Abstract
Poly(I:C) is a kind of chemosynthetic double-stranded RNA (dsRNA) analogue which could act as TLR3 agonist and induce IFN production. It is widely applied in anti-virus treatment and immunoregulation, as well as vaccine adjuvant in farmed animals. However, whether poly(I:C) could activate innate immune response to defense against bacterial infection remains unclear. In this study, we established a feeding trial model with different dose of poly(I:C) in turbot larvae, then challenged with Edwardsiella piscicida after 3–7 weeks resting period. The results show that feeding turbot with poly(I:C) exhibited a stronger inflammatory response and antioxidant stress ability, and significantly elevated the survival rate within the decreased bacterial loads. Importantly, the bacterial infection-induced white feces in hindgut of turbot were significantly alleviated after poly(I:C) feeding, and this administration induced protection could last for about 7 weeks. Taken together, these findings indicate that feeding turbot with poly(I:C) could enhance a long-term intestinal mucosal immunity in response to bacterial infection, suggesting that poly(I:C) might be a promising immunostimulant in aquaculture.
Keywords: Poly(I:C), Anti-oxidant stress, Intestinal immune responses, Bacterial-infection, turbot
1. Introduction
Polyriboinosinic-polyribocytidilic acid (poly(I:C)) is a kind of chemosynthetic double-stranded RNA (dsRNA) analogue [1], which could mimic the structure of virus RNA and as a potent ligand of TLR3 to induce IFN production [2]. It has been widely applied as an effective immunotherapy for human diseases, as well as the adjuvant utilized in vaccines and cancer treatment [3, 4]. The role of poly(I:C) in activating antiviral immune response has been revealed in teleost fish. For example, poly(I:C) vaccination could effectively protect grouper from nervous necrosis virus infection [5], as well as promote the anti-viral response in zebrafish liver cells [6]. Moreover, it was suggested that poly(I:C) could induce the expression of IFNa3 through the TLR/TRIF and RLRs/MAVS pathways in the macrophages of large yellow croaker [7]. However, as a potent immunostimulant, whether poly(I:C) could protect host from bacterial infection remains largely unknown.
In recent years, the farmed turbot (Scophthalmus maximus) is mainly threatened by the bacterial pathogens (e.g., the Edwardsiella piscicida (previously known as Edwardsiella tarda) [8] and Aeromonas salmonicida [9]), causing an enormous economic loss in this industry. To avoid the antibiotics resistant, and develop an effective immuno-therapy to prevent the diseases is urgent. To this end, the effective vaccine design and the immunostimulants administration are the promising strategies. For example, we have successfully developed the live vaccine against Edwardsiella tarda, and the genetically engineered live vaccine against Vibro anguillarum in turbot [10, 11]. However, these vaccines’ inoculation should be utilized as intraperitoneal injection, which is limited by the weight of the fish. Thus, the immunostimulants development should be a promising supplement of vaccination.
There are several kinds of immunostimulants, which have been applied as the diet additive to improve the innate immune responses of fish. For example, dietary feeding with β-glucan could elevate the innate immune responses and improve the disease resistance effects in Nile tilapia [12]. Administrated with probiotics could modulate the immune response against tilapia lake virus infection [13]. Moreover, dietary feeding with Spirulina platensis could activate the mucosal immune responses in rainbow trout [14]. In this study, we proposed that poly(I:C) could be utilized as dietary additive for turbot, and developed a short-term, low-dose feeding of poly(I:C) strategy, and revealed its anti-bacterial infection effects, through provoking the non-specific and long-lasting protection, as well as maintaining the intestinal health of turbot. Taken together, these results suggest that feeding with poly(I:C) might be a promising immunopotentiator in aquaculture.
2. Materials and methods
2.1. Ethical statement
All animal experiments in this study were conformed to the guidelines for the care and use of laboratory animals and ethically approved by the Laboratory Animal Ethical Committee of East China University of Science and Technology (Protocol number 2,006,272). Turbot larvae were anesthetized using tricaine (200 μg/ml, Aladdin, China) before dissection, and all efforts were made to minimize suffering.
2.2. Turbot feeding trial and maintaining condition
Turbot larvae (3–5 g) were used for feeding trial. Poly(I:C) (NiujiaBio, China) was emulsified with 10% fish oil and 3% phosphatidylcholine in water to generate emulsion, then mixed into the basic pellet diet to the final dose of 0.005% (FD-H) and 0.00125% (FD-L), the control group fed the emulsion mixed pellet diet without poly(I:C) (FD-N). After 4-weeks daily feeding, the turbot larvae were maintained as the control groups. For the maintenance of turbot larvae, the temperature was kept at 18 °C, total ammonia-nitrogen was 〈 0.5 mg/L and dissolved oxygen was 〉 5.0 mg/L.
2.3. Bacterial challenge
At 3-, 6- and 7-weeks post feeding (wpf), turbot larvae were challenged with Edwardsiella piscicida. The bacteria were cultured overnight in trypticase yeast broth (TYB) medium with 50 μg/ml Colistin (Col) at 30 °C, 200 rpm shaker. Then the bacteria were inoculated into fresh medium as the ratio of 1:100 and cultured overnight. The concentration of the bacteria was calculated by NanoDrop (Thermo, UAS). Thirty fish from each group were randomly picked and immersed with 2 × 108 CFU/ml bacteria for 2 h in 6-L tank. The survival rate of turbot larvae was observed every day after bacterial challenge.
2.4. Bacteria enumeration
After 3 wpf, nine larvae from corresponding groups were randomly picked at 7 dpi of bacterial challenge and divided triplet, the tissues were collected and sufficiently grinded in 200 μl PBS. Then pipetting 20 μl grinding fluid to 180 μl PBS for a 10-fold dilution and series dilution were prepared in the same way. For bacterial enumeration, 10 μl of serial diluent were plated on the deoxycholate hydrogen sulfide lactose (DHL) agar plate (Colr) and enumerated the black colonies after culturing in 37 °C incubator for 24 h.
2.5. RNA isolation and q-PCR
After 3 wpf, nine turbot larvae were randomly picked from corresponding feeding group after bacterial challenge and divided triplet, the tissues, including gill, intestine, liver, spleen and kidney, were collected from the larvae and placed into 200 μl RNAstore Reagent (Tiangen, China) and stored at −80 °C. RNA of individual sample was isolated by Trizol (Invitrogen, USA) and chloroform. The cDNA was synthesized by FastQuant RT Kit (Tiangen), then analyzed the gene expression with QuantStudio 3 (Thermofisher, USA) by using SuperReal PreMix Plus (SYBR Green) (Monard, China) according to procedures. The results were analyzed by 2−ΔΔCt method. Primers used in this study were listed in Table 1. The reference gene was gapdh.
Table 1.
q-PCR primers used in this study.
| Primers | Forward sequence (5′−3′) | Reverse sequence (5′−3′) |
|---|---|---|
| il-1β | CTTCCCCAACTGGTACATCAG | CCCATTCCACCTTCCACTTT |
| il-6 | CTTCCCAAACCTGCCTCTAC | GGAAGCATTTGACAGCAACTC |
| tnfα | CCTTTACTTCGTCTACAGCCAG | GAGTACCGCCATATCCTGTG |
| hk1 | AGAATATGACCGTGCTGTGG | CATGCCGCTACACATTTTCTC |
| pfk | GGCACCTTCTCCCTTTGATAG | ATGCTGTATCTGGTGAGTTGG |
| ldh | ACTGTTCCTGTTATGCCTGTG | TCATCCTCAGTCCCCATTTTG |
| gapdh | AAGGGCATTCTGGGATACAC | CATGACACCAGCTTGACAAAG |
2.6. MDA, SOD and CAT activity assay
After 3 wpf, nine turbot larvae were randomly picked at 7 dpi of bacterial challenge and divided triplet, the tissues including intestine, liver and spleen were collected and added 200 μl RIPA lysis buffer (Beyotime, China) to lyse the tissues by grinding. Total protein concentration in different tissues were measured by Enhanced BCA Protein Assay Kit (Beyotime). The contents of superoxide dismutase (SOD), catalase (CAT) and Malondialdehyde (MDA) were measured through commercial assay kits (Beyotime) according to manufacturer's recommendations. The absorbance of the reaction mixture was measured by Spectra Max M5 micro plate reader (Molecular Devices, USA).
2.7. LZM activity assay
Lysozyme (LZM) activity was measured by Micrococcus Lysodeikticus Cells (Sangon Biotech, China). The Micrococcus Lysodeikticus Cells suspension was prepared by PBS and the final concentration was 0.2 g/L. 200 μl suspension and 10 μl cell lysis solution or 10 μl RIPA lysis buffer was added to 96-well plate and immediately mixed by pipetting. Lysozyme activity was calculated through the decrease of absorbance in A450 nm within 3 min, using the formula applied in previous study [15]: U = (ΔA450 nm/3/0.001) × dilution ratio.
2.8. Histopathological analysis
After 3 wpf and 7 wpf, the tissues including liver, spleen and intestine from 3 fish at 7 dpi in different groups were randomly collected and fixed with 4% paraformaldehyde (PFA), then dehydrated using ethanol and vitrificated by dimethylbenzene. The organs were embedded in paraffine blocks and sliced with paraffine slicing hematoxylin and eosin (H&E) staining and stereomicroscopy were used for histopathological observation.
2.9. Statistical analysis
The results were performed by Prism 7.0 (Graphpad, USA). The data were presented as mean ± SD with three independent experiments. The statistical significance was analyzed by two-way ANOVA or Students’ t-test. * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001.
3. Results
3.1. Poly(I:C) feeding elevates the gene expression of innate immune responses during bacterial infection
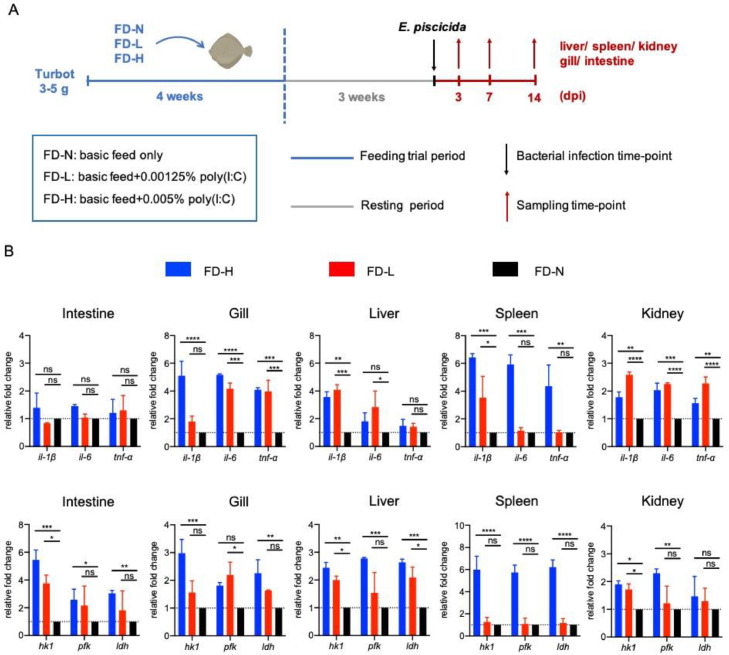
To evaluate the protective effects of poly(I:C) as dietary additive for turbot, we performed a 4-weeks feeding trial with different dose of poly(I:C) (FD-L and FD-H) in turbot larvae, then the bacterial challenge experiments were performed after 3 weeks of resting period (Fig. 1A). We analyzed the expression of several inflammatory cytokines (including il-1β, il-6 and tnf-α) in different tissues at 3 dpi, as well as the enzymes involved in aerobic glycolysis pathways (including hk1, pfk and ldh) which has been reported to mediate immune response through enhancing the transcription of cytokines [16]. We found that the transcriptional levels of metabolic genes (hk1, pfk and ldh) in FD-H and FD-L larvae were remarkably up-regulated in all immuno-organs, comparing with that in FD-N larvae (Fig. 1B). Meanwhile, the expression of inflammatory cytokines (il-1β, il-6 and tnf-α) in FD-H and FD-L larvae were also dynamically up-regulated in most of the immuno-organs, but not in intestine (Fig. 1B). Taken together, these results suggest that poly(I:C) feeding could enhance the innate immune response during bacterial infection in turbot.
Fig. 1.
Poly(I:C) feeding elevates the gene expression of innate immune responses during bacterial infection. (A) Schematic of the poly(I:C) feeding trial and experimental design. (B) q-PCR analysis of cytokine genes (including il-1β, il-6 and tnf-α) and metabolic enzymes related genes (including hk1, pfk and ldh) transcripts in different tissues (including gill, intestine, liver, spleen and kidney) of infected turbot larvae (including FD-H, FD-L and FD-N group) at 3dpi. Data are presented as mean ± SD of three independent experiments; ns, no significance, * p<0.05, ** p<0.01, *** p<0.001 and **** p<0.0001.
3.2. Poly(I:C) feeding elevates the anti-oxidative and bactericidal enzymatic activity
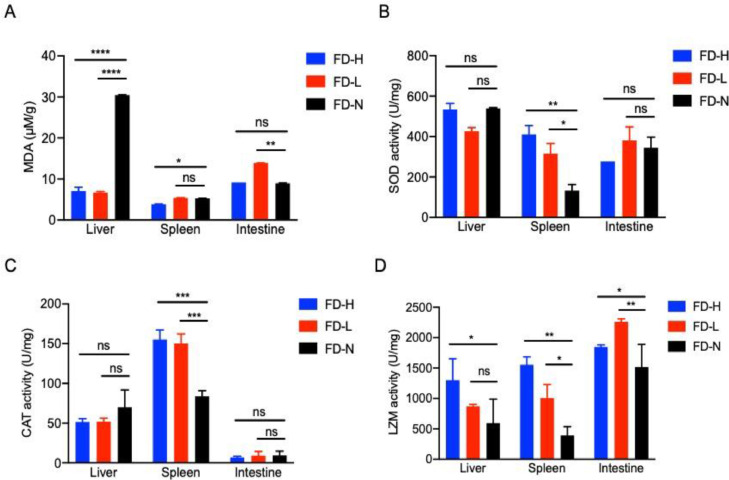
Since the cytotoxic malondialdehyde (MDA) could be accumulated during oxidative stress, while the superoxide dismutase (SOD) and catalase (CAT) could inhibit the overwhelming of oxidative stress [17], we further analyzed the anti-oxidative stress and bactericidal enzymatic activity during bacterial challenge with/without poly(I:C) feeding in turbot. We found that the content of MDA in the liver of FD-H and FD-L larvae was significantly less than that in FD-N larvae (Fig. 2A). Moreover, the enzyme activity of SOD and CAT in the spleen of FD-H and FD-L larvae were apparently higher than that in FD-N larvae, but not in tested liver and intestine (Fig. 2B and 2C). In addition, the LZM activity in different immuno-organs of FD-H and FD-L larvae are all comparatively higher than that in FD-N larvae, especially in intestine (Fig. 2D). These results suggest that poly(I:C) feeding could improve the anti-oxidative stress activity in liver and spleen, and elevate the bactericidal activity in intestine.
Fig. 2.
Poly(I:C) feeding elevates the anti-oxidative and bactericidal enzymatic activity. The content of lipid peroxidation marker (A), anti-oxidant enzymes (B and C) and lysozyme (D) in different tissues (including liver, spleen and intestine) of infected turbot larvae (including FD-H, FD-L and FD-N group) were measured at 7 dpi. Data are presented as mean ± SD of three independent experiments; ns, no significance, * p<0.05, ** p<0.01, *** p<0.001 and **** p<0.0001.
3.3. Poly(I:C) feeding alleviates the immuno-organ injury and strengthen the anti-infection ability
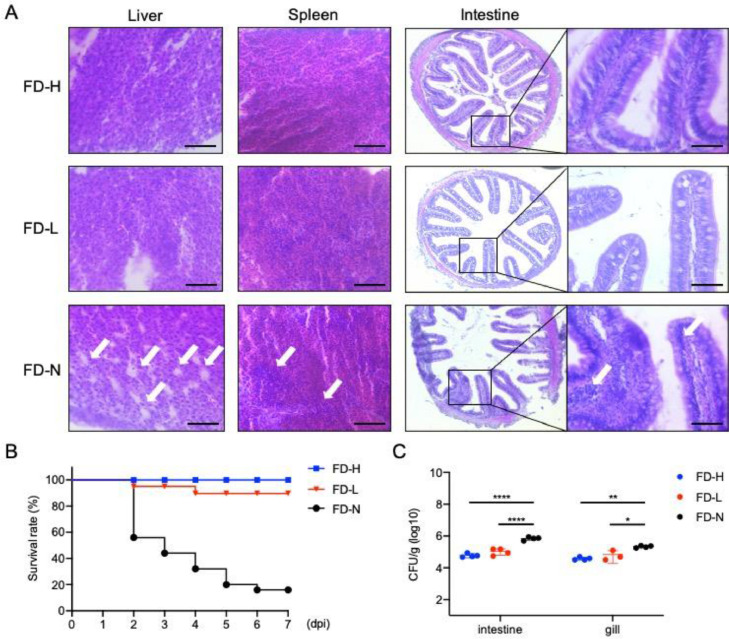
Furthermore, we analyzed the histopathological changes in different immuno-organs, and found that the liver vacuolization caused by oxidative stress after bacterial infection was obviously alleviated in FD-H and FD-L larvae, which is consistent with the result in Fig. 2A. Besides, the inflammatory infiltration in spleen and intestine was also relieved in FD-H and FD-L larvae compared to FD-N larvae, and intestinal microvilli in FD-H larvae were more intact and thicker than that in FD-N larvae (Fig. 3A). Moreover, to verify whether poly(I:C) feeding could improve the anti-bacterial effects in turbot, we analyzed the bacterial infection-induced survival rate and bacterial loads, and found that the survival rate in FD-H and FD-L larvae were higher than that in FD-N larvae (Fig. 3B), and the bacterial colonization in intestine and gills were significantly decreased in FD-H and FD-L larvae (Fig. 3C). Taken together, these results suggest that poly(I:C) feeding could play an important role in maintaining intestinal integrity, which is essential for anti-bacterial infection in turbot.
Fig. 3.
Poly(I:C) feeding alleviates the immuno-organ injury and strengthen the anti-infection ability. (A) Images of paraffin section in different tissues (including liver, spleen and intestine) of infected turbot larvae (including FD-H, FD-L and FD-N group) at 7 dpi. The white arrows indicated the histopathological injury, scale bar, 20 μm. (B) Percent survival of turbot larvae fed with poly(I:C) after E. piscicida infection. (C) Bacterial burden in gill and intestine of turbot larvae at 7 dpi of E. piscicida infection. * p<0.05, ** p<0.01 and **** p<0.0001.
3.4. Poly(I:C) feeding induces long-term effects in protecting turbot from bacterial infection-induced white feces
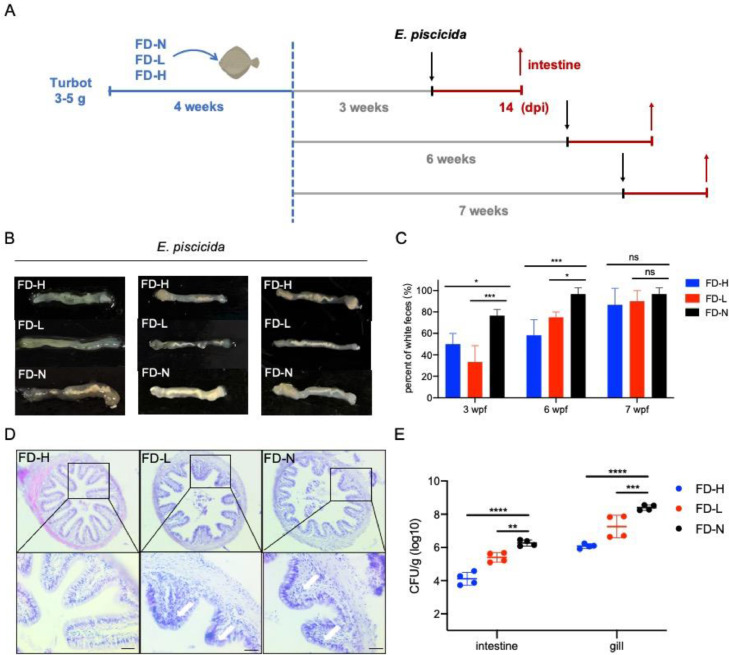
Based on the results above, we further analyzed the long-lasting protective effects of poly(I:C) feeding in turbot, through extending the resting period of poly(I:C) feeding to 6- and 7-weeks post administration (Fig. 4A). Firstly, The intestinal white feces [18], a common symptom of enteritis caused by bacterial infection, was detected at 14 dpi to evaluate the long-term effects of poly(I:C) feeding. We found that bacterial infection caused white feces in hindgut was significantly decreased in FD-H and FD-L larvae, until 6-weeks post poly(I:C) feeding (Fig. 4B and 4C). However, at 7-wpf, although the bacterial infection-induced white feces in FD-H and FD-L larvae exhibited no difference with FD-N larvae, the intestinal histopathological changes in FD-H larvae remains more intact, and exhibits less inflammatory infiltration, comparing with that in FD-N larvae (Fig. 4D). Furthermore, the bacterial loads in FD-H and FD-L larvae remain less than that in FD-N larvae during E. piscicida infection at 7-wpf (Fig. 4E). Taken together, these results suggest that poly(I:C) feeding as dietary additive is an effective way to keep turbot larvae from bacterial infection with a long-term protection.
Fig. 4.
Poly(I:C) feeding induces long-term effects in protecting turbot from bacterial infection-induced white feces. (A) Schematic of the experimental design to evaluate the long-term effects of poly(I:C) feeding. (B) Images of white feces in the hindgut of infected turbot larvae during E. piscicida infection at 3 wpf, 6 wpf and 7 wpf. (C) The quantitative statistics of the white feces indicated in (B) (n = 10). (D) Images of intestinal paraffin section in turbot larvae during E. piscicida infection at 7 wpf. The white arrows indicated the histopathological injury, scale bar, 20 μm. (E) The bacterial burden in the intestine and gill of turbot larvae during E. piscicida infection at 7 wpf. ns, no significance, * p<0.05, ** p<0.01, *** p<0.001 and **** p<0.0001.
4. Discussion
Mucosal immunity plays critical roles in immune defense of fish. Mucosal tissues including gills, skin and intestine, are the important immune barriers against microbial infection [19]. Among these organs, the intestinal health of fish is a major concern in aquaculture. Intestinal oxidation stress and inflammatory injury would cause high mortality in farmed fish [20], thus maintaining the homeostasis of intestine is crucial for healthy farming. Intact intestinal barrier could prevent the bacteria invade into the circulation and cause systemic infection [21], and intestinal epithelium could release antimicrobial peptides and alarmins onto the mucosal surface to kill the microbial directly or recruit the immune cells [22]. Moreover, the intestinal microbiota might also contribute to anti-bacterial infection process [23]. In our study, we found that feeding with poly(I:C) could not only elevate the immune response in peripheral immune organs, including liver and spleen, but also alleviate the intestinal oxidation stress and inflammatory symptoms caused by bacterial infection in mucosal organs. These results suggest that poly(I:C) feeding could effectively activate the mucosal immunity in turbot, which provide a feasible strategy to maintain the intestinal health of aquaculture animals. However, the molecular mechanisms of poly(I:C) feeding induced mucosal immune responses remains to be elucidated.
Trained immunity (TI), a kind of innate immune memory has been increasingly recognized in recent years [24]. Specifically, the stimulants, including β-glucan and bacille Calmette-Guerin (BCG), could be recognized by the cell membrane receptors (e.g., dectin-1 and NOD2) to activate the host innate immunity through epigenetic and metabolic reprogramming, exhibiting a long-term, non-specific immune responses during secondary challenge [25, 26]. Interestingly, in this study, we found that low-dose of poly(I:C) feeding could provoke a long-lasting innate immune response against bacterial infection in turbot larvae, and this phenomenon is quite similar with that in TI activation. Thus, we speculate that poly(I:C) treatment might trigger the TI activation in this teleost fish, and clarification of these key points will contribute to better guide the usage of immunopotentiators in aquaculture.
In summary, our study demonstrates that low-dose and short-period poly(I:C) addition in fish diet could confer turbot with long-term protection, and alleviate the white feces syndrome. Moreover, our results also suggest a potential role of poly(I:C) against bacterial infection, which might possess a promising application in biological control in aquaculture disease.
Authors contribution
D. Yang conceived the study; Z. Wang and J. He conducted the majority of experiments with help from Y. Zhao and J. Yang; Y. Zhang and Q. Liu. provided expert advice and critical review of the manuscript. D. Yang and Z. Wang wrote the manuscript; all authors discussed the results and commented on the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the Natural Science Foundation of Shanghai (20ZR1415500), the Innovation Group Project of Southern Marine Science and Engineering Guangdong Laboratory (Zhuhai) (311020005), and the Fundamental Research Funds for the Central Universities (222201211729).
References
- 1.Davies D.R. Polyinosinic plus polycytidylic acid: a crystalline polynucleotide complex. Nature. 1960;186:1030–1031. doi: 10.1038/1861030a0. [DOI] [PubMed] [Google Scholar]
- 2.Matsumoto M., Seya T. TLR3: interferon induction by double-stranded RNA including poly(I:C) Adv. Drug. Deliv. Rev. 2008;60:805–812. doi: 10.1016/j.addr.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Ammi R., De Waele J., Willemen Y., Van Brussel I., Schrijvers D.M., Lion E., Smits E.L. Poly(I:C) as cancer vaccine adjuvant: knocking on the door of medical breakthroughs. Pharmacol. Ther. 2015;146:120–131. doi: 10.1016/j.pharmthera.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 4.Hafner A.M., Corthesy B., Merkle H.P. Particulate formulations for the delivery of poly(I:C) as vaccine adjuvant. Adv. Drug. Deliv. Rev. 2013;65:1386–1399. doi: 10.1016/j.addr.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Oh M.J., Takami I., Nishizawa T., Kim W.S., Kim C.S., Kim S.R., Park M.A. Field tests of Poly(I:C) immunization with nervous necrosis virus (NNV) in sevenband grouper, Epinephelus septemfasciatus (Thunberg) J. Fish Dis. 2012;35:187–191. doi: 10.1111/j.1365-2761.2011.01334.x. [DOI] [PubMed] [Google Scholar]
- 6.Ruyra A., Torrealba D., Morera D., Tort L., MacKenzie S., Roher N. Zebrafish liver (ZFL) cells are able to mount an anti-viral response after stimulation with Poly (I:C) Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2015;182:55–63. doi: 10.1016/j.cbpb.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Li Q., Wu M., Cui K., Zhu S., Mai K., Ai Q. Characterization of antiviral immune response induced by poly(I:C) in macrophages of farmed large yellow croaker (Larimichthys crocea) Fish Shellfish Immunol. 2020;104:663–672. doi: 10.1016/j.fsi.2020.05.066. [DOI] [PubMed] [Google Scholar]
- 8.Wang X., Wang Q., Xiao J., Liu Q., Wu H., Xu L., Zhang Y. Edwardsiella tarda T6SS component evpP is regulated by esrB and iron, and plays essential roles in the invasion of fish. Fish Shellfish Immunol. 2009;27:469–477. doi: 10.1016/j.fsi.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 9.Wang P., Li J., He T.T., Li N., Mo Z.L., Nie P., Xie H.X. Pathogenic characterization of Aeromonas salmonicida subsp. masoucida turbot isolate from China. J. Fish Dis. 2020;43:1145–1154. doi: 10.1111/jfd.13224. [DOI] [PubMed] [Google Scholar]
- 10.Gao Y., Wu H., Wang Q., Qu J., Liu Q., Xiao J., Zhang Y. A live attenuated combination vaccine evokes effective immune-mediated protection against Edwardsiella tarda and Vibrio anguillarum. Vaccine. 2014;32:5937–5944. doi: 10.1016/j.vaccine.2014.08.074. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y., Wang Q., Yang W., Liu B., Zhang Y. Functional characterization of Edwardsiella tarda twin-arginine translocation system and its potential use as biological containment in live attenuated vaccine of marine fish. Appl. Microbiol. Biotechnol. 2013;97:3545–3557. doi: 10.1007/s00253-012-4462-9. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto F.Y., Sutili F.J., Hume M., Gatlin D.M., 3rd The effect of beta-1,3-glucan derived from Euglena gracilis (AlgamuneTM) on the innate immunological responses of Nile tilapia (Oreochromis niloticus L.) J. Fish Dis. 2018;41:1579–1588. doi: 10.1111/jfd.12871. [DOI] [PubMed] [Google Scholar]
- 13.Waiyamitra P., Zoral M.A., Saengtienchai A., Luengnaruemitchai A., Decamp O., Gorgoglione B., Surachetpong W. Probiotics modulate tilapia resistance and immune response against tilapia lake virus infection. Pathogens. 2020;9:919. doi: 10.3390/pathogens9110919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheikhzadeh N., Mousavi S., Hamidian G., Firouzamandi M., Oushani A.K., Mardani K. Role of dietary Spirulina platensis in improving mucosal immune responses and disease resistance of rainbow trout (Oncorhynchus mykiss) Aquaculture. 2019;510:1–8. [Google Scholar]
- 15.Koch J.F.A., de Oliveira C.A.F., Zanuzzo F.S. Dietary beta-glucan (MacroGard®□) improves innate immune responses and disease resistance in Nile tilapia regardless of the administration period. Fish Shellfish Immunol. 2021;112:56–63. doi: 10.1016/j.fsi.2021.02.014. [DOI] [PubMed] [Google Scholar]
- 16.Cheng S.C., Quintin J., Cramer R.A., Shepardson K.M., Saeed S., Kumar V., Giamarellos-Bourboulis E.J., Martens J.H., Rao N.A., Aghajanirefah A., Manjeri G.R., Li Y., Ifrim D.C., Arts R.J., van der Veer B.M., Deen P.M., Logie C., O’Neill L.A., Willems P., van de Veerdonk F.L., van der Meer J.W., Ng A., Joosten L.A., Wijmenga C., Stunnenberg H.G., Xavier R.J., Netea M.G. mTOR- and HIF-1alpha-mediated aerobic glycolysis as metabolic basis for trained immunity. Science. 2014;345 doi: 10.1126/science.1250684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mousa A.A., Ramachandran R., Ozdemir O., Karsi A., Abdelhamed H. Dietary trans-cinnamaldehyde improves oxidative stress response of channel catfish (Ictalurus punctatus) following Edwardsiella ictaluri infection. Aquaculture. 2021;532 [Google Scholar]
- 18.Liu Z., Wang Y., Sun X. Correlation between bacteria in feed pellets and diseases of cultured turbot Scophthalmus maximus. South China Fish. Sci. 2009;5:13–21. [Google Scholar]
- 19.Somamoto T., Nakanishi T. Mucosal delivery of fish vaccines: local and systemic immunity following mucosal immunisations. Fish Shellfish Immunol. 2020;99:199–207. doi: 10.1016/j.fsi.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Lopez Nadal A., Ikeda-Ohtsubo W., Sipkema D., Peggs D., McGurk C., Forlenza M., Wiegertjes G.F., Brugman S. Feed, microbiota, and gut immunity: using the zebrafish model to understand fish health. Front. Immunol. 2020;11:114. doi: 10.3389/fimmu.2020.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schug H., Yue Y., Krese R., Fischer S., Kortner T.M., Schirmer K. Time- and concentration-dependent expression of immune and barrier genes in the RTgutGC fish intestinal model following immune stimulation. Fish Shellfish Immunol. 2019;88:308–317. doi: 10.1016/j.fsi.2019.02.036. [DOI] [PubMed] [Google Scholar]
- 22.Allaire J.M., Crowley S.M., Law H.T., Chang S.Y., Ko H.J., Vallance B.A. The intestinal epithelium: central coordinator of mucosal immunity. Trends Immunol. 2018;39:677–696. doi: 10.1016/j.it.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Yap Y.A., Marino E. An insight into the intestinal web of mucosal immunity, microbiota, and diet in inflammation. Front. Immunol. 2018;9:2617. doi: 10.3389/fimmu.2018.02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Netea M.G., Quintin J., van der Meer J.W. Trained immunity: a memory for innate host defense. Cell Host Microbe. 2011;9:355–361. doi: 10.1016/j.chom.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 25.Quintin J., Saeed S., Martens J.H.A., Giamarellos-Bourboulis E.J., Ifrim D.C., Logie C., Jacobs L., Jansen T., Kullberg B.J., Wijmenga C., Joosten L.A.B., Xavier R.J., van der Meer J.W.M., Stunnenberg H.G., Netea M.G. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe. 2012;12:223–232. doi: 10.1016/j.chom.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arts R.J.W., Moorlag S., Novakovic B., Li Y., Wang S.Y., Oosting M., Kumar V., Xavier R.J., Wijmenga C., Joosten L.A.B., Reusken C., Benn C.S., Aaby P., Koopmans M.P., Stunnenberg H.G., van Crevel R., Netea M.G. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe. 2018;23:89–100. doi: 10.1016/j.chom.2017.12.010. [DOI] [PubMed] [Google Scholar]