Abstract
Background:
Aberrant Motor Behaviour (AMB) is a neuropsychiatric symptom (NPS) prevalent in Alzheimer’s Disease (AD), known to cause great distress to both patients and caregivers. APOE4 is the most important genetic predictor of AD, and it has been associated with high NPS prevalence.
Objective:
To investigate the neuropathological substrates and risk factors associated with AMB in AD patients.
Methods:
Cases with Braak stage I-I and CERAD 0-1 were classified as Low AD (LAD), while Braak stage III-IV and CERAD 2 were grouped as Intermediate AD (IAD). Cases with Braak stage V-VI and CERAD 3 were classified as High AD (HAD) in accordance with NIA-Reagan criteria. All cases were stratified by APOE genotype, yielding No ε4, ε4 and ε4/ε4 groups depending on ε4 copy number within APOE. Presence of AMB was assessed using NPI-Q.
Results & Conclusion:
AMB increased in parallel with CERAD and Braak & Braak scores. Hypercholesterolemia, but no other Cardiovascular Risk Factors, was associated with AMB in HAD. AMB prevalence in HAD was significantly increased in the presence of two APOE ε4 alleles as compared to No ε4 & ε4. The relationship between homozygous APOE4 and AMB was strongly associated with the presence of both LB and CAA pathologies in both sexes.
Keywords: Alzheimer’s Disease, Neuropsychiatric symptoms, Aberrant Motor Behaviour, neuropathology, APOE gene, Lewy Bodies, Cerebral Amyloid Angiopathy
INTRODUCTION
Aberrant Motor Behaviour (AMB) is characterized by engagement in repetitive movements, inability to keep still and irregular pacing [1] among others. As a symptom, AMB has been strongly associated with Alzheimer’s Disease (AD) [2-3], dementia and its several subtypes [4]. AMB is also one of the twelve neuropsychiatric symptoms (NPS) assessed through the Neuropsychiatric Inventory questionnaire (NPI) [2]. As a symptom, several studies report higher AMB prevalence scores in clinically diagnosed severe AD [3,5], with an overall 24.3% [6] to 32% prevalence [7]. AMB has also been associated with medium to high levels of caregiver burden and patient distress [8]. The ε4 allele of the Apolipoprotein E (APOE) gene is the most important genetic predictor of late onset Alzheimer’s Disease [9]. APOE4 has been associated with several neuropathological and clinical features of AD such as Aβ plaque formation, disruption of synaptic plasticity [10-11] and accelerated dementia in a dose dependent manner [12]. In addition, APOE4 genotype has been repeatedly associated with a higher prevalence of all NPS [13]. Focused studies on depression [14], psychosis and its subtypes in severe AD have also reported an association with APOE4 [15]. In the aforementioned study, APOE4 effect on psychosis was found to be mediated by the presence of comorbid Lewy Body pathology (LB) [15].
Despite its prevalence and distressing effects on AD patients, the underlying mechanisms responsible for AMB are poorly understood. The goal of this study is to investigate the role of AD load and comorbid neuropathological substrates such as LB and Cerebrovascular Pathology play in the development of AMB. Furthermore, we seek to investigate the relationship between AMB prevalence and genetic and cardiovascular risk factors.
MATERIALS AND METHODS
Data and subject criteria
Data was obtained from the National Alzheimer’s Coordinating Centre (NACC) dataset, which contains clinical and neuropathological data from Alzheimer’s Disease Centres in the United States funded by the National Institute of Aging (NIA). The Uniform Data Set (UDS), the Neuropathological Data Set (NP), as well as the Research Data Dictionary-Genetic Data set (RDD-Gen) were used throughout the study. Demographical and clinical data, including gender and Cardiovascular Risk Factors such as Hypertension, Hypercholesterolemia, Diabetes and Smoking History were obtained from the UDS. AMB presence was assessed using the Neuropsychiatric Inventory, Quick version (NPI-Q), evaluating the presence of motor disturbance within the month prior to a clinical visit. Criteria for motor disturbance within NPI-Q comprises, yet is not limited to, engaging in irregular pacing, repetitive set of movements and overall inability to keep still.
The NP was used to evaluate AD severity using neuropathological criteria. CERAD scores and Braak & Braak stage were used to assess density of neuritic plaques and neurofibrillary pathology respectively. NP was also used to gauge the presence of comorbid Lewy Body pathology (LB), as determined by positive immunohistochemical staining for alpha-synuclein at any cerebral site. Further LB quantification was not used. Cerebrovascular disease was assessed through the presence of Ischemic, Haemorrhagic and overall Vascular Pathology (IHVP), Cerebral amyloid angiopathy (CAA - including mild, moderate and severe CAA) and Subcortical arteriosclerotic leukocephalopathy (SAL).
The RDD- Gen was used to confirm patient’s genetic background. The number of ε4 alleles within APOE gene was used to classify cases according to their APOE4 genotype.
Cases with other primary etiological diagnoses such as Traumatic Brain Injury (TBI), CNS neoplasm, Down Syndrome, Huntington’s Disease, Progressive Supranuclear Palsy (PSP), Corticobasal Degeneration (CBD), Prion Disease and Frontotemporal Dementia with or without ALS were excluded. Cases with Braak & Braak stage I & II and CERAD 1 and 0 were classified as Low AD pathological load (LAD), while Braak stage III & IV and CERAD 2 cases were classified as Intermediate AD pathological load (IAD), and cases with Braak stage V & VI and CERAD 3 were classified as High AD load (HAD), in accordance with NIA-Reagan criteria [16]. Cases with incongruent CERAD and Braak & Braak scores were excluded. Regarding APOE ε4 status, cases were classified as No ε4, presence of one (ε4) or two copies (ε4/ε4) of the ε4 allele; cases with unassessed APOE4 genotype were also excluded.
Statistical analysis
Statistical analysis was performed using SPSS 23.0 software. Unadjusted logistic regression models were used to assess the relationship between AD pathological loads, gender, APOE4 genotype, preliminary associations, comorbid neuropathological substrates and AMB. Logistic regression results were reported as odds ratio (OR) with associated 95% confidence intervals (CI), as well as p-value. One-way ANOVA was used for direct comparisons between continuous data such as preliminary neuropathological associations coupled with risk estimate. Results were reported as p value, followed by OR with associated 95% CI. All statistical analyses were conducted using a α = 0.05 significance.
RESULTS
Subject demographics
We assessed AMB prevalence in an initial sample of 2959 cases in the NACC NP dataset. Cases were then classified using CERAD and Braak & Braak scores as described [16], yielding 831 LAD, 584 IAD and 1544 HAD cases. Because of their scarcity of ε4/ε4 subjects, the LAD and IAD subsets were grouped into a Low to Intermediate AD group (LIAD). After excluding those with unassessed APOE4 genotype, we identified 1218 LIAD and 1333 HAD cases. Further information regarding the demographic and clinical characteristics of LIAD and HAD groups can be found in Table 1.
TABLE 1.
Demographic and Clinical characteristics
| AD pathological load | ||
|---|---|---|
| LIAD N = 1218 N/Mean |
HAD N = 1333 N/Mean |
|
| Sex | ||
| Male | 648 | 727 |
| Female | 570 | 606 |
| Age at last clinical visit | 83.45 | 78.38 |
| Age at death | 84.57 | 79.86 |
| Years of education | 15.73 | 15.78 |
| APOE4 status | ||
| No ε4 | 858 | 506 |
| ε4 | 323 | 632 |
| ε4/ε4 | 37 | 195 |
Aberrant Motor Behaviour across CERAD, Braak & Braak scores and overall AD load
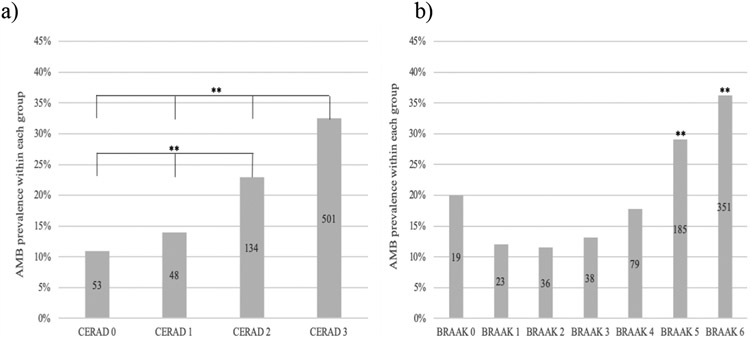
First we assessed the prevalence of AMB in relation to AD pathology load, separately for plaques and neurofibrillary pathology, and then jointly. AMB prevalence significantly increased with CERAD scores (p=0.001, Figure 1a). CERAD 3 and CERAD 2 cases were significantly more likely to report present AMB compared to CERAD 0 (OR=3.928, 95% CI 2.897-5.326, p=0.001; OR=2.433, 95% CI 1.724-3.433, p=0.01 respectively). AMB prevalence increased along Braak & Braak stages as well (p=0.001, Figure 1.b), with Braak VI being significantly more likely to report AMB than Braak 0 (OR=2.268, 95% CI 1.349-3.813, p=0.002). Interestingly, Braak II was significantly less likely to report AMB than Braak 0 (OR=0.520, 95%CI 0.282-0.958, p=0.036). As seen in Figure 1.b, those 19 Braak 0 cases reported present AMB in an off-trend fashion, with their primary diagnosis being Lewy Body Disease (26.3%) and Cognitive impairment with unknown cause (26.3%).
FIGURE 1. AMB prevalence increases across neuropathological substrates.
Each column represents % of cases with present AMB in each pathological substrate group. Logistic regression was used to assess AMB prevalence differences between groups. Number of cases within group reflected in each bar. ** refers to p=0.001, * refers to p < 0.050 significance. AMB prevalence increases across a) CERAD scores and b) Braak & Braak stages (except Braak 0).
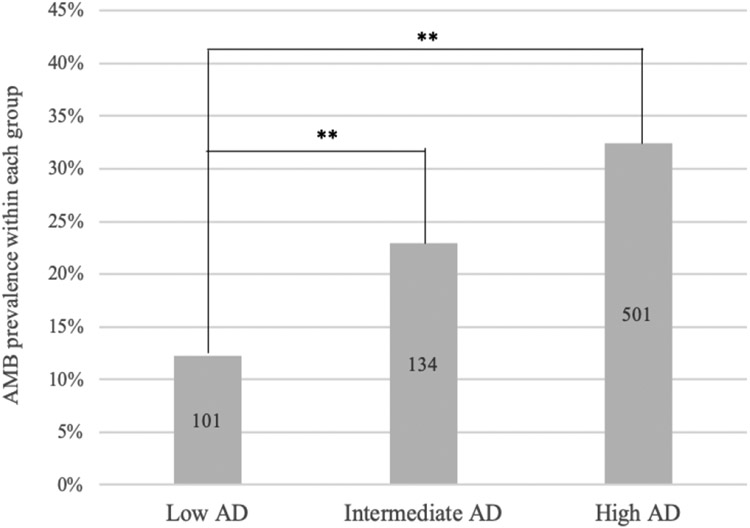
AMB significantly increased across AD load (p=0.001, Figure 2), with HAD being significantly higher than LAD (OR=3.473, 95% CI 2.748-4.386, p=0.001). AMB differences between IAD and LAD were also significant (OR=2.152, 95% CI 1.621-2.858, p=0.001). Throughout AD load, gender did not influence AMB prevalence, as females were not more likely to report AMB compared to males (OR=0.878, 95% CI 0.742-1.039, p=0.129).
FIGURE 2. AMB prevalence increases significantly across AD pathological load.
Each column represents percent of cases with present AMB in each AD load group. AMB prevalence differences were assessed with an unadjusted logistic regression model using LAD as an indicator. Number of cases within group reflected in each bar. ** refers to p=0.001 significance.
APOE4 genotype is associated with AMB in HAD but not in LIAD load
APOE4 genotype did not significantly influence AMB within LIAD (p=0.120, Table 3). Compared to No ε4, ε4/ε4 cases were not significantly more likely to report AMB in LIAD (OR=1.356, 95% CI 0.583-3.153, p=0.480). Only 7 cases homozygous for APOE ε4 genotype reported present AMB in LIAD, and thus we did not perform any further analysis in the LIAD group.
TABLE 3.
Regression analysis of the relationship between APOE4 genotype and presence of AMB in LIAD and High AD load
| Predictors | Odds Ratio (95% CI) |
P value |
|---|---|---|
| LIAD | 0.120 | |
| No ε4 (indicator) | ||
| ε4 | 1.408 (1.008-1.966) |
0.045 |
| ε4/ε4 | 1.356 (0.583-3.153) |
0.480 |
| HAD | ||
| No ε4 (indicator) | 0.001 | |
| ε4 | 1.377 (1.065-1.781) |
0.015 |
| ε4/ε4 | 2.124 (1.504-2.998) |
0.001 |
| ε4 only (indicator) | ||
| ε4/ε4 | 1.542 (1.111-2.140) |
0.010 |
| No ε4 & ε4 (indicator) | ||
| ε4/ε4 | 1.769 (1.297-2.411) |
0.001 |
Table representing the relationship between APOE4 genotype and AMB prevalence in different AD pathological loads. Predictors referred to the presence of one (ε4) or two (ε4/ε4) copies of the ε4 allele of the APOE gene in cases with Low to Intermediate AD load (LIAD) or High AD load (HAD). Numerical values within each column represent Odds Ratio (OR) with associated 95% Confidence Interval (95% CI) and p value. The relationship between APOE4 genotype and AMB prevalence was assessed in an unadjusted logistic regression model.
In HAD, APOE4 genotype did influence AMB prevalence (p=0.001, Table 3). Compared to No ε4 only, ε4/ε4 cases were significantly more likely to report AMB in HAD (OR=2.124, 95% CI 1.504-2.998, p=0.001, Table 3). ε4 cases were also more likely to report present AMB compared to No ε4 (OR=1.377, 95% CI 1.065-1.781, p=0.015).
APOE ε4/ε4 is associated with AMB in HAD cases
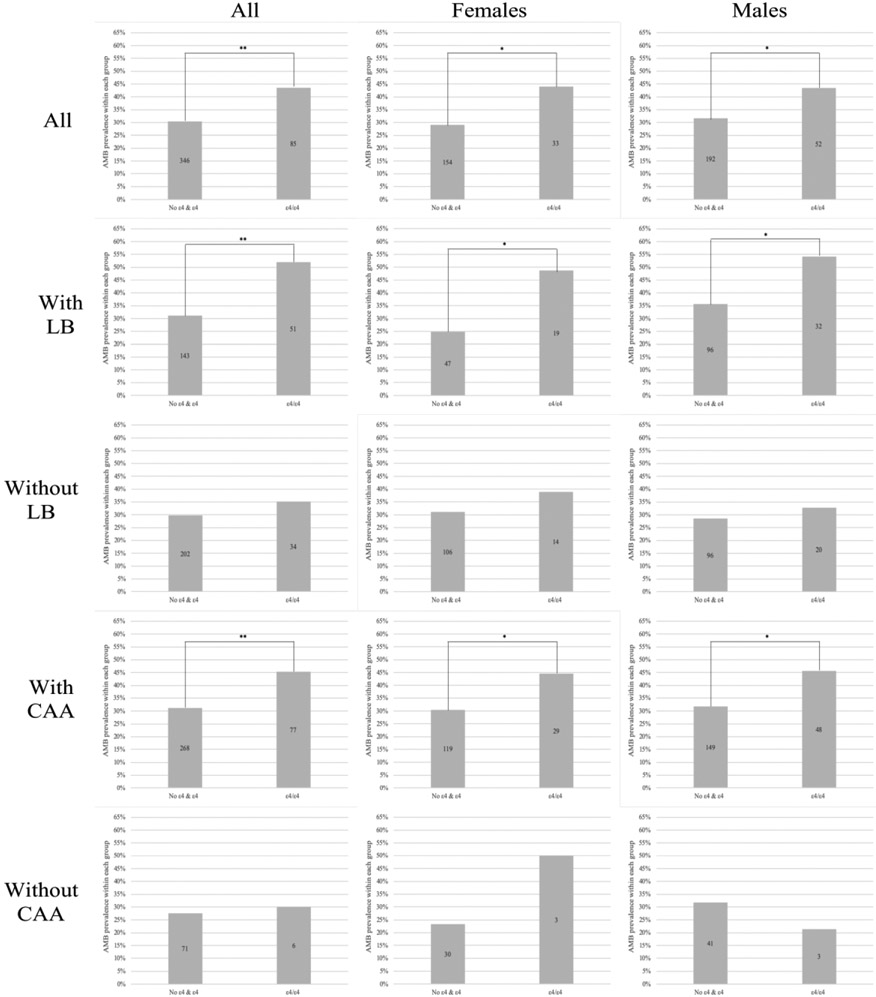
We then performed a regression analysis of the relationship between the presence of two copies of ε4 in APOE4 and AMB prevalence in HAD. ε4/ε4 cases were more likely to report AMB when analysed against one ε4 copy only (OR=1.542, 95% CI 1.111-2.140, p=0.010, Table 3) and No ε4 & ε4 (OR=1.769, 95% CI 1.297-2.411, p=0.001; Figure 3, 1.a). Given these results, the impact of APOE ε4 allele on AMB prevalence was further assessed by grouping HAD cases into No ε4 & ε4 and ε4 homozygous (ε4/ε4) groups. ε4/ε4 was more likely to report present AMB in both females (OR=1.923, 95% CI 1.175-3.149, p=0.009; Table 4, Figure 3, 1.b) and males (OR=1.653, 95% CI 1.108-2.465, p=0.014; Table 4, Figure 3, 1.c) compared to No ε4 & ε4 in HAD.
FIGURE 3. APOE ε4/ε4 significantly increases AMB prevalence in the presence of individual neuropathological substrates in both genders in HAD.
Each column represents percent of cases with present AMB according to its APOE4 genotype-HAD group. AMB prevalence differences were assessed with an unadjusted logistic regression model using No ε4 & ε4 as an indicator. Number of cases within group reflected in each bar. First row represents all HAD cases, 2nd row represents HAD cases with comorbid LB, 3rd row represents HAD cases with absent LB, 4th row represents HAD cases with comorbid CAA and 5th row represents HAD cases with absent CAA. Column A represents both females and males, column B represents females only and column C represents males only. Each individual graph is labeled as Figure 5 number.letter, with number referring to row and letter referring to column. ** refers to p=0.001, * refers to p < 0.050 significance.
TABLE 4.
Regression analysis of the relationship between APOE ε4/ε4 genotype and presence of AMB with comorbid neuropathological substrates in HAD
| Predictors | Odds Ratio (95% CI) |
P value |
|---|---|---|
| HAD females | ||
| No ε4 & ε4 (indicator) | ||
| ε4/ε4 | 1.923 (1.175-3.149) |
0.009 |
| HAD males | ||
| No ε4 & ε4 (indicator) | ||
| ε4/ε4 | 1.653 (1.108-2.465) |
0.014 |
| HAD with LB | ||
| No ε4 & ε4 (indicator) | ||
| ε4/ε4 | 2.390 (1.535-3.722) |
0.001 |
| HAD with LB, females | ||
| No ε4 & ε4 (indicator) | ||
| ε4/ε4 | 2.870 (1.412-5.834) |
0.004 |
| HAD with LB, males | ||
| No ε4 & ε4 (indicator) | ||
| ε4/ε4 | 2.136 (1.208-3.775) |
0.009 |
| HAD without LB | ||
| No ε4 & ε4 (indicator) | ||
| ε4/ε4 | 1.269 (0.810-1.987) |
0.298 |
| HAD without LB, females | ||
| No ε4 & ε4 (indicator) | ||
| ε4/ε4 | 1.405 (0.692-2.852) |
0.347 |
| HAD without LB, males | ||
| No ε4 & ε4 (indicator) | ||
| ε4/ε4 | 1.225 (0.683-2.197) |
0.497 |
| HAD with CAA | ||
| No ε4 & ε4 (indicator) | ||
| ε4/ε4 | 1.826 (1.306-2.552) |
0.001 |
| HAD with CAA, females | ||
| No ε4 & ε4 (indicator) | ||
| ε4/ε4 | 1.835 (1.075-3.131) |
0.026 |
| No ε4 & ε4 (indicator) | ||
| ε4/ε4 | 1.809 (1.176-2.781) |
0.007 |
| HAD without CAA | ||
| No ε4 & ε4 (indicator) | ||
| ε4/ε4 | 1.129 (0.417-3.052) |
0.811 |
| HAD without CAA, females | ||
| No ε4 & ε4 (indicator) | ||
| ε4/ε4 | 3.300 (0.633-17.211) |
0.157 |
| HAD without CAA, males | ||
| No ε4 & ε4 (indicator) | ||
| ε4/ε4 | 0.585 (0.155-2.212) |
0.430 |
Table representing LB and CAA’s impact as separate comorbid neuropathological substrates on the relationship between APOE4 and AMB in HAD. Predictors referred to presence of two (ε4/ε4) copies of the ε4 allele of the APOE gene in HAD cases with or without Lewy Bodies (LB), as well as with or without Cerebral Amyloid Angiopathy (CCA). Numerical values within each column represent Odds Ratio (OR) with associated 95% Confidence Interval (95% CI) and p value. The relationship between APOE4 genotype and AMB prevalence was assessed in an unadjusted logistic regression model.
Hypercholesterolemia is the only vascular risk factor or lesion directly associated with AMB in HAD
Prevalence of vascular lesions or risk factors in cases with AMB was then investigated. No direct significant associations were found between AMB and IHVP (p=0.603, OR=1.285, 95% CI 0.499-3.307), CAA (p=0.065, OR=1.317, 95% CI 0.982-1.765) and SAL (p=0.733, OR=1.059, 95% CI 0.761-1.474) in HAD. Hypercholesterolemia was the only Cardiovascular Risk Factor significantly associated with AMB in HAD (p=0.035, OR=1.279, 95% CI 1.017-1.607). AMB prevalence did not significantly differ in cases with Hypertension (p=0.506, OR=0.927, 95% CI 0.741-1.159), Diabetes (p=0.968, OR=1.007, 95% CI 0.704-1.442) or past smoking history (p=0.618, OR=1.021, 95% CI 0.942-1.106) in HAD.
In HAD, APOE ε4/ε4 is associated with AMB in cases with LB pathology in both sexes
Lewy Body pathology was not directly associated with higher AMB prevalence in HAD (p=0.091, OR=1.222, 95% CI 0.969-1.541). However, LB pathology was significantly associated with APOE ε4 allele’s copy number in HAD (p=0.006), with homozygous APOE4 being significantly more likely to report present LB (OR=1.704, 95% CI 1.220-2.378, p=0.002). Therefore, APOE4 effect on AMB was re-assessed upon stratification by LB. In HAD cases with comorbid LB, the presence of ε4/ε4 in APOE significantly influenced AMB prevalence when analysed against No ε4 & ε4 (OR=2.390, 95% CI 1.535-3.722, p=0.001; Table 4, Figure 3, 2.a). This association was maintained when females (OR=2.870, 95% CI 1.412-5.834, p=0.004; Figure 3, 2.b) and males (OR=2.136, 95% CI 1.208-3.775, p=0.009; Figure 3, 2.c) were analysed separately. In the absence of LB, AMB prevalence differences between ε4/ε4 and No ε4 & ε4 were not significant (OR=1.269, 95% CI 0.810-1.987, p=0.298; Table 4, Figure 3, 3,a). The association remained non-significant in both females (OR=1.405, 95% CI 0.692-2.852, p=0.347; Figure 3, 3.b) and males (OR=1.225, 95% CI 0.683-2.197, p=0.497; Figure 3, 3.c).
In HAD, APOE ε4/ε4 is associated with AMB in cases with CAA in both sexes
APOE4 genotype was not significantly associated with either IHVP or SAL in HAD (p=0.113 and p=0.555 respectively). In contrast, ε4 copy number in APOE was significantly associated with CAA in HAD (p=0.001), with homozygous APOE4 being significantly more likely to report CAA (OR=4.212, 95% CI 2.556-6.940, p=0.001). The association between APOE4 and AMB was then re-assessed upon stratification by CAA. In the presence of comorbid CAA, ε4/ε4 in APOE significantly influenced AMB prevalence when analysed against No ε4 & ε4 (OR=1.826, 95% CI 1.306-2.552, p=0.001; Table 4, Figure 3, 4.a). This association was maintained in both females (OR=1.835, 95% CI 1.075-3.131, p=0.026; Figure 3, 4.b) and males (OR=1.809, 95% CI 1.176-2.781, p=0.007; Figure 3, 4.c).
In the absence of CAA, we found no significant differences in AMB prevalence based on APOE4 genotype (OR=0.811, 95% CI 0.417-3.052, p=0.811; Table 4, Figure 3, 5a). This relationship remained non-significant in both females (OR=3.300, 95 % CI 0.633-17.211, p=0.157; Figure 3, 5.b) and males (OR=0.585, 95% CI 0.155-2.212, p=0.430; Figure 3, 5.c).
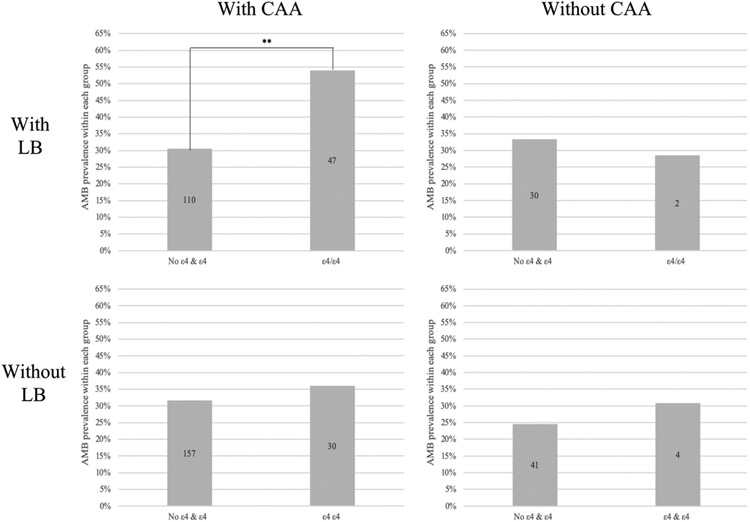
In HAD, APOE ε4/ε4 is associated with AMB in cases with concurrent CAA and LB only
Given our previous results on the relationship between APOE4 and both LB and CAA, the association between APOE4 and AMB was re-assessed upon double stratification by CAA and LB. In cases with presence of both LB and CAA, AMB prevalence in ε4/ε4 was significantly greater than in No ε4 & ε4 (OR=2.681, 95% CI 1.663-4.322, p=0.001; Table 5, Figure 4.a). We found no significant differences in AMB prevalence between ε4/ε4 and No ε4 & ε4 in HAD cases with comorbid LB but absent CAA (OR=0.800, 95% CI 0.147-4.368, p=0.797; Figure 4.b). The same trend was found in cases with absent LB but present CAA (OR=1.226, 95% CI 0.754-1.993, p=0.412; Figure 4.c), as well as cases with both LB and CAA absent (OR=1.366, 95% CI 0.399-4.670, p=0.619; Figure 4.d).
TABLE 5.
Regression analysis of the relationship between APOE ε4/ε4 genotype and presence of AMB with concurrent LB and CAA pathology in HAD
| Predictors | Odds Ratio (95% CI) |
P value |
|---|---|---|
| HAD with LB and CAA | ||
| No ε4 & ε4 (indicator) | ||
| ε4/ε4 | 2.681 (1.663-4.322) |
0.001 |
| HAD with LB & without CAA | ||
| No ε4 & ε4 (indicator) | ||
| ε4/ε4 | 0.800 (0.147-4.368) |
0.797 |
| HAD without LB & with CAA | ||
| No ε4 & ε4 (indicator) | ||
| ε4/ε4 | 1.226 (0.754-1.993) |
0.412 |
| HAD without LB & CAA | ||
| No ε4 & ε4 (indicator) | ||
| ε4/ε4 | 1.366 (0.399-4.670) |
0.619 |
Table representing the effect of LB and CAA as concurrent neuropathological substrates on the relationship between APOE4 and AMB in HAD. Predictors referred to presence of two (ε4/ε4) copies of the ε4 allele of the APOE gene in HAD cases with or without Lewy Bodies (LB), as well as with or without Cerebral Amyloid Angiopathy (CCA). Numerical values within each column represent Odds Ratio (OR) with associated 95% Confidence Interval (95% CI) and p value. The relationship between APOE4 genotype and AMB prevalence was assessed in an unadjusted logistic regression model.
FIGURE 4. APOE ε4/ε4 is associated with higher AMB prevalence only in cases with comorbid LB and CAA pathology in HAD.
Each column represents percent of cases with present AMB according to its APOE4 genotype-HAD group. AMB prevalence differences were assessed with an unadjusted logistic regression model using No ε4 & ε4 as an indicator. Number of cases within group reflected within each bar. a) The presence of two copies of ε4 within APOE is significantly associated with AMB prevalence in HAD cases with comorbid LB and CAA (top, left). The presence of two copies of APOE ε4 does is not significantly associated with higher AMB prevalence in HAD cases with b) present LB but absent CAA (top, right), c) absent LB but present CAA (bottom, left) and d) absent LB and CAA pathology (bottom, right). ** refers to p=0.001 significance.
DISCUSSION
We found Aberrant Motor Behaviour prevalence to significantly increase along with AD pathological load. These results are consistent with others reporting an association between AMB and neurofibrillary pathology [17], although that study focused on AMB with comorbid agitation. Neurofibrillary tangle burden has also been linked to other NPS such as apathy [18] and psychosis [19]. We found SAL not significantly associated with either AMB or APOE genotype, unlike the findings of a study focused on psychosis [20]. However, there is mixed evidence regarding the relationships between specific NPS and white matter rarefaction in AD [21,22]. In High AD, the ε4 allele of the APOE gene significantly influenced AMB prevalence in a strong recessive pattern. The literature offers mixed evidence regarding APOE4’s role in NPS development, with several studies reporting a non-significant [23], mixed [16] and a significant relationship between them [17]. This discrepancy could be explained by the reliance on clinical AD characterization [16,23], whereas our study used neuropathological post-mortem analysis. Interestingly, the relationship between NPS and APOE has mostly been assessed in APOE ε4 carriers [17]. However, we found the association between homozygous APOE4 and AMB to be significant when analysed against cases with no copies of ε4, as well as cases with one copy of ε4, and both no copies and one copy together. These results agree with others reporting a specific APOE4 recessive effect in delusions [24] and anxiety prevalence [25] in AD.
We found Lewy Body pathology significantly associated with high AMB prevalence in homozygous APOE4 cases, thus agreeing with others on the importance of comorbid LB in the association between APOE4 genotype and NPS prevalence [26]. However, LB development has been associated with multiple genetic variants [27], and so the association between APOE4 and AMB might not be entirely mediated by LB. This observation is supported by our own findings on CAA, which was found to be significantly associated with a homozygous APOE4 effect on AMB. Although APOE4 has been extensively associated with CAA in AD [28,29], the relationship between CAA and NPS is not understood. Up to date, CAA has only been associated with psychosis in AD [30], as well as being reported in a clinical case with a patient presenting psychosis and depression [31]. To our knowledge, our study is the first one reporting Cerebral Amyloid Angiopathy presence as an additional pathological feature possibly mediating the association between APOE4 genotype and any NPS development.
Most importantly, our results suggest that the proven association between APOE4 and NPS is possibly influenced by several pathological substrates. A strong association between APOE4 homozygosity and AMB prevalence was only reported in the presence of both LB and CAA. If either of those pathological substrates was absent, differences in AMB prevalence became non-significant. High CAA prevalence has been previously associated with Lewy Body Disease [32] and Lewy Body Variant of AD [33]; however, no studies have focused on the functional consequences of CAA and LB comorbidity. To our knowledge, our study is the first one reporting comorbid LB and CAA as a pathological substrate possibly mediating the association between APOE4 homozygosity and any AD-related clinical outcome.
However, there are limitations to our study. The use of NIA-Reagan criteria to characterize High AD load could also be considered a limitation, for we excluded cases with discordant CERAD and Braak scores. The use of NPI-Q is also limiting in that it only considers a standardized recording of AMB within the last clinical visit, and thus data on AMB course was not reported. Additionally, presence of AMB was dependant on caregiver input, leaving room for possible confounds. It should also be noted that the NACC database is a voluntary sample set, and thus it might not be representative of the entire population suffering from AD. In summary, we demonstrate that Aberrant Motor Behaviour prevalence significantly increases with AD load, and that APOE4 genotype influenced AMB in a recessive pattern. This association was only found in the presence of concurrent LB and CAA pathology in both sexes, supporting the existence of multiple pathological substrates possibly mediating the association between APOE4 genotype and AMB prevalence. Further research is needed to clarify whether comorbid CAA and LB pathology influence other clinical manifestations of the disease.
TABLE 2.
Regression analysis of the relationship between AMB prevalence and AD neuropathological load
| Predictors | Odds Ratio (95% CI) |
P value |
|---|---|---|
| CERAD 0 (indicator) | ||
| CERAD 1 | 1.325 (0.873-2.011) |
0.187 |
| CERAD 2 | 2.433 (1.724-3.433) |
0.001 |
| CERAD 3 | 3.928 (2.897-5.326) |
0.001 |
| BRAAK 0 (indicator) | ||
| BRAAK 1 | 0.548 (0.282-1.065) |
0.076 |
| BRAAK 2 | 0.520 (0.282-0.958) |
0.036 |
| BRAAK 3 | 0.606 (0.330-1.112) |
0.106 |
| BRAAK 4 | 0.868 (0.497-1.518) |
0.620 |
| BRAAK 5 | 1.644 (0.967-2.797) |
0.066 |
| BRAAK 6 | 2.268 (1.349-3.813) |
0.002 |
| LAD (indicator) | ||
| IAD | 2.152 (1.621-2.858) |
0.001 |
| HAD | 3.472 (2.748-4.386) |
0.001 |
Table representing the relationship between AD pathological load, separately and jointly, and AMB prevalence. Predictors referred to presence of neuritic plaques (CERAD score), neurofibrillary pathology (Braak & Braak score) or joint AD pathological load (LAD-HAD). Numerical values within each column represent Odds Ratio (OR) with associated 95% Confidence Interval (95% CI) and p value. The relationship between AD neuropathological load and AMB was assessed in an unadjusted logistic regression model.
ACKNOWLEDGMENTS
The NACC database is funded by NIA/NIH Grant U01 AG016976. NACC data are contributed by the NIA-funded ADCs: P30 AG019610 (PI Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P50 AG047266 (PI Todd Golde, MD, PhD), P30 AG010133 ( PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Steven Ferris, PhD), P30 AG013854 (PI M. Marsel Mesulam, MD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennet, MD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG016570 (PI Marie-Francoise Chesselet, MD, PhD), P50 AG005131 (PI Douglas Galasko, MD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russel Swerdlow, MD), P30 AG028383 ( PI Linda Van Eldik, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P50 AG005136 (PI Thomas Montine, MD, PhD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), P50 AG005681 (PI John Morris, MD) and P50 AG047270 (PI Stephen Strittmatter, MD, PhD).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- [1].Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J (1994) The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology 44, 2308. 10.1212/WNL.44.12.2308 [DOI] [PubMed] [Google Scholar]
- [2].Mega MS, Cummings JL, Fiorello T, Gornbein J (1996) The spectrum of behavioral changes in Alzheimer’s disease. Neurology 46, 130–135. 10.1212/WNL.46.1.130 [DOI] [PubMed] [Google Scholar]
- [3].Lyketsos CG, Carrillo MC, Ryan JM, Khachaturian AS, Trzepacz P, Amatniek J, Cedarbaum J, Brashear R, Miller DS (2011) Neuropsychiatric symptoms in Alzheimer’s disease. Alzheimers Dement 7, 532–539. 10.1016/j.jalz.2011.05.2410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Steinberg M, Sheppard JM, Tschanz JT, Norton MC, Steffens DC, Breitner JCS, Lyketsos CG (2003) The incidence of mental and behavioural disturbances in dementia: the cache county study. J Neuropsychiatry Clin Neurosci 15, 340–345. 10.1176/jnp.15.3.340 [DOI] [PubMed] [Google Scholar]
- [5].Siafarikas N, Selbaek G, Fladby T, Saltyte Benth J, Auning E, Aarsland D (2018) Frequency and subgroups of neuropsychiatric symptoms in mild cognitive impairment and different stages of dementia in Alzheimer’s disease. Int Psychogeriatr 30, 103–113. 10.1017/S1041610217001879 [DOI] [PubMed] [Google Scholar]
- [6].Fernandez-Martinez M, Castro J, Molano A, Zarranz JJ, Rodrigo RM, Ortega R (2008) Prevalence of neuropsychiatric symptoms in Alzheimer’s disease and vascular dementia. Curr Alzheimer Res 5, 61–69. 10.2174/156720508783884585 [DOI] [PubMed] [Google Scholar]
- [7].Ikeda M, Fukuhara R, Shigenobu K, Hokoishi K, Maki N, Nebu A, Komori K, Tanabe H (2004) Dementia associated mental and behavioural disturbances in elderly people in the community: findings from the first Nakayama study. J Neurol Neurosurg Psychiatry 75, 146–148. [PMC free article] [PubMed] [Google Scholar]
- [8].Shin IS, Carter M, Masterman D, Fairbanks L, Cummings JL (2005) Neuropsychiatric symptoms and quality of life in Alzheimer disease. Am J Geriatr Psychiatry 13, 469–474. 10.1176/appi.ajgp.13.6.469 [DOI] [PubMed] [Google Scholar]
- [9].Poirier J, Davignon J, Bouthillier D, Kogan S, Bertrand P, Gauthier S (1993) Apolipoprotein E polymorphism and Alzheimer’s disease. Lancet 342, 697–699. 10.1016/0140-6736(93)91705-q [DOI] [PubMed] [Google Scholar]
- [10].Liu CC, Kanekiyo T, Xu H, Bu G (2013) Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol 9, 106–118. 10.1038/nrneurol.2012.263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].van der Lee SJ, Wolters FJ, Ikram MK, Hofman A, Ikram MA, Amin N, van Dujin CM (2018) The effect of APOE and other common genetic variants on the onset of Alzheimer’s disease and dementia: a community-based cohort study. Lancet Neurol 17, 434–444. 10.1016/S1474-4422(18)30053-X [DOI] [PubMed] [Google Scholar]
- [12].Panza F, Frisardi V, Seripa D, D’Onofrio G, Santamato A, Masullo C, Logroscino G, Solfrizzi G, Pilotto A (2012) Apolipoprotein E genotypes and neuropsychiatric symtoms and síndromes in late-onset Alzheimer’s Disease. Ageing Res Rev 11, 87–103. 10.1016/j.arr.2011.06.005 [DOI] [PubMed] [Google Scholar]
- [13].D’Onofrio G, Panza F, Seripa D, Sancarlo D, Paris F, Cascavilla L, Urbano M, Gravina C, Fontana A, Solfrizzi V, Pellegrini F, Piotto A (2011) The APOE polymorphism in Alzheimer’s disease patients with neuropsychiatric symptoms and syndromes. Int J Geriatr Psychiatry 26, 1062–1070. 10.1002/gps.2644 [DOI] [PubMed] [Google Scholar]
- [14].Geda YE, Knopman DS, Mrazek DA, Jicha GA, Smith GE, Negash S, Boeve BF, Ivnik RJ, Petersen RC, Pankratz VS, Rocca WA (2006) Depression, apolipoprotein E genotype, and the incidence of mild cognitive impairment: a prospective cohort study. Arch Neurol 63, 435–440. 10.1001/archneur.63.3.435 [DOI] [PubMed] [Google Scholar]
- [15].Kim J, Fischer CE, Schweizer TA, Munoz DG (2017) Gender and pathology-specific effect of Apolipoprotein E genotype on Psychosis in Alzheimers Disease. Curr Alzheimer Res 14, 834–840. 10.2174/1567205014666170220150021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hyman BT, Trojanowski JQ (1997) Consensus recommendations for the post-mortem diagnosis of Alzheimer disease from the National Institute of Aging and the Reagan Institute working group on diagnostic criteria for the neuropathological assessment of Alzheimer Disease. J Neuropathol Exp Neurol 56, 1095–1097. 10.1097/00005072-199710000-00002 [DOI] [PubMed] [Google Scholar]
- [17].Tekin S, Mega MS, Masterman DM, Chow T, Garakian J, Vinters HV, Cummings JL (2001) Orbitofrontal and Anterior Cingulate Cortex Neurofibrillary Tangle Burden is Associated with Agitation in Alzheimer Disease. Ann Neurol 49, 355–361. 10.1002/ana.72 [DOI] [PubMed] [Google Scholar]
- [18].Marshall GA, Fairbanks LA, Tekin S, Vinters HV, Cummings JL (2006) Neuropathologic correlates of apathy in Alzheimer’s disease. Dement Geriatr Cogn Disord 21, 144–147. 10.1159/000090674 [DOI] [PubMed] [Google Scholar]
- [19].Farber NB, Rubin EH, Newcomer JW, Kinscherf BA, Miller JP, Morris JC, Olney JW, McKeel DW Jr (2000) Increased Neocortical Neurofibrillary Tangle Density in subjects With Alzheimer Disease and Psychosis. Arch Gen Psychiatry 57, 1165–1173. 10.1001/archpsyc.57.12.1165 [DOI] [PubMed] [Google Scholar]
- [20].Fischer CE, Qian W, Schweizer TA, Millikin CP, Ismail Z, Smith EE, Lix LM, Shelton P, Munoz DG (2016) Lewy Bodies, Vascular Risk Factors, and Subcortical Arteriosclerotic Leukoencephalopathy, but not Alzheimer Pathology, are Associated with Development of Psychosis in Alzheimer’s Disease. J Alzheimer’s Dis 50, 283–295. 10.3233/JAD-150606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tu MC, Huang WH, Hsu YH, Lo CP, Deng JF, Huang CF (2017) Comparison of neuropsychiatric symptoms and diffusion tensor imaging correlates among patients with subcortical ischemic vascular disease and Alzheimer’s disease. BMC Neurol 17, 144. 10.1186/s12883-017-0911-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wu MK, Lu YT, Huang CW, Lin PH, Chen NC, Lui CC, Chang WN, Lee CC, Chang YT, Chen SF, Chang CC (2015) Clinical Significance of Cerebrovascular Biomarkers and White Matter Tract Integrity in Alzheimer Disease: Clinical correlations with Neurobehavioral Data in Cross-Sectional and After 18 Months Follow-ups. Medicine (Baltimore) 94, e1192. 10.1097/MD.0000000000001192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hirono N, Mori E, Yasuda M, Imamura T, Shimomura T, Hashimoto M, Tanimukai S, Kazui H, Yamashita H (1999) Lack of effect of Apolipoprotein E E4 allele on Neuropsychiatric Manifestations in Alzheimer’s Disease. J Neuropsychiatry Clin Neurosci 11, 66–70. 10.1176/jnp.11.1.66 [DOI] [PubMed] [Google Scholar]
- [24].van der Flier WM, Staekenborg S, Pijnenburg YA, Gillissen F, Romkes R, Kok A, Bouwman FH, Scheltens P (2007) Apolipoprotein E genotype influences presence and severity of delusions and aggressive behavior in Alzheimer Disease. Dement Geriatr Cogn Dis 23,42–44. 10.1159/000096682 [DOI] [PubMed] [Google Scholar]
- [25].Robertson J, Curley J, Kaye J, Quinn J, Pfankuch T, Raber J (2005) ApoE isoforms and measures of anxiety in probable AD patients and Apoe −/− mice. Neurobiol Aging 26, 637–643. 10.1016/j.neurobiolaging.2004.06.003 [DOI] [PubMed] [Google Scholar]
- [26].Chung EJ, Babulal GM, Monsell SE, Cairns NJ, Roe CM, Morris JC (2015) Clinical features of Alzheimer Disease with and without Lewy Bodies. JAMA Neurol 72, 789–796. 10.1001/jamaneurol.2015.0606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wider C, Ross OA, Nishioka K, Heckman MG, Vilariño-Guell C, Jasinka-Myga B, Erketin-Taner N, Rademakers R, Graff-Radford NR, Mash DC, Papapetropoulus S, Duara R, Uchikado H, Wszolek ZK, Farrer MJ, Dickson DW (2012) An evaluation of the impact MAPT, SNCA and APOE on the burden of Alzheimer and Lewy Body pathology. J Neurol Neurosurg Psychiatry 83, 424–429. 10.1136/jnnp-2011-301413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Rannikmae K, Kalaria RN, Greenberg SM, Chui HC, Schmitt FA, Samarasekera N, Salman RA-S, Sudlow CL (2014) APOE associations with severe CAA-associated vasculopathic changes: collaborative meta-analysis. J Neurol Neurosurg Psychiatry 85, 300–305. 10.1136/jnnp-2013-306485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Shinohara M, Murray ME, Frank RD, Shinohara M, DeTure M, Yamazaki Y, Tachibana M, Atagi Y, Davis MD, Liu CC, Zhao N, Painter MM, Petersen RC, Fryer JD, Crook JE, Dickson DW, Bu G, Kanekiyo T (2016) Impact of sex and APOE4 on cerebral amyloid angiopathy in Alzheimer’s Disease. Acta Neuropathol 132, 225–234. 10.1007/s00401-016-1580-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Vik-Mo AO, Bencze J, Ballard C, Hortobagyi T, Aarsland D (2019) Advanced cerebral amyloid angiopathy and small vessel disease are associated with psychosis in Alzheimer’s Disease. J Neurol Neurosurg Psychiatry 90, 728–730. 10.1136/jnnp-2018-318445 [DOI] [PubMed] [Google Scholar]
- [31].Gleason A, Hayhow B, Emmanuel J, Gaillard F (2014) Cerebral amyloid angiopathy presenting with neuropsychiatric symptoms. Aust N Z J Psychiatry 48, 779–80. 10.1177/0004867414525843 [DOI] [PubMed] [Google Scholar]
- [32].Jellinger KA, Attens J (2008) Cerebral amyloid angiopathy in Lewy body disease. J Neural Transm 115, 473–482. 10.1007/s00702-007-0856-8 [DOI] [PubMed] [Google Scholar]
- [33].Jellinger KA (2010) Prevalence and impact of cerebrovascular lesions in Alzheimer and Lewy Body Diseases. Neurodegener Dis 7, 112–115. 10.1159/000285518 [DOI] [PubMed] [Google Scholar]