Highlights
-
•
C. irritans can be immobilized by untreated serum but not heat-treated serum from pearl gentian grouper.
-
•
The expression level of PGC3 was increased in infected grouper serum.
-
•
PGC3 covered on the surface of C. irritans trophont.
Keywords: Pearl gentian grouper, Complement C3, Cryptocaryon irritans
Abstract
Pearl gentian grouper is a new aquacultural hybrid resulted from breeding of female tiger grouper (Epinephelus fuscogutattus) and male giant grouper (E. lanceolatus). Our preliminary study found that pearl gentian grouper exhibits less susceptible to the primary infection of Cryptocaryon irritans, which is an important parasitic ciliate in marine aquaculture, indicated that pearl gentian grouper might own a strong innate immune system. Complement system play key roles in innate immunity, whether pearl gentian grouper's complement component contribute for the defensing against the C. irritans infection remain unclear. In the present study, we found that C. irritans can be immobilized by untreated serum but not heat-treated serum from pearl gentian grouper, suggested that the heat-labile components in serum are responsible for the immobilization of C. irritans. Moreover, we cloned and characterized the encoding sequence of pearl gentian grouper complement C3 (PGC3), a key component in complement system. We also found that the expression level of PGC3 was increased in infected grouper serum when compared with that of control grouper. Furthermore, the binding of PGC3 on the surface of C. irritans trophonts located on the grouper skin was detected. These data suggested that pearl gentian grouper's complement system indeed play roles in the immune response against the C. irritans infection.
1. Introduction
Complements are the most important heat-labile innate immune factors, which play multiple roles in both innate and adaptive immunity against infection, participates in an array of physiological and pathological processes, such as immune cell activation, inflammation, immune complex clearance, phagocytosis and microbial killing [1]. To date, there are more than 35 soluble and membrane-bound proteins have been identified as the components of complement system [2, 3]. Complement activation is known to occur through three major pathways: the classical pathway, the alternative pathway and the lectin pathway [4]. The classical pathway is triggered activation by an antigen-antibody complex; the alternative pathway is activated by the certain structures on the surface of microorganisms; and the lectin pathway is initiated by the binding of mannose-binding lectin to carbohydrates on microbial surfaces [5, 6]. Once the complement system is activated, the three activation pathways converge at formation of C3 convertases and cleavage of C3, resulting in C3a and C3b fragment. Afterward, the C3a is involved in inflammatory responses, while the C3b acts as either a potent effective or as a recruiter for other proteins [7]. These protein fragments ultimately are mediated by lytic pathway to form perforin-like proteins or membrane attack complexes (MAC), which can directly lyse microbial cells [6].
C3 is the most abundant complement protein in the blood and plays a central role in complement activation which is first discovered in 1912, and then its function has been extensively studied in mammals and other higher vertebrate species [8, 9], considerably less in lower vertebrates, including teleosts. Although the homologous genes of C3 have been identified from many teleost species, such as Cyprinus carpio [10], Danio rerio [11], E. coioides [12], Labeo rohita [13], Oncorhynchus mykiss [14], Salmo salar [15], to our knowledge, the roles of C3 factor during disease responses have not yet been studied thoroughly, in particular the role in defensing against parasite infection.
Cryptocaryon irritans is a pathogenic ectoparasitic ciliate that invades the epithelium of marine fish skin, gills, fins and even eyes, causing cryptocaryoniasis or “white spot disease” [16, 17]. It is reported that the parasite with low degree of host specificity, which can infect an array of marine teleosts in tropical and subtropical areas [16, 18]. However, variability in the degree of susceptibility to C. irritans was found among different fish species with several studies [19, 20]. Hybrid pearl gentian groupers is a new aquacultural species resulted from breeding of female tiger grouper (E. fuscogutattus) and male giant grouper (E. lanceolatus). Our previous study had found that pearl gentian grouper exhibits less susceptible to C. irritans infection and the mRNA expression level of C3 molecular was significant upregulation post the infection [21]. However, whether pearl gentian grouper's complement system contributing for the defense of C. irritans infection remains unknow. In this study, the serum immobilization titer of infected pearl gentian grouper to C. irritans theronts was higher than that of control grouper. Nevertheless, after treatment at 56 °C, the serum of both species lost their immobilization activity, implying that the anti-parasitic components were labile to heat. To further assess whether C3 is involved in host defenses against C. irritans infection, a C3 cDNA sequence was identified in pearl gentian grouper and its expression pattern as well as immunohistochemistry of C3 were analyzed in infected fish. These results contribute to broaden the knowledge of interactions between C3 and C. irritans, which will eventually help in the development of novel intervention strategies for farming fish.
2. Materials and methods
2.1. C. irritans challenge and sampling
Healthy pearl gentian groupers (54.6 ± 4.8 g) and golden pompano Trachinotus ovatus (255 ± 18.5 g), were purchased from local farm in Dapeng Bay, Guangdong, China, and maintained at 28 °C in a flow-through water system (300 L) as previously described [22]. C. irritans were originally isolated from an infected golden pompano obtained from a local farm in Daya Bay, Guangdong Province, China, and cultured using T. ovatus as hosts, as described previously [23]. Briefly, tomonts were harvested from a collective unit and incubated in seawater for 3 d at 28 °C. Theronts collected within 2 h of excystment were re-infected to T. ovatus (10,000 theronts per fish) for 2 h. Then, T. ovatus were transferred into the collection unit for the next cycle of propagation. Both groupers and pompanos were acclimated at least for two weeks and feeding with commercial feed twice every day.
The challenged procedure was performed as previously described [24]. Briefly, thirty groupers were challenged with C. irritans theronts at a dose of 20,000 theronts per fish (∼370 theronts per gram) for 3 h. Meanwhile, the uninfected fish were also transferred. At 3 day post the infection (dpi), the control and infected groupers were anesthetized with MS-222, cutaneous mucus and serum samples were collected and stored at −20 °C. Skin near the dorsal fin was sampled and fixed in 4% paraformaldehyde at room temperature. Liver tissue for RNA extraction was collected and immediately frozen in liquid nitrogen and stored at −80 °C.
2.2. Immobilization assay
The immobilization assay was performed as described in [25]. Briefly, fifty μL mucus or serum from pearl gentian grouper were serial two-fold diluted with seawater in a 96-well plate. Approximately 500 theronts (in 50 μL volume) were added into each well and incubated for 30 min at room temperature. The last well showing 50% of theronts were immobilized considered as endpoint titer.
2.3. Cloning of pearl gentian grouper C3 (PGC3) gene sequences
Total RNA from all pearl gentian grouper's liver tissue was extracted using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA) according to manufacturer's instructions. First Strand cDNA was synthesized from total RNA from liver tissue using the ReverTra Ace-a-Kit (Toyobo, Katata, Ohtsu, Japan) with oligo dT as primer. An expressed sequence tag (EST) sequences of C3 was identified from a pearl gentian grouper transcriptome data (SRR11912784, SRR11912785) by BLASTx program. The open reading frame (ORF) of pearl gentian grouper C3 was amplified using the following primers C3 ORF F: 5′CCTGTGGCCACAGTTCACTGAAGCTCC 3′, and C3 ORF R: 5′ ACTCAATCTTTGTGTGCCCACTAATT 3′. The amplification protocol was performed as follows: (98 °C, 10 s; 58 °C, 15 s; 72 °C, 2 min) × 35 cycles, and 72 °C, 5 min for one cycle. The amplification products were purified and ligated to pEASY-Blunt Cloning Vector (TRANS, Beijing, China) for sequencing.
2.4. Protein structure and phylogenetic analysis
The theoretical pI (isoelectric point) and Mw (molecular weight) of PGC3 were predicted using Compute pI/Mw tool (http://web.expasy.org/compute_pi/). The conserved domains of PGC3 were predicted in CDD server (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) and SMART program (http://smart.embl-heidelberg.de/). The 3D model of PGC3 was built by using SWISS-MODEL server (https://swissmodel.expasy.org/interactive). Phylogenic analysis of PGC3 was performed using MEGA 5.04 program and illustrated by iTol server (https://itol.embl.de/itol.cgi). Silhouette images of fish provided from PhyloPic (http://www.phylopic.org).
2.5. Western blotting
The reduced serum samples were mixed with SDS sample buffer by boiling in the presence of β-mercaptoethanol for 10 min, and the non-reduced samples were incubated with SDS sample buffer without β-mercaptoethanol at room temperature for 30 min. Samples of theront Equal amounts of the protein samples were electrophoresed in a 6% SDS-PAGE gel and transferred to a polyvinylidene fluoride (PVDF) membrane. The PVDF membranes were blocked in 5% dried milk (dilute in PBST) for 1 hour at room temperature, the membranes were washed with PBST for three times and then incubate with anti-grouper C3 polyclone antibody (prepared by immunized rabbit with rC3α from orange-spotted grouper, 1 μg/ml, dilute in PBST contain 5% BSA) overnight at 4 °C, followed by incubation with HRP-conjugated anti-rabbit IgG (1 μg/ml, dilute in PBST contain 5% BSA) for 1 hour at room temperature. After washing with PBST, the membranes were incubated with the SuperSignal West Pico Chemiluminescent Substrate and then exposed and analyzed using Tanon 5200 chemiluminescence imaging analysis system (Tanon).
2.6. Histochemistry and immunohistochemistry (IHC) staining
The fixed skin samples were washed with tap water, dehydrated with gradient ethanol, washed with xylene, and infiltrated with wax. After sectioning by a microtome at 7 um, sections were dewaxed and rehydrated. For histochemistry, the sections were stained with hematoxylin and eosin (HE). For IHC, antigen retrieval was performed by treated with 0.5% trypsin at 37 °C for 20 min. Peroxides were inactivated by incubation with 3% H2O2 (diluted in methyl alcohol) for 15 min. The sections were blocked with 10% goat serum at 37 °C for 1 h, and incubate with anti-grouper C3 polyclone antibody (1 μg/ml, dilute in PBST contain 2.5% BSA and 5% goat serum), anti-C. irritans polyclone antibody (1 μg/ml), or pre-bleed rabbit serum (1:1000 dilution) overnight at 4 °C. The sections were washed with PBST for three times and then with HRP-conjugated anti-mouse or anti-rabbit IgG (1 μg/ml) and the peroxide activity was developed using an enhenced DAB kit. The nucleus was stained with hematoxylin and mounted in neutral resins. All slides were photographed under a NIH-Elements System (Nikon).
3. Results and discussion
3.1. Immobilization of serum from control or infected pearl gentian grouper
A large amount of immune-related proteins, such as antibody, lysozyme, complement, peroxide, alkaline phosphatase and esterase, were contained in the fish mucus and blood, which had an extremely strong antimicrobial effect [26, 27]. Immobilization assay is the major indicator used to assess the efficacy of anti-C. irritans substances in mucus or serum [28]. In this study, we initially assessed the immobilization of control and infected pearl gentian grouper mucus and serum with or without inactive treatment (Fig. 1). The results showed that all mucus samples did not show any immobilization of C. irritans (data not shown). However, the immobilization titers (∼3.3) were detected in control grouper serum, and exhibited a strengthening trend in the infected grouper (∼6.0) (P > 0.05). It should be noted that both control and infected serum loss the immobilization ability after bathed at 56 °C for 30 min. These results exclude the possibility of antibodies and imply that the anti-parasitic components were labile to heat. These results reflect those of Yin et al. who also found that the heat-labile complement components of spotted maigre (Nibea albiflora) take part in killing C. irritans by the complement activity inhibition assays [20]. Moreover, our previous research the complement components were significantly upregulated in infected pearl gentian grouper skin than the uninfected grouper by a transcriptome analysis [21]. In addition, complement components are significantly upregulated post infecting C. irritans also found other fish, such as sea bass (Lates calcarifer) [29], orange-spotted grouper [30], false kelpfish (Sebastiscus marmoratus) [31], large yellow croaker (Larimichthys crocea) [32], rabbitfish (Siganus oramin) [33]. These experiments supported that the grouper's components might play a major role in against C. irritans infection.
Fig. 1.
Immobilization of serum from control or infected pearl gentian grouper. Different letter represents significantly different (p <0.05).
3.2. Characteristics of pearl gentian grouper C3 and phylogenetic analysis
C3 is the central protein to form perforin-like proteins or membrane attack complexes [1], which may be the key complement component of grouper against C. irritans infection. In this study, complement component 3 from pearl gentian grouper (PGC3) was cloned. Similar gene structure of PGC3 was identified in pearl gentian grouper's parents (E. fuscogutatus♀, E. lanceolatus♂), whose both contain 44 exons (Fig. 2a). The open reading frame (ORF) of PGC3 (GenBank no. MZ802969) was 5388 bp, which encoded sequences of 1795 amino acids with a theoretical pI of 6.44 and molecular mass of 200.59 kDa (Fig. S1). The deduced amino acid sequence analysis revealed that the conserved motifs of C3 found in higher vetebrates also identified in PGC3, including a signal peptide, four Macroglobulin domains (MGs), an Alpha-2-macroglobulin family N-terminal region, an Anaphylatoxin homologous domain, an Alpha-2-macroglobulin family domain, an A-macroglobulin receptor domain, and an NTR domain (Fig. 2b). In addition, five potential N-glycosylation sites and a conserved region of proteinase-binding alpha-macroglobulin (Thiol-ester-cl) were also predicted in PGC3 sequence. PGC3 shared above 97% amino acid identity with its parent's C3 (predicted from their released genome sequence). Homology analysis showed that PGC3 had 38%–79% amino acid identity across vertebrates’ C3 beside groupers, and shares the highest amino acid identity (79%) with Perca fluviatilis C3 (Tab. S1). The 3D homology modeling of PGC3 presents a similar structure with human C3 molecular which maturation protein contains α and β chain and linked by disulfide bond (Sequence identity is 38.27%, GMQE value is 0.57, QMEANDisCo value is 0.6 ± 0.05) (Fig. 2c). These conserved motifs suggested that the PGC3 played the basically physiological functions of C3.
Fig. 2.
Gene and protein structure of pearl gentian grouper C3.
(A) Genomic structure of C3 gene in E. fuscogutattus and E. lanceolatus.
(B) Conserved domain predicted in PGC3.
(C) The 3D homology model of PGC3 was performed using the human C3 model as a template.
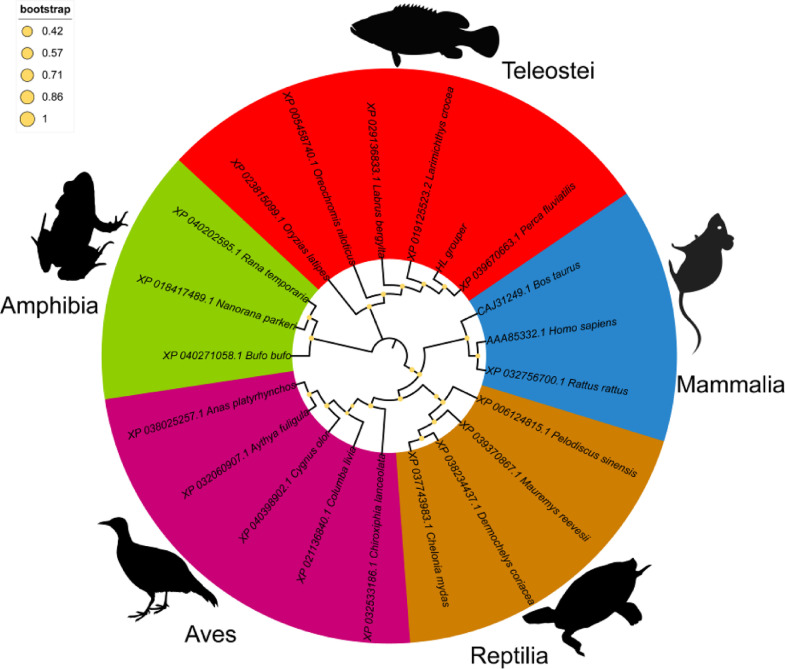
A phylogenetic tree was constructed to demonstrate the evolutional status of PGC3 based on amino acid sequences by phylogenetic analysis. As shown in Fig. 3, five clades are formed in the phylogenetic tree, which represent mammalia C3, aves C3, reptilia C3, amphibia C3, and teleostei C3. Consistent with traditional taxonomy and phylogeny, the PGC3 firstly clustered with the C3 of P. fluviatilis, following grouped with other teleostei C3, such as L.crocea, Labrus bergylta, Oreochromis niloticus, Oryzias latipes into one sole clade.
Fig. 3.
Phylogenetic tree of vertebrated C3.
A phylogenetic tree illustrating the relationship across vertebrate C3 molecules using the neighbor-joining method within the MEGA program.
3.3. Detection of PGC3 in pearl gentian grouper
To detected the protein level of C3 in pearl gentian grouper, we used anti-orange-spotted grouper C3 polyantibody (prepared by our laboratory) for the following immune blot and IHC analysis. In mammals, maturation C3 molecular is a glycoprotein and contains α and β chain which linked by interchain disulfide bonds [6]. By using this anti-grouper C3 polyclone antibody, an ∼240 kDa band was easily detected from pearl gentian grouper serum sample under non-reduced condition, which represents the molecular weight of C3α and C3β chain (Fig. 4). In addition, as the anti-grouper C3 polyclone antibody was generated by immunized the C3α chain from orange-spotted grouper, this antibody could well recognize an ∼122 kDa band from reduced pearl gentian grouper serum sample, which represents the molecular weight of C3α chain alone (Fig. 4). These data confirmed that the anti-orange-spotted grouper C3 can well recognize the PGC3, which also indicated that, similar to its mammals homolog, maturation PGC3 molecular also exhibits a consered interchain disulfide bonding structure.
Fig. 4.
Detection of pearl gentian grouper C3 under reducing or non-reducing condition.
M: protein marker, Lane 1: non-reducing grouper serum sample. Lane 2: reducing grouper serum sample.
3.4. Increment of PGC3 in infected pearl gentian grouper
Previous studies had determined that an increment of orange-spotted grouper C3 in transcriptional level post C. irritans infection [30]. Our latest result also confirmed that an up-regulation of C3 transcript in C. irritans infected skin tissue from pearl gentian grouper (data in publishing). In this study, western blotting showed an enhance level of PGC3 protein in infected serum when compared with those in control pearl gentian grouper (Fig. 5), which corroborate the findings of the previous that an up-regulation of PGC3 transcript post C. irritans infection. However, we detected the protein level of C3 in pearl gentian grouper cutaneous mucus, but there is no signal from both the control or infected cutaneous mucus samples (data not shown). Results suggested that PGC3 is not the main immune factor expressed in the skin mucus, but is essentially and closely related to the immune response in blood of grouper post C. irritans infection.
Fig. 5.
The level of C3 in control or infected pearl gentian grouper.
(A) Immunoblot and (B) densitometric analysis of C3 in serum from control and infected grouper at 3 dpi (n = 3).
Under normal physiological conditions, most of the complement proteins are synthesised as inactive precursors, and can sequentially cleaved and activated upon activation by certain foreign substances (i.e., viruses, bacteria, fungi, parasites) [34]. They activation is known to occur three pathways: the classical complement activation pathway, the alternative complement pathway and the lectin complement pathway, and all the pathways will converge at C3, resulting in the formation of the activation products [4, 35]. In this study, serum loss the immobilization ability after the complement inactivation treatment, suggested that not formed antigen-antibody complex. Previous study shown that C. irritans cells can cause up regulation of alternative complement activity in orange-spotted grouper blood [36]. Most of up-regulated complement genes were also found in alternative pathways in orange-spotted grouper and large yellow croaker post C. irritans infection [30, 32]. The alternative complement pathway could be triggered by carbohydrates, lipids and proteins on the surface of microorganisms [6]. Hence, the PGC3 may be activated by the alternative complement pathway in the first C. irritans infection.
3.5. PGC3 covered on the surface of C. irritans trophont
The alternative complement pathway is activated directly by viruses, bacteria, parasites or fungi and is independent of antibody [6]. Once the pathway is activated, the C3 convertase and cleavage by a series of proteins (Factor B, D, properdin), then formation of the activation products, C3a, C3b, C5a and the membrane attack complex (C5b-9) [4, 6]. To further determine whether C. irritans trophont could recruit the host C3 or its activation products to the parasite surface, IHC and HE stain of infected pearl gentian grouper skin was carried out (Fig. 6). HE stained of the infected grouper skin clearly showed that the large C. irritans trophonts (∼200 μm in size) parasitize between the layer of epidermis and melanophores. More importantly, abundant C3 positive signal was detected on the trophonts surface which suggested that PGC3 was activated and able to cover over the parasite during the infection. An anti-C. irritans polyclone antibody and pre-bleed rabbit serum were also used for the detection of C. irritans and considered as positive and negative control. In view of the above immobilization result, these finding suggested that pearl gentian grouper complement system is involved in defensing against C. irritans infection.
Fig. 6.
Detection of pearl gentian grouper C3 coating on the surface of C. irritans.
IHC of infected grouper skin using anti-grouper C3, anti-C. irritans, or pre-bleed rabbit serum. Positive signal results in brown. HE stain of infected grouper skin section. Ep: Epidermis, Sc: Scales, Me: Melanophores, N: Nuclei. Scale bar is 25 μm.
4. Conclusion
In this study, we found that heat-treated serum from pearl gentian grouper loss the immobilization of C. irritans, and the expression level of PGC3 increases in infected grouper serum as well as the C. irritans is covered by PGC3 in the skin tissue. These findings suggested that the complement system from pearl gentian grouper play roles in the defensing against C. irritans infection.
Author contributions
Conceptualization: Y.L., X.D., B.J., and Z.M.; Investigation: Z.M., Xueli Lai, H.W.; Methodology: Z.M., and H.W.; Project administration: X.D., Xiaochun Luo, and Y.L.; Resources: A.L.; Supervision: X.D. and Xiaochun Luo; Visualization: Z.M.; Writing - original draft: Z.M., B.J., and Y.L.; Writing - review & editing: X.D., Y.L., and Z.M. All authors read and approved the final manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (U20A20102, 42006100, 41876162), the Guangdong Basic and Applied Basic Research Foundation (2019A1515110424), the China Postdoctoral Science Foundation (2019M662938), and the Guangdong Provincial Special Fund for Modern Agriculture Industry Technology Innovation Teams (2019KJ141), China Modern Agricultural Industry Technology System (The Control of Parasites Infection on Marine Fish, CARS-47-18).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.fsirep.2021.100032.
Contributor Information
Xueming Dan, Email: dxm72@scau.edu.cn.
Yanwei Li, Email: yanweili@scau.edu.cn.
Appendix. Supplementary materials
References
- 1.Holland M.C.H., Lambris J.D. The complement system in teleosts. Fish Shellfish Immunol. 2002;12(5):399–420. doi: 10.1006/fsim.2001.0408. [DOI] [PubMed] [Google Scholar]
- 2.Sunyer J.O., Boshra H., Lorenzo G., Parra D., Freedman B., Bosch N. Evolution of complement as an effector system in innate and adaptive immunity. Immunol. Res. 2003;27(2–3):549–564. doi: 10.1385/IR:27:2-3:549. [DOI] [PubMed] [Google Scholar]
- 3.Wang L.L., Zhang H., Wang L.L., Zhang D.X., Lv Z., Liu Z.Q., Wang W.L., Zhou Z., Qiu L.M., Wang H., Li J., Song L.S. The RNA-seq analysis suggests a potential multi-component complement system in oyster Crassostrea gigas. Dev. Comp. Immunol. 2017;76:209–219. doi: 10.1016/j.dci.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Dunkelberger J.R., Song W.C. Complement and its role in innate and adaptive immune responses. Cell Res. 2010;20(1):34–50. doi: 10.1038/cr.2009.139. [DOI] [PubMed] [Google Scholar]
- 5.Qu H., Ricklin D., Lambris J.D. Recent developments in low molecular weight complement inhibitors. Mol. Immunol. 2009;47(2–3):185–195. doi: 10.1016/j.molimm.2009.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarma J.V., Ward P.A. The complement system. Cell Tissue Res. 2011;343(1):227–235. doi: 10.1007/s00441-010-1034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dishaw L.J., Smith S.L., Bigger C.H. Characterization of a C3-like cDNA in a coral: phylogenetic implications. Immunogenetics. 2005;57(7):535–548. doi: 10.1007/s00251-005-0005-1. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y.X., Xu K.D., Li J.J., Wang X., Ye Y.Y., Qi P.Z. Molecular characterization of complement component 3 (C3) in Mytilus coruscus improves our understanding of bivalve complement system. Fish Shellfish Immunol. 2018;76:41–47. doi: 10.1016/j.fsi.2018.02.044. [DOI] [PubMed] [Google Scholar]
- 9.Zhou Z.C., Sun D.P., Yang A.F., Dong Y., Chen Z., Wang X.Y., Guan X.Y., Jiang B., Wang B. Molecular characterization and expression analysis of a complement component 3 in the sea cucumber (Apostichopus japonicus) Fish Shellfish Immunol. 2011;31(4):540–547. doi: 10.1016/j.fsi.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 10.Nakao M., Mutsuro J., Obo R., Fujiki K., Nonaka M., Yano T. Molecular cloning and protein analysis of divergent forms of the complement component C3 from a bony fish, the common carp (Cyprinus carpio): presence of variants lacking the catalytic histidine. Eur. J. Immunol. 2000;30(3):858–866. doi: 10.1002/1521-4141(200003)30:3<858::AID-IMMU858>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 11.Wang Z.P., Zhang S.C., Wang G.F. Response of complement expression to challenge with lipopolysaccharide in embryos/larvae of zebrafish Danio rerio: acquisition of immunocompetent complement. Fish Shellfish Immunol. 2008;25(3):264–270. doi: 10.1016/j.fsi.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Qi Z.H., Liu Y.F., Wang W.N., Wu X., Xin Y., Lu Y.F., Wang A.L. Molecular characterization and functional analysis of a complement C3 molecule in the orange-spotted grouper (Epinephelus coioides) Fish Shellfish Immunol. 2011;31(6):1284–1290. doi: 10.1016/j.fsi.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 13.Mishra J., Sahoo P.K., Mohanty B.R., Das A. Sequence information, ontogeny and tissue-specific expression of complement component C3 in Indian major carp, Labeo rohita (Hamilton) Indian J. Exp. Biol. 2009;47(8):672–678. [PubMed] [Google Scholar]
- 14.Lovoll M., Kilvik T., Boshra H., Bogwald J., Sunyer J.O., Dalmo R.A. Maternal transfer of complement components C3-1, C3-3, C3-4, C4, C5, C7, Bf, and Df to offspring in rainbow trout (Oncorhynchus mykiss) Immunogenetics. 2006;58(2–3):168–179. doi: 10.1007/s00251-006-0096-3. [DOI] [PubMed] [Google Scholar]
- 15.Lovoll M., Johnsen H., Boshra H., Bogwald J., Sunyer J.O., Dalmo R.A. The ontogeny and extrahepatic expression of complement factor C3 in Atlantic salmon (Salmo salar) Fish Shellfish Immunol. 2007;23(3):542–552. doi: 10.1016/j.fsi.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Colorni A., Burgess P. Cryptocaryon irritans Brown 1951, the cause of ‘white spot disease’ in marine fish: an update. Aquarium Sci. Conserv. 1997;1(4):217–238. [Google Scholar]
- 17.Li Y.W., Jiang B.A., Mo Z.Q., Li A.X., Dan X.M. Cryptocaryon irritans (Brown, 1951) is a serious threat to aquaculture of marine fish. Rev. Aquacult. 2021 [Google Scholar]
- 18.Luo X.C., Xie M.Q., Zhu X.Q., Li A.X. Some characteristics of host-parasite relationship for Cryptocaryon irritans isolated from South China. Parasitol. Res. 2008;102(6):1269–1275. doi: 10.1007/s00436-008-0904-9. [DOI] [PubMed] [Google Scholar]
- 19.Wang F.H., Li R.J., Xie M.Q., Li A.X. The serum of rabbitfish (Siganus oramin) has antimicrobial activity to some pathogenic organisms and a novel serum l-amino acid oxidase is isolated. Fish Shellfish Immunol. 2011;30(4–5):1095–1108. doi: 10.1016/j.fsi.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Yin F., Liu W.C., Bao P.B., Jin S., Qian D., Wang J.T., Tang B.J. Comparison of the susceptibility and resistance of four marine perciform fishes to Cryptocaryon irritans infection. Fish Shellfish Immunol. 2018;77:298–303. doi: 10.1016/j.fsi.2018.03.052. [DOI] [PubMed] [Google Scholar]
- 21.Mo Z.-.Q., Wu H.-.C., Hu Y.-.T., Lu Z.-.J., Lai X.-.L., Chen H.-.P., He Z.-.C., Luo X.-.C., Li Y.-.W., Dan X.-.M. Transcriptomic analysis reveals innate immune mechanisms of an underlying parasite-resistant grouper hybrid (Epinephelus fuscogutatus × Epinephelus lanceolatus) Fish Shellfish Immunol. 2021;119:67–75. doi: 10.1016/j.fsi.2021.09.041. [DOI] [PubMed] [Google Scholar]
- 22.Mo Z.Q., Wang J.L., Yang M., Ni L.Y., Wang H.Q., Lao G.F., Li Y.W., Li A.X., Luo X.C., Dan X.M. Characterization and expression analysis of grouper (Epinephelus coioides) co-stimulatory molecules CD83 and CD80/86 post Cryptocaryon irritans infection. Fish Shellfish Immunol. 2017;67:467–474. doi: 10.1016/j.fsi.2017.05.064. [DOI] [PubMed] [Google Scholar]
- 23.Dan X.M., Li A.X., Lin X.T., Teng N., Zhu X.Q. A standardized method to propagate Cryptocaryon irritans on a susceptible host pompano Trachinotus ovatus. Aquaculture. 2006;258(1–4):127–133. [Google Scholar]
- 24.Mo Z.Q., Han Q., Zeng Y.L., Wang J.L., Li X.Z., Li Y.W., Sun H.Y., Li A.X., Luo X.C., Dan X.M. Molecular characterization and function analysis of grouper (Epinephelus coioides) Bruton’s tyrosine kinase BTK. Fish Shellfish Immunol. 2018;77:91–99. doi: 10.1016/j.fsi.2018.03.039. [DOI] [PubMed] [Google Scholar]
- 25.Mo Z.Q., Xu S., Cassidy-Hanley D.M., Li Y.W., Kolbin D., Fricke J.M., Li A.X., Clark T.G., Dan X.M. Characterization and immune regulation role of an immobilization antigen from Cryptocaryon irritans on groupers. Sci. Rep. 2019;9(1):1029. doi: 10.1038/s41598-018-25710-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cordero H., Cuesta A., Meseguer J., Esteban M.A. Changes in the levels of humoral immune activities after storage of gilthead seabream (Sparus aurata) skin mucus. Fish Shellfish Immunol. 2016;58:500–507. doi: 10.1016/j.fsi.2016.09.059. [DOI] [PubMed] [Google Scholar]
- 27.Guardiola F.A., Cuesta A., Abellan E., Meseguer J., Esteban M.A. Comparative analysis of the humoral immunity of skin mucus from several marine teleost fish. Fish Shellfish Immunol. 2014;40(1):24–31. doi: 10.1016/j.fsi.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 28.Luo X.C., Xie M.Q., Zhu X.Q., Li A.X. Protective immunity in grouper (Epinephelus coioides) following exposure to or injection with Cryptocaryon irritans. Fish Shellfish Immunol. 2007;22(4):427–432. doi: 10.1016/j.fsi.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 29.Khoo C.K., Abdul-Murad A.M., Kua B.C., Mohd-Adnan A. Cryptocaryon irritans infection induces the acute phase response in Lates calcarifer: a transcriptomic perspective. Fish Shellfish Immunol. 2012;33(4):788–794. doi: 10.1016/j.fsi.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Hu Y., Li A., Xu Y., Jiang B., Lu G., Luo X. Transcriptomic variation of locally-infected skin of Epinephelus coioides reveals the mucosal immune mechanism against Cryptocaryon irritans. Fish Shellfish Immunol. 2017;66:398–410. doi: 10.1016/j.fsi.2017.05.042. [DOI] [PubMed] [Google Scholar]
- 31.Yin F., Qian D. Transcriptomic analysis reveals the key immune-related signalling pathways of Sebastiscus marmoratus in response to infection with the parasitic ciliate Cryptocaryon irritans. Parasit. Vectors. 2017:10. doi: 10.1186/s13071-017-2508-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yin F., Gao Q.X., Tang B.J., Sun P., Han K.H., Huang W.Q. Transcriptome and analysis on the complement and coagulation cascades pathway of large yellow croaker (Larimichthys crocea) to ciliate ectoparasite Cryptocaryon irritans infection. Fish Shellfish Immunol. 2016;50:127–141. doi: 10.1016/j.fsi.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 33.Jiang B., Du J.J., Li Y.W., Ma P., Hu Y.Z., Li A.X. Transcriptome analysis provides insights into molecular immune mechanisms of rabbitfish, Siganus oramin against Cryptocaryon irritans infection. Fish Shellfish Immunol. 2019;88:111–116. doi: 10.1016/j.fsi.2019.02.039. [DOI] [PubMed] [Google Scholar]
- 34.Boshra H., Li J., Sunyer J.O. Recent advances on the complement system of teleost fish. Fish Shellfish Immunol. 2006;20(2):239–262. doi: 10.1016/j.fsi.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 35.Flierl M.A., Rittirsch D., Nadeau B.A., Day D.E., Zetoune F.S., Sarma J.V., Huber-Lang M.S., Ward P.A. Functions of the complement components C3 and C5 during sepsis. FASEB J. 2008;22(10):3483–3490. doi: 10.1096/fj.08-110595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dan X.M., Zhang T.W., Li Y.W., Li A.X. Immune responses and immune-related gene expression profile in orange-spotted grouper after immunization with Cryptocaryon irritans vaccine. Fish Shellfish Immunol. 2013;34(3):885–891. doi: 10.1016/j.fsi.2012.12.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.