. All the histological procedures were followed as detailed by Bullock [42]. The tissues on the glass slides were analysed through the light microscope (Olympus, BX3-25ND25, Japan) to observe the gut associated lymphoid tissue (GALT).
2.13. Statistical analysis
All statistical analysis was performed using IBM SPSS version 20 software. All of the results were analyzed using one-way analysis of variance (ANOVA) and Duncan's multiple range tests after being represented as means ±SE of three replications. Differences were determined and P < 0.05 was used to determine whether they were statistically significant.
3. Results
3.1. Characterization of biofilms through light microscopy and FE-SEM
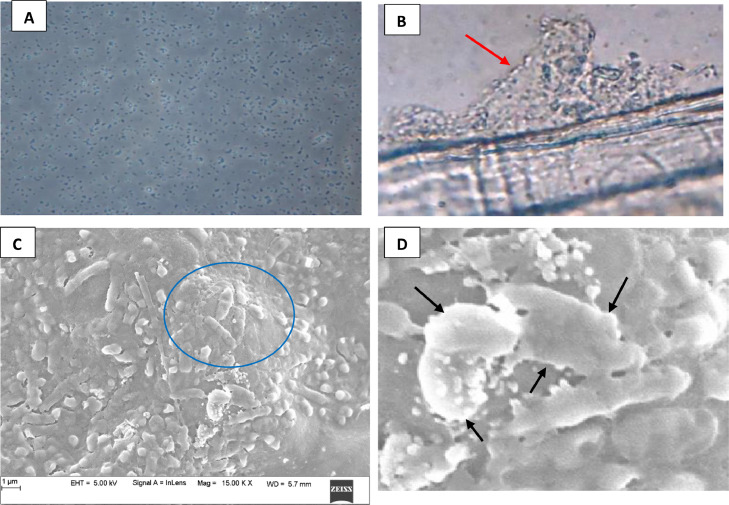
Light microscopy and Field Emission Scanning Electron Microscopy (FE-SEM) revealed projection of biofilm and encapsulation of bacteria, saline features for biofilm bacteria (Fig. 1A–D).
Fig. 1.
A: Free cells of A. hydrophila from the one day cultured broth (100X); B: Microphotograph of the 4-day old biofilm cell of A. hydrophila, note that the fingerlike projections (red arrow) of the glycocalyx (100X) C: FE-SEM of 4-day old biofilm cell of A. hydrophila. Note that the clumps of bacterial cell (circle) (15000X) D: Computer blown up image of biofilm of A. hydrophila showing the A. hydrophila inside the glycocalyx matrix (arrow).
3.2. Effects of A. hydrophila biofilm oral vaccine on growth of P. hypophthalmus
Significantly improved weight gain was achieved in BF fed fishes (46.91 ± 0.59 g) compared to FC (35.94 ± 0.21 g) and control group (34.92 ± 0.35 g) (P < 0.05). Significant lower but best FCR was observed in BF vaccine containing diet (1.190 ± 0.02) in comparison of FC (1.54 ± 0.01) and control diet (1.58 ± 0.03) is presented in Table 2 (P < 0.05).
Table 2.
Growth and feed utilization of P. hypophthalmus recorded under vaccinated and control groups.
| Parameters | Treatments | ||
|---|---|---|---|
| BF | FC | Control | |
| Initial weight (g) | 12.60* ± 0.15a | 12.33 ± 0.33a | 12.34 ± 0.32a |
| Final weight (g) | 59.51 ± 0.62a | 46.93 ± 1.74b | 47.26 ± 0.64b |
| Weight gain (g) | 46.91 ± 0.59a | 35.94 ± 0.21b | 34.92 ± 0.35b |
| % weight gain | 372.32 ± 6.17a | 291.80 ± 5.94b | 283.21 ± 5.36b |
| Average daily gain (g/day) | 0.59 ± 0.01a | 0.45 ± 0.00b | 0.44 ± 0.00b |
| Specific growth rate (% day −1) | 1.94 ± 0.02a | 1.71 ± 0.02b | 1.68 ± 0.02b |
| Feed conversion ratio (FCR) | 1.19 ± 0.02b | 1.54 ± 0.01a | 1.58 ± 0.03a |
| Protein efficiency ratio (PER) | 2.58 ± 0.07a | 2.03 ± 0.02b | 1.98 ± 0.03b |
| Feeding rate (FR) | 1.97 ± 0.06b | 2.15 ± 0.03a | 2.25 ± 0.05a |
| Survival (%) | 100 ± 0.00 a | 100 ± 0.00 a | 100 ± 0.00 a |
*Data are expressed as mean ± standard error (M ± SE). The values with different superscript letters in the same row are significantly different (P < 0.05)
Weight gain (g) = Mean final weight (g) – Mean initial weight (g)
% Weight gain =
ADG (g/day) =
SGR (% day −1) =
FCR =
PER=
FR=
Survival (%) =
3.3. Disease resistance against A. hydrophila
3.3.1. Challenge with A. hydrophila
Levels of protection with biofilm and free cell oral vaccination were assessed through homologous challenge with A. hydrophila. After 80 days (at 60 dpv) of the vaccine trial, fish from all the three groups including positive control were injected with 0.2 ml of bacterial suspension at 4.5 × 107 CFU/ml. Fishes were injected with 0.2 ml PBS consider as negative control. Relative percent survival (Table 3, Fig. 2) in BF vaccinated group was higher (84.21 ± 1.49%) compared with that in FC (33.33 ± 1.21%). Several clinical signs has appeared in all treatments group including sluggish movement, tail and fins rot, haemorrhagic lesions on the dorsal peduncle, eyes and skin-muscles. Sloughed off epidermis, abrasion, abdominal distention and dropsy also observed in the challenged fishes. However, these clinical symptoms were apparently less in vaccinated fish. Moreover, the total fish mortality after challenge with A. hydrophila decrease significantly (P < 0.05) in BF vaccinated group compared to FC and control. A. hydrophila from blood and kidney of moribund fish were collected aseptically for the confirmation of the death.
Table 3.
Protection level in vaccinated P. hypophthalmus challenged with A. hydrophila at 4.5 × 107 CFU/ml.
| Treatments | No. of fish challenged | No. of fish survived | No. of fish dead | % cumulative mortality | % survival | RPS (%) | Independent T test |
|---|---|---|---|---|---|---|---|
| Biofilm vaccine | 78 ± 0.60 | 69 ± 0.60 | 9 ± 0.58 | 11.54 ± 0.63c | 88.46 ± 2.10a | 84.21 ± 1.49a | T< 0.05 |
| Free cell vaccine | 78 ± 0.58 | 40 ± 1.20 | 38 ± 1.20 | 48.72 ± 1.18b | 51.28 ± 1.85b | 33.33 ± 1.21b | |
| Control | 78 ± 0.60 | 21 ± 1.20 | 57 ± 1.73 | 73.07 ± 1.88a | 26.92 ± 0.71c | - |
Values are expressed as mean ± SE, in the columns with different superscripts differ significantly (P < 0.05).
Fig. 2.
Relative percent survival of P. hypophthalmus upon challenge with A. hydrophila
3.4. Serum antibody response of P. hypophthalmus
3.4.1. IgM purification and characterization
The SDS-PAGE gel of extracted pangasius IgM displayed a 24 kDa band for the light chain (Fab) and a second 84 kDa band for the heavy chain (Fc) (Fig. 3).
Fig. 3.
SDS – PAGE (A) and western blot (B) analysis of the purified pangasius immunoglobulin (Ig): Lane 1: Molecular weight marker Lane 2: Affinity purified serum, Lane 3: Whole serum of pangasius, Lane 4 and 5: western blots showing reactivity of the PAb to the heavy chain of the purified Ig.
3.4.2. Measurements of serum antibody by polyclonal antibody (PAb) based ELISA
The serum antibody activity of the pangasius catfish respond strongly to vaccination. Serum antibody titer (two fold dilution) in ELISA of the treatments and control from 0th to 60th dpv, are depicted in Fig. 4. Higher antibody titer was observed in the BF group compared that with the FC and control groups.
Fig. 4.
ELISA antibody titer in P. hypophthalmus orally vaccinated with BF and FC of A. hydrophila.
3.5. Haematological parameters
Haematological profiles (TEC, Hb, PCV, TLC and Platelets) and indices (MCV, MCH, and MCHC) of vaccinated and unvaccinated P. hypophthalmus fingerlings are recorded in Table 4. The results exhibited that there were significant increase in total erythrocyte counts (TEC) in vaccinated fishes compared to control group on 0 day post vaccination (dpv). Moreover, TEC in biofilm vaccinated (BF) were significantly higher than FC vaccinated group on 0 dpv (P < 0.05). TEC were more excelled (P < 0.05) in BF treated fish compared to both vaccinated FC and control group, on 10 dpv. However BF group had the higher but no meaningful variations in total RBC counts at 30, 40, 50 and 60 dpv (Table 4). Higher haemoglobin were recorded in BF vaccinated fishes (Table 4). Significant increment of PCV was noted in BF vaccinated fishes compared to both vaccinated FC and unvaccinated fishes at 10 and 20 dpv. Mean cell volume (MCV) were found stable at 0, 10, 50 and 60 dpv in all the treatments. In general higher level of MCV were detected in BF treated fishes compared to both FC and control group, except 10, 20 and 60 dpv. The level of MCH and MCHC were higher at 0 to 10 dpv but no significant differences were noted. In general, except 10 dpv both MCH and MCHC were found similar among the treatments (P>0.05) (Table 4).
Table 4.
Haematological profiles, total erythrocyte count (TEC), haemoglobin content (Hb), packed cell volume (PCV), mean cell volume (MCV), mean cell haemoglobin (MCH), mean cell haemoglobin concentration (MCHC), total leukocyte count (TLC) and Platelets of oral vaccinated and control P. hypophthalmus.
| Parameters | Treatments | 0 dpv | 10 dpv | 20 dpv | 30 dpv | 40 dpv | 50 dpv | 60 dpv |
|---|---|---|---|---|---|---|---|---|
| TEC (106 mm−3) | BF | 2.64 ± 0.05a | 2.60 ± 0.13a | 2.52 ± 0.14a | 2.54 ± 0.12a | 2.80 ± 0.09a | 2.62 ± 0.10a | 2.94 ± 0.09a |
| FC | 1.80 ± 0.05b | 1.78 ± 0.09b | 2.24 ± 0.04ab | 2.48 ± 0.12a | 2.65 ± 0.13a | 2.34 ± 0.148a | 2.63 ± 0.15a | |
| Control | 1.79 ± 0.08b | 1.82 ± 0.10b | 2.18 ± 0.07bc | 2.36 ± 0.11a | 2.87 ± 0.07a | 2.39 ± 0.12a | 2.90 ± 0.05a | |
| Hb (g dL−1) | BF | 11.60 ± 0.15a | 11.20 ± 0.37a | 11.50 ± 0.21a | 13.60 ± 0.37a | 13.20 ± 0.14a | 11.60 ± 1.55a | 14.30 ± 0.55a |
| FC | 7.40 ± 0.46b | 8.10 ± 0.57b | 9.40 ± 1.04a | 12.10 ± 0.84a | 12.0 ± 0.68a | 9.40 ± 0.58a | 14.10 ± 0.86a | |
| Control | 7.90 ± 0.17b | 8.20 ± 1.04b | 9.70 ± 1.44a | 12.10 ± 0.31a | 12.50 ± 1.79a | 9.80 ± 0.23a | 14.20 ± 0.51a | |
| PCV (%) | BF | 33.60 ± 1.21a | 33.70 ± 1.21a | 34.40 ± 0.46a | 35.77 ± 2.26a | 39.0 ± 2.19a | 39.70 ± 2.70a | 41.10 ± 2.10a |
| FC | 21.60 ± 0.57b | 23.60 ± 2.25b | 28.90 ± 038a | 30.40 ± 3.11a | 36.0 ± 0.57a | 36.50 ± 0.57a | 38.20 ± 1.21a | |
| Control | 22.10 ± 2.36b | 24.10 ± 0.57b | 30.90 ± 2.67a | 31.20 ± 1.09a | 38.0 ± 1.15a | 38.0 ± 4.04a | 40.0 ± 5.84a | |
| MCV (in fl) | BF | 127.27 ± 2.73a | 129.62 ± 1.15a | 136.51 ± 1.21a | 140.83 ± 2.79a | 139.40 ± 1.5a | 151.53 ± 2.30a | 139.80 ± 2.42a |
| FC | 120.00 ± 2.30a | 132.58 ± 3.53a | 129.02 ± 1.15b | 122.58 ± 1.73b | 135.85 ± 0.57ab | 155.98 ± 1.57ab | 145.4 ± 1.78a | |
| Control | 123.46 ± 2.62a | 132.42 ± 1.08a | 141.74 ± 1.10c | 132.20 ± 2.17c | 132.5 ± 1.15bc | 158.99 ± 1.15bc | 137.93 ± 4.25a | |
| MCH (pg) | BF | 43.94 ± 1.15a | 43.08 ± 1.73a | 46.0 ± 1.85a | 53.54 ± 1.15a | 47.14 ± 1.15a | 44.27 ± 1.15a | 48.64 ± 1.15a |
| FC | 41.11 ± 1.05a | 44.94 ± 2.93a | 41.96 ± 4.04a | 48.79 ± 0.51a | 45.28 ± 1.15a | 39.17 ± 2.88a | 53.61 ± 2.30a | |
| Control | 44.13 ± 1.15a | 45.05 ± 1.73a | 44.49 ± 3.46a | 51.27 ± 2.35a | 43.55 ± 1.15a | 39.04 ± 1.15a | 48.96 ± 1.23a | |
| MCHC (%) | BF | 34.52 ± 1.15a | 33.23 ± 1.73a | 33.43 ± 1.15a | 38.02 ± 1.15a | 33.85 ± 1.15a | 29.22 ± 1.15a | 34.79 ± 1.73a |
| FC | 34.4 ± 2.30a | 33.90 ± 0.61a | 32.52 ± 1.73a | 39.80 ± 1.73a | 33.33 ± 0.57a | 25.75 ± 1.15a | 36.65 ± 2.30a | |
| Control | 35.75 ± 2.88a | 34.02 ± 0.94a | 31.39 ± 2.30a | 38.78 ± 1.15a | 32.89 ± 2.3a | 25.79 ± 1.15a | 35.50 ± 1.73a | |
| TLC (103 mm−3) | BF | 163.24 ± 2.30a | 167 ± 1.73ab | 167.8 ± 1.73ac | 192 ± 1.15a | 193.5 ± 1.15a | 188.7 ± 3.17a | 174.53 ± 2.30a |
| FC | 161.65 ± 1.15a | 163.90 ± 2.30bc | 156.9 ± 2.30b | 173.1 ± 1.15b | 169.5 ± 3.17b | 168.1 ± 1.15b | 162 ± 1.73b | |
| Control | 159.47 ± 1.73a | 161.30 ± 2.30c | 161.3 ± 2.30bc | 163 ± 1.73c | 167.5 ± 0.57b | 165 ± 1.73b | 163 ± 2.30b | |
| Platelets (103 mm−3) | BF | 72.09 ± 1.73a | 62.67 ± 1.70a | 49 ± 1.73a | 66 ± 1.15ab | 64 ± 1.73ab | 87 ± 1.15a | 87 ± 2.88a |
| FC | 73.09 ± 1.73a | 59 ± 1.76a | 42 ± 1.15a | 62 ± 1.73b | 60 ± 0.57b | 62 ± 0.57b | 61 ± 2.30b | |
| Control | 76.26 ± 2.30a | 72 ± 1.70b | 51 ± 5.57b | 70 ± 2.30ac | 68.50 ± 1.15ac | 60 ± 1.15b | 60 ± 1.15b |
Values are expressed as mean ± SE (n = 9) in the columns with different superscripts differ significantly (p < 0.05).
Total leukocyte count (TLC) plays an important role in defence mechanism. The level of TLC were significantly higher in BF group fishes on 40, 50 and 60 dpv compared with the FC and control group fishes. Despite the fact that vaccinated fish showed increased TLC, there was no noticeable difference between the treatments at 0 and 10 dpv. Platelets count were raised at 0 dpv in case of control fishes, moreover, significantly higher at 10 and 20 dpv than both the vaccinated treatments (P < 0.05). Surprisingly, at 50 and 60 dpv control fishes had significant lower platelets count compared to BF vaccinated fishes (Table 4).
3.6. Biochemical parameters
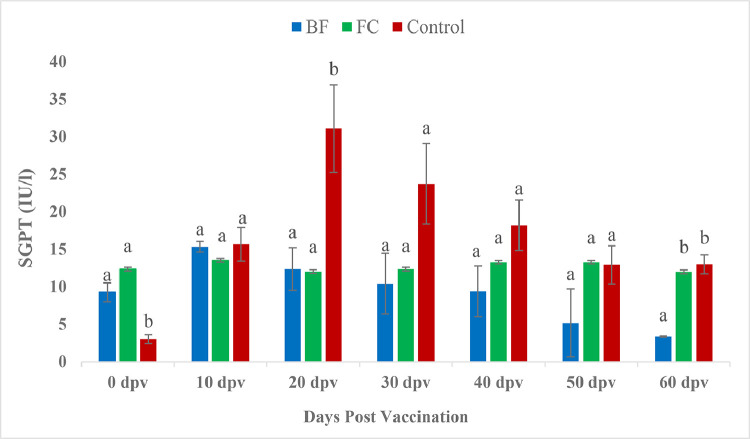
At 0 dpv, vaccinated groups showed significantly higher SGPT levels than the unvaccinated group (Fig. 5). In contrary, at 20 dpv, SGPT showed significant increase in control fishes than the vaccinated fishes. No significant difference noted among the treatments at 10, 30, 40 and 50 dpv (P > 0.05). However, at 60 dpv SGPT had reached significant lower level in BF vaccinated fishes compared to both FC vaccinated and control fishes. The SGOT level (Fig. 6) followed the same trend as SGPT in the in the present study. The ALP level in the FC marked a significant raise at 50 dpv, in comparison to the corresponding data for control (P < 0.05) (Fig. 7).
Fig. 5.
Comparison of SGPT (IU/l) between treatment groups.
Fig. 6.
Comparison of SGOT (IU/l) between treatment groups. Same letters indicate no significant differences (P>0.05) between groups (Mean ± SE, n = 9).
Fig. 7.
Comparison of alkaline phosphate (IU/l)) between treatment groups.
The plasma cholesterol meaningfully onward in the vaccinated fishes than the unvaccinated control fishes in the beginning i.e. at 0 and 10 dpv. However, biofilm vaccinated fishes showed significant lower cholesterol level than control fishes by 30, 40 and 50 dpv (Fig. 8). Significantly higher serum triglyceride values were observed in vaccinated groups at 0 and 10 days than control group. However, at 60 dpv, biofilm vaccinated fishes registered significant lower triglyceride value in contrast with both FC and control group fishes (P < 0.05) (Fig. 9). Similar trend were also found in blood glucose level (Fig. 10).
Fig. 8.
Comparison of cholesterol (mg/dl) between treatment groups.
Fig. 9.
Comparison of triglycerides (mg/dl) between treatment groups. Same letters indicate no significant differences (P > 0.05) between groups (Mean ± SE, n = 9).
Fig. 10.
Comparison of glucose (mg/dl) between treatment groups. Different letters indicate significant differences (P < 0.05) between groups (Mean ± SE, n = 9).
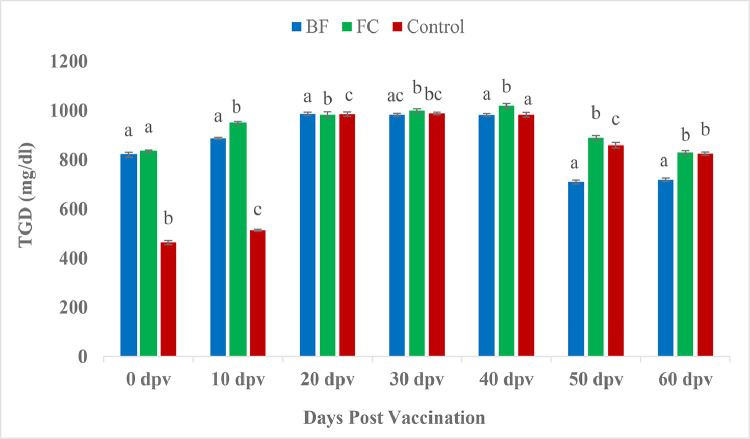
Vaccinated BF group recorded a significant upward activity of total protein parallel to both the FC and control group throughout the study period except 20 dpv (P < 0.05) (Table 5). A level of albumin was significantly elevated 40 d post vaccination in the vaccinated group in comparison to the control group (Table 5). Oral vaccination of A. hydrophila antigens had strong respond to globulin on day 0, 20 and 40 post vaccination (P < 0.05). Yet, no higher deviation was recorded between vaccinated FC and unvaccinated control group at 10, 30, 50 and 60 dpv, while in vaccinated BF samples, globulins level stayed significantly higher on those days (Table 5).
Table 5.
Biochemical parameters, total protein, albumin, globulin, A:G ratio and total immunoglobulin of oral vaccinated and control P. hypophthalmus.
| Parameters | Treatments | 0 dpv | 10 dpv | 20 dpv | 30 dpv | 40 dpv | 50 dpv | 60 dpv |
|---|---|---|---|---|---|---|---|---|
| Total protein (g/dl) | BF | 5.19 ± 1.27a | 5.72 ± 1.01a | 5.61 ± 0.96a | 6.93 ± 0.67a | 6.90 ± 0.65a | 5.97 ± 0.24a | 5.37 ± 0.69a |
| FC | 3.92 ± 1.15b | 3.79 ± 1.39b | 3.80 ± 0.98a | 4.07 ± 0.68b | 4.56 ± 0.78b | 3.34 ± 1.14b | 3.91 ± 0.66b | |
| Control | 2.97 ± 1.02c | 3.73 ± 0.75b | 4.67 ± 0.52a | 3.85 ± 0.83b | 3.87 ± 0.91c | 3.43 ± 0.65b | 3.85 ± 0.34b | |
| Albumin (g/dl) | BF | 0.44 ± 0.58a | 0.54 ± 0.01a | 0.59 ± 0.05a | 0.82 ± 0.04a | 1.11 ± 0.11a | 0.65 ± 0.06a | 0.51 ± 0.04a |
| FC | 0.42 ± 0.12a | 0.53 ± 0.02a | 0.57 ± 0.16a | 0.75 ± 0.06a | 1.02 ± 0.08a | 0.55 ± 0.06a | 0.47 ± 0.02a | |
| Control | 0.43 ± 0.07a | 0.51 ± 0.06a | 0.53 ± 0.07a | 0.79 ± 0.1a | 0.68 ± 0.10b | 0.56 ± 0.01a | 0.41 ± 0.05a | |
| Globulin (g/dl) | BF | 4.75 ± 0.12a | 5.18 ± 0.10a | 4.14 ± 0.03a | 6.11 ± 0.11a | 5.79 ± 0.12a | 5.32 ± 0.17a | 4.86 ± 0.08a |
| FC | 3.50 ± 0.23b | 3.26 ± 0.15b | 3.23 ± 0.12b | 3.32 ± 0.17b | 3.54 ± 0.11b | 2.87 ± 0.12b | 3.44 ± 0.11b | |
| Control | 2.54 ± 0.12c | 3.22 ± 0.12b | 2.02 ± 0.06c | 3.06 ± 0.02b | 3.19 ± 0.06c | 2.79 ± 0.08b | 3.44 ± 0.12b | |
| A:G ratio | BF | 0.09 ± 0.00c | 0.10 ± 0.01b | 0.12 ± 0.01b | 0.13 ± 0.01b | 0.19 ± 0.01c | 0.12 ± 0.01b | 0.11 ± 0.02b |
| FC | 0.12 ± 0.00b | 0.16 ± 0.02a | 0.18 ± 0.02a | 0.23 ± 0.02a | 0.21 ± 0.01b | 0.20 ± 0.01a | 0.14 ± 0.01a | |
| Control | 0.17 ± 0.01a | 0.16 ± 0.02a | 0.13 ± 0.01b | 0.26 ± 0.03a | 0.29 ± 0.01a | 0.20 ± 0.01a | 0.12 ± 0.02ab | |
| Total Immunoglobulin (g/dl) | BF | 1.55 ± 0.30a | 1.89 ± 0.18a | 2.18 ± 0.30a | 2.22 ± 0.33a | 2.86 ± 0.22a | 1.85 ± 0.15a | 1.65 ± 0.25a |
| FC | 1.35 ± 0.07b | 1.76 ± 0.08a | 1.63 ± 0.22b | 1.33 ± 0.35b | 1.15 ± 0.5b | 0.97 ± 0.13b | 1.27 ± 0.25a | |
| Control | 0.71 ± 0.05b | 0.75 ± 0.15b | 1.04 ± 0.25c | 1.10 ± 0.07b | 0.75 ± 0.35b | 1.19 ± 0.05b | 1.25 ± 0.12a |
Values are expressed as mean ± SE (n = 9) in the columns with different superscripts differ significantly (P < 0.05)
Highest A/G ratio was registered in the unvaccinated fishes compared to vaccinated groups at 0, 10, 30 and 40 dpv, whereas least values were recorded in the BF vaccinated group (P < 0.05). Significantly elevated total immunoglobulin (Ig) activity were found in the BF, and FC groups compared to the control group. However, BF group was exhibited meaningfully increased (P < 0.05) Ig level than that of FC and unvaccinated group throughout the study period (Table 5).
3.7. Validation of gut associated lymphoid tissues (GALT)
Transvers section of mid gut in biofilm (BF) vaccinated fishes showed higher aggregation of GALT (Fig. 11 A). Moreover, FC group fishes showed less aggregation of lymphoid cells (Fig. 11 B). Lymphoid cells aggregation was not detected in the intestinal part of mid gut of the control group fishes (Fig. 11 C).
Fig. 11.
Histomorphological analysis of mid gut of P. hypophthalmus fed vaccinated and unvaccinated diets. A: Transvers section of BF vaccinated fishes showing two focal aggregations of lymphoid tissue (GALT) in lamina propria B: Gut histology of FC vaccinated fishes showing less aggregation of lymphoid cells near the lamina propria (red arrow) and C: Unvaccinated fishes showing no aggregation of lymphoid tissue (GALT) in either lamina propria or mucosal tissues (arrows) as compared with BF and FC group fishes.
4. Discussion
The goal of this research was to assess the efficacy of oral immunization with biofilm of Aeromonas hydrophila vaccine on growth, haemato-biochemical and gut morphology of striped catfish, P. hypophthalmus.
4.1. Effect of biofilm oral vaccine on growth of pangasius juveniles
Present study successfully demonstrated growth-related effects in pangasius catfish vaccinated with A. hydrophila biofilm based oral vaccine. The study indicates that vaccination had positive effects on the growth of pangasius catfish. Biofilm fed fishes displayed significantly higher weight gain and decreased FCR (p < 0.05) (Table 2). Identical results compared to present studies were observed in ornamental goldfish, recorded highest weight gain at 25 dpv and 50 dpv in biofilm plus immunoadjuvant group fishes [43]. Oral route of vaccination considered as stress-free administration can play vital role in protecting a bacterial [44] and viral disease [45] suggested that the oral immunization had no effect on healthy fish growth. The non-stressful method of immunization described by Chair et al. 46] using encapsulated V. anguillarum bacterin within Artemia nauplii increased weight gain and feed conversion in European sea bass (Dicentrarchus labrax), which is also evident in the current investigation. The elevated growth in BF fishes might be due to the polymetric extracellular matrix of biofilm of A. hydrophila. A bacterial biofilm is a self-contained population of bacterium cells through extracellular polymeric substances (EPS) [47] that would facilitate the nutrient uptake [48]. Biofilms are bioactive substances and dietary stimulants that can help aquaculture organisms grow faster [49]. African catfish, Clarias batrachus vaccinated with feed based A. hydrophila GPl-04 vaccine did not adversely affect the growth [50]. Earlier studies were conducted with the employing of natural substrate based biofilm, achieved higher biomass compare to control at the end of the experiments [[51], [52], [53]].
4.2. Immune response and diseases resistance upon biofilm oral vaccine
An onward level of protection was registered in biofilm vaccinated pangasius in comparison with vaccinated FC and control fishes when injected with homologous intramuscular (i.m.) challenge (Table 3). Survival was significantly raised in BF vaccinated group (88.46 ± 2.10%) compared to both vaccinated FC group (51.28 ± 1.85%) and control group (26.92 ± 0.71%). As a result, the biofilm vaccine is thought to be more effective than other treatments since the relative percent survival (RPS) was 84.21 ± 1.49 % which is notably soaring than free cell vaccine (P < 0.05) (33.33 ± 1.21%) (Fig. 2). The present study are in accordance with previous authors who reported similar findings with biofilm vaccine in Indian major carps [[25], [26], [27]], Tilapia [28,54] gold fish [55] and walking catfish [26]. A higher protection level (85.4%) was achieved through the oral vaccine against V. anguillarum biofilm was compared to the free cell vaccine (27%) in Asian seabass, Lates calcarifer [29]. Findings of the present study closely agree with the similar study conducted in Taiwan [56,57]. They had reported significantly higher RPS in biofilm vaccines against Lactococcus garvieae infection in Mullet (77%) and in Photobacterium damselae infection in giant grouper (62%). Moreover, cross protection of different geographical A. hydrophila isolates were tested and found meaningful RPS (p < 0.05) rate of 81.5% in the vaccinated fish compared to unvaccinated fish (21%) [58]. The better protection of biofilm in oral vaccination could be attributed to the nature of glycocalyx, which protects vaccine antigen from digestion in the stomach, as demonstrated in the current work.
4.3. Antibody titre of Polyclonal antibody based ELISA
The biofilm vaccinated P. hypophthalmus had higher antibody titre than those vaccinated with free cell on all days of post vaccination except 0 and 20 dpv. On 0th dpv, the antibody titer was similar to all the experimental groups. The presence of natural antibodies in the fish against the widespread A. hydrophila could explain the high level of antibodies found in the unvaccinated control group. Previous research have also reported similar observations by antibody-agglutination titre method [25,26,28] as well as in MAb based ELISA [27,58,59,]. Better performance with higher antibody titer in BF groups than in FC and control groups, may be attributed to biofilm glycocalyx [60] that protect the A. hydrophila antigens from acid degradation in the stomach of pangasius could facilitates the bunch of antigens to the posterior gut to be traced and processed by the memory cells, and finally, the immune response could activated [18].
4.4. Effect of haemato-biochemical parameters
A range of blood parameters were evaluated to assess the effect of vaccination on fish [59,61]. In the present study significant increase in TEC in BF vaccinated fishes compared to both vaccinated FC and unvaccinated control group on 0 and 10 day post vaccination (dpv) were observed. Furthermore, on 20 dpv, significant higher TEC were noted on BF fed fishes than the basal diet fed fishes (Table 4). Similarly, significantly higher erythrocytes count were reported on day 21 in orally vaccinated Nile tilapia [61]. Red blood cell (RBC) count were 0.86 (× 106 mm–3) at 25 dpv in the control group, and this significantly increased to 1.35 (x106 mm–3) with biofilm plus adjuvant (BF2) group fishes (P < 0.05) [43]. At 14 days after vaccination against A. hydrophila, Irianto et al. [55] noticed an increase in erythrocyte count in goldfish (C. auratus). Studies have shown that a decrease in the number of erythrocytes in the blood as well as a decrease in the hematocrit percentage can be indicators of bacterial infection [62,63]. Nile tilapia (Oreochromis niloticus) naturally invaded with E. tarda reported significant reduction in erythrocytes and haemoglobin level [62]. The total erythrocyte count, haemoglobin content were significantly enhanced in pangasius catfish fed dietary carotenoid supplementation diet [64] and biofilm vaccinated gold fish [43]. Immunostimulants have the ability to enhance RBC and haemoglobin levels in general, which could have been induced by biofilm supplementation in this study.
Haematological indices including PCV, MCV, MCH and MCHC can be regarded as appropriate indicators to find out possible physiological response in fish. Current experiment showed elevated level of hematocrit (PCV) and MCV in BF group in comparison to both free cell and control groups at different timeline. The level of MCH and MCHC in the current work were higher but no remarkable changes were noted in all the experimental groups except 10 dpv. The past study showed significantly higher hematocrit percentage from orally vaccinated fish than in the unvaccinated control fish Silva et al. 61].
Total leukocyte counts (TLC) plays an important role in defence mechanism. On day 30 after vaccination, the TLC was markedly higher in BF group than that of both FC and control group, and it reached a maximum value of 193.5 ± 1.15 (x 103 mm–3) on day 40 dpv but decreasing trend at 50 and 60 dpv although level of TLC significantly higher than other groups. The lower level of TLC in FC group in this study might be due to the degradation of antigen in acid stomach of fish, is also evident as GALT was less aggregated in FC vaccinated fish. The findings are in accordance with Silva et al. in 2009 [61] and Sirimanapong et al. in 2014 [59], noted significant higher leukocyte counts over the course of the research. Biofilm fed fishes in the present study showed increased WBCs might be due to the improved general health condition by better immune responses and various actions of the specific cell-mediated immune systems. Result of the present study correlated with the findings of the research of Monir et al. [65] and Rahman et al. [66]. In their studies, they found that the leukocyte, lymphocytes, monocytes, granulocytes counts were boosted substantially (p < 0.05) by the feed-based bi-valent vaccines in red tilapia and catfish respectively. Thrombocytes counts were significantly higher in BF group fishes at 50 and 60 dpv. Silva et al. 61] reported similar results in the orally immunized tilapia where number of thrombocytes were up regulated to 40.78 (x 103 mm−1) than the control {31.11 (x 103 mm−1)} on day 21 after vaccination.
The evaluation and profiling of serum biochemical characteristics is thought to be a useful technique in clinical pathology for detecting tissue or organ damage, disease progression, and making appropriate medical decisions [67,68]. Serum glutamate pyruvate transaminase (SGPT) and serum glutamate oxaloacetate transaminase (SGOT) activities are associated with hepatic pathology [69]. In the present study, significantly elevated SGPT was recorded in vaccinated groups in comparison to the control group at 0 dpv. However, at 20 dpv, SGPT level were significantly decrease in both the vaccinated groups over control. Moreover, BF group had down regulated (P < 0.05) SGPT level compared to both FC and control group on 60 dpv indicating normal health status of biofilm vaccinated fishes. Similarly, SGOT level in the present study followed the same trend of SGPT. Aeromonas infection in goldfish [70] and rainbow trout [71] resulted increased SGPT and SGOT. Previous reports showed elevated SGPT and SGOT following monogenean infestation [72] and toxicological challenge in Salmo gairdneri [73] and Parophrys vetulus [69]. A significant increase in the SGPT and SGOT levels is caused by any acute injuries or necrotic lesions in the liver [67,74]. The severity of histopathology in the liver, gut, and trunk kidney was linked positively with SGPT and SGOT, according to Chen et al. [67]. The histopathological findings in the present study, in BF fed P. hypophthalmus, showed normal architecture however, several histopathological abnormalities were detected in gills, liver and kidney of FC and control group fishes [75]. Our results also corroborate the finding of Biswas et al. [74], where extensive degenerated and necrotized head kidney tissues were observed in all the non-vaccinated fish, L. rohita against A. hydrophila vaccine. The ALP activity of the pangasius catfish did not respond strongly to vaccination except on 50 dpv (Fig. 7). But the levels of serum enzymes including SGPT, SGOT and ALP in BF vaccinated fishes were lower than other treatments. The decline in such blood enzymes in the present study could explain that the extracellular polymeric substances of biofilm of A. hydrophila have the potential to benefit the health of fish by improving liver function.
The concentration of serum glucose and triglycerides is commonly utilized as a health indicator in fish [76]. The plasma cholesterol, triglycerides and glucose level significantly increased in the vaccinated fishes than the unvaccinated control fishes in the beginning of the analysed time point however at the end of the trial, biofilm (BF) vaccinated fishes showed significant lower activity. This might be due to the capability of biofilm of A. hydrophila to reduce the effect of stressors. Similar observation were noticed in pangasius catfish fed with fucoidan rich sea weed extract [77]. Herbal extracts are well known suitable immunostimulants to boost the non-specific immune system as well as to keep the aquaculture organisms stress free [78]. Rainbow trout fed with both heat killed and freeze dried probiotic bacteria, L. rhamnosus at 1011CFU g−1 had significant upregulated cholesterol and triglycerides level [79].
The presence of significant total protein, albumin, and globulin levels in the serum proteins suggests that high amounts are likely due to the improvement of the non-specific immune response of fish [67,80]. In the present study, at 40 dpv the serum albumin activity of both vaccinated groups were reached a maximum value of 1.11 ± 0.11 (g/dl) and 1.02 ± 0.08 (g/dl) for BF and FC respectively than that of the control (0.68 ± 0.10 (g/dl) (Table 5). Compared to other treatments vaccinated BF group documented a notably (P < 0.05) higher activity of total protein for any time points analysed and reached at peak at 30 dpv (6.93 ± 0.67 g/dl). The finding in the present study are in agreement of Viji et al. [43] reported higher albumin level in biofilm groups than that of control group. An increase in albumin and globulin levels, as well as a drop in the A/G ratio, is thought to indicate a strong innate reaction in fish [72]. In the present study, an increment in globulin content and the decrement in A/G ratio were found in BF vaccinated group. It was observed that globulin level was increased steadily with BF vaccinated fishes and peaked at 40 dpv (6.11 ± 0.11 g/dl) indicating increased phagocytic and leukocytes counts in this experiment (Table 5). The present study's findings are in line with Prabu et al. [77] showed significant higher globulin (6.28 g/dl) and lower A/G ratio (0.24 g/dl) in striped catfish, P. hypophthalmus fed with 3% fucoidan rich sea weed extract (FRSE) diet. Earlier studies demonstrated in an increment of total protein, albumin, globulin and decreased A/G ratio in different finfishes [43,81]. In general, BF group was exhibited higher albumin, total serum protein and total immunoglobulins than that of FC and control group throughout the study period.
4.5. Validation of gut associate lymphoid tissue
Oral vaccination has many advantages in addition to the ability to stimulate both adaptive and local responses [82]. The gut-associated lymphoid tissue (GALT) of vertebrates plays an important role in eliciting both systemic and mucosal immune response [28,83]. Earlier studies demonstrated GALT especially in second segment of carp [84] and Nile tilapia [28,54]. Present study, for the first time demonstrating the GALT in pangasius catfish employing biofilm of A. hydrophila oral vaccine (Fig. 11 A). Findings of the present study closely agree with a similar study conducted in Malaysia [85]. They found higher level of GALT development and lymphocytes association in the hindgut of vaccinated red hybrid tilapia, while those remain unchanged in the tissue of unvaccinated control group. In another study Kahieshesfandiari et al. [28] experimented biofilm vaccine of S. agalactiae in protecting tilapia from streptococcosis and demonstrated GALT in the intestine of Nile tilapia. Moreover, present study has validated the presence of GALT in mid gut of BF treated pangasius even after 60 dpv compared to 2 weeks post vaccination (wpv) in tilapia, an indication of longer retention of antigens in the gut. The second gut segment has approximately four times the number of intraepithelial macrophages (per enterocyte) as the first [83]. Antigens may be transferred from the second segment's enterocytes to lymphoid cells (B and T), macrophages, and dendritic cells, causing them to respond correctly [18,84]. Similarly, an increase in blood lymphocytes is likely due to the oral vaccine activating mucosal immunity in the gut's lymphoid tissues. The current investigation found anti- A. hydrophila antibodies in pangasius blood serum, which is consistent with the notion that the GATL in teleost fish can stimulate both local and systemic humoral immune responses. However, such claim requires further investigation on whole cell biofilm oral vaccine on striped catfish.
5. Conclusions
The findings suggest that present research approaches to fish health, such as nutritional, haematological, biochemical, immunological, and gut associate lymphoid tissue, could be useful in determining the effects of oral vaccines on fish. The present study indicated that feeding with biofilm vaccine significantly enhanced the pangasius growth. Importantly, the oral administration of A. hydrophila biofilm vaccination enhanced both non-specific humoral and cellular immune responses in pangasius, as measured in this study. Furthermore, specific immunoglobulin (IgM) level measured by polyclonal antibody was also increased by oral administration biofilm of A. hydrophila vaccines in parallel with the better health condition of P. hypophthalmus. Intestinal gut of the biofilm vaccinated fishes expressed GALT even at 60 dpv, convincingly confirming the presence of antigens in the posterior gut. All of these findings suggested that biofilm vaccine could be one of the promising vaccines for A. hydrophila infection in pangasius.
Funding source
The first two authors warmly recognize the Indian Council of Agricultural Research for sponsoring their Ph.D. through the Netaji Subhas-ICAR International Fellowship 2015-16.
Declaration of Competing Interest
There are no competing interests stated by the authors.
Data availability
Data will be made available on request.
References
- 1.FAO, Aquaculture. http://www.fao.org/fishery/aquaculture/en, 2018 (accessed on 20 Dec 2020).
- 2.Ferguson W.H., Turnbull J.F., Shinn A., Thompson K., Dung T.T., Crumlish M. Bacillary necrosis in farmed Pangasius hypophthalmus (Sauvage, 1878) from the Mekong Delta, Vietnam. J. Fish Dis. 2001;24:509–513. doi: 10.1046/j.1365-2761.2001.00308.x. [DOI] [Google Scholar]
- 3.Yuasa K., Edy B.K., Panigoro N., Hatai K. First isolation of Edwardsiella ictaluri from cultured striped catfish Pangasius hypophthalmus in Indonesia. Fish Pathol. 2003;38:181–183. doi: 10.3147/jsfp.38.181. [DOI] [Google Scholar]
- 4.Subagja J., Slembrouck J., Hung L.T., Legendre L. Larval rearing of an Asian catfish Pangasius hypophthalmus (Siluroidei Pangasiidae): analysis of precocious mortality and proposition of appropriate treatments. Aquat. Living Resour. 1999;12:37–44. doi: 10.1016/S0990-7440(99)80013-8. [DOI] [Google Scholar]
- 5.Crumlish M., Thanh P.C., Koesling J., Tung V.T., Gravningen K. Experimental challenge studies in Vietnamese catfish, Pangasianodon hypophthalmus (Sauvage), exposed to Edwardsiella ictaluri and Aeromonas hydrophila. J. Fish Dis. 2010;33:717–722. doi: 10.1111/j.1365-2761.2010.01173.x. [DOI] [PubMed] [Google Scholar]
- 6.Lakshmi B., Viswanath B., Sai Gopal D.V.R. Probiotics as antiviral agents in shrimp aquaculture. J. Pathog. 2013 doi: 10.1155/2013/424123. e424123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaur P., Peterson E. Antibiotic resistance mechanisms in bacteria: relationships between resistance determinants of antibiotic producers, environmental bacteria, and clinical pathogens. Front. Microbiol. 2018;9:e2928. doi: 10.3389/fmicb.2018.02928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nayak S.K. Current status of Aeromonas hydrophila vaccine development in fish: an Indian perspective. Fish Shellfish Immunol. 2020;100:283–299. doi: 10.1016/j.fsi.2020.01.064. [DOI] [PubMed] [Google Scholar]
- 9.Sommerset I., Krossøy B., Biering E., Frost P. Vaccines for fish in aquaculture. Expert Rev. Vaccines. 2005;4:89–101. doi: 10.1586/14760584.4.1.89. [DOI] [PubMed] [Google Scholar]
- 10.Brudeseth B.E., Wiulsrød R., Fredriksen B.N., K Lindmo, Løkling K.E., Bordevik M., Gravningen K. Status and future perspectives of vaccines for industrialised fin-fish farming. Fish Shellfish Immunol. 2013;35:1759–1768. doi: 10.1016/j.fsi.2013.05.029. [DOI] [PubMed] [Google Scholar]
- 11.Kayansamruaj P., Areechon N., Unajak S. Development of fish vaccine in Southeast Asia: a challenge for the sustainability of SE Asia aquaculture. Fish Shellfish Immunol. 2020;103:73–87. doi: 10.1016/j.fsi.2020.04.031. [DOI] [PubMed] [Google Scholar]
- 12.Embregts C.W., Forlenza M. Oral vaccination of fish: lessons from humans and veterinary species. Dev. Comp. Immunol. 2016;64:118–137. doi: 10.1016/j.dci.2016.03.024. [DOI] [PubMed] [Google Scholar]
- 13.Mutoloki S., Alexandersen S., Evensen Ø. Sequential study of antigen persistence and concomitant inflammatory reactions relative to side-effects and growth of Atlantic salmon (Salmo salar L.) following intraperitoneal injection with oil-adjuvanted vaccines. Fish Shellfish Immunol. 2004;16:633–644. doi: 10.1016/j.fsi.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Midtlyng P.J., Reitan L.J., Speilberg L. Experimental studies on the efficacy and side-effects of intraperitoneal vaccination of Atlantic salmon (Salmo salar L.) against furunculosis. Fish Shellfish Immunol. 1996;6:335–350. doi: 10.1006/fsim.1996.0034. [DOI] [Google Scholar]
- 15.Sørum U., Damsgård B. Effects of anaesthetization and vaccination on feed intake and growth in Atlantic salmon (Salmo salar L.) Aquaculture. 2004;232:333–341. doi: 10.1016/S0044-8486(03)00529-5. [DOI] [Google Scholar]
- 16.Berg A., Rødseth O.M., Tangerås A., Hansen T. Time of vaccination influences development of adhesions, growth and spinal deformities in Atlantic salmon Salmo salar. Dis. Aquat. Org. 2006;69:239–248. doi: 10.3354/dao069239. [DOI] [PubMed] [Google Scholar]
- 17.Villumsen K.R., Neumann L., Ohtani M., Strøm H.K., Raida M.K. Oral and anal vaccination confers full protection against enteric redmouth disease (ERM) in rainbow trout. PLoS One. 2014;9:e93845. doi: 10.1371/journal.pone.0093845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rombout J.W.H.M., Lamers C.H.J., Helfrich M.H., Dekker A., Taverne-Thiele A.J. Uptake and transport of intact macromolecules in the intestinal epithelium of carp (Cyprinus carpio L.) and the possible immunological implications. Cell Tissue Res. 1985;239:519–530. doi: 10.1007/BF00219230. [DOI] [PubMed] [Google Scholar]
- 19.Mutoloki S., Munanģandu H.M., Evensen Ø. Oral vaccination of fish–antigen preparations, uptake, and immune induction. Front. Immunol. 2015;6:519. doi: 10.3389/fimmu.2015.00519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piganelli J.D., Zhang J.A., Christrnsen J.M., Kattari S.C. Enteric coated microsphere as an oral method for antigen delivery to salmonids. Fish Shellfish Immunol. 1994;4:179–188. doi: 10.1006/fsim.1994.1017. [DOI] [Google Scholar]
- 21.Dalmo R.A., Leifson R.M., Bogwald J. Microspheres as antigen carriers; studies on intestinal absorption and tissue localization of polystyrene microspheres in Atlantic salmon, Salmo salar L. J. Fish Dis. 1995;18:87–91. doi: 10.1111/j.1365-2761.1995.tb01270.x. [DOI] [Google Scholar]
- 22.Yasumoto S., Yoshimura T., Miyazaki T. Oral immunization of common carp with a liposome vaccine containing Aeromonas hydrophila antigen. Fish Pathol. 2006;41:45–49. doi: 10.3147/jsfp.41.45. [DOI] [Google Scholar]
- 23.Wong G., Kaattari S.L., Christensen J.M. Effectiveness of oral enteric coated Vibrio vaccines for use in salmonlid fish. Immunol. Investig. 1992;10:341–351. doi: 10.3109/08820139209069375. [DOI] [PubMed] [Google Scholar]
- 24.Yang H.L. Proceedings of the 3rd International Symposium on Fish Vaccinology. 2003. Fish oral vaccine with recombinant E. coli encapsulated in brine shrimp. April 9–11Bergen, Norway. [Google Scholar]
- 25.Azad I.S., Shankar K.M., Mohan C.V., Kalita B. Biofilm vaccine of Aeromonas hydrophila – standardization of dose and duration for oral vaccination of carps. Fish Shellfish Immunol. 1999;9:519–528. doi: 10.1006/fsim.1998.0206. [DOI] [Google Scholar]
- 26.Nayak D.K., Asha A., Shankar K.M., Mohan C.V. Evaluation of biofilm of Aeromonas hydrophila for oral vaccination of Clarias batrachus a carnivore model. Fish Shellfish Immunol. 2004;16:613–619. doi: 10.1016/j.fsi.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 27.Siriyappagouder P., Shankar K.M., Naveen Kumar B.T., Patil R., Byadgi O.V. Evaluation of biofilm of Aeromonas hydrophila for oral vaccination of Clarias batrachus – a carnivore model. Fish Shellfish Immunol. 2014;41:581–585. doi: 10.1016/j.fsi.2014.09.021. [DOI] [PubMed] [Google Scholar]
- 28.Kahieshesfandiari M., Sabri M.Y., Ina-Salwany M.Y., Hassan M.D., Noraini O., Ajadi A.A., Isiaku A.I. Streptococcosis in Oreochromis sp.: is feed-based biofilm vaccine of Streptococcus agalactiae effective? Aquac. Int. 2019;27:817–832. doi: 10.1007/s10499-019-00372-8. [DOI] [Google Scholar]
- 29.Ram M.K., Naveen Kumar B.T., Poojary S.R., Abhiman P.B., Patil P., Ramesh K.S., Shankar K.M. Evaluation of biofilm of Vibrio anguillarum for oral vaccination of Asian seabass, Lates calcarifer (Bloch, 1790) Fish Shellfish Immunol. 2019;94:746–751. doi: 10.1016/j.fsi.2019.09.053. [DOI] [PubMed] [Google Scholar]
- 30.Sharma S.K., Shankar K.M., Sathyanarayana M.L., Sahoo A.K., Patil R., Narayanaswamy H.D., Rao S. Evaluation of immune response and resistance to diseases in tiger shrimp, Penaeus monodon fed with biofilm of Vibrio alginolyticus. Fish Shellfish Immunol. 2010;29(5):724–732. doi: 10.1016/j.fsi.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 31.Sen S.S., Giri S.S., Sukumaran V. Immune responses and protection in rohu vaccinated against Aeromonas hydrophila infection. Aquac. Int. 2014;22:1637–1648. doi: 10.1007/s10499-014-9770-x. [DOI] [Google Scholar]
- 32.Saikia D., Kamilya D. Immune responses and protection in catla (Catla catla) vaccinated against epizootic ulcerative syndrome. Fish Shellfish Immunol. 2012;32:353–359. doi: 10.1016/j.fsi.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 33.Azad I.S., Shankar K.M., Mohan C.V., Kalita B. Uptake and processing of biofilm and free-cell vaccines of Aeromonas hydrophila in Indian major carps and common carp following oral vaccination antigen localization by a monoclonal antibody. Dis. Aquat. Org. 2000;43:103–108. doi: 10.3354/dao043103. [DOI] [PubMed] [Google Scholar]
- 34.Mamun M.A.A., Nasren S., Abhiman P.B., Rathore S.S., Sowndarya N.S., Ramesh K.S., Shankar K.M. Investigation of production, formation and characterization of biofilm cells of Aeromonas hydrophila for oral vaccination of fish. J. Exp. Zool. 2019;22:1115–1123. [Google Scholar]
- 35.Azad I.S., Shankar K.M., Mohan C.V. Evaluation of an Aeromonas hydrophila biofilm oral vaccination of carps. Proceedings of the Diseases in Asian Aquaculture III, Fish Health Section; Manila; Asian Fisheries Society; 1997. pp. 181–186. M.I. Flegal TW (Ed.) [Google Scholar]
- 36.Rathore S.S., Murthy H.S., Mamun M.A.A., Nasren S., Rakesh K., Kumar B.T.N., Khandagale A.S. Nano-selenium supplementation to ameliorate nutrition physiology, immune response, antioxidant system and disease resistance against Aeromonas hydrophila in monosex Nile tilapia (Oreochromis niloticus) Biol. Trace Elem. Res. 2021;199:3073–3088. doi: 10.1007/s12011-020-02416-0. [DOI] [PubMed] [Google Scholar]
- 37.Reed L.J., Muench H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938;27:493–497. doi: 10.1093/oxfordjournals.aje.a118408. [DOI] [Google Scholar]
- 38.D.F. Amend, Potency testing of fish vaccines. fish biologics: serodiagnostics and vaccines, (49) (1981) 447-454.
- 39.Babu P.S., Shankar K.M., Honnananda B.R., Swamy H.V.K., Shama K.P., Suryanarayana V.V.S, Dechamma H.J. Isolation and characterization of immunoglobulin of the Indian major carp, rohu (Labeo rohita) Fish Shellfish Immunol. 2008;24:779–783. doi: 10.1016/j.fsi.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 40.Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 41.Furuta T., Anichnam T., Zakaguchi J., Akaba Y., Ashi H. Indirect Enzyme Linked Immunosorbant Assay (ELISA) for the detection of antibody in serum of Japanese flounder. Fish Sci. 1995;61:663–667. doi: 10.2331/fishsci.61.663. [DOI] [Google Scholar]
- 42.Bullock A.M., Roberts R.J. Fish Pathology. Bailliere Tindall; London: 1989. Laboratory methods; pp. 374–406. [Google Scholar]
- 43.Viji V.T., Deepa K., Velmurugan S., Donio M.B.S., Jenifer J.A., Babu M.M., Citarasu T. Vaccination strategies to protect goldfish Carassius auratus against Aeromonas hydrophila infection. Dis. Aquat. Org. 2013;104:45–57. doi: 10.3354/dao02581. [DOI] [PubMed] [Google Scholar]
- 44.Tobar J.A., Jerez S., Caruffo M., Bravo C., Contreras F., Bucarey S.A., Harel M. Oral vaccination of Atlantic salmon (Salmo salar) against salmonid rickettsial septicaemia. Vaccine. 2011;29:2336–2340. doi: 10.1016/j.vaccine.2010.12.107. [DOI] [PubMed] [Google Scholar]
- 45.Reyes M., Ramírez C., Ñancucheo I., Villegas R., Schaffeld G., Kriman L., Oyarzun P. A novel “in-feed” delivery platform applied for oral DNA vaccination against IPNV enables high protection in Atlantic salmon (Salmon salar) Vaccine. 2017;35:626–632. doi: 10.1016/j.vaccine.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 46.Chair M., Gapasin R.S.J., Dehasque M., Sorgeloos P. Vaccination of European sea bass fry through bioencapsulation of Artemia nauplii. Aquac. Int. 1994;2:254–261. doi: 10.1007/BF00123434. [DOI] [Google Scholar]
- 47.Khatoon Z., McTiernan C.D., Suuronen E.J., Mah T.F., Alarcon E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon. 2018;4 doi: 10.1016/j.heliyon.2018.e01067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hall-Stoodley L., Costerton J.W., Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004;2:95. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 49.Pandey P.K., Bharti V., Kumar K. Biofilm in aquaculture production. Afr. J. Microbiol. Res. 2014;8:1434–1443. doi: 10.5897/AJMR2013.6445. [DOI] [Google Scholar]
- 50.Mulia D.S., Utomo T., Isnansetyo A. The efficacy of Aeromonas hydrophila GPl-04 feed-based vaccine on African catfish (Clarias gariepinus) Biodivers. J. Biol. Divers. 2022;23:1505–1510. doi: 10.13057/biodiv/d230339. [DOI] [Google Scholar]
- 51.Ramesh M.R., Shankar K.M., Mohan C.V., Varghese T.J. Comparison of three plant substrates for enhancing carp growth through bacterial biofilm. Aquac. Eng. 1999;19:119–131. doi: 10.1016/S0144-8609(98)00046-6. [DOI] [Google Scholar]
- 52.Thompson F.L., Abreu P.C., Wasielesky W. Importance of biofilm for water quality and nourishment in intensive shrimp culture. Aquaculture. 2002;203:263–278. doi: 10.1016/S0044-8486(01)00642-1. [DOI] [Google Scholar]
- 53.Mamun M.A.A., Hossain M.A., Hossain M.S., Ali M.L. Effects of different types of artificial substrates on nursery production of freshwater prawn, Macrobrachium rosenbergii (de Man) in recirculatory system. J. Bangladesh Agric. Univ. 2010;8:333–340. doi: 10.3329/jbau.v8i2.7946. [DOI] [Google Scholar]
- 54.Firdaus-Nawi M., Yusoff S.M., Yusof H., Abdullah S.Z, Zamri-Saad M. Efficacy of feed-based adjuvant vaccine against Streptococcus agalactiae in Oreochromis spp. in Malaysia. Aquac. Res. 2013;45:87–96. doi: 10.1111/j.1365-2109.2012.03207.x. [DOI] [Google Scholar]
- 55.Irianto A., Robertson P.A.W., Austin B. Oral administration of formalin-inactivated cells of Aeromonas hydrophila A3-51 controls infection by atypical A. salmonicida in goldfish, Carassius auratus (L.) J. Fish Dis. 2003;26:117–120. doi: 10.1046/j.1365-2761.2003.00439.x. [DOI] [PubMed] [Google Scholar]
- 56.Su F.J., Chen M.M. Protective efficacy of novel oral biofilm vaccines against Lactococcus garvieae infection in mullet, Mugil cephalus. Vaccines. 2021;9(2021):844. doi: 10.3390/vaccines9080844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Su F.J., Chen M.M. Protective Efficacy of novel oral biofilm vaccines against Photobacterium damselae subsp. damselae Infection in giant grouper, Epinephelus lanceolatus. Vaccines. 2022;10(2022):207. doi: 10.3390/vaccines10020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaur B., Kumar B.N., Tyagi A., Holeyappa S.A., Singh N.K. Identification of novel vaccine candidates in the whole-cell Aeromonas hydrophila biofilm vaccine through reverse vaccinology approach. Fish Shellfish Immunol. 2021;114(2021):132–141. doi: 10.1016/j.fsi.2021.04.019. [DOI] [PubMed] [Google Scholar]
- 59.Sirimanapong W., Thompson K.D., Kledmanee K., Thaijongrak P., Collet B., Ooi E.L., Adams A. Optimisation and standardisation of functional immune assays for striped catfish (Pangasianodon hypophthalmus) to compare their immune response to live and heat killed Aeromonas hydrophila as models of infection and vaccination. Fish Shellfish Immunol. 2014;40:374–383. doi: 10.1016/j.fsi.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 60.Costerton J.W., Irwin R.T., Cheng K.J. The bacterial glycocalyx in nature and disease. Annu. Rev. Microbiol. 1981;35:299–324. doi: 10.1146/annurev.mi.35.100181.001503. [DOI] [PubMed] [Google Scholar]
- 61.Silva B.C., Martins M.L., Jatobá A., Buglione Neto C.C., Vieira F.N., Pereira G.V., Mouriño J.L.P. Hematological and immunological responses of Nile tilapia after polyvalent vaccine administration by different routes. Pesqui. Vet. Bras. 2009;29(11):874–880. doi: 10.1590/S0100-736X2009001100002. [DOI] [Google Scholar]
- 62.Benli A.C.K., Yildiz H.Y. Blood parameters in Nile tilapia (Oreochromis niloticus L.) spontaneously infected with Edwardsiella tarda. Aquac. Res. 2004;35:1388–1390. doi: 10.1111/j.1365-2109.2004.01158.x. [DOI] [Google Scholar]
- 63.Shoemaker C.A., Lim C., Yildirim-Aksoy M., Welker T.L., Klesius P. Growth response and acquired resistance of Nile tilapia, Oreochromis niloticus (L.) that survived Streptococcus iniae infection. Aquac. Res. 2006;37:1238–1245. doi: 10.1111/j.1365-2109.2006.01555.x. b. [DOI] [Google Scholar]
- 64.Gopan A., Ande M.P., Varghese T., Sahu N.P., Lalappan S., Srivastava P.P., Jain K.K. Dietary carotenoid supplementation improves fillet appearance, antioxidant status and immuneresponses in striped catfish (Pangasianodon hypophthalmus) nevertheless the growth performance. Turk. J. Fish Aquat. Sci. 2018;18:1303–1313. doi: 10.4194/1303-2712-v18_11_07. [DOI] [Google Scholar]
- 65.Monir M.S., Yusoff S.B.M., Mohamad A., Ngoo M.S.B.M.H., Ina-Salwany M.Y. Haemato-immunological responses and effectiveness of feed-based bivalent vaccine against Streptococcus iniae and Aeromonas hydrophila infections in hybrid red tilapia (Oreochromis mossambicus× O. niloticus) BMC Vet. Res. 2020;16(2020):1–14. doi: 10.1186/s12917-020-02443-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rahman M.M., Rahman M.A., Hossain M.T., Siddique M.P., Haque M.E., Khasruzzaman A.K.M., Islam M.A. Efficacy of bi-valent whole cell inactivated bacterial vaccine against Motile Aeromonas Septicemia (MAS) in cultured catfishes (Heteropneustes fossilis, Clarias batrachus and Pangasius pangasius) in Bangladesh. Saudi J. Biol. Sci. 2022;29(5):3881–3889. doi: 10.1016/j.sjbs.2022.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen C.Y., Wooster G.A., Bowser P.R. Comparative blood chemistry and histopathology of tilapia infected with Vibrio vulnificus or Streptococcus iniae or exposed to carbon tetrachloride, gentamicin, or copper sulphat. Aquaculture. 2004;239:421–443. doi: 10.1016/j.aquaculture.2004.05.033. [DOI] [Google Scholar]
- 68.Del Rio-Zaragoza O.B., Fajer-Avila E.J., Almazán-Rueda P. Haematological and gill responses to an experimental infection of dactylogyrid monogeneans on the spotted rose snapper Lutjanus guttatus (Steindachner 1869) Aquac. Res. 2010;4:1592–1601. doi: 10.1111/j.1365-2109.2009.02471.x. [DOI] [Google Scholar]
- 69.Casillas E., Myers M., Ames W.E. Relationship of serum chemistry values to liver and kidney histopathology in English sole (Parophrys vetulus) after acute exposure to carbon tetrachloride. Aquat. Toxicol. 1983;3:61–78. doi: 10.1016/0166-445X(83)90007-3. [DOI] [Google Scholar]
- 70.Brenden R.A., Hulzlngat H.W. Pathophysiology of experimental Aeromonas hydrophila infection in gold fish, Carassius auratus. J. Fish Dis. 1986;9:163–167. doi: 10.1111/j.1365-2761.1986.tb00999.x. [DOI] [Google Scholar]
- 71.Racicot J.G., Gaudet M., Leray C. Blood and liver enzymes in rainbow trout (Salmo gairdneri Rich.) with emphasis on their diagnostic use: study with CCl4 toxicity and a case of Aeromonas infection. J. Fish Biol. 1975;7:825–835. doi: 10.1111/j.1095-8649.1975.tb04653.x. [DOI] [Google Scholar]
- 72.Kumar S., Raman R.P., Prasad K.P., Srivastava P.P., Kumar S., Rajendran K.V. Modulation of innate immune responses and induction of oxidative stress biomarkers in Pangasianodon hypophthalmus following an experimental infection with dactylogyrid monogeneans. Fish Shellfish Immunol. 2017;63:334–343. doi: 10.1016/j.fsi.2017.02.033. [DOI] [PubMed] [Google Scholar]
- 73.Statham C.N., Croft W.A., Lech J.J. Uptake, distribution, and effects of carbon tetrachloride in rainbow trout (Salmo gairdneri) Toxicol. Appl. Pharmacol. 1978;45:131–140. doi: 10.1016/0041-008X(78)90034-0. [DOI] [PubMed] [Google Scholar]
- 74.Biswas A., Dash G., Mali P., Joardar S.N., Dey B., Roy A., Karmakar S. Histopathology of head kidney tissues in challenged rohu, Labeo rohita Hamilton after vaccinating with Aeromonas hydrophila antigens. Fish Shellfish Immunol. Rep. 2021;2 doi: 10.1016/j.fsirep.2021.100025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mamun M.A.A., Nasren S., Abhiman P.B., Rathore S.S., Sowndarya N.S., Ramesh K.S., Shankar K.M. Effect of biofilm of Aeromonas hydrophila oral vaccine on growth performance and histopathological changes in various tissues of striped catfish, Pangasianodon hypophthalmus (Sauvage1878) Indian J. Anim. Res. 2020;54:563–569. doi: 10.18805/ijar.B-3814. [DOI] [Google Scholar]
- 76.Kavitha C., Malarvizhi A., Senthil Kumaran S., Ramesh M. Toxicological effects of arsenate exposure on hematological, biochemical and liver transaminases activity in an Indian major carp, Catla catla. Food Chem. Toxicol. 2010;48:2848–2854. doi: 10.1016/j.fct.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 77.Prabu D.L., Sahu N.P., Pal A.K., Dasgupta S., Narendra A. Immunomodulation and interferon gamma gene expression in sutchi cat fish, Pangasianodon hypophthalmus: effect of dietary fucoidan rich seaweed extract (FRSE) on pre and post challenge period. Aquac. Res. 2016;47:199–218. doi: 10.1111/are.12482. [DOI] [Google Scholar]
- 78.Sahu S., Das B.K., Pradhan J., Mohapatra B.C., Mishra B.K., Sarangi N.N. Effect of Mangifera indica kernel as a feed additive on immunity and resistance to Aeromonas hydrophila in Labeo rohita fingerlings. Fish Shellfish Immunol. 2007;23:109–118. doi: 10.1016/j.fsi.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 79.Panigrahi A., Kiron V., Satoh S., Watanabe T., bacteria Probiotic. Lactobacillus rhamnosus influences the blood profile in rainbow trout Oncorhynchus mykiss (Walbaum) Fish Physiol. Biochem. 2010;36:969–977. doi: 10.1007/s10695-009-9375-x. [DOI] [PubMed] [Google Scholar]
- 80.Harikrishnan R., Kim M.C., Kim J.S., Balasundaram C., Heo M.S. Protective effect of herbal and probiotics enriched diet on haematological and immunity status of Oplegnathus fasciatus (Temminck and Schlegel) against Edwardsiella tarda. Fish Shellfish Immunol. 2011;30:886–893. doi: 10.1016/j.fsi.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 81.Tahmasebi-Kohyani A., Keyvanshokooh S., Nematollahi A., Mahmoudi N., Pasha-Zanoosi H. Effects of dietary nucleotides supplementation on rainbow trout (Oncorhynchus mykiss) performance and acute stress response. Fish Physiol. Biochem. 2012;38:431–440. doi: 10.1007/s10695-011-9524-x. [DOI] [PubMed] [Google Scholar]
- 82.Chen L., Klaric G., Wadsworth S., Jayasinghe S., Kuo T.Y., Evensen Ø., Mutoloki S. Augmentation of the antibody response of Atlantic salmon by oral administration of alginate-encapsulated IPNV antigens. PLoS One. 2014;9 doi: 10.1371/journal.pone.0109337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rombout J.H.W.M., Berg V.D.A.A. Immunological importance of the second gut segment of carp. I. Uptake and processing of antigens by epithelial cells and macrophages. J. Fish Biol. 1989;35:13–22. doi: 10.1111/j.1095-8649.1989.tb03388.x. [DOI] [Google Scholar]
- 84.Rombout J.H.W.M., Blok L.J., Lamers C.H.J., Egberts E. Immunization of carp with Vibrio anguillarum bacterin: indications for a common mucosal immune system. Dev. Comp. Immunol. 1986;10:341–351. doi: 10.1016/0145-305X(86)90024-8. [DOI] [PubMed] [Google Scholar]
- 85.Hayat M., Mohd Yusoff M.S., Samad M.J., Abdul Razak I.S., Md Yasin I.S., Thompson K.D., Hasni K. Efficacy of feed-based formalin-killed vaccine of Streptococcus iniae stimulates the gut-associated lymphoid tissues and immune response of red hybrid tilapia. Vaccines. 2021;9:51. doi: 10.3390/vaccines9010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.