Highlights
-
•
Zebrafish as a suitable model to study mitochondrial dynamics in immunological research
-
•
Recent findings on immunimetabolism concerning zebrafish are summarized
-
•
Advantages and disadvantages of different strategies applied in zebrafish model to understand signaling pathways in mitochondria
-
•
Which transgenic lines of zebrafish may help to elucidate doubts about the association between the immune system and mitochondrial function
Keywords: Mitochondria, Mitochondrial functions, Metabolism, Immunology, Immuno metabolism, Fish
Abstract
Mitochondria are organelles commonly associated with adenosine triphosphate (ATP) formation through the oxidative phosphorylation (OXPHOS) process. However, mitochondria are also responsible for functions such as calcium homeostasis, apoptosis, autophagy, and production of reactive oxygen species (ROS) that, in conjunction, can lead to different cell fate decisions. Mitochondrial morphology changes rely on nutrients’ availability and the bioenergetics demands of the cells, in a process known as mitochondrial dynamics, which includes both fusion and fission. This organelle senses the microenvironment and can modify the cells to either a pro or anti-inflammatory profile. The zebrafish has been increasingly used to research mitochondrial dynamics and its connection with the immune system since the pathways and molecules involved in these processes are conserved on this fish. Several genetic tools and technologies are currently available to analyze the behavior of mitochondria in zebrafish. However, even though zebrafish presents several similar processes known in mammals, the effect of the mitochondria in the immune system has not been so broadly studied in this model. In this review, we summarize the current knowledge in zebrafish studies regarding mitochondrial function and immuno metabolism.
1. Introduction
The mitochondria have been historically known as the “powerhouse of the cell” because of their primary function of generating energy through the oxidation of metabolites in the tricarboxylic acid (TCA) cycle, which culminates in the synthesis of ATP in the electron transport chain (ETC) (Fig. 1) [1,2]. In this process, pyruvate or fatty acids are oxidized to Acetyl Coenzyme A (Acetyl-CoA) by pyruvate dehydrogenase (PDH) and then Acetyl-CoA is condensed with oxaloacetate to compose citrate [3]. The TCA cycle synthesizes FADH2 and NADH that are responsible for providing electrons for ETC, which in turn, transfer these electrons to molecular oxygen while pumping protons across the inner mitochondrial membrane. This process creates a proton-driving force that is used to generate ATP by the ATP synthase complex. Mitochondria that fail to induce membrane potential for ATP generation are targets of destruction by mitophagy [4]. Metabolites from the TCA cycle can also be used for the formation of macromolecules, such as proteins, DNA, RNA, and lipids [5]. The generation of energy assembled as ATP, and the biosynthesis of macromolecules, must be thoughtfully balanced to support certain demands of each cell. Tumor cells, for example, use glycolysis to suppress the high energy demand due to its rapid proliferation, even in oxygen-rich environments, which is known as the “Warburg effect” [6]. Therefore, the mitochondria can be considered as the metabolic hubs within the cells that change their functions to suit cellular needs [7,8].
Fig. 1.
The metabolites once inside the mitochondrial matrix are converted to acetyl-CoA through the tricarboxylic acid (TCA) cycle. The oxidation of fatty acids and pyruvate to Acetyl-CoA in the TCA cycle happens through pyruvate dehydrogenase (PDH), followed by the reduction of NAD+ to NADH and production of H+ on the Complex I (I) [3]. Complex II (II) oxidizes the succinate to fumarate and converts FAD to FADH2[2]. The oxidation process of metabolites allows the synthesis of ATP in the electron transport chain (ETC) as the formation of FADH2 and NADH render electrons to Complex I and II [1]. The electrons provided by the TCA cycle (red arrow) goes throughout ETC, composed by the Complex I, II, III and IV, this process allows the pumping of protons (H+) of the matrix to the inter membrane space, creating a proton-driving force. In Complex IV, the electron is used to build HO (2 H++ 1/2O2) [2]. The high proton concentration on the inter membrane space is essential to the Complex V- ATP synthase complex- function. Once activated, the ATP synthase complex can join the phosphatase molecule (P) to the adenosine diphosphate (ADP) to generate adenosine triphosphate (ATP) [2,3].
Nowadays it is known that the mitochondria are not only related to the production of energy but participates in processes like development, proliferation, differentiation, activation, and death of different cell types, including immune cells [8]. These processes are influenced by the cell environment and alter mitochondria morphology and position within the cell, causing changes in cell metabolism [8,9]. The mitochondria metabolism and its influence on the immune system have been classically analyzed in mammalian cells but new studies have used the zebrafish, a non-mammalian vertebrate, as a new option for mitochondrial analysis. The inclusion of the fish model in the bioenergetics research field has allowed the generation of new insights, mainly by using these animal models to understand the effects of chemicals, such as metals, herbicides, and fungicides, in mitochondria metabolism, the process of ROS production, ion homeostasis, etc [10].
The zebrafish (Danio rerio) has been widely used in different research fields in the last 30 years. This tropical teleost fish was brought to light as an experimental model by George Streisinger in 1981, who pointed out the several advantages of the use of this vertebrate model in genetic manipulation [11], [12], [13]. Originally from Southeast Asia, this freshwater fish lives for 4 to 5 years, with females being capable of laying 200-300 eggs per week, with its small size (3–4 cm) facilitating the maintenance and reducing the breeding costs in research facilities [14,15]. The zebrafish larva has a rapid development and is optically transparent, which facilitates the visualization for in vivo experiments [14,16]. Besides that, the zebrafish has 70% gene homology with humans [17]. Zebrafish have been used to simulate diverse human diseases, enabling the improvement of medical research, been applied in several areas, including behavioral biology, drug testing, genetics, immunology, metabolism, oncology, among others [13,16,[18], [19], [20], [21]], due to its anatomical, physiological and metabolic similarities with mammals [22].
The study of energy metabolism has grown considerably in recent years [23]. In this sense, the use of zebrafish as a tool that assists in a better understanding of mitochondrial dynamics and its metabolism has come into focus, consequently, several methodologies are being developed to understand the physiology of this organelle.
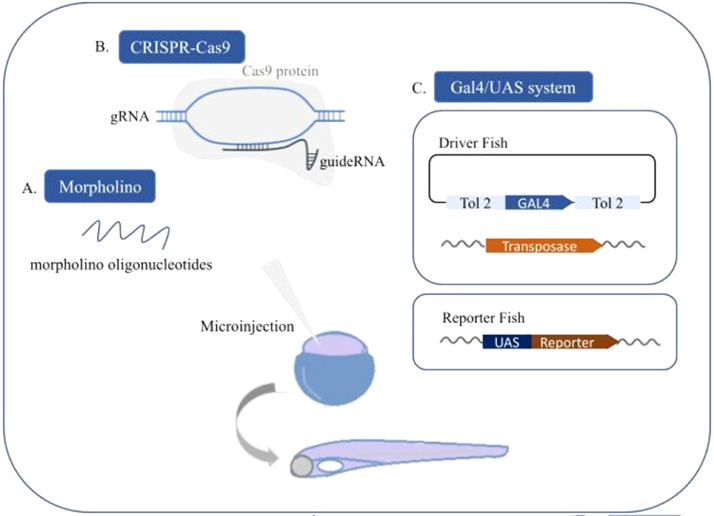
From a genetic point of view, studies show that in humans, mitochondrial proteins have two origins: mitochondrial DNA (mtDNA) and nuclear DNA. Mutations in these proteins can lead to heterogeneous clinical presentations, causing failure in organs that require a lot of energy, such as the heart and nervous system [24]. Given this, the creation of transgenic lines from genomic editing techniques is extremely advantageous to study cell metabolism. The use of genome editing in zebrafish is less complicated than in mammals and is possible to generate transgenic and mutant strains in a feasible manner by different techniques (Fig. 2) [22,25,26]. The achievable genetic manipulation and the fact that most organs are functionally active at the first 5 days after fertilization makes the analyses of specific genetic functions altered in vivo accessible in the early days of life in the model [27]. Through microinjection of morpholino oligonucleotides, a transient knockdown technique used in zebrafish, it is possible to decrease the expression of a particular gene product (Fig. 2A) [25]. On the opposite side, with the addition of processed mRNA, a gene product can be highly expressed [25]. The microinjection method, performed at the stage of one cell, is fast and effective, resulting in hundreds of embryos injected in a matter of hours. CRISPR/Cas9 is another genomic editing technique commonly used on zebrafish because of its high efficiency and low off-target effects (Fig. 2B) [26]. Also, new studies focus on the alteration of mitochondrial DNA, which already showed that the system is efficient for the deletion of mtDNA [28].
Fig. 2.
Microinjection method at the stage of one cell [25]. A- Microinjection of morpholino oligonucleotides can reduce the expression of the selected gene or, by adding processed mRNA, enhance the expression of the select gene [25]. B- CRISPR/Cas9 is a DNA editing technique that consists of the microinjection of the Cas9 protein/mRNA and a guide RNA that will induce a double-strand break on the DNA causing a mutation in the target gene. Novel versions are used to add genes to the genome using this technique [26]. C- The Gal4/UAS system allows tissue-specific editing of the genome. The technique consists of a two-machinery program: the “driver fish” with a Tol2 transposition system: a specific mRNA synthesized of a Gal4 tissue-specific gene among two transposable elements- Tol2; and the “reporter fish” a transgenic fish carrying a reporter gene together with the UAS. Once crossed the “driver” and “reporter” fish it generates a linage with Gal4 as a transcription factor for UAS. [24, 29, 31, 32].
Transgenic mice with fluorescent mitochondria make it possible to analyze the life cycle of this organelle in vivo. However, in cases of neurological diseases related to mitochondrial abnormalities, microscopy access becomes a challenge in murine models [29]. The zebrafish can be used as an option to avoid this problem, because of its optical transparency, as well as the great availability of transgenic strains. For example, the Tg (dat:tom20 MLS-mCherry) zebrafish strain allows the visualization of mitochondria in dopaminergic neurons [30]. This approach allows the monitoring of mitochondrial changes in vivo, analyzing the expression of mCherry fluorescence [30]. The generation of a tissue-specific line using the Gal4/UAS system (Fig. 2C), the “MitoFish”, allowed not only the study of mitochondria in specific cells such as neurons but offers a tool for the creation of new tissue-specific expression lines by creating a fish carrier of a cell/tissue-specific GAL4-driver [24,29,31]. The MitoDsRed transgenic strain also uses the Gal4/UAS system, making it possible to monitor the morphology and motility of mitochondria during axon degeneration on zebrafish in vivo [32].
Most mitochondrial metabolic pathways are conserved between teleost and mammals but still, there's a lack of information about the effect of mitochondrial changes in immune cell fate in this animal model [33], [34], [35]. In this manuscript, we reunite the last research regarding immunometabolism of mitochondria using the zebrafish experimental model.
2. Mitochondrial Metabolism
The signal transduction mechanisms between the cell and mitochondria are several, such as anterograde and retrograde signaling. Retrograde signaling departs from the mitochondria to the cytosol, mainly represented by the production of mitochondrial reactive oxygen species (mtROS), including peroxides, superoxide, and hydroxyl radical, among others. The ETC is in the inner mitochondrial membrane with four-electron transport complexes and the ATP synthase (Fig. 1). It can synthesize superoxide significantly through complexes I and III, which can further be converted by superoxide dismutase (SOD) into hydrogen peroxide and released into the cytosol, where the hydrogen peroxide can cause the oxidation of thiol groups of proteins [36,37]. An interesting study investigated the differences between the functioning of mitochondrial metabolism in endothermic and ectothermic fish. Since endothermic fish maintain temperatures close to 21°C and constantly swim for breathing, it is suggested that mitochondria play an important role in replenishing the ATP consumed by muscle contractions. Consequently, in addition to the production of ATP by aerobic metabolism, there is also the synthesis of reactive oxygen species [38]. On the other hand, it has been previously shown that ectothermic fish have a lower efflux of hydrogen peroxide in isolated mitochondria compared to mitochondrial rates in rats [39]. Since the exacerbated production of ROS can cause tissue damage, it was hypothesized that together with the appearance of endothermy in fish, a compensatory mechanism was developed to minimize these effects. However, the study did not find a compensatory mechanism in Pacific Bluefin tuna, an endothermic fish, compared to the studied ectothermic fish species. However, this compensatory mechanism exists in endothermic mammals, which present less fraction electron leakage [38].
Mitochondrial ROS, which is similarly produced both in mammals and fish, can regulate the activation of the hypoxia-inducible transcription factor 1 (HIF-1), which is a transcription factor with an important role revolving around the adaptation of cells to lower oxygen levels [40], [41], [42] Vertebrates possess three functional HIF-1α isoforms: HIF-1α, HIF-2α, and HIF-3α. HIF-1 is comprised of two subunits, an oxygen-regulated subunit (HIF-1α) and a constitutively expressed subunit (HIF-1β). A role for HIF-1α has been defined in physiological phenotypes concerning metabolism, mitochondrial function, inflammatory cell recruitment, etc. [40,41]. In zebrafish and other teleosts, there are two copies of each HIF-α isoforms, because of whole-genome duplication events during evolution, a study investigating the role of hif1aa and hif1ab on mitochondria regulation revealed that both paralogous provide distinct functions: hif1aa is responsible for the regulation of Ca2+ concentration, ROS production, transcriptional levels of citrate synthase and for the increase of mitochondrial membrane potential, whereas hif1ab promotes OXPHOS by increasing activities of complexes I, III and IV, and promoting ATP generation, as well. Additionally, both hif1aa and hif1ab promote mitochondrial biogenesis [43]. Other retrograde signaling functions of the mitochondria include the influence of signaling pathways by changing the availability of TCA cycle intermediates such as succinate, Acetyl-CoA, α-ketoglutarate, and fumarate [44].
On the other hand, mitochondrial can communicate with the cell by anterograde signals from the cytosol to the mitochondria. Anterograde signals can be seen in response to high cytosolic calcium levels, which causes the rapid sequestration of calcium into the mitochondrial matrix. The influx of calcium can modulate OXPHOS and influence oxygen consumption and ATP formation by the activation of dehydrogenases from the TCA cycle [45,46]. This also leads to the production of nitric oxide by the mitochondrial nitric oxide synthase (mtNOS) that regulates the rates of oxygen consumption due to competitive inhibition of cytochrome oxidase. This negative feedback mechanism allows a smoother gradient of oxygen to the rest of the cell [46]. Another study demonstrated that the calcium influx increases the conductance of complexes I, III, IV, ATP production/transport, and fuel transport/dehydrogenases of the OXPHOS cascade in skeletal muscle cells, supporting, even more, the idea that Ca2+ activates this pathway [47]. In zebrafish, there are some studies regarding calcium uptake and calcium influx in hair cells [48]. It is known that Ca2+ is a vital element, especially for vertebrates. In the case of terrestrial vertebrates, the main source of this element is food, with the intestines serving as the primary site for its uptake. Distinctly, aquatic animals’ environment is susceptible to variable concentrations of Ca2+ in the water. Due to that, the gills become the main site of Ca2+ uptake in fish. However, in fish early embryos and larvae the calcium uptake occurs mainly through skin mitochondria-rich cells. In this sense, the epithelial Ca2+ channel (ECaC) was demonstrated to play a physiological role in the fish Ca2+ uptake mechanism, but a lot is still unknown [49].
Cellular processes such as the OXPHOS chain and the synthesis of mtROS are conserved in zebrafish and have been increasingly studied with different techniques, presented in Table 1 [33]. Otten and collaborators analyzed the impact on the development of the mitochondrial transcription factor A (TFAM) using a KO approach [50]. In zebrafish, TFAM is responsible for the formation of mtRNA, as in mammals. Without TFAM the zebrafish have impaired development, fewer copies of mtRNA, and display lower OXPHOS activity [50].
Table 1.
Zebrafish techniques and tools available to study mitochondrial aspects.
| Techniques and Tools | Utility | Advantage | Disadvantage | References |
|---|---|---|---|---|
| Fluorescence Detection Techniques | ||||
| Mito-MQ | Identify intracellular homocysteine and cysteine, reporting ROS production | Live image in vivo and fixed samples | Cytotoxicity of the probe | [47], [48], [49] |
| MitoSOX ™ | Detection of mitochondrial superoxide production and intracellular ROS | Live image of in vivo and in vitro | Debatable specificity of the marker | [50,51] |
| CellROX ™ | Detection of intracellular ROS | Live image of in vivo and in vitro | Debatable specificity of the marker | [51,52] |
| Antisense RNA probe | Analysis of the expression of nucleotides or proteins | Temporal analysis of gene expression in adults, whole embryo, and early larva | The probe penetrates superficially into later stages of development | [53] |
| Metabolic Assays | ||||
| Seahorse Technology | Analysis of mitochondrial respiration rate, glycolysis, activation of intracellular pathways, and the use of substrates by the cell | Live zebrafish embryos and larvae Inhibitors can be used in different mammals’ models | Cell individualization necessary for adult zebrafish analysis | [54], [55], [56], [57] |
| Oroboros Oxygraph-2k System | Analysis of ROS production, respiratory rates, or changes in respiratory chain complexes | Different tissue analysis | Due to equipment sensitivity, a large number of samples are needed | [36] |
3. Mitochondrial Dynamics
The mitochondrial morphology varies from cell to cell and is closely related to mitochondrial function in response to extra- and intracellular signals [45]. In this sense, the mitochondria undergo events of fission, fusion, and trafficking that cycle depending on the metabolic demands of the cell [51]. These processes together compose the mitochondrial dynamics and they have demonstrated to be needed for mitochondrial biogenesis, as these organelles are not created de novo but from the expansion in size, replication of the mtDNA, and subsequent fragmentation to distribute the organelles during cell division [52]. The elimination of organelles through autophagy and the specific process of mitochondria elimination through mitophagy is also related to mitochondrial dynamics and will be discussed later.
The main proteins report to participate in mitochondrial dynamics are part of the dynamin guanosine triphosphatases (GTPases) family and include mitofusin 1 (Mfn1) and 2 (Mfn2), optic atrophy 1 (Opa1), and dynamin-related protein 1 (Drp1). Both Mfn1 and Mfn2 are located in the outer mitochondrial membrane (OMM) with their amino (N-) and carboxyl (C-) terminal towards the cytosol, while Opa1 is found in the inner mitochondrial membrane (IMM). In contrast, Drp1 is mostly localized in the cytosol but can be recruited by the mitochondria [8, 51]. In mammals, mitochondrial fusion is initiated by the physical approximation of two mitochondria followed by the GTP-independent tethering of Mnf1/Mnf2 and the GTP-dependent fusion of OMMs. Next, the fusion of the IMMs is coordinated by Opa1 [51]. It is believed that the main function of mitochondrial fusion is the homogenization of molecular components such as mtDNA, proteins, and lipids [52,53], but evidence has shown that fusion also protects the mitochondria from degradation during starvation periods [54]. Mitochondrial fusion also occurs in cells with high OXPHOS activity [55].
On the other hand, mitochondria fission is orchestrated in the first place by interactions between the endoplasmic reticulum (ER) and the mitochondria, which will mark the site of future fission on the OMM [53,56]. Drp1 and Dynamin-2 (Dnm2) will be recruited from cytosolic locations to bind to the OMMs anchor proteins: fission protein 1 (Fis1), mitochondrial fission factor (MFF), and mitochondrial dynamics proteins of 49 and 51 kDa (MiD49 and MiD51) [53,57,58]. MFF, MiD49, and MiD51 will induce Drp1 oligomerization and the formation of a ring-like structure around the mitochondria, that will constrict the membranes to finally be cut by Dnm2 [58]. Mitochondrial fission has been observed in nutrient-rich environments and has been related to functions such as the higher expedition of mitochondria transport across the cell, the elimination of damaged mitochondria by mitophagy, and the releasing of cytochrome C into the cytosol during apoptosis [57,[59], [60], [61], [62], [63], [64]]. For the accomplishment of all these functions, mitochondria must be transported to regions where metabolic requirements are higher. Mfn1 and Mfn2 mediate this transport by forming a complex with the OMM proteins Miro1 and Miro2 and the adaptor protein Milton that interacts with Dynein and Kinesin circulating in microtubules [9]. In this sense, it was seen that smaller mitochondria are easier to transport than larger ones [59,65].
Proteins involved in mitochondrial dynamics are highly conserved in zebrafish, displaying genes that are also present in humans, including mnf1, mfn2, opa1, dnml-1, among others [66]. In general, fish have a special composition of DAP kinases. In one study, six species of teleosts, which had two gene loci of drp1, were analyzed. These two drp1 subtypes are likely the result of a whole-genome duplication event. One of the subtypes has the same genomic structure as the DRP-1 gene of other vertebrates, however, in zebrafish, this same gene does not have the C-terminal ZIPk-like exon. Thus, it is suggested that drp1 in these fish is still evolving, or has already developed, in two distinct genes. The function of these duplicated genes could be similar to the different transcripts of the single DRP-1 of other vertebrates [67].
Although mitochondrial protein identities between humans and zebrafish fluctuate between 35 to 90%, it is possible to observe higher conservation in domains important for function [66]. For instance, the alignment of the PINK1 protein has 54% of identity, while the alignment of the kinase domain only results in 71% of identity [66]. In the last decade, zebrafish have been used as a model for mitochondrial studies and a variety of mutants and transgenic lines have been created for different purposes. For instance, knockdown using morpholinos of GDAP1 and OPA1 show defects in mitochondrial fission and network morphology, respectively, in sensitive neurons [68]. Pharmacological studies have been also important in the zebrafish because of the possibility of using mitochondria disrupting drugs and pharmacological modulators directly in the water and observe the results in vivo [29,69].
Studies have demonstrated that adult zebrafish retina expresses seven sirtuins (sirt1-7), proteins related to the protection of the mitochondria from oxidative damage, and also opa1, in greater intensity than the liver of the fish [70]. The treatment with resveratrol, an activator of sirtuin 1 (SIRT1) increased the gene and protein expressions of both sirt1 and opa1, and also enhanced the expression of sirt4 with an improvement of DNA repair in the adult retina [71]. Another study showed lower mtDNA integrity and copy number, lower expression of mitochondrial fusion regulators, lower mitophagy activity, and expression of antioxidant genes in aging retinas from adult zebrafish [70]. Treatment with resveratrol increased mitochondrial quality and function, up-regulating the Ampk/Sirt1/Pgc1α pathway and down-regulating the Akt/mTOR pathway [71]. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) is another chemical model that has also been used in zebrafish to induce Parkinson's disease in vivo [72]. MPTP can lead to neuron damage and cellular stress by the generation of ROS once inside the mitochondrial membrane of neurons [72]. Zebrafish model provided insights into how certain drugs, such as TPP-PEG-ADA (a polyethylene glycol system tagged with triphenylphosphonium and adamantane) and hyaluronic acid- CB(7)-HA- together, might protect against MPTP effects and neurodegenerative diseases by inducing mitochondrial fusion and against mitochondrial fragmentation/fission [73]. All these studies show the versatility that zebrafish can provide to mitochondrial dynamic research [71,73,74].
4. Autophagy
Autophagy is the process where cellular components are broken down and arrested inside double-membrane structures named auto phagosomes, which will fuse with lysosomes and breaked by lysosomal enzymes. This catabolic process will provide macromolecular precursors for other metabolic processes and will help in the elimination of defective cellular components [75]. Autophagy is performed at low rates in basal conditions but is increased during starvation periods of metabolic stress, being mediated by autophagy-related proteins (Atg) and membrane trafficking proteins [76]. Starvation induces mitochondrial fusion that will protect mitochondria from autophagy [62]. This happens because the mitochondrial fragmentation process is inhibited by the phosphorylation of Drp1 at S637 by protein kinase A (PKA). PKA is activated by the high amounts of cyclic adenosine monophosphate (cAMP) in starving cells and by the releasing of Drp1 from syntaxin 17 (Stx17), a protein at the ER-mitochondria interface [62,77,78]. Some evidence has emerged involving mitochondria and autophagy, including the finding that isolation membranes (also known as phagophoros), the first membrane structure in the autophagy process, can be formed at the mitochondria-associated membrane (MAM) of the ER. Several auto phagosomal markers have been localized in mitochondria, including Atg9 (in yeast), Beclin-1, Atg5, and Bif-1 [79,80]. Hayley and colleagues demonstrated that tail-anchored OMMs proteins also mark the membranes of auto phagosomes and that lipids are capable to transfer from mitochondria to auto phagosomes [81]. These autophagy events did not happen in absence of the OMM protein Mnf2, also involved in mitochondrial fusion [78].
The molecular mechanisms involved in autophagy are conserved in yeast and metazoans [82]. Zebrafish is not the exception and many reporter lines for genes related to autophagy are already available for study in these animals including two yeast Atg8 homologs: light-chain 3 (lc3) and GABA type A receptor-associated protein (gabarap) [81]. These transgenic lines helped to reveal that zebrafish lc3 suffers post-translational modifications during the pharyngula stage of embryonic development and that Gabarap accumulates in lysosomes after autophagy induction [81]. Moreover, studies in zebrafish have revealed that autophagy is highly active in zebrafish embryos and the use of rapamycin, an inhibitor of the mTOR pathway, and calpeptin, an inhibitor of the calpain protein, have resulted in increased activity of this process [81]. Varga and collaborators showed that the genetic and pharmaceutical inhibition of autophagy compromised cell regeneration in an amputated caudal fin model [83]. It was showed that up regulation of autophagy in the regeneration zone protected the cells from going into apoptosis by acting downstream of MAPK/ERK pathway, demonstrating that autophagy is an important process to tissue renewal during caudal fin regeneration in zebrafish [83]. On the other hand, different studies have pointed to autophagy as a protective process for neurodegenerative diseases with the accumulation of mis-folded proteins like amyotrophic lateral sclerosis (ALS), as well as Parkinson's (PD), Alzheimer's (AD), and Huntington's (HD) diseases [84], [85], [86], [87], [88], [89]. Nowadays there are different models of these diseases in the zebrafish that resume specific characteristics of each condition (see Wager et al. 2013 for review) and that can be of great utility in the research of autophagy as a therapeutic approach [68,73].
5. Mitophagy
Mitophagy is the process of elimination of defective mitochondria by autophagy. This is because mitochondria can suffer from damage generated from stress and self-generated ROS [76]. Mitophagy also controls the mitochondrial mass in physiological states [83]. To date, at least two different pathways of mitophagy have been related to mammalian cells. The first one involves the PTEN-induced putative kinase 1 (PINK1), a serine/threonine kinase that is normally imported from the cytosol to the inter membrane space (IMS) of the mitochondria in a membrane potential-dependent way [76,90]. In steady-state conditions, PINK1 is cleaved inside the mitochondria by the protease presenilin-associated rhomboid-like protein (PARL), retrotranslocation, and degraded by E3 ubiquitin ligases in the cytosol [91,92]. Whereas in damaged mitochondria, there is a loss of membrane potential, PINK1 attaches to the OMM, enabling the binding and activation of the E3 ubiquitin ligase Parkin. Some authors defend that Parkin is activated by binding to a PINK1-phosphorylated Mfn2 while others believe that Parkin is activated directly by PINK1 [93,94]. When Parkin is activated, it mediates the ubiquitylation of several proteins on the OMM, recruiting the autophagosome and leading to the degradation of this organelle [52]. Autosomal recessive mutations in PINK1 and Parkin have been involved with Parkinson's disease in humans, proving evidence that mitochondrial damage is related to this disease [93].
Studies in zebrafish have identified two mitochondrial matrix proteins called the 4-Nitrophenylphosphatase domain and non-neuronal SNAP25-like protein homolog 1 (NIPSNAP1) and 2 (NIPSNAP2), that gather on the OMM due to mitochondrial depolarization and recruit proteins for PARKIN-dependent mitophagy [95]. Moreover, Nipsnap1-deficient zebrafish present accumulation of ROS, reduction of the number of dopaminergic neurons, and alterations in locomotion, characteristic of a PD phenotype [95]. The pink1-mutant is an established model of PD in zebrafish and can be rescued by inactivation of Mitochondrial Calcium Uni-porter (MCU) in dopaminergic neurons [96]. Zebrafish has been also used as a model of Methyl-Malonic Acidemia (MMA), due to the lack of methylmalonyl-CoA mutase (MMUT), a mitochondrial enzyme that participates in the metabolism of fatty acids, such as propionate, and amino acids like isoleucine, methionine, valine, and threonine [97]. The MMUT-deficient zebrafish displayed an accumulation of MMA in liver and kidney cells, with altered mitochondrial morphology, impaired bioenergetics, and high oxidative stress response [98]. These authors also demonstrated that the accumulation of MMA compromised the PINK1-PARKIN pathway affecting the mitophagy system [98].
Another pathway described in mammals involves the OMM protein FUN14 domain-containing protein 1 (FUNDC1) that contains an LC3-interacting region (LIR) that binds to LC3 in auto phagosomes [99]. During hypoxia FUNDC1 expression is increased and dephosphorylated by PGAM5, which allows its association with LC3, leading to auto phagosome engulfment and degradation of mitochondria [99]. Zebrafish Fundc1 is also involved in mitophagy and has been shown to be crucial for body axis development [100]. This way, zebrafish fundc1 knockdown results in deregulation of genes related to autophagy and apoptosis, in addition to reduced expression of some neural genes [100]. BDN2 gene is also involved in mitophagy processes in zebrafish. BDN2 is a conserved gene responsible for the formation of siderophores to facilitate the transport of iron inside the mitochondria [101,102]. Moreover, BDH2 expression acts during erythroid by averting mitophagy processes, as inactivation of bdh2 leads to increased erythroid immaturity in zebrafish embryos, and heme deficient cells [101,102].
6. Mitochondria and Zebrafish Immune System
The zebrafish immune system has shown different temporal activation in which the innate immune system is active 48 h post-fertilization (HPF) while the adaptive immune system is completely mature around 4–6 weeks post-fertilization (wpf). The maturation of cells differs from zebrafish to human: while in mammals the hematopoietic stem cells (HSCs) develop in the yolk sac and later on the bone marrow [103,104], in the zebrafish the HSCs develops on the ventral endothelial cells of the dorsal aorta, then migrate to the posterior cardinal vein, afterward, moves to the caudal hematopoietic tissue (CHT), around 48 hpf, where starts to proliferate. The HSCs then moves from the CHT to pronephros and thymus, and by the sixth-day post fertilization (dpf) the HSCs development moves to its final and definitive place: the kidney marrow] [105,106]. The development of the adaptive immune system begins around 3 dpf with V(D)J rag1–2 dependent mechanism, but T- and B-cells are activated and circulating at 4–6 weeks post-fertilization (WPF) [107], [108], [109]. Moreover, zebrafish B cells do not undergo class switching and express only IgM, IgD, and IgZ/T subtypes, being the last a teleost exclusive immunoglobulin [110], [111], [112]. Thus, the temporal distance between the appearances of the first innate immune cells and the first adaptive immune cells allows compartmentalization of the study of the immune system in the zebrafish.
The immune system consists of a series of specialized cell types that adapt in response to various danger signals from both pathogens and the host itself, to maintain homeostasis. Similarly, the metabolism of these cells also needs to adapt, responding to the availability of substrates and the changing metabolic needs of each cell to perform their effector functions [113]. Innate immune cells, especially macrophages and neutrophils, have been extensively studied concerning the contribution of cellular metabolism to the exercise of their functions in a given tissue in inflammatory and infectious contexts [114,115]. Macrophages are highly plastic cells, comprising a vastly heterogeneous population [116]. Their main functions are the recognition of molecular patterns associated with damage and pathogens (DAMPs and PAMPs, respectively), the elimination of pathogens through the synthesis of ROS and by phagocytic mechanisms, the removal of cellular debris, and the production of cytokines [117], [118], [119]. The mitochondrial ROS production is essential in cases of infection in zebrafish, Mycobacterium marinum- infected macrophages have increased ROS production and, as consequence, the necrosis pathway is activated once induced by TNF-α [120]. In this sense, the mtROS production can be evaluated by MitoTracker Red CM-H2Xros, as mitochondrion upon oxidation fluoresces red (Table 1) [120,121]. The mtROS production was assessed as well by Wu and collaborators, demonstrating that zebrafish macrophages were injected with monosodium urate (MSU) crystals and treated with liposomes loaded with L-Etomoxir and L-MitoTEMPO have inhibited mtROS, seen with MitoSOX technique (Table 1). Moreover, the macrophages treated with L-Etomoxir and L-MitoTEMPO had lower interleukin (IL) -1β and TNF-α expression and narrowed the neutrophil recruitment [122].
Furthermore, they can promote the synthesis of molecules that act on tissue remodeling, leading to tissue regeneration, repair, or fibrosis [123]. When activated, these cells may show different profiles, depending on the microenvironment in which they are inserted [124].
In in vitro models, M0 macrophages are polarized to the pro-inflammatory macrophages (also known as M1) or classically activated when stimulated by IFN-γ or lipopolysaccharide (LPS), whereas macrophages are converted to an anti-inflammatory or pro-resolving phenotype (M2) when stimulated by cytokines such as IL-4 or IL-13. In addition, macrophage polarization can be regulated by intracellular signals, soluble proteins, and transcription factors, such as when Toll-like receptor signaling activates the signal transducer and activator of transcription (STAT) 1 protein which leads macrophages towards an M1-like phenotype [125]. Despite being a somewhat simplistic nomenclature when dealing with in vivo models, this is a means of studying metabolism and other biological processes and correlates it with the phenotypic profile of these cells [126]. This polarization process has also been described in teleosts, characterized by inducible nitric oxide synthase (iNOS) and arginase 2 (Arg2) as markers of M1 and M2-like cells, respectively [127]. Intriguingly, in vitro studies have been carried out, and TLR4, the mammalian receptor of LPS, may not be functional in these fishes [128]. In this sense, it is suggested that LPS may be sensed through distinct mechanisms since this microbial stimulus is thought to be an important immune stimulator [129]. Importantly, mammals retain single IFN-γ molecules which are important to skew M1 macrophages. In contrast, many teleosts display multiple IFN-γ that have distinct effects, some eliciting the production of strictly ROS, but not nitric oxide (NO), whilst others NO and limited ROS [130,131]. Pro-inflammatory macrophages undergo a metabolic program using glycolysis, pentose-phosphate pathway (PPP), and fatty acid synthesis. M1 macrophages show a decrease in oxygen consumption compared to M0 cells, suggesting little dependence on mitochondrial metabolism, with the synthesis of ATP mainly through glycolysis [132,133]. In opposition to that, anti-inflammatory macrophages have an enhanced OXPHOS metabolism besides FAO, glutaminolysis, and tryptophan catabolism [124,134]
7. Relationship between mitochondrial metabolism and immune system in zebrafish
Mitochondrial metabolic processes have been associated with inflammatory responses and tissue damage [135,136]. After tissue damage, an inflammatory response is triggered and blood leukocytes pass through the endothelium and go to the injured area [137]. The first cells to participate in the inflammatory response are neutrophils, followed by macrophages, which have the function of cleaning the surrounding debris through efferocytosis [138]. Biological processes in the mitochondria are involved in phagocytosis, specifically, through the participation of the mitochondrial membrane protein (UCP) decoupling protein 2. A study by Park and collaborators showed that the loss of Ucp2 reduces the phagocytic capacity of the mouse macrophage, and the increase of Ucp2 expression is associated with increased macrophage phagocytization [139]. Carassius auratus, a teleost fish, demonstrated a unique response upon a challenge with zymosan. This fish possesses <5% of neutrophils at the total fraction of circulating leukocytes during homeostasis, but when this animal model was challenged it represented up to 50% of the total. In this study, neutrophils were isolated at 18 and 48 h post-injection, time-points equivalent to pro-inflammatory and pro-resolving phases, respectively. Interestingly, these cells displayed different phenotypes, with the first cells having a robust respiratory burst, releasing leukotriene B4 and boosting macrophage ROS production. Contrastingly, the cells isolated during the pro-resolving phase produced low levels of ROS, released lipoxin A4, which is a pro-resolving lipid mediator that contributed to the uptake of apoptotic cells by macrophages [140].
Mitochondrial fission, mediated by Drp1 protein, is also required in efferocytosis processes. Drp1 induces the release of calcium from the endoplasmic reticulum, a process necessary for the formation of phagosomes and elimination of apoptotic cells by macrophages [141]. Therefore, mitochondrial fission allows macrophages to eliminate several apoptotic bodies and avoid pathological consequences of not eliminating these apoptotic remains. A failure in efferocytosis can lead to important diseases, such as autoimmune diseases and atherosclerosis [141]. Another efferocytosis-related disease is Covid-19. The SARS-CoV-2 virus induces impaired immune responses and elevated levels of inflammatory cytokines that can inhibit the expression of efferocytic receptors, causing damage to continuous macrophage efferocytosis. It was also shown that monocytes from patients who developed severe COVID-19 also expressed reduced levels of efferocytic receptors and did not capture apoptotic cells. This dysfunctional efferocytosis of cell bodies infected with SARS-CoV-2 impairs the anti-inflammatory role of macrophages and the efficiency in tissue repair. Studies of this relationship between mitochondria and efferocytosis processes in zebrafish are still very scarce [142].
Studies in teleost fish evaluated the metabolism of M1 and M2 macrophages in basal and under stress conditions. By real-time measurements of oxygen consumption and lactate acidification, it was found that M1 macrophages have an increase in nitric oxide synthesis and expression of immuno responsive gene 1 (irg1), in addition to alteration of the glycolytic pathway. In M2 macrophages, an increase in arginase activity was demonstrated, with glycolytic and OXPHOS pathways similar to the control group. These results suggest that metabolic reprogramming is related to different macrophage phenotypes, similar to what happens in mammals [143]. Studies involving zebrafish irg1 have revealed changes in glucose and lipid metabolism of immune cells during infection. In response to Salmonella typhimurium infection, macrophages showed an increased expression of irg1, a mitochondria-related enzyme, which induces the catabolism of fatty acids to sustain OXPHOS leading to the production of mtROS [144]. This contributed to the killing of the bacteria during phagocytosis, demonstrating that this enzyme is a critical component of the immuno metabolism axis, relating infection, cell metabolism, and macrophage effector function [144]. In a zebrafish model of tuberculosis, the excess of TNF induces mtROS formation in infected macrophages, increasing their micro bicidal activity but also inducing necroptosis, allowing the mycobacteria to be released to the extracellular medium [120]. This pathway is activated by the mitochondrial cyclophilin D and the production of ceramide mediated by acid sphingomyelinase. When this pathway is chemically inhibited, the effect is reversed [120,145]. This exposes the potential of zebrafish for therapeutic compound testing in mitochondria-related conditions.
Another study related the mitochondrial function to the effector function of macrophages against the bacterial activity, by demonstrating, in mouse, that the activation of the Toll-like receptors 1, 2, and 4 (TLR1, TLR2, and TLR4) lead to the recruitment of mitochondria to the phagosomes on these cells, increasing the production of mtROS [146]. Also, the response has been shown to involve translocation of the TLR signaling adapter tumor necrosis factor receptor-associated factor 6 (TRAF6) into the mitochondria, where TRAF6 joins to the cytosolic adaptor protein evolutionarily conserved signaling intermediate in Toll pathways (ECSIT), a protein related to the formation of the mitochondrial respiratory chain [146,147]. The interaction of this protein with TRAF6 leads to the ubiquitination of ECSIT, which results in increased synthesis of cellular and mitochondrial ROS. In the absence of TRAF6 or ECSIT in macrophages, the ability of these cells to eliminate intracellular pathogens is reduced, due to the lower capacity for generating ROS mediated by TLR. [146]. Different studies have demonstrated functional conservation of TRAF6 in zebrafish, but still, there are no reports of its translocation to the mitochondria in this model [148,149].
The mitochondria are signaling centers for apoptosis and inflammation leading to the generation of mtROS in the form of by-products of the ETC, which, in turn, impacts significantly in cellular signaling pathways [150]. Peterman and collaborators demonstrated the role of the antioxidant enzyme SOD2 in the regulation of mtROS in zebrafish larvae. In response to an infection by Pseudomonas aeruginosa, sod2-deficient zebrafish exhibited a greater bacterial burden, increased mortality, and decreased the number of phagocytes, suggesting that sod2 increase innate immune function by protecting macrophages and neutrophils against the negative effects of superoxide mitochondrial [150]. Another study showed that sod1−/- zebrafish, knockout with CRISP/Cas9 technology (fig. 2B), resulted in an alteration of the motility of neutrophils. Also, the specific disruption of mitochondrial DNA polymerase (polg) in neutrophils, significantly reduced the speed of their interstitial migration. The inhibition of ETC or the enzymes that reduce reactive oxygen species also caused effects on phagocyte motility, suggesting that mitochondria influence the behavior of immune cells through their redox status [151]. Mammalian adaptive immune cells that do not undergo activation, such as naïve T-cells, or that have to survive for long terms like memory T-cells, or have a regulatory profile like regulatory T cells, have a metabolism predominated by OXPHOS and/or FAO [152]. In contrast, effector Th1, Th2, and Th17 cells express high levels of the glucose transporter Glut1 and are highly glycolytic [153]. In the case of CD8+ T-cells, when they are at the naïve stage, they rely mainly on OXPHOS for their metabolic sustain and turn towards aerobic glycolysis, PPP, and glutaminolysis when activated [154]. However, this shift is not total as OXPHOS levels increase in parallel to glycolysis in CD8+ T-cells [154]. On the other hand, memory CD8+ T-cells are reduced in glycolysis and show a higher OXPHOS capacity than naïve cells and rely on FAO as other memory T-cells with a larger mitochondrial mass than effector cells [155,156]. However, those metabolic changes seen in T-cells have yet to be confirmed in the zebrafish model.
There is still much to be assessed regarding the role of mitochondrial metabolism in the performance of immune system cells. In vivo models like zebrafish can be a powerful tool to be used in this field and give answers to the study of mitochondrial functions in physiological and pathological contexts.
8. Conclusions
The data exposed here demonstrate how adaptable the zebrafish is to achieve different levels of mitochondrial function analysis. The zebrafish appears an interesting model to elucidate several questions regarding immuno metabolism and mitochondrial processes because of its genetic convergence with the human and conservation of signaling pathways with mammals [1,3,4,8,45,107,108,110,118]. These factors and the considerable availability of disease models and transgenic lines can help elucidate key questions for the mitochondria research field using an in vivo model, using different techniques, such as genetic tools, transcriptomic and proteomic data networks, metabolic assays, fluorescence detection techniques, and many others [[25], [26], [27], [28], [29], [30], [31], [32],37,84,[157], [158], [159], [160], [161], [162], [163], [164], [165], [166], [167]]. These techniques allowed the study of GDAP1 and OPA1 gene function in the zebrafish mitochondrial fission [68]. Other important genes for mitochondrial dynamics, such as Mnf1, mfn2, opa1, dnml-1 were identified in zebrafish [66]. But genes, such as Drp1, are yet to be studied [67].
The mitochondria function is involved as well in immune functions in zebrafish, being associated with the macrophages profile. Similar to mammals, zebrafish present macrophages with a different metabolic set up in the nitric oxide synthesis, glycolytic and OXPHOS pathways, increased production of mtROS in response to infections [120,143,145]. The mtROS production is linked with the antioxidant enzyme SOD2, while SOD1 is associated with neutrophil motility [150,151].
Even though the studies about zebrafish are increasing daily, the literature still lacks important matters concerning the immunology field. The differences between immune responses in zebrafish, and other experimental models are still to be elucidated. This review aims to improve the research and guide the researcher through the discoveries and techniques made so far in the zebrafish field. However, it is important to know the advantages and disadvantages of each model and technique combination depending on the objectives. The development and refinement of these tools will help researchers to improve zebrafish mitochondrial studies and create new options to analyze mitochondrial dysfunctions and diseases.
Author Contributions
Mariana A. Amaral: Writing – Original Draft, Review & Editing, Visualization. Lais C. Paredes: Writing – Original Draft, Review & Editing. Barbara N. Padovani: Writing – Original Draft, Review & Editing. Juliana M. M. Gomes: Writing – Original Draft, Review & Editing. Luan F. Montes: Writing - Review & Editing. Niels O. S. Câmara: Writing – Review, Supervision. Camila M. Fénero: Writing – Original Draft, Review & Editing, Review, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We kindly thank Paulo José Basso and Bruno Ghirotto for the suggestions and comments on the manuscript. This work was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP- grants n 2017/05264-7, 2015/21644-9), the Conselho Nacional de desenvolvimento Científico e Tecnológico (CNPq), and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Brasil (CAPES) – Financial Code 001.
Contributor Information
Mariana Abrantes do Amaral, Email: amaral.mariana@unifesp.br.
Camila Morales Fénero, Email: ci.moralesfe@alumni.usp.br.
References
- 1.Mookerjee S.A., Goncalves R.L.S., Gerencser A.A., Nicholls D.G., Brand M.D. The contributions of respiration and glycolysis to extracellular acid production. Biochim. Biophys. Acta. Feb 2015;1847(2):171–181. doi: 10.1016/j.bbabio.2014.10.005. (in eng) [DOI] [PubMed] [Google Scholar]
- 2.Nolfi-Donegan D., Braganza A., Shiva S. Mitochondrial electron transport chain: oxidative phosphorylation, oxidant production, and methods of measurement. Redox. Biol. 2020;37 doi: 10.1016/j.redox.2020.101674. (in eng) 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akram M. Citric acid cycle and role of its intermediates in metabolism. Cell Biochem. Biophys. Apr 2014;68(3):475–478. doi: 10.1007/s12013-013-9750-1. (in eng) [DOI] [PubMed] [Google Scholar]
- 4.Twig G., Shirihai O.S. The interplay between mitochondrial dynamics and mitophagy. Antioxid. Redox. Signal. May 2011;14(10):1939–1951. doi: 10.1089/ars.2010.3779. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spinelli J.B., Haigis M.C. The multifaceted contributions of mitochondria to cellular metabolism. Nat Cell Biol. 2018;20(7):745–754. doi: 10.1038/s41556-018-0124-1. (in eng) 07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WARBURG O. On the origin of cancer cells. Science. Feb 1956;123(3191):309–314. doi: 10.1126/science.123.3191.309. (in eng) [DOI] [PubMed] [Google Scholar]
- 7.Chandel N.S. Mitochondria as signaling organelles. BMC Biol. May 2014;12:34. doi: 10.1186/1741-7007-12-34. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Souza Breda C.N., Davanzo G.G., Basso P.J., Câmara N.O.S., Moraes-Vieira P.M.M. Mitochondria as central hub of the immune system. Redox Biol. 2019;26 doi: 10.1016/j.redox.2019.101255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wai T., Langer T. Mitochondrial dynamics and metabolic regulation. Trends Endocrinol. Metab. 2016;27(2):105–117. doi: 10.1016/j.tem.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Azevedo R.D.S., Falcão K.V.G., Amaral I.P.G., Leite A.C.R., Bezerra R.S. Mitochondria as targets for toxicity and metabolism research using zebra fish. Biochim. Biophys. Acta Gen. Subj. 2020;1864(8) doi: 10.1016/j.bbagen.2020.129634. (in eng) 08. [DOI] [PubMed] [Google Scholar]
- 11.Streisinger G., Walker C., Dower N., Knauber D., Singer F. Production of clones of homozygous diploid zebra fish (Brachydanio rerio) Nature. 1981;291(5813):293–296. doi: 10.1038/291293a0. [DOI] [PubMed] [Google Scholar]
- 12.Streisinger G., Coale F., Taggart C., Walker C., Grunwald D.J. Clonal origins of cells in the pigmented retina of the zebrafish eye. Dev. Biol. Jan 1989;131(1):60–69. doi: 10.1016/s0012-1606(89)80038-7. (in eng) [DOI] [PubMed] [Google Scholar]
- 13.Privat M., Sumbre G. Naturalistic behavior: the zebra fish larva strikes back. Curr. Biol. 2020;30(1):R27–R29. doi: 10.1016/j.cub.2019.11.014. [DOI] [PubMed] [Google Scholar]
- 14.Hanyang L., et al. Application of zebra fish models in inflammatory bowel disease. Front. Immunol. 2017;8:501. doi: 10.3389/fimmu.2017.00501. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Z. R, "Um peixe modelo," vol. Jul:16-21., G. M., Ed., ed. Guimarães M.: Pesquisa fapesp, 2013.
- 16.Poureetezadi S.J., Wingert R.A. Little fish, big catch: zebra fish as a model for kidney disease. Kidney Int. 2016;89(6):1204–1210. doi: 10.1016/j.kint.2016.01.031. (in eng) 06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howe K., et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature. Apr 2013;496(7446):498–503. doi: 10.1038/nature12111. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varshney G.K., Burgess S.M. Mutagenesis and phenotyping resources in zebrafish for studying development and human disease. Brief. Funct. Genom. 2014;13(2):82–94. doi: 10.1093/bfgp/elt042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu S., Leach S.D. Zebra fish models for cancer. Annu. Rev. Pathol.: Mech. Dis. 2011;6:71–93. doi: 10.1146/annurev-pathol-011110-130330. [DOI] [PubMed] [Google Scholar]
- 20.Gerlai R., Lahav M., Guo S., Rosenthal A. Drinks like a fish: zebra fish (Danio rerio) as a behavior genetic model to study alcohol effects. Pharmacol. Biochem. Behav. 2000;67(4):773–782. doi: 10.1016/s0091-3057(00)00422-6. [DOI] [PubMed] [Google Scholar]
- 21.Best J., Alderton W.K. Zebrafish: an in vivo model for the study of neurological diseases. Neuropsychiatric Dis. Treat. 2008;4(3):567. doi: 10.2147/ndt.s2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santoriello C., Zon L.I. Hooked! modeling human disease in zebra fish. J. Clin. Invest. 2012;122(7):2337–2343. doi: 10.1172/JCI60434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Focusing on mitochondrial form and function. Nat Cell Biol. 2018;20(7):735. doi: 10.1038/s41556-018-0139-7. (in eng) 07. [DOI] [PubMed] [Google Scholar]
- 24.Fichi G., et al. Fishing in the cell powerhouse: zebra fish as a tool for exploration of mitochondrial defects affecting the nervous system. Int. J. Mol. Sci. May 2019;20(10) doi: 10.3390/ijms20102409. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bill B.R., Petzold A.M., Clark K.J., Schimmenti L.A., Ekker S.C. A primer for morpholino use in zebrafish. Zebrafish. Mar 2009;6(1):69–77. doi: 10.1089/zeb.2008.0555. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cornet C., Di Donato V., Terriente J. Combining zebra fish and CRISPR/Cas9: toward a more efficient drug discovery pipeline. Front. Pharmacol. 2018;9:703. doi: 10.3389/fphar.2018.00703. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosen J.N., Sweeney M.F., Mably J.D. Microinjection of zebra fish embryos to analyze gene function. J. Vis. Exp. Mar 2009;(25) doi: 10.3791/1115. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bian W.P., Chen Y.L., Luo J.J., Wang C., Xie S.L., Pei D.S. Knock-in strategy for editing human and zebra fish mitochondrial DNA Using Mito-CRISPR/Cas9 System. ACS Synth. Biol. 2019;8(4):621–632. doi: 10.1021/acssynbio.8b00411. (in eng) 04. [DOI] [PubMed] [Google Scholar]
- 29.Plucińska G., et al. In vivo imaging of disease-related mitochondrial dynamics in a vertebrate model system. J. Neurosci. Nov 2012;32(46):16203–16212. doi: 10.1523/JNEUROSCI.1327-12.2012. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noble S., Godoy R., Affaticati P., Ekker M. Transgenic zebra fish expressing m-cherry in the mitochondria of dopaminergic neurons. Zebrafish. Oct 2015;12(5):349–356. doi: 10.1089/zeb.2015.1085. (in eng) [DOI] [PubMed] [Google Scholar]
- 31.Kawakami K., Asakawa K., Hibi M., Itoh M., Muto A., Wada H. Gal4 driver transgenic zebra fish: powerful tools to study developmental biology, organogenesis, and neuroscience. Adv. Genet. 2016;95:65–87. doi: 10.1016/bs.adgen.2016.04.002. (in eng) [DOI] [PubMed] [Google Scholar]
- 32.O'Donnell K.C., Vargas M.E., Sagasti A. WldS and PGC-1α regulate mitochondrial transport and oxidation state after axonal injury. J. Neurosci. Sep 2013;33(37):14778–14790. doi: 10.1523/JNEUROSCI.1331-13.2013. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Artuso L., et al. Mitochondrial DNA metabolism in early development of zebra fish (Danio rerio) Biochim. Biophys. Acta. Jul 2012;1817(7):1002–1011. doi: 10.1016/j.bbabio.2012.03.019. (in eng) [DOI] [PubMed] [Google Scholar]
- 34.Steele S.L., Prykhozhij S.V., Berman J.N. Zebra fish as a model system for mitochondrial biology and diseases. Transl. Res. Feb 2014;163(2):79–98. doi: 10.1016/j.trsl.2013.08.008. (in eng) [DOI] [PubMed] [Google Scholar]
- 35.Broughton R.E., Milam J.E., Roe B.A. The complete sequence of the zebra fish (Danio rerio) mitochondrial genome and evolutionary patterns in vertebrate mitochondrial DNA. Genome Res. Nov 2001;11(11):1958–1967. doi: 10.1101/gr.156801. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muller F. The nature and mechanism of superoxide production by the electron transport chain: its relevance to aging. J. Am. Aging Assoc. Oct 2000;23(4):227–253. doi: 10.1007/s11357-000-0022-9. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Müller T.E., et al. Neurochemical mechanisms underlying acute and chronic ethanol-mediated responses in zebrafish: The role of mitochondrial bioenergetics. Neurochem. Int. 2019;131 doi: 10.1016/j.neuint.2019.104584. (in eng) 12. [DOI] [PubMed] [Google Scholar]
- 38.Wiens L., et al. Comparison of mitochondrial reactive oxygen species production of ectothermic and endothermic fish muscle. Front. Physiol. 2017;8:704. doi: 10.3389/fphys.2017.00704. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Banh S., Wiens L., Sotiri E., Treberg J.R. Mitochondrial reactive oxygen species production by fish muscle mitochondria: potential role in acute heat-induced oxidative stress. Comp. Biochem. Physiol. B Biochem. Mol. Biol. Jan 2016;191:99–107. doi: 10.1016/j.cbpb.2015.10.001. (in eng) [DOI] [PubMed] [Google Scholar]
- 40.Chandel N.S., Maltepe E., Goldwasser E., Mathieu C.E., Simon M.C., Schumacker P.T. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc. Natl. Acad. Sci. U S A. Sep 1998;95(20):11715–11720. doi: 10.1073/pnas.95.20.11715. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Slemc L., Kunej T. Transcription factor HIF1A: downstream targets, associated pathways, polymorphic hypoxia response element (HRE) sites, and initiative for standardization of reporting in scientific literature. Tumour Biol. Nov 2016;37(11):14851–14861. doi: 10.1007/s13277-016-5331-4. (in eng) [DOI] [PubMed] [Google Scholar]
- 42.Filho D.Wilhelm. Reactive oxygen species, antioxidants and fish mitochondria. Front. Biosci. Jan 01 2007;12:1229–1237. doi: 10.2741/2141. (in eng) [DOI] [PubMed] [Google Scholar]
- 43.Chen J., Guan L., Zou M., He S., Li D., Chi W. Specific cyprinid HIF isoforms contribute to cellular mitochondrial regulation. Sci. Rep. 2020;10(1):17246. doi: 10.1038/s41598-020-74210-w. (in eng) 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Metallo C.M., Vander Heiden M.G. Metabolism strikes back: metabolic flux regulates cell signaling. Genes. Dev. Dec 2010;24(24):2717–2722. doi: 10.1101/gad.2010510. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wai T., Langer T. Mitochondrial dynamics and metabolic regulation. Trends Endocrinol. Metab. Feb 2016;27(2):105–117. doi: 10.1016/j.tem.2015.12.001. (in eng) [DOI] [PubMed] [Google Scholar]
- 46.Traaseth N., Elfering S., Solien J., Haynes V., Giulivi C. Role of calcium signaling in the activation of mitochondrial nitric oxide synthase and citric acid cycle. Biochim. Biophys. Acta. Jul 2004;1658(1-2):64–71. doi: 10.1016/j.bbabio.2004.04.015. (in eng) [DOI] [PubMed] [Google Scholar]
- 47.Glancy B., Willis W.T., Chess D.J., Balaban R.S. Effect of calcium on the oxidative phosphorylation cascade in skeletal muscle mitochondria. Biochemistry. Apr 2013;52(16):2793–2809. doi: 10.1021/bi3015983. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin L.Y., Yeh Y.H., Hung G.Y., Lin C.H., Hwang P.P., Horng J.L. Role of Calcium-Sensing Receptor in Mechanotransducer-Channel-Mediated Ca. Front. Physiol. 2018;9:649. doi: 10.3389/fphys.2018.00649. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pan T.C., Liao B.K., Huang C.J., Lin L.Y., Hwang P.P. Epithelial Ca(2+) channel expression and Ca(2+) uptake in developing zebra fish. Am. J. Physiol. Regul. Integr. Comp. Physiol. Oct 2005;289(4):R1202–R1211. doi: 10.1152/ajpregu.00816.2004. (in eng) [DOI] [PubMed] [Google Scholar]
- 50.Otten A.B., et al. Tfam knockdown results in reduction of mtDNA copy number, OXPHOS deficiency and abnormalities in zebra fish embryos. Front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dorn G.W. Evolving concepts of mitochondrial dynamics. Annu. Rev. Physiol. 2019;81:1–17. doi: 10.1146/annurev-physiol-020518-114358. [DOI] [PubMed] [Google Scholar]
- 52.Labbé K., Murley A., Nunnari J. Determinants and functions of mitochondrial behavior. Annu. Rev. Cell Dev. Biol. 2014;30:357–391. doi: 10.1146/annurev-cellbio-101011-155756. [DOI] [PubMed] [Google Scholar]
- 53.Ramachandran R. Vol. 76. Elsevier; 2018. Mitochondrial dynamics: the dynamin superfamily and execution by collusion; pp. 201–212. (Seminars in Cell & Developmental Biology). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rambold A.S., Kostelecky B., Elia N., Lippincott-Schwartz J. Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation. Proc. Natl. Acad. Sci. 2011;108(25):10190–10195. doi: 10.1073/pnas.1107402108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yao C.-H., Wang R., Wang Y., Kung C.-P., Weber J.D., Patti G.J. Mitochondrial fusion supports increased oxidative phosphorylation during cell proliferation. E life. 2019;8:e41351. doi: 10.7554/eLife.41351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Friedman J.R., Lackner L.L., West M., DiBenedetto J.R., Nunnari J., Voeltz G.K. ER tubules mark sites of mitochondrial division. Science. 2011;334(6054):358–362. doi: 10.1126/science.1207385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xie J.H., Li Y.Y., Jin J. The essential functions of mitochondrial dynamics in immune cells. Cell Mol. Immunol. 2020;17(7):712–721. doi: 10.1038/s41423-020-0480-1. (in eng) 07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kraus F., Ryan M.T. The constriction and scission machineries involved in mitochondrial fission. J. Cell Sci. Sep 2017;130(18):2953–2960. doi: 10.1242/jcs.199562. (in eng) [DOI] [PubMed] [Google Scholar]
- 59.Hollenbeck P.J., Saxton W.M. The axonal transport of mitochondria. J. Cell Sci. 2005;118(23):5411–5419. doi: 10.1242/jcs.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liesa M., Shirihai O.S. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab. 2013;17(4):491–506. doi: 10.1016/j.cmet.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Youle R.J., Narendra D.P. Mechanisms of mitophagy. Nat. Rev. Mol. Cell Biol. 2011;12(1):9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gomes L.C., Di Benedetto G., Scorrano L. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat. Cell Biol. May 2011;13(5):589–598. doi: 10.1038/ncb2220. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kary C. Splitting up for mitophagy. Nat Cell Biol. 2018;20(3):224. doi: 10.1038/s41556-018-0058-7. (in eng) 03. [DOI] [PubMed] [Google Scholar]
- 64.Estaquier J., Arnoult D. Inhibiting Drp1-mediated mitochondrial fission selectively prevents the release of cytochrome c during apoptosis. Cell Death Differ. Jun 2007;14(6):1086–1094. doi: 10.1038/sj.cdd.4402107. (in eng) [DOI] [PubMed] [Google Scholar]
- 65.Lewis S.C., Uchiyama L.F., Nunnari J. ER-mitochondria contacts couple mtDNA synthesis with mitochondrial division in human cells. Science. 2016;353(6296) doi: 10.1126/science.aaf5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wager K., Russell C. Mitophagy and neuro degeneration: the zebra fish model system. Autophagy. Nov 2013;9(11):1693–1709. doi: 10.4161/auto.25082. (in eng) [DOI] [PubMed] [Google Scholar]
- 67.Shoval Y., Berissi H., Kimchi A., Pietrokovski S. New modularity of DAP-kinases: alternative splicing of the DRP-1 gene produces a ZIPk-like isoform. PLoS One. Mar 08 2011;6(2):e17344. doi: 10.1371/journal.pone.0017344. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Eijkenboom I., et al. A zebrafish model to study small-fiber neuropathy reveals a potential role for GDAP1. Mitochondrion. 2019;47:273–281. doi: 10.1016/j.mito.2019.01.002. (in eng) 07. [DOI] [PubMed] [Google Scholar]
- 69.Kim M.J., Kang K.H., Kim C.H., Choi S.Y. Real-time imaging of mitochondria in transgenic zebra fish expressing mitochondrially targeted GFP. BioTechniques. Sep 2008;45(3):331–334. doi: 10.2144/000112909. (in eng) [DOI] [PubMed] [Google Scholar]
- 70.Sheng W., et al. Effect of Resveratrol on Sirtuins, OPA1, and Fis1 expression in adult Zebra fish Retina. Invest. Ophthalmol. Vis. Sci. 2018;59(11):4542–4551. doi: 10.1167/iovs.18-24539. (in eng) 09. [DOI] [PubMed] [Google Scholar]
- 71.Wang N., et al. Exploration of age-related mitochondrial dysfunction and the anti-aging effects of resveratrol in zebra fish retina. Aging (Albany NY) 2019;11(10):3117–3137. doi: 10.18632/aging.101966. (in eng) 05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vaz R.L., Outeiro T.F., Ferreira J.J. Zebra fish as an animal model for drug discovery in Parkinson's Disease and other movement disorders: a systematic review. Front Neurol. 2018;9:347. doi: 10.3389/fneur.2018.00347. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sun C., Wang Z., Yue L., Huang Q., Cheng Q., Wang R. Supra-molecular induction of mitochondrial aggregation and fusion. J. Am. Chem. Soc. 2020;142(39):16523–16527. doi: 10.1021/jacs.0c06783. (in eng) 09. [DOI] [PubMed] [Google Scholar]
- 74.Bohovych I., et al. Metalloprotease OMA1 fine-tunes mitochondrial bio-energetic function and respiratory super-complex stability. Sci. Rep. Sep 2015;5:13989. doi: 10.1038/srep13989. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rabinowitz J.D., White E. Autophagy and metabolism. Science. Dec 2010;330(6009):1344–1348. doi: 10.1126/science.1193497. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tagaya M., Arasaki K. Regulation of mitochondrial dynamics and autophagy by the mitochondria-associated membrane. Adv. Exp. Med. Biol. 2017;997:33–47. doi: 10.1007/978-981-10-4567-7_3. (in eng) [DOI] [PubMed] [Google Scholar]
- 77.Arasaki K., et al. A role for the ancient SNARE syntaxin 17 in regulating mitochondrial division. Dev. Cell. Feb 2015;32(3):304–317. doi: 10.1016/j.devcel.2014.12.011. (in eng) [DOI] [PubMed] [Google Scholar]
- 78.Hailey D.W., et al. Mitochondria supply membranes for auto-phagosome biogenesis during starvation. Cell. May 2010;141(4):656–667. doi: 10.1016/j.cell.2010.04.009. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reggiori F., Shintani T., Nair U., Klionsky D.J. Atg9 cycles between mitochondria and the pre-auto-phagosomal structure in yeasts. Autophagy. Jul 2005;1(2):101–109. doi: 10.4161/auto.1.2.1840. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kang R., Zeh H.J., Lotze M.T., Tang D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. Apr 2011;18(4):571–580. doi: 10.1038/cdd.2010.191. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.He C., Bartholomew C.R., Zhou W., Klionsky D.J. Assaying autophagic activity in transgenic GFP-Lc3 and GFP-Gabarap zebrafish embryos. Autophagy. May 2009;5(4):520–526. doi: 10.4161/auto.5.4.7768. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yorimitsu T., Klionsky D.J. Autophagy: molecular machinery for self-eating. Cell Death Differ. Nov 2005;12(Suppl 2):1542–1552. doi: 10.1038/sj.cdd.4401765. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hamacher-Brady A., Brady N.R. Mitophagy programs: mechanisms and physiological implications of mitochondrial targeting by autophagy. Cell Mo.l Life Sci. Feb 2016;73(4):775–795. doi: 10.1007/s00018-015-2087-8. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nah J., Yuan J., Jung Y.K. Autophagy in neurodegenerative diseases: from mechanism to therapeutic approach. Mol. Cells. May 2015;38(5):381–389. doi: 10.14348/molcells.2015.0034. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fujikake N., Shin M., Shimizu S. Association between autophagy and neurodegenerative diseases. Front Neurosci. 2018;12:255. doi: 10.3389/fnins.2018.00255. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Marrone L., et al. FUS pathology in ALS is linked to alterations in multiple ALS-associated proteins and rescued by drugs stimulating autophagy. Acta Neuropathol. 2019;138(1):67–84. doi: 10.1007/s00401-019-01998-x. (in eng) 07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hou X., Watzlawik J.O., Fiesel F.C., Springer W. Autophagy in Parkinson's Disease. J. Mol. Biol. 2020;432(8):2651–2672. doi: 10.1016/j.jmb.2020.01.037. (in eng) 04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu J., Li L. Targeting autophagy for the treatment of Alzheimer's disease: challenges and opportunities. Front. Mol. Neurosci. 2019;12:203. doi: 10.3389/fnmol.2019.00203. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Croce K.R., Yamamoto A. A role for autophagy in Huntington's disease. Neurobiol. Dis. 2019;122:16–22. doi: 10.1016/j.nbd.2018.08.010. (in eng) 02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lin W., Kang U.J. Characterization of PINK1 processing, stability, and subcellular localization. J. Neurochem. Jul 2008;106(1):464–474. doi: 10.1111/j.1471-4159.2008.05398.x. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Deas E., et al. PINK1 cleavage at position A103 by the mitochondrial protease PARL. Hum. Mol. Genet. Mar 2011;20(5):867–879. doi: 10.1093/hmg/ddq526. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yamano K., Youle R.J. PINK1 is degraded through the N-end rule pathway. Autophagy. Nov 2013;9(11):1758–1769. doi: 10.4161/auto.24633. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pickrell A.M., Youle R.J. The roles of PINK1, Parkin, and mitochondrial fidelity in Parkinson's disease. Neuron. Jan 2015;85(2):257–273. doi: 10.1016/j.neuron.2014.12.007. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen Y., Dorn G.W. PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science. Apr 2013;340(6131):471–475. doi: 10.1126/science.1231031. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Abudu Y.P., Pankiv S., Mathai B.J., Lamark T., Johansen T., Simonsen A. NIPSNAP1 and NIPSNAP2 act as "eat me" signals to allow sustained recruitment of autophagy receptors during mitophagy. Autophagy. 2019;15(10):1845–1847. doi: 10.1080/15548627.2019.1637642. (in eng) 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Soman S.K., Bazała M., Keatinge M., Bandmann O., Kuznicki J. Restriction of mitochondrial calcium overload by. Biol Open. Oct 2019;8(10) doi: 10.1242/bio.044347. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ballhausen D., Mittaz L., Boulat O., Bonafé L., Braissant O. Evidence for catabolic pathway of propionate metabolism in CNS: expression pattern of methylmalonyl-CoA mutase and propionyl-CoA carboxylase alpha-subunit in developing and adult rat brain. Neuroscience. Dec 2009;164(2):578–587. doi: 10.1016/j.neuroscience.2009.08.028. (in eng) [DOI] [PubMed] [Google Scholar]
- 98.Chen Z., Berquez M., Luciani A. Mitochondria, mitophagy, and metabolic disease: towards assembling the puzzle. Cell Stress. May 2020;4(6):147–150. doi: 10.15698/cst2020.06.222. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu L., et al. Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat. Cell Biol. Jan 2012;14(2):177–185. doi: 10.1038/ncb2422. (in eng) [DOI] [PubMed] [Google Scholar]
- 100.Xu G., et al. Fundc1 is necessary for proper body axis formation during embryogenesis in zebrafish. Sci Rep. 2019;9(1):18910. doi: 10.1038/s41598-019-55415-0. (in eng) 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pant D.C., Nazarko T.Y. Selective autophagy: the rise of the zebra fish model. Autophagy. Dec 2020:1–9. doi: 10.1080/15548627.2020.1853382. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Davuluri G., et al. Inactivation of 3-hydroxybutyrate dehydrogenase 2 delays zebra fish erythroid maturation by conferring premature mitophagy. Proc. Natl. Acad. Sci. U S A. Mar 2016;113(11):E1460–E1469. doi: 10.1073/pnas.1600077113. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Palis J., Yoder M.C. Yolk-sac hematopoiesis: the first blood cells of mouse and man. Exp. Hematol. 2001;29(8):927–936. doi: 10.1016/s0301-472x(01)00669-5. [DOI] [PubMed] [Google Scholar]
- 104.Detrich H., 3rd, et al. Intraembryonic hematopoietic cell migration during vertebrate development. Proc. Natl. Acad. Sci. 1995;92(23):10713–10717. doi: 10.1073/pnas.92.23.10713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Herbomel P., Thisse B., Thisse C. Ontogeny and behavior of early macrophages in the zebra fish embryo. Development. 1999;126(17):3735–3745. doi: 10.1242/dev.126.17.3735. [DOI] [PubMed] [Google Scholar]
- 106.Zhang P., Liu F. In vivo imaging of hematopoietic stem cell development in the zebra fish. Front Med. Sep 2011;5(3):239–247. doi: 10.1007/s11684-011-0123-0. (in eng) [DOI] [PubMed] [Google Scholar]
- 107.Willett C.E., Zapata A.G., Hopkins N., Steiner L.A. Expression of Zebra fishr agGenes during early development identifies the thymus. Dev. Biol. 1997;182(2):331–341. doi: 10.1006/dbio.1996.8446. [DOI] [PubMed] [Google Scholar]
- 108.C. A. Painter and C. Ceol, "Adaptive immunity in a zebra fish model of melanoma," ed: AACR, 2013.
- 109.Lam S.H., Chua H.L., Gong Z., Lam T.J., Sin Y.M. Development and maturation of the immune system in zebra fish, Danio rerio: a gene expression profiling, in situ hybridization and immunological study. Dev. Comp. Immunol. Jan 2004;28(1):9–28. doi: 10.1016/s0145-305x(03)00103-4. (in eng) [DOI] [PubMed] [Google Scholar]
- 110.Sültmann H., et al. Conservation of MHC class III region synteny between zebra fish and human as determined by radiation hybrid mapping. J. Immunol. 2000;165(12):6984–6993. doi: 10.4049/jimmunol.165.12.6984. [DOI] [PubMed] [Google Scholar]
- 111.Trede N.S., Langenau D.M., Traver D., Look A.T., Zon L.I. The use of zebra fish to understand immunity. Immunity. 2004;20(4):367–379. doi: 10.1016/s1074-7613(04)00084-6. [DOI] [PubMed] [Google Scholar]
- 112.Mashoof S., Criscitiello M.F. Fish immuno-globulins. Biology. 2016;5(4):45. doi: 10.3390/biology5040045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hall C.J., Sanderson L.E., Crosier K.E., Crosier P.S. Mitochondrial metabolism, reactive oxygen species, and macrophage function-fishing for insights. J. Mol. Med. (Berl) Nov 2014;92(11):1119–1128. doi: 10.1007/s00109-014-1186-6. (in eng) [DOI] [PubMed] [Google Scholar]
- 114.Thapa B., Lee K. Metabolic influence on macrophage polarization and pathogenesis. BMB Rep. Jun 2019;52(6):360–372. doi: 10.5483/BMBRep.2019.52.6.140. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stunault M.I., Bories G., Guinamard R.R., Ivanov S. Metabolism plays a key role during macrophage activation. Mediators. Inflamm. 2018;2018 doi: 10.1155/2018/2426138. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hu K., Jin Y., Chroneos Z., Han X., Liu H., Lin L. Macrophage functions and regulation: roles in diseases and implications in therapeutics. J. Immunol. Re. 2018;2018 doi: 10.1155/2018/7590350. (in eng) 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang X., Mosser D.M. Macrophage activation by endogenous danger signals. J. Pathol. Jan 2008;214(2):161–178. doi: 10.1002/path.2284. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mogensen T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. Apr 2009;22(2):240–273. doi: 10.1128/CMR.00046-08. (in eng) Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Aderem A., Underhill D.M. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. (in eng) [DOI] [PubMed] [Google Scholar]
- 120.Roca F.J., Ramakrishnan L. TNF dually mediates resistance and susceptibility to mycobacteria via mitochondrial reactive oxygen species. Cell. Apr 25 2013;153(3):521–534. doi: 10.1016/j.cell.2013.03.022. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kweon S.M., et al. Real-time measurement of intracellular reactive oxygen species using Mito tracker orange (CMH2TMRos) Biosci. Rep. Jun 2001;21(3):341–352. doi: 10.1023/a:1013290316939. (in eng) [DOI] [PubMed] [Google Scholar]
- 122.Wu Z., et al. Liposome-mediated drug delivery in larval zebra fish to manipulate macrophage function. Zebrafish. Apr 2019;16(2):171–181. doi: 10.1089/zeb.2018.1681. (in eng) [DOI] [PubMed] [Google Scholar]
- 123.Gordon S., Martinez-Pomares L. Physiological roles of macrophages. Pflugers Arch. 2017;469(3-4):365–374. doi: 10.1007/s00424-017-1945-7. (in eng) 04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Viola A., Munari F., Sánchez-Rodríguez R., Scolaro T., Castegna A. The metabolic signature of macrophage responses. Front. Immunol. 2019;10:1462. doi: 10.3389/fimmu.2019.01462. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sica A., Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. Mar 2012;122(3):787–795. doi: 10.1172/JCI59643. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mosser D.M., Edwards J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. Dec 2008;8(12):958–969. doi: 10.1038/nri2448. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wiegertjes G.F., Wentzel A.S., Spaink H.P., Elks P.M., Fink I.R. Polarization of immune responses in fish: The 'macrophages first' point of view. Mol. Immunol. Jan 2016;69:146–156. doi: 10.1016/j.molimm.2015.09.026. (in eng) [DOI] [PubMed] [Google Scholar]
- 128.Sepulcre M.P., et al. Evolution of lipopolysaccharide (LPS) recognition and signaling: fish TLR4 does not recognize LPS and negatively regulates NF-kappaB activation. J Immunol. 2009;182(4):1836–1845. doi: 10.4049/jimmunol.0801755. (in eng) Feb 15. [DOI] [PubMed] [Google Scholar]
- 129.Meng Z., Zhang X.Y., Guo J., Xiang L.X., Shao J.Z. Scavenger receptor in fish is a lipopolysaccharide recognition molecule involved in negative regulation of NF-κB activation by competing with TNF receptor-associated factor 2 recruitment into the TNF-α signaling pathway. J. Immunol. 2012;189(8):4024–4039. doi: 10.4049/jimmunol.1201244. (in eng) Oct 15. [DOI] [PubMed] [Google Scholar]
- 130.Grayfer L., Kerimoglu B., Yaparla A., Hodgkinson J.W., Xie J., Belosevic M. Mechanisms of Fish Macrophage Antimicrobial Immunity. Front. Immunol. 2018;9:1105. doi: 10.3389/fimmu.2018.01105. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lu X.J., Chen J. Specific function and modulation of teleost monocytes/macrophages: polarization and phagocytosis. Zool Res. May 18 2019;40(3):146–150. doi: 10.24272/j.issn.2095-8137.2019.035. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Haschemi A., et al. The sedoheptulose kinase CARKL directs macrophage polarization through control of glucose metabolism. Cell Metab. Jun 2012;15(6):813–826. doi: 10.1016/j.cmet.2012.04.023. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Huang S.C., et al. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat. Immunol. Sep 2014;15(9):846–855. doi: 10.1038/ni.2956. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Paredes L.C., Olsen Saraiva Camara N., Braga T.T. Understanding the metabolic profile of macrophages during the regenerative process in zebra fish. Front. Physiol. 2019;10:617. doi: 10.3389/fphys.2019.00617. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pan H., et al. Autophagy-associated immune responses and cancer immunotherapy. Oncotarget. Apr 2016;7(16):21235–21246. doi: 10.18632/oncotarget.6908. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bordi M., Nazio F., Campello S. The close interconnection between mitochondrial dynamics and mitophagy in cancer. Front. Oncol. 2017;7:81. doi: 10.3389/fonc.2017.00081. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sugimoto M.A., Vago J.P., Perretti M., Teixeira M.M. Mediators of the resolution of the inflammatory response. Trends Immunol. 2019;40(3):212–227. doi: 10.1016/j.it.2019.01.007. (in eng) 03. [DOI] [PubMed] [Google Scholar]
- 138.Watanabe S., Alexander M., Misharin A.V., Budinger G.R.S. The role of macrophages in the resolution of inflammation. J. Clin. Invest. 2019;129(7):2619–2628. doi: 10.1172/JCI124615. (in eng) 05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Park D., et al. Continued clearance of apoptotic cells critically depends on the phagocyte Ucp2 protein. Nature. Aug 2011;477(7363):220–224. doi: 10.1038/nature10340. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Havixbeck J.J., Rieger A.M., Wong M.E., Hodgkinson J.W., Barreda D.R. Neutrophil contributions to the induction and regulation of the acute inflammatory response in teleost fish. J. Leukoc. Biol. Feb 2016;99(2):241–252. doi: 10.1189/jlb.3HI0215-064R. (in eng) [DOI] [PubMed] [Google Scholar]
- 141.Wang Y., et al. Mitochondrial fission promotes the continued clearance of apoptotic cells by macrophages. Cell. Oct 2017;171(2):331–345. doi: 10.1016/j.cell.2017.08.041. (in eng) e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.dos-Santos D., et al. Efferocytosis of SARS-CoV-2-infected dying cells impairs macrophage anti-inflammatory programming and continual clearance of apoptotic cells. medRxiv. 2021 doi: 10.7554/eLife.74443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wentzel A.S., Janssen J.J.E., de Boer V.C.J., van Veen W.G., Forlenza M., Wiegertjes G.F. Fish macrophages show distinct metabolic signatures upon polarization. Front. Immunol. 2020;11:152. doi: 10.3389/fimmu.2020.00152. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hall C.J., et al. Immuno responsive gene 1 augments bactericidal activity of macrophage-lineage cells by regulating β-oxidation-dependent mitochondrial ROS production. Cell Metab. Aug 2013;18(2):265–278. doi: 10.1016/j.cmet.2013.06.018. (in eng) [DOI] [PubMed] [Google Scholar]
- 145.Torraca V., Masud S., Spaink H.P., Meijer A.H. Macrophage-pathogen interactions in infectious diseases: new therapeutic insights from the zebra fish host model. Dis. Model. Mech. Jul 2014;7(7):785–797. doi: 10.1242/dmm.015594. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.West A.P., et al. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature. Apr 2011;472(7344):476–480. doi: 10.1038/nature09973. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Vogel R.O., et al. Cytosolic signaling protein Ecsit also localizes to mitochondria where it interacts with chaperone NDUFAF1 and functions in complex I assembly. Genes. Dev. Mar 2007;21(5):615–624. doi: 10.1101/gad.408407. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Phelan P.E., Mellon M.T., Kim C.H. Functional characterization of full-length TLR3, IRAK-4, and TRAF6 in zebra fish (Danio rerio) Mol. Immunol. May 2005;42(9):1057–1071. doi: 10.1016/j.molimm.2004.11.005. (in eng) [DOI] [PubMed] [Google Scholar]
- 149.Stockhammer O.W., Rauwerda H., Wittink F.R., Breit T.M., Meijer A.H., Spaink H.P. Transcriptome analysis of Traf6 function in the innate immune response of zebra fish embryos. Mol. Immunol. 2010;48(1-3):179–190. doi: 10.1016/j.molimm.2010.08.011. (in eng) Nov-Dec 2010. [DOI] [PubMed] [Google Scholar]
- 150.Peterman E.M., Sullivan C., Goody M.F., Rodriguez-Nunez I., Yoder J.A., Kim C.H. Neutralization of mitochondrial superoxide by superoxide dismutase 2 promotes bacterial clearance and regulates phagocyte numbers in zebra fish. Infect. Immun. Jan 2015;83(1):430–440. doi: 10.1128/IAI.02245-14. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhou W., Cao L., Jeffries J., Zhu X., Staiger C.J., Deng Q. Neutrophil-specific knockout demonstrates a role for mitochondria in regulating neutrophil motility in zebra fish. Dis. Model Mech. 2018;11(3) doi: 10.1242/dmm.033027. (in eng) 03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang R., Green D.R. Metabolic reprogramming and metabolic dependency in T cells. Immunol. Rev. Sep 2012;249(1):14–26. doi: 10.1111/j.1600-065X.2012.01155.x. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Michalek R.D., et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J. Immunol. Mar 2011;186(6):3299–3303. doi: 10.4049/jimmunol.1003613. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Konjar Š., Veldhoen M. Dynamic metabolic state of tissue resident CD8 T Cells. Front. Immunol. 2019;10:1683. doi: 10.3389/fimmu.2019.01683. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.van der Windt G.J., et al. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. Jan 2012;36(1):68–78. doi: 10.1016/j.immuni.2011.12.007. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pearce E.L., et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. Jul 2009;460(7251):103–107. doi: 10.1038/nature08097. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yue P., et al. A mitochondria-targeted ratiometric two-photon fluorescent probe for detecting intracellular cysteine and homocysteine. Talanta. Feb 2018;178:24–30. doi: 10.1016/j.talanta.2017.08.085. (in eng) [DOI] [PubMed] [Google Scholar]
- 158.Zhang M., et al. A highly selective fluorescence turn-on sensor for cysteine/homocysteine and its application in bioimaging. J. Am. Chem. Soc. Aug 2007;129(34):10322–10323. doi: 10.1021/ja073140i. (in eng) [DOI] [PubMed] [Google Scholar]
- 159.A. Rissone and F. Candotti, "Detection of reactive oxygen species using MitoSOX and CellROX Green in Zebra fish," 2016.
- 160.Kulkarni A.A., et al. An In Vivo zebrafish model for interrogating ros-mediated pancreatic β-cell injury, response, and prevention. Oxid. Med. Cell. Longevity. 2018;2018 doi: 10.1155/2018/1324739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lackmann C., et al. Novel procedures for whole organism detection and quantification of fluorescence as a measurement for oxidative stress in zebra fish (Danio rerio) larvae. Chemosphere. Apr 2018;197:200–209. doi: 10.1016/j.chemosphere.2018.01.045. (in eng) [DOI] [PubMed] [Google Scholar]
- 162.Chitramuthu B.P., Bennett H.P. High resolution whole mount in situ hybridization within zebrafish embryos to study gene expression and function. J. Vis. Exp. Oct 2013;(80):e50644. doi: 10.3791/50644. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Stackley K.D., Beeson C.C., Rahn J.J., Chan S.S. Bioenergetic profiling of zebrafish embryonic development. PLoS One. 2011;6(9):e25652. doi: 10.1371/journal.pone.0025652. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bond S.T., McEwen K.A., Yoganantharajah P., Gibert Y. Live metabolic profile analysis of zebra fish embryos using a seahorse XF 24 extracellular flux analyzer. Methods Mol. Biol. 2018;1797:393–401. doi: 10.1007/978-1-4939-7883-0_21. (in eng) [DOI] [PubMed] [Google Scholar]
- 165.Zhang J., Zhang Q. Using seahorse machine to measure OCR and ECAR in Cancer Cells. Methods Mol Biol. 2019;1928:353–363. doi: 10.1007/978-1-4939-9027-6_18. (in eng) [DOI] [PubMed] [Google Scholar]
- 166.Gibert Y., McGee S.L., Ward A.C. Metabolic profile analysis of zebra fish embryos. J. Vis. Exp. Jan 2013;(71):e4300. doi: 10.3791/4300. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Thisse C., Thisse B. High-resolution in situ hybridization to whole-mount zebra fish embryos. Nat Protoc. 2008;3(1):59–69. doi: 10.1038/nprot.2007.514. (in eng) [DOI] [PubMed] [Google Scholar]