Highlights
-
•
β-glucan fed sturgeon were not protected against columnaris.
-
•
Greatest mortality occurred in glucan fed treatment infected with F. columnare.
-
•
Mortality associated with higher acute phase protein transcripts in gills.
Keywords: β-glucan, Flavobacterium columnare, White sturgeon
Abstract
Flavobacterium columnare represent one of the most important bacterial pathogens of cultured sturgeon. However, at present there are no commercially available vaccines to prevent infection and treatment options are limited. β-glucans have been shown to be potent immunostimulants that can provide fish protection against infectious disease. In this study, the effects of dietary β-glucan supplementation on disease susceptibility were examined by exposing 0.3% β-glucan-fed white sturgeon (Acipenser transmontanus) to Flavobacterium columnare in laboratory-controlled challenges. Morbidity and mortality were monitored for 15 days post-challenge (dpc). Additionally, transcript levels for pro-inflammatory cytokines, regulatory cytokines and acute phase proteins (APP) were investigated in the spleen and gills at different time points post-challenge. No evidence of protection was observed in β-glucan-fed fish challenged with the bacteria. Moreover, significantly greater mortalities were observed in β-glucan-fed fish challenged with F. columnare (p<0.05), likely associated with acute inflammatory response as haptoglobin and serotransferrin transcripts in the gills were significantly higher in fish within this group at 1 dpc. Transcript levels for all tested cytokines and APP in the spleen were similar amongst treatment groups. The results from this study suggest that β-glucan supplementation at the concentration and rate investigated provides no-benefit to white sturgeon against F. columnare.
Abbreviations
- dpc
days post-challenge
- Fc
F. columnare
- FDA
U.S. Food and Drug Administration
- USDA
United States Department of Agriculture
- MSA
modified-Shieh agar
- PBS
phosphate buffered saline
- FCGM
F. columnare growth medium
- MS-222
buffered tricaine methanesulfonate
- SAA
serum amyloid A
- TNF-α
tumor necrosis factor alpha
- TGF-β
transforming growth factor beta
- IL-17
interleukin 17 predicted protein
- IRF8
interferon regulatory factor 8
- MHCII
major histocompatibility class II
- PCR
polymerase chain reaction
- RT-qPCR
reverse transcription quantitative real-time PCR
1. Introduction
Sturgeon (Acipenser spp.) aquaculture has become a rapidly expanding industry mainly focused on restocking fry and fingerlings into natural reservoirs and commercialization of caviar and meat for human consumption [1,2]. The main cultured species are white sturgeon (Acipenser transmontanus), Siberian sturgeon (Acipenser baerii), Adriatic sturgeon (Acipenser naccarii) and Russian sturgeon (A. gueldenstaedtii) [3]. Along with reducing pressure on wild-caught fisheries, sturgeon culture is a multi-million dollar industry generating considerable producer revenue and employment opportunities [1], [2], [3]. However, with the rapid expansion of intensive sturgeon culture, infectious diseases have also emerged, causing significant challenges to the sturgeon industry, typically resulting in high morbidity and mortality and serious economic losses [3], [4], [5].
Flavobacterium columnare is a filamentous Gram-negative bacterium responsible for significant economic losses in several cultured freshwater fish including white sturgeon [6], rainbow trout Oncorhynchus mykiss [7], Atlantic salmon Salmo salar L. and coho salmon O. kisutch [8], tilapia (Oreochromis spp) [9], and channel catfish Ictalurus punctatus [10]. Flavobacterium columnare strains have been assigned into four different genetic groups (GG) likely representing distinct bacterial species with some fish-host association [11]. Recently, LaFrentz, et al. [12] identified that these four GG of F. columnare represent four different species with the names F. columnare, F. covae sp. nov., F. davisii sp. nov., and F. oreochromis sp. nov., representing genetic groups 1, 2, 3, and 4, respectively. Columnaris disease has been described as both a primary and secondary infection [13]. Generally, fry and fingerling are most sensitive to columnaris infection and clinical signs include frayed fins or fin rot, necrotic lesions on the mouth and gills and depigmented ‘saddleback’ lesions on the skin [14].
Currently, few effective vaccines and antibiotics are available to combat columnaris in cultured fish. Additionally, development of antimicrobial resistant bacterial strains and the high costs and low efficacy that have been associated with immunization and treatment highlight the need for other prophylactic tools against these infectious diseases [15,16].
Immunostimulants are emerging as an alternative tool to antibiotics and vaccines to combat infectious disease in aquaculture [17]. This includes β-glucans, which are high molecular-weight polysaccharides extracted from D-glucose building blocks that are common components of the cell walls of bacteria, fungi, algae, and plants [18]. As one of the most well-studied immunostimulants, β-glucans are broadly used to improve the health of both domesticated animals and humans [19]. Various studies in aquaculture models have confirmed β-glucans as a potent immunostimulant to enhance the immune defense system of fish and to reduce the spread of infectious diseases [20]. Specifically, β-glucans have been shown to enhance both humoral and cellular immunity inducing short-lived and long-lived effects on the fish immune response resulting in protection against several infectious diseases [21]. Additionally, it has been reported that administration of β-glucans can result in stress-reduction [20], growth enhancement [22] and anti-toxin effects [23] to additionally support food fish production. In sturgeon, feed supplementation with β-glucans have been shown to help prevent losses due to the fungus Veronaea botryosa [24,25].
Few studies have investigated the immune responses of sturgeon to bacterial pathogens or characterized the effects of immunostimulants on systemic response to disease. To date, no study has investigated the effects of immunostimulants on F. columnare infections in white sturgeon. Therefore, the objectives of this study were to evaluate the protections conferred to white sturgeon fed β-glucans and challenged with F. columnare and to examine cytokine and acute-phase protein transcript expression in the gills and spleen of white sturgeon after β-glucan immunostimulation in feed.
2. Materials and methods
2.1. Fish and husbandry conditions
A total of 250 white sturgeon fingerling (53.2 ± 15.5 g; 21.6 ± 2.2 cm) (mean ± SD) were obtained from a local producer in Northern California, USA and acclimated to laboratory conditions for two weeks. Ten fish were subjected to clinical examination and bacterial culture was performed on the posterior kidney prior to beginning the experiment to ascertain that the population was non-infected before challenge. Fish (n = 240/tank) were maintained in an outdoor, enclosed, 1000-L freshwater tanks with flow-through, 18 ± 1 °C well water. Fish were maintained in this tank for 30 days and provided a “β-glucan feed” or “normal feed” regimen as described below. After this period, fish were transferred to 130-liter tanks (20 fish/tank, three replicatetanks per treatment) with fresh, flow-through well water at a rate of four liters per minute at 18 ± 2.0 °C.
2.2. Diets
Fish were fed a basal commercial feed (Skretting, Tooele, UT, USA) at 1% body weight per day. The β-glucan diet included the basal feed supplemented with 0.3% β-glucan (3 g of β-glucan per 1 kg basal diet) derived from the yeast Saccharomyces cerevisiae (MacroGard®, Biorigin, Sao Paulo, Brazil) along with vegetable oil (10 ml/kg food) mixed for 10 min manually. The non β-glucans diet was prepared by mixing the feed with vegetable oil only [24]. The pellets were dried overnight at room temperature and stored at 4 °C until used. All diets were prepared weekly.
2.3. Bacteria
The F. columnare isolate was cultured from an outbreak of columnaris in Lahontan cutthroat trout Oncorhynchus clarkii henshawi in California in 2018 and identified as F. columnare by de Alexandre Sebastião, et al. [6]. Flavobacterium columnare was grown on modified-Shieh agar (MSA) [26] supplemented with tobramycin (1 μg/ml) [27], and incubated at 28 °C for 48 h.
2.4. Laboratory controlled challenges
All challenges were conducted under protocols approved by the University of California, Davis Institutional Animal Care and Use Committee. Forty-eight hours pre-challenge, F. columnare colonies were inoculated into 10 mL of F. columnare growth medium (FCGM) broth [28] in a 50 mL conical tube and incubated at 28 °C for 17–18 h with shaking at 180 rpm. The broth culture was then inoculated into 100 mL of FCGM broth in a 500 mL Erlenmeyer flask and incubated at 28 °C for ∼15–18 h with shaking at 170 rpm until reaching an optical density of 0.901 at 600 nm to produce ∼108 CFU/ml. The bacterial suspension was diluted ten-fold using PBS and spread on MSA to estimate the number of CFUs used in the F. columnare challenge.
Flavobacterium columnare challenge was performed by immersion as in Soto, et al. [29] with some modifications. Flavobacterium columnare suspension was inoculated into tank water to obtain a challenge concentration of 1.1 × 106 CFU/mL for 2 h in static conditions with aeration. Two hours post challenge, the flow of water into the tanks was resumed. The F. columnare control group was immersed with sterile FCGM broth and treated in a similar manner. Morbidity and mortality were recorded daily for 15 days following F. columnare challenge. Fish presenting with two or more clinical signs of poor body condition, skin ulceration, hyperemia, loss of balance, lethargy, anorexia, scale protrusion, and/or exophthalmia were euthanized using 500 mg/L of buffered MS-222. Gills of 3–5 moribund and dead fish per tank were cultured to verify the cause of mortality.
At 1, 5 and 10 days post-challenge (dpc), two fish per tank (six fish per group) were euthanized with MS-222 and ∼30 mg of gill and spleen were collected. Tissues were immediately preserved in RNAlater (Qiagen) and stored at −80 °C until analysis.
At the end of the challenge, nine survivors (3 fish/tank) were cultured from each treatment group to determine carrier status. Posterior kidney and gills were streaked on MSA plates and incubated for 48 h at 28 °C. Flavobacterium columnare was morphologically identified as golden-yellow, flat, rhizoid colonies with irregular margins, tightly adherent to the agar.
2.5. Transcript expression analysis using quantitative reverse transcriptase PCR (RT-qPCR)
Quantitative analysis of haptoglobin, serotransferrin, serum amyloid A (saa), tumor necrosis factor alpha (tnfa), transforming growth factor beta (tgfb), interleukin 17 predicted protein (il17), interferon regulatory factor 8 (irf8), and major histocompatibility class II (mhcII) transcript expression in the gills and spleen tissues was investigated using RT-qPCR [24]. The primer sequences used in this study were published and validated in previous studies and are listed in Table 1.
Table 1.
Primers used in this study for transcript expression analysis.
| Gene | Oligo name | Primer sequences (5′−3′) | Refs. |
|---|---|---|---|
| beta-actin | Sturact60F | CATTGTCACCAACTGGGATGAC | Roy, et al. [31] |
| Sturact125R | ACACGCAGCTCATTGTAGAAGGT | Roy, et al. [31] | |
| elongation factor | Ef F | GGACTCCACTGAGCCACCT | Akbarzadeh, et al. [32] |
| Ef R | GGGTTGTAGCCGATCTTCTTG | Akbarzadeh, et al. [32] | |
| haptoglobin | AcHp-3′ F | ACATGTTCTGCACTGAGGTCAC | Soto, et al. [33] |
| AcHp-3′R | AGGTACCATGCACCATCCTG | Soto, et al. [33] | |
| serotransferrin | AcSTF-1 M-F | CCAGGTTCTTTTCTGCAAGC | Soto, et al. [33] |
| AcSTF-1 M-R | TAAGGTTCCGTGTGTGAACG | Soto, et al. [33] | |
| serum amyloid A | AcSAA-1F | GGCCAATTATATCGGTGCAG | Soto, et al. [33] |
| AcSAA-1R | AAACTCTGCCAAGCTTCACG | Soto, et al. [33] | |
| tnfα | TNFaIP-5′F | AAGCCCAGATGGACCAAAAG | Soto, et al. [33] |
| TNFaIP-5′R | TTGAGCTGCTCTTGTTTCCC | Soto, et al. [33] | |
| tgfβ | TGFI-5′F | TGACTGAACTGGACCACCTG | Soto, et al. [33] |
| TGFI-5′R | GGAACTCCTTCAGCTTCTCG | Soto, et al. [33] | |
| interleukin 17 | IL17A-5′F | TAACCCTCCCTCATGTCAGC | Soto, et al. [33] |
| IL17A-5′R | AATTCCCCCTACCAAAAACG | Soto, et al. [33] | |
| irf 8 | IRF8–5′F | CGGACTCTTGTGGGAAAATG | Soto, et al. [33] |
| IRF8–5′R | ATGGAAGCGTCGATTTCTTG | Soto, et al. [33] | |
| mhc class II | AcMHC-IIb F | TCTGCTACGTCATTGGCTTC | Soto, et al. [33] |
| AcMHC-IIb | TAGGATACATCAGCCGTCACC | Soto, et al. [33] |
Total RNA was isolated using the RNeasy®Plus Mini Kit (QIAGEN, Hilden, Germany) according to the manufacturer's instructions. RNA concentrations and purity, determined by 260/280 absorbance, were measured with a spectrophotometer (NanoDrop ND-1000; Thermo Fisher Scientific). Reverse transcription of 1 µg extracted RNA in 20 µL reactions was performed using the QuantiTect® Reverse Transcription Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. After reverse transcription, the samples were used as templates for RT-qPCR. The PCR mixture contained 5 µL of template cDNA, 1X Power SYBR® Green PCR Master Mix (Applied Biosystems by Thermo Fisher Scientific, Woolston Warrington, UK), and 0.2 µM of the appropriate forward and reverse primers (Invitrogen, Carlsbad, CA, USA). Conditions for the RT-qPCR were as follows: 1 cycle at 50 °C 2 min, 1 cycle at 95 °C 10 min, and 40 cycles of amplification at 95 °C for 15 s and annealing at 60 °C for 1 min. Negative (DEPC-treated H2O) controls were included in each run. An RT-qPCR was performed on each sample from all fish and each sample was run in duplicate.
Relative transcript expression was calculated using the 2−ΔΔCt method and normalized against the expression of the average of β-actin and elongation factor reference genes [30]. Tissues from control fish were used as calibrators for all genes.
2.6. Histopathology
Tissues from six F. columnare challenged fish that died between two and five dpc in the glucan-fed group and six fish that died from the normal-fed group were preserved in 10% buffered formalin and submitted for histopathologic analysis. Additionally, six survivors from each challenged group and three fish from each control group were euthanized at the end of the experiment for histological analysis. Sections of gill, heart, liver, spleen, and cranial kidney were collected from each fish and processed routinely, sectioned at 5 µm, and stained with hematoxylin and eosin (H&E). Tissues were examined blindly by a single pathologist. Based on preliminary examination of tissues, each organ was semi-quantitatively scored for the amount of inflammatory infiltrates, cellular necrosis, and bacteria (Table 2).
Table 2.
Scoring details of histopathology analysis in this study.
| Observed Tissue Change | Scoring | |||
|---|---|---|---|---|
| 0 | 1 (Mild) | 2 (Moderate) | 3 (Severe) | |
| Inflammatory cell infiltrates | Not present | Infiltration by ≤ 10 mononuclear cells within a 20x field. | Infiltration of 11–50 mononuclear cells within a 20x field. | Infiltration of ≥ 51 mononuclear cells within a 20x field. |
| Cellular necrosis | Not present | Rare pyknosis and/or karyorrhexis of cells affecting ≤ 5% of the entire tissue. | Pyknosis and/or karyorrhexis of cells affecting 6–50% of the entire tissue. | Widespread pyknosis and/or karyorrhexis affecting ≥ 51% of the entire tissue. |
| Bacteria | Not present | Bacteria are present within ≤ two inflammatory cells within a 40x field. | Bacteria are present within 3–5 inflammatory cells and/or in a single extracellular aggregates within a 40x field. | Bacteria are present within more 5 inflammatory cells and within multiple extracellular aggregates within a 40x field. |
2.7. Statistical analysis
Survival was analyzed using SigmaPlot 11.0, Kaplan-Meier Survival Analysis. Multiple comparisons were performed using the Holm-Sidak method. Histological scores for each variable were compared between groups and between tissues using a one-way ANOVA. Transcript expression differences between the different treatments at each time point, and within the same treatment at different time points were compared using a two-way ANOVA and Tukey's multiple comparisons test. Histologic scoring and transcript expression analysis was performed using GraphPad Prism 9 (San Diego California, USA).
3. Results
3.1. Survival and transcript expression analysis
No mortalities or other adverse effects were observed during the 30 days pre-challenge glucan feeding period. Significantly greater mortality was observed in the F. columnare exposed treatments when compared to non-exposed treatments (p < 0.001) (Fig. 1). Additionally, glucan-fed fish challenged with F. columnare presented significantly greater mortality than normal-fed fish challenged with F. columnare (p = 0.025). Mortalities began two dpc and reached 76.2% on six dpc for the glucan-fed and F. columnare exposed treatment group. Mortality reached 42.9% by 10 dpc for the normal-fed and F. columnare exposed treatment. Flavobacterium columnare was reisolated from the gills of all dead and moribund fish sampled. Cultures did not yield F. columnare from the gills of the surviving fish (n = 36) in all four treatment groups at 15 dpc.
Fig. 1.
Survival curves of white sturgeon fingerlings challenged by immersion with 1.1 × 106 CFU/mL of Flavobacterium columnare or FCGM media (control groups) (n = 20 fish/tank, 3 tanks/group). Different letters indicate significant differences in survival (Survival analysis, SigmaPlot 11.0, p < 0.05).
Similar transcript levels of tnfa, tgfb, il17, irf8, and mhcII were quantified in the gills of control and treatment groups at 1 dpc, 5 dpc, and 10 dpc (Fig. 2). However, haptoglobin, saa and serotransferrin transcripts were significantly higher in β-glucan-fed fish challenged with F. columnare when compared to glucan-fed non-exposed control fish at 1 dpc (p < 0.05, two-way ANOVA followed by Tukey's multiple comparisons test). Associated with peak of morbidity and mortality in normal-fed fish challenged with F. columnare, higher expression of the acute-phase proteins haptoglobin, saa and serotransferrin were observed 5 dpc; however, only serotransferrin transcript level was significantly greater to those quantified in glucan-fed fish challenged with F. columnare (Fig. 2). By 10 dpc, the transcript number of most cytokines had decreased to similar levels of control treatments; however, transcript levels of tnfa significantly increased in β-glucan-fed fish challenged with F. columnare (Fig. 2). Within the spleen, higher expression of saa, serotransferrin, tnfa, il17, irf8 and mhcII transcripts were observed 5 dpc when compared to 1 and 10dpc, although most were not significantly different (Fig. 3).
Fig. 2.
Transcript expression profiles in the gills of white sturgeon (Acipenser transmontanum) challenged with Flavobacterium columnare after 30 d of β-glucan (0.3% yeast, Saccharomyces cerevisiae) or normal diet feeding. Expression levels of haptoglobin (A), serum amyloid A (B), serotransferrin (C), tumor necrosis factor alpha (D), interleukin 17 (E), interferon regulator factor 8 (F), major histocompatibility complex II (G) and transforming growth factor beta (H) transcripts are shown as log-fold change for β-glucan-fed fish compared to the control-normal-fed fish for each gene. Data represents mean ± SE (n = 6). Statistically significant differences between treatments at each time point or within the same treatment at different time points is indicated by asterisk (p < 0.05, two-way ANOVA followed by Tukey's multiple comparisons test).
Fig. 3.
Transcript expression profiles in the spleens of white sturgeon (Acipenser transmontanum) challenged with Flavobacterium columnare after 30 d of β-glucan (0.3% yeast, Saccharomyces cerevisiae) or normal diet feeding. Expression levels of haptoglobin (A), serum amyloid A (B), serotransferrin (C), tumor necrosis factor alpha (D), interleukin 17 (E), interferon regulator factor 8 (F), major histocompatibility complex II (G) and transforming growth factor beta (H) transcrips are shown as log-fold change for β-glucan-fed fish compared to the control-normal-fed fish for each gene. Data represents mean ± SE (n = 6). Statistically significant differences between treatments at each time point or within the same treatment at different time points is indicated by asterisk (p < 0.05, two-way ANOVA followed by Tukey's multiple comparisons test).
3.2. Histopathology
Histopathologic changes were consistent between mortalities from β-glucan and normal-fed challenged groups with no significant differences in semi-quantified scores for inflammatory infiltrates, cellular necrosis, or bacterial infiltration of the tissues. The most common finding in mortalities was a moderate to severe, diffuse, necrotizing interstitial nephritis (7/12, 57.4%) and a mild to moderate, multifocal to diffuse necrotizing branchitis (6/12, 58.3%) rarely associated with extracellular filamentous bacteria consistent with F. columnare in H&E stained sections. The severity of cellular necrosis and bacterial infiltrates were significantly higher in both kidney and gill compared to liver, heart, and spleen (p = 0.03) (Fig. 4). One β-glucan-fed mortality had a mild, multifocal lymphoplasmacytic myocarditis. The levels of inflammatory infiltrates and tissue necrosis were significantly higher in challenged fish compared to recovered and non-challenged-control fish (p = 0.001). Tissues from fish that were challenged but recovered from infection were relatively uneventful. The only finding was a mild, multifocal, branchial hyperplasia and fusion in one normal-fed challenged fish.
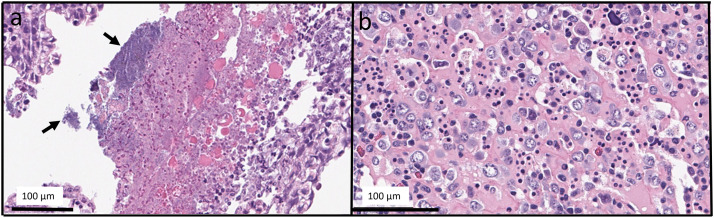
Fig. 4.
Representative histopathologic changes observed in white sturgeon (Acipenser transmontanum) challenged with Flavobacterium columnare. (a) Focal necrosis of gill lamellae and filaments characterized by loss of cellular detail and replacement by acellular debris associated with rafts of filamentous bacteria consistent with F. columnare (arrows). (b) Area of hematopoietic cell necrosis, hemorrhage, and edema within the interstitium of the kidney.
4. Discussion
Results of this study showed no evidence of improved protection in fish fed 0.3% β-glucan supplemented feed for 30 days and challenged with F. columnare. Moreover, significantly greater mortalities associated with higher gill transcript levels of several acute phase proteins were observed in glucan-fed fish challenged with F. columnare compared to the control diet of normal-fed fish. Although β-glucan administration has been shown to improve protection in fish against a number of pathogens, this is not the first study to demonstrate negative outcomes associated with β-glucan supplementation [34]. These data highlight that immunomodulation of fish requires a more in-depth understanding at both the basic and applied level before they can be used consistently to improve fish health.
There are a number of potentially overlapping factors that may have resulted in the outcomes of this study. This includes sturgeon-specific response to immunostimulation, route of administration, and the study's environmental parameters. Twenty-one day supplementation of β-glucan has been shown to provide protection in white sturgeon challenged with Veronaea botryosa, a systemic fungal disease resulting in phaeohyphomycosis [24]. This indicates effective immunomodulation does occur in sturgeon. The dosing timeline was shorter than the 30-day feeding trial described herein which suggests shorter immunostimulation periods may be more optimal for white sturgeon. However, it is difficult to directly compare results as systemic fungal infection may require different protective immune responses to combat V. botryosa compared to that associated with a largely mucosal bacterial infection such as columnaris. Alternative β-glucan administration routes may provide different levels of protection in mucosal tissues. Specifically, immersion administration of β-glucans has been found to increase wound healing in carp skin [35] and provide surface protection to chum salmon eggs against Saprolegnia spp. [36]. This is similar to immersion vaccination in fish which has been found to induce more localized mucosal immune responses compared to systemic responses elicited with vaccine that is distributed by feed or injection [37]. There is also evidence that feeding β-glucan in fish held at cooler water temperatures stimulates greater immunomodulatory effects of some immune factors [38]. Along with exploration of various administration routes, the effects of environmental parameters during the feeding and challenge time periods warrant further investigation.
The effectiveness of β-glucans has also been shown to be affected by the concentration, frequency and duration of administration. Positive protective responses have been achieved in salmonids against Aeromonas hydrophila with β-glucan dosage ranges between 0.1% [39] and 0.5% [40]. Although the 0.3% supplementation dosage used in this study falls within this range, species-specific, fish life stage, and infection pathogenesis may influence the protection received [41]. Decreased effectiveness of β-glucans has been associated with inappropriate treatment duration leading to stress induction and immunosuppression [15]. It has been hypothesized that extended β-glucans administration down regulates the immune system by negative feedback regulation [42]. In rainbow trout, fish supplemented with 0.2% β-glucans for 15 days showed higher expression of immune-related genes of il1b and il10 in spleen, tgfb in kidney and HSP70 in gills as compared to treatments fed the same diet for 30 days [43]. In addition, Amphan, et al. [44] suggested that optimal frequency of feeding β-glucans to tilapia is every-other-week to provide better protection against A. hydrophila in comparison with continuous feeding for 2 and 4 weeks.
Branchial and splenic levels of haptoglobin, serotransferrin, saa, cathelicidin, tnfa, il17, irf8, tgfb and mhcII were chosen to investigate pro-inflammatory, regulatory, innate and adaptive immune responses within this challenge model [24, 43]. Although transcript levels of most inflammatory mediators were similar between treatments, transcript expression of acute phase proteins were higher in the gills of β-glucan-fed fish challenged with F. columnare at one dpc compared to other treatments. Serum amyloid A and haptoglobin are some of several acute-phase proteins generated early in the initial immune response to infection [45,46] which coincides with the one dpc increase observed in this study. Previous studies have showed increasing levels of haptoglobin expression after bacterial infection suggesting an important role preventing iron loss as a hemoglobin scavenger [47]. Similarly, saa’s role early in infection as pro-inflammatory regulator is well studied in mammals and fish [46,48]. Serotransferrin plays a crucial role in regulating availability of iron during infection and immunostimulation of macrophages and other inflammatory responses [39]. Increased acute phase protein transcript levels are consistent with trends found in V. botryosa infected white sturgeon compared to non-exposed controls 6 weeks post-challenge [24] suggesting sustained responses of these inflammatory mediators during infection.
The association between high acute phase protein transcript expression and increased mortality in β-glucan-fed fish challenged with F. columnare suggests that the supplementation regimen may have contributed to an excessive initial inflammatory response within gills contributing to mortality. Although immune response overstimulation has not been well characterized in fish, this phenomenon is well described in mammals [49]. Necrotizing branchitis was a predominant histopathologic finding which is consistent with what is described during natural infections as a leading contributor to morbidity and mortality [14].
Haptoglobin, saa and serotransferrin levels were not simultaneously increased in the gill and spleen which may suggest that β-glucan regulation of cytokine expression is organ-dependent. For example, Falco, et al. [50] described up-regulation of tnfa2 transcript expression in the head kidney and concurrent down-regulation of tnfa2 transcript expression in the gut of A. salmonicida infected fish. These findings indicate that organ-specific responses to β-glucan may be important for optimal immune response, especially with pathogens such as F. columnare that have a predilection for the gills and other mucosal sites.
5. Conclusion
This study suggests feed supplementation with β-glucans at the concentration and rate investigated has no-benefit to help prevent losses due to F. columnare in cultured white sturgeon. Interestingly, significantly greater mortalities were observed in fish immunostimulated and challenged with F. columnare, associated with a significant increase in the acute inflammatory mediators haptoglobin, saa, and/or serotransferrin transcripts in the gills. Future research investigating various β-glucan supplemental protocols to explore alternate dosages and feeding duration of β-glucans are warranted for examine their use in the management of these important pathogens and to optimize husbandry within the white sturgeon industry. Furthermore, the combinational use of β-glucans with one or more immunostimulants in fish diet can be considered to boost the white sturgeon immune system.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the USDA National Institute of Food and Agriculture, HATCH project CA-V-VME-4091-H and by the St. George's University, School of Veterinary Medicine and University of California and Davis School of Veterinary Medicine Postdoctoral Scholars Program. There is no conflict of interest declared in this article.
References
- 1.Nelson T.C., Robichaud D., Challenger W., English K.K., Mochizuki T., Thibault T., Rissling J., Gazey W.J. 2019. Report prepared for the Fraser River Sturgeon Conservation Society. Vancouver. [Google Scholar]
- 2.Birstein V.J. Sturgeons and paddlefishes: threatened fishes in need of conservation. Conserv. Biol. 1993;7:773–787. doi: 10.1046/j.1523-1739.1993.740773.x. [DOI] [Google Scholar]
- 3.Radosavljević V., Milićević V., Maksimović-Zorić J., Veljović L., Nešić K., Pavlović M., Pelić D.L., Marković Z. Sturgeon diseases in aquaculture. Arch. Vet. Med. 2019;12:5–20. doi: 10.46784/e-avm.v12i1.34. [DOI] [Google Scholar]
- 4.Soto E., Richey C., Stevens B., Yun S., Kenelty K., Reichley S., Griffin M., Kurobe T., Camus A. Co-infection of Acipenserid herpesvirus 2 (AciHV-2) and Streptococcus iniae in cultured white sturgeon Acipenser transmontanus. Dis. Aquat. Org. 2017;124:11–20. doi: 10.3354/dao03108. [DOI] [PubMed] [Google Scholar]
- 5.Pierezan F., Shahin K., Heckman T.I., Ang J., Byrne B.A., Soto E. Outbreaks of severe myositis in cultured white sturgeon (Acipenser transmontanus L.) associated with Streptococcus iniae. J. Fish Dis. 2020;43:485–490. doi: 10.1111/jfd.13145. [DOI] [PubMed] [Google Scholar]
- 6.de Alexandre Sebastião F., Shahin K., Heckman T.I., LaFrentz B.R., Griffin M.J., Loch T.P., Mukkatira K., Veek T., Richey C., Adkison M. Genetic characterization of Flavobacterium columnare isolates from the pacific Northwest, USA. Dis. Aquat. Org. 2021;144:151–158. doi: 10.3354/dao03588. [DOI] [PubMed] [Google Scholar]
- 7.Avendaño-Herrera R., Gherardelli V., Olmos P., Godoy M., Heisinger A., Fernandez J. Flavobacterium columnare associated with mortality of salmonids farmed in Chile: a case report of two outbreaks. Bull. Eur. Assoc. Fish Pathol. 2011;31:36–44. [Google Scholar]
- 8.Staroscik A., Nelson D. The influence of salmon surface mucus on the growth of Flavobacterium columnare. J. Fish Dis. 2008;31:59–69. doi: 10.1111/j.1365-2761.2007.00867.x. [DOI] [PubMed] [Google Scholar]
- 9.Dong H., LaFrentz B., Pirarat N., Rodkhum C. Phenotypic characterization and genetic diversity of Flavobacterium columnare isolated from red tilapia, Oreochromis sp., in Thailand. J. Fish Dis. 2015;38:901–913. doi: 10.1111/jfd.12304. [DOI] [PubMed] [Google Scholar]
- 10.García J., LaFrentz B., Waldbieser G., Wong F., Chang S. Characterization of atypical Flavobacterium columnare and identification of a new genomovar. J. Fish Dis. 2018;41:1159–1164. doi: 10.1111/jfd.12778. [DOI] [PubMed] [Google Scholar]
- 11.LaFrentz B.R., García J.C., Waldbieser G.C., Evenhuis J.P., Loch T.P., Liles M.R., Wong F.S., Chang S.F. Identification of four distinct phylogenetic groups in Flavobacterium columnare with fish host associations. Front. Microbiol. 2018;9:452. doi: 10.3389/fmicb.2018.00452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LaFrentz B.R., Králová S., Burbick C.R., Alexander T.L., Phillips C.W., Griffin M.J., Waldbieser G.C., García J.C., de Alexandre Sebastião F., Soto E. The fish pathogen Flavobacterium columnare represents four distinct species: flavobacterium columnare, Flavobacterium covae sp. nov., Flavobacterium davisii sp. nov. and Flavobacterium oreochromis sp. nov., and emended description of Flavobacterium columnare. Syst. Appl. Microbiol. 2022;45 doi: 10.1016/j.syapm.2021.126293. [DOI] [PubMed] [Google Scholar]
- 13.Shoemaker C., LaFrentz B. Lack of association between Flavobacterium columnare genomovar and virulence in hybrid tilapia Oreochromis niloticus (L.) × Oreochromis aureus (Steindachner) J. Fish Dis. 2015;38:491–498. doi: 10.1111/jfd.12262. [DOI] [PubMed] [Google Scholar]
- 14.LaFrentz B., LaPatra S., Shoemaker C., Klesius P. Reproducible challenge model to investigate the virulence of Flavobacterium columnare genomovars in rainbow trout Oncorhynchus mykiss. Dis. Aquat. Org. 2012;101:115–122. doi: 10.3354/dao02522. [DOI] [PubMed] [Google Scholar]
- 15.Ching J.J., Shuib A.S., Majid N.Abdul, Taufek N.Mohd. Immunomodulatory activity of β-glucans in fish: relationship between β-glucan administration parameters and immune response induced. Aquac. Res. 2021;52:1824–1845. doi: 10.1111/are.15086. [DOI] [Google Scholar]
- 16.Xing J., Xu H., Wang Y., Tang X., Sheng X., Zhan W. Protective efficacy of six immunogenic recombinant proteins of Vibrio anguillarum and evaluation them as vaccine candidate for flounder (Paralichthys olivaceus) Microb. Pathog. 2017;107:155–163. doi: 10.1016/j.micpath.2017.03.027. [DOI] [PubMed] [Google Scholar]
- 17.Liao K., Meng R., Ran Z., Cheng G., Wang Y., Xu J., Xu S., Yan X. Short-term starvation in silver pomfret (Pampus argenteus): molecular effects on lipid mobilization and utilization. Aquac. Res. 2017;48:4874–4885. [Google Scholar]
- 18.Rodrigues M.V., Zanuzzo F.S., Koch J.F.A., de Oliveira C.A.F., Sima P., Vetvicka V. Development of fish immunity and the role of β-glucan in immune responses. Molecules. 2020;25:5378. doi: 10.3390/molecules25225378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fusté N.P., Guasch M., Guillen P., Anerillas C., Cemeli T., Pedraza N., Ferrezuelo F., Encinas M., Moralejo M., Garí E. Barley β-glucan accelerates wound healing by favoring migration versus proliferation of human dermal fibroblasts. Carbohydr. Polym. 2019;210:389–398. doi: 10.1016/j.carbpol.2019.01.090. [DOI] [PubMed] [Google Scholar]
- 20.Do Huu H., Sang H.M., Thuy N.T.T. Dietary β-glucan improved growth performance, Vibrio counts, haematological parameters and stress resistance of pompano fish, Trachinotus ovatus Linnaeus, 1758. Fish Shellfish Immunol. 2016;54:402–410. doi: 10.1016/j.fsi.2016.03.161. [DOI] [PubMed] [Google Scholar]
- 21.Rodríguez F.E., Valenzuela B., Farías A., Sandino A.M., Imarai M. β-1, 3/1, 6-Glucan-supplemented diets antagonize immune inhibitory effects of hypoxia and enhance the immune response to a model vaccine. Fish Shellfish Immunol. 2016;59:36–45. doi: 10.1016/j.fsi.2016.10.020. [DOI] [PubMed] [Google Scholar]
- 22.D. Hoang, H.S. Lam, and C. Nguyen, "Efficiency of dietary β-glucan supplementation on growth, body composition, feed, and nutrient utilization in juveniles of pompano fish (Trachinotus ovatus, Linnaeus, 1758)," (2018).
- 23.Dawood M.A., Abdo S.E., Gewaily M.S., Moustafa E.M., SaadAllah M.S., AbdEl-Kader M.F., Hamouda A.H., Omar A.A., Alwakeel R.A. The influence of dietary β-glucan on immune, transcriptomic, inflammatory and histopathology disorders caused by deltamethrin toxicity in Nile tilapia (Oreochromis niloticus) Fish Shellfish Immunol. 2020;98:301–311. doi: 10.1016/j.fsi.2020.01.035. [DOI] [PubMed] [Google Scholar]
- 24.Soto E., Coleman D., Yazdi Z., Purcell S.L., Camus A., Fast M.D. Analysis of the white sturgeon (Acipenser transmontanus) immune response during immunostimulation and Veronaea botryosa infection. Comp. Biochem. Physiol. D Genom. Proteom. 2021;40 doi: 10.1016/j.cbd.2021.100950. [DOI] [PubMed] [Google Scholar]
- 25.Aramli M.S., Kamangar B., Nazari R.M. Effects of dietary β-glucan on the growth and innate immune response of juvenile Persian sturgeon, Acipenser persicus. Fish Shellfish Immunol. 2015;47:606–610. doi: 10.1016/j.fsi.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 26.LaFrentz B.R., Klesius P.H. Development of a culture independent method to characterize the chemotactic response of Flavobacterium columnare to fish mucus. J. Microbiol. Methods. 2009;77:37–40. doi: 10.1016/j.mimet.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 27.Decostere A., Haesebrouck F., Devriese L.A. Shieh medium supplemented with tobramycin for selective isolation of Flavobacterium columnare (Flexibacter columnaris) from diseased fish. J. Clin. Microbiol. 1997;35:322–324. doi: 10.1128/jcm.35.1.322-324.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farmer B.D. LSU Master's Theses Louisiana State University; 2004. Improved Methods For the Isolation and Characterization of Flavobacterium columnare. [Google Scholar]
- 29.Soto E., Mauel M.J., Karsi A., Lawrence M. Genetic and virulence characterization of Flavobacterium columnare from channel catfish (Ictalurus punctatus) J. Appl. Microbiol. 2008;104:1302–1310. doi: 10.1111/j.1365-2672.2007.03632.x. [DOI] [PubMed] [Google Scholar]
- 30.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 31.Roy N.K., Walker N., Chambers R.C., Wirgin I. Characterization and expression of cytochrome P4501A in Atlantic sturgeon and shortnose sturgeon experimentally exposed to coplanar PCB 126 and TCDD. Aquat. Toxicol. 2011;104:23–31. doi: 10.1016/j.aquatox.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akbarzadeh A., Farahmand H., Mahjoubi F., Nematollahi M.A., Leskinen P., Rytkönen K., Nikinmaa M. The transcription of l-gulono-gamma-lactone oxidase, a key enzyme for biosynthesis of ascorbate, during development of Persian sturgeon Acipenser persicus. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2011;158:282–288. doi: 10.1016/j.cbpb.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 33.Soto E., Fast M.D., Purcell S.L., Coleman D.D., Yazdi Z., Kenelty K., Yun S., Camus A. Expression of immune markers of white sturgeon (Acipenser transmontanus) during Veronaea botryosa infection at different temperatures. Comp. Biochem. Physiol. D Genom. Proteom. 2022;41 doi: 10.1016/j.cbd.2021.100950. [DOI] [PubMed] [Google Scholar]
- 34.Refstie S., Baeverfjord G., Seim R.R., Elvebø O. Effects of dietary yeast cell wall β-glucans and MOS on performance, gut health, and salmon lice resistance in Atlantic salmon (Salmo salar) fed sunflower and soybean meal. Aquaculture. 2010;305:109–116. doi: 10.1016/j.aquaculture.2010.04.005. [DOI] [Google Scholar]
- 35.Przybylska-Diaz D., Schmidt J., Vera-Jimenez N., Steinhagen D., Nielsen M.E. β-glucan enriched bath directly stimulates the wound healing process in common carp (Cyprinus carpio L.) Fish Shellfish Immunol. 2013;35:998–1006. doi: 10.1016/j.fsi.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 36.Kiseleva M., Balabanova L., Elyakova L., Rasskazov V., Zvyagintseva T. Effect of treatment of chum salmon Oncorhynchus keta (Walbaum) eggs with 1, 3; 1, 6-β-d-glucans on their development and susceptibility to Saprolegnia infection. J. Fish Dis. 2014;37:3–10. doi: 10.1111/jfd.12043. [DOI] [PubMed] [Google Scholar]
- 37.Munang'andu H.M., Mutoloki S., Evensen Ø. A review of the immunological mechanisms following mucosal vaccination of finfish. Front. Immunol. 2015;6:427. doi: 10.3389/fimmu.2015.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bagni M., Romano N., Finoia M., Abelli L., Scapigliati G., Tiscar P.G., Sarti M., Marino G. Short-and long-term effects of a dietary yeast β-glucan (Macrogard) and alginic acid (Ergosan) preparation on immune response in sea bass (Dicentrarchus labrax) Fish Shellfish Immunol. 2005;18:311–325. doi: 10.1016/j.fsi.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 39.Nikl L., Evelyn T.T., Albright L. Trials with an orally and immersion-administered β-1, 3 glucan as an immunoprophylactic against Aeromonas salmonicida in juvenile chinook salmon Oncorhynchus tshawytscha. Dis. Aquat. Org. 1993;17:191–196. doi: 10.3354/dao017191. [DOI] [Google Scholar]
- 40.El-Boshy M.E., Ahmed M., AbdelHamid F.M., Gadalla H.A. Immunomodulatory effect of dietary Saccharomyces cerevisiae, β-glucan and laminaran in mercuric chloride treated Nile tilapia (Oreochromis niloticus) and experimentally infected with Aeromonas hydrophila. Fish Shellfish Immunol. 2010;28:802–808. doi: 10.1016/j.fsi.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 41.Meena D., Das P., Kumar S., Mandal S., Prusty A., Singh S., Akhtar M., Behera B., Kumar K., Pal A. Beta-glucan: an ideal immunostimulant in aquaculture (a review) Fish Physiol. Biochem. 2013;39:431–457. doi: 10.1007/s10695-012-9710-5. [DOI] [PubMed] [Google Scholar]
- 42.Sabioni R.E., Zanuzzo F.S., Gimbo R.Y., Urbinati E.C. β-Glucan enhances respiratory activity of leukocytes suppressed by stress and modulates blood glucose levels in pacu (Piaractus mesopotamicus) Fish Physiol. Biochem. 2020;46:629–640. doi: 10.1007/s10695-019-00739-x. [DOI] [PubMed] [Google Scholar]
- 43.Douxfils J., Fierro-Castro C., Mandiki S., Emile W., Tort L., Kestemont P. Dietary β-glucans differentially modulate immune and stress-related gene expression in lymphoid organs from healthy and Aeromonas hydrophila-infected rainbow trout (Oncorhynchus mykiss) Fish Shellfish Immunol. 2017;63:285–296. doi: 10.1016/j.fsi.2017.02.027. [DOI] [PubMed] [Google Scholar]
- 44.Amphan S., Unajak S., Printrakoon C., Areechon N. Feeding-regimen of β-glucan to enhance innate immunity and disease resistance of Nile tilapia, Oreochromis niloticus Linn., against Aeromonas hydrophila and Flavobacterium columnare. Fish Shellfish Immunol. 2019;87:120–128. doi: 10.1016/j.fsi.2018.12.062. [DOI] [PubMed] [Google Scholar]
- 45.Gauldie J., Baumann H. Cytokines and acute phase protein expression. Cytokines and inflammation. 1991;275 [PubMed] [Google Scholar]
- 46.Castellano M., Silva-Álvarez V., Aversa-Marnai M., Lamas-Bervejillo M., Quartiani I., Perretta A., Villarino A., Ferreira A.M. Serum amyloid A is a positive acute phase protein in Russian sturgeon challenged with Aeromonas hydrophila. Sci. Rep. 2020;10:1–14. doi: 10.1038/s41598-020-79065-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cordero H., Li C.H., Chaves-Pozo E., Esteban M.Á., Cuesta A. Molecular identification and characterization of haptoglobin in teleosts revealed an important role on fish viral infections. Dev. Comp. Immunol. 2017;76:189–199. doi: 10.1016/j.dci.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 48.Kania P.W., Chettri J.K., Buchmann K. Characterization of serum amyloid A (SAA) in rainbow trout using a new monoclonal antibody. Fish Shellfish Immunol. 2014;40:648–658. doi: 10.1016/j.fsi.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 49.Eberl G. Immunity by equilibrium. Nat. Rev. Immunol. 2016;16:524–532. doi: 10.1038/nri.2016.75. [DOI] [PubMed] [Google Scholar]
- 50.Falco A., Frost P., Miest J., Pionnier N., Irnazarow I., Hoole D. Reduced inflammatory response to Aeromonas salmonicida infection in common carp (Cyprinus carpio L.) fed with β-glucan supplements. Fish Shellfish Immunol. 2012;32:1051–1057. doi: 10.1016/j.fsi.2012.02.028. [DOI] [PubMed] [Google Scholar]