Highlights
-
1
Mast cells and eosinophilic granule cells in Oncorhynchus mykiss.
-
2
Are they similar or the same?
-
3
We compare the cytomorphological characteristics and titorial affinities of both cells.
-
4
Electron microscopy shows two different cells.
-
5
Mast cells were positive for CD117 (c-kit), while eosinophilic granular cells were negative.
-
6
They are similar but not the same cells.
Keywords: Eosinophilic granular cells, Mast cells, Immunohistochemistry, Oncorhynchus mykiss, Immune response
Abstract
Mast cells are important in inflammatory processes and in the nonspecific immune response, and there are also indications that these cells are associated with the effectors of the specific immune response. Eosinophilic granular cells are frequently compared to mast cells, and some authors maintain that they are the same cells. In this study, we take a fresh look at the similarities and differences between these two cell types in Oncorhynchus mykiss. We evaluated the cytomorphology of each cell type with optical microscopy, their staining affinities, and their ultrastructure. We observed that mast cells were positive for CD117 (c-kit), while eosinophilic granular cells were negative for this marker. We propose that these two cell types have certain common characteristics but represent well-differentiated populations distributed in several Oncorhynchus mykiss tissues.
1. Introduction
Mast cells (MCL) were described by Paul Ehrlich in 1878. The first studies on these cells were morphological, focusing particularly on their staining characteristics. Ehrlich described MCLs as metachromatic granule cells. Later, in both mammals and humans, these cells were considered allergy effectors. MCLs contain histamine A central finding regarding the physiology of these cells was the discovery of their production of histamine, which is why these cells are associated with hypersensitivity and anaphylaxis pathologies [1]. Ontogenetically, MCLs originate from undifferentiated mesenchymal cells [2]. Kitamura et al. in 1977 [3] he demonstrated the hematopoietic origin of MCLs. Bone marrow transplantation into irradiated mice and subsequent normal animal marrow transplantation revealed that MCLs appeared in the marrow and tissues of recipient mice. According to some authors, eosinophilic granule cells (EGC) are similar to mammalian MCLs, and these cells produce histamine, which has been demonstrated directly or indirectly with antihistamine drugs such as diphenhydramine [4,5,6].
MCLs, or phylogenetically similar cells, are found in some invertebrates and amphibians [7,8,9,10]. They are cells of mesenchymal origin, possibly originating as granulocytes of hematopoietic tissue [11,12,13]. In this work, we found two cell types in various Oncorhynchus mykiss tissues, some very similar to mammalian MCLs and others to the typical EGCs described in various fish species. We studied different Oncorhynchus mykiss tissues and found differences in terms of morphology, staining affinity, metachromaticity and positivity for the binding of anti-CD117 antibody (c-kit) in MCL and EGC. Finally, we find differences in the ultrastructural characteristics of these cell types. Due to their cytomorphological characteristics, staining affinities, immunohistochemical affinity, and ultrastructure, we suggest that these cells are homologous but not similar and represent two distinct populations of cells.
2. Materials and methods
A total of 1249 O. mykiss were sampled over a two-year period in the Alicura reservoir, built on the Limay River, Patagonia, Argentina, to examine the eventual occurrence of notifiable salmonid diseases according to the OIE. Samples were taken each season and consisted of 75 fish from pens from eight farms (8–10 fish per farm) and 75 live fish caught by fishing around net cages, resulting in 600 fish from farms and 600 live fish that were caught free, In addition, 49 fish supplied by sport fishermen from the region were added. The mean total length of the farmed fish was 34.9 ± 2.3 cm and that of the fish caught around the pens was 29.7 ± 1.7 cm. Archived tissue samples were studied. The total tissues of the necropsies of the 1249 specimens of rainbow trout were reviewed, also new paraplast block cuts were made and samples of liver, stomach, various regions of the intestine, anterior and posterior kidney, brain, heart, tegument, and muscle were taken for histopathological study. These tissues were fixed in 10% buffered formalin. The samples were processed in an automatic tissue processor (Leica TP1020) that performed dehydration, rinsing, and embedding in Paraplast (Sigma-Aldrich). Subsequently, the tissues were sectioned on a microtome (Leica RM2245) with a thickness of 4 mm. Sections of all mentioned were stained with hematoxylin and eosin and Giemsa for tissues [14]. In thirty wild fish and thirty farmed fish immunohistochemical studies were performed using the avidin-biotin complex method as previously described [15]. The tissue slides were deparaffinized by rinsing with xylol and then rehydrated with alcohols of different strengths (absolute ethanol, 90, 80, 70, 50%). Endogenous peroxidase activity was blocked by incubating the slides for 30 min in 0.3% H2O2 in a 5% methanol solution. After washing the slides in water and 0.05% PBS solution, they were subsequently incubated in 1/100 normal serum (Vectastain Universal Elite, BC Kit, Vector) in a 10% PBS solution of bovine serum albumin (BSA) at normal temperature for 30 min in a humid chamber. After incubation, primary anti-CD117 (c-kit) (Sigma, Argentina, Clonality: 104D2) was applied at a dilution of 1:400, and the slides were incubated overnight in a humid chamber. The slides were then rinsed in PBS and incubated for 7 min in a solution of 50 ml of 30,3-diaminobenzidine (DAB, Sigma-Aldrich) containing 1% PBS-BSA in 50 ml of H2O2. Tissue sections were used as a negative control in which the primary antibodies were replaced by normal rabbit serum at a similar dilution [16].
In 25 wild and 25 cultured Oncorhynchus mykiss studies were performed with electron microscopy, small tissue fragments were cut into 1 mm blocks and immediately fixed in phosphate-buffered glutaraldehyde (pH 6.9 at 4 °C), washed in Millonig's solution and subsequently postfixed in 1% osmium tetroxide. The tissue blocks were then dehydrated in a graded ethanol-acetone series, dipped in propylene oxide, and embedded in Durcupan ACNI (Fluka Chemie A.G., Switzerland). Thin sections were cut with an LKB ultramicrotome and stained twice with uranyl acetate and lead citrate before examination on a Jeol JEM-8T electron microscope (Jeol, Tokyo, Japan).
3. Results
With light microscopy, we observed two types of well-differentiated cells, one very similar to mammalian and human mast cells that we called mast cells (MCLs), which were round or oval-shaped cells with a hyperchromatic central nucleus and little cytoplasm containing small granules with varying degrees of metachromacy, usually low metachromacy, with Giemsa staining. The definition of metachromacy we use is the one described by Bergeron and Singer in 1958, defined as a process that consists of obtaining a color different from that of the colorant used [17]. Of all the tissue samples belonging to the 1249 specimens of O. mykiss, MCLs were found in soft tissue and connective tissue belonging to the samples of 950 fish. In the cardiac cone arteriosus, MCLs were found in hearts belonging to 156 fish. In all these fish and in the remaining 143 MCLs were found at the base of the brachial cavity (Fig. 1). On the other hand, EGCs, observed as round or oval-shaped cells with a peripheral nucleus and multiple granules of different sizes with marked Giemsa-stained metachromacy, were observed in the cytoplasm. These cells were present mostly in fibroconnective tissue, in the submucosa of the digestive tract, in the swim bladder, in the liver, in the gills and in some inflammatory processes (Fig. 2). Of the 1249 specimens of O. mykiss, 1145 had intestinal and peritoneal acantocephalosis that generated inflammatory processes in the intestine and peritoneal cavity. ECGs were observed in these inflammatory processes, although also in some nonspecific inflammatory foci.
Fig. 1.
A: Branchial chamber showing cartilage (C) and vascular structures (V) surrounded by mast cells (arrows). H-E Bar: 200 µm. B: An accumulation of mast cells is observed (arrow) surrounding vascular structures (arrow). H-E. Bar: 100 µm. C: Mast cells observed with Giemsa staining. Bar: 200 µm. Insert: Giemsa-positive mast cells with little metachromacy. Bar: 20 µm. D: Two mast cells in the cone arteriosus tissue, exhibiting granules with partial metachromacy, are observed. Giemsa. Bar: 20 µm.
Fig. 2.
Vascularized fibroconnective tissue (V) with abundant eosinophilic granular cells. H-E: Bar: 50 µm. Insert: Eosinophilic granular cell with abundant metachromatic granules (arrow). Giemsa 10 µm.
According to immunohistochemistry, MCLs were positive for anti-CD117 (c-kit) labeling, while EGCs were negative (Fig. 3).
Fig. 3.
Positive anti-CD117 mast cells (c-kit). Note that the labeling is primarily on the cell membrane (arrows) Bar: 50 µm.
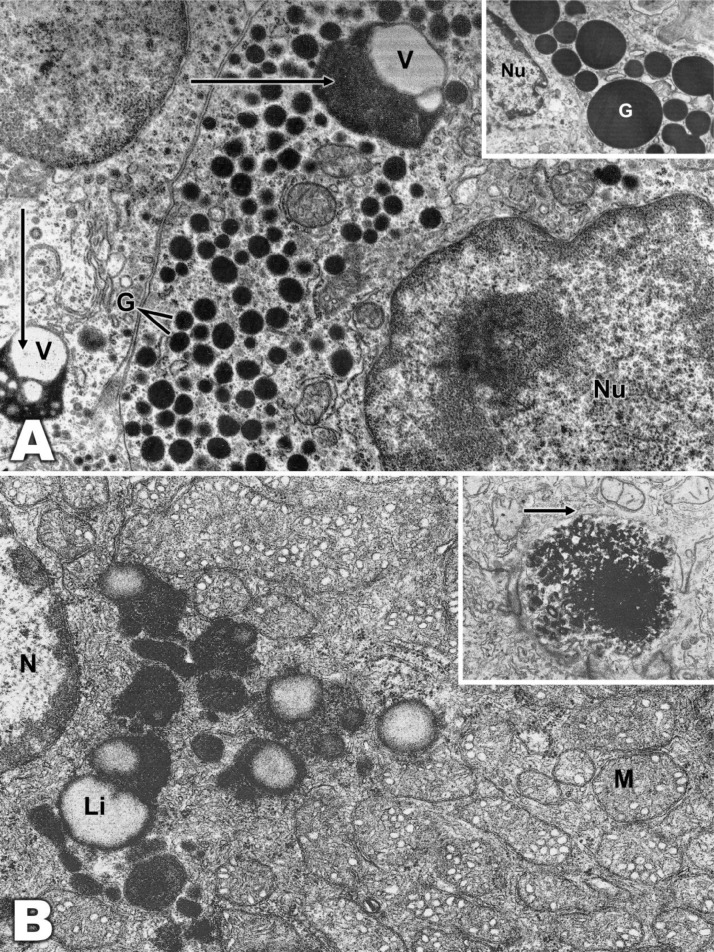
Transmission electron microscopy showed many differences between these cells. The ultrastructure of the MCLs resembled that of mammalian and human mast cells. The nucleus was oval-shaped and unilobed with a small open matrix and relatively large amounts of condensed peripheral chromatin. The nucleoli were not prominent. The cytoplasm was filled with granules of relatively uniform size but variable shape. The internal structure of the granules was complex, and some had regular parallel lamellar arrangements or multiple curves, exhibiting distinctive periodicity. The lamellar structures in some granules consisted of parallel arrangements enclosing fine regular stripes. Some granules were filled with fine or coarse granular material. Many granules contained mixtures of these standards. Therefore, we classified the granules as G1 encompassing coarse granules, G2 with fine granules, and G3 with curved lamellae (Figs. 4, 5). The ultrastructure of EGCs differed from that of MCLs. The nucleus was generally round or oval-shaped and located in the periphery with condensed peripheral chromatin, the granules were conserved in the cytoplasm and varied greatly in number, size and shape, and many granules were electron-dense and composed of amorphous material, without any internal structure. In some cases, lysosomes surrounded by amorphous material were observed in sectors, acquiring a vacuolar appearance. There was also a well-developed Golgi complex and abundant mitochondria (Figs. 6, 7).
Fig. 4.
A: A mast cell with numerous lysosomes (L). There are large vacuoles, which in at least one case appear to represent a phagosome (P). Mixed heteromorphic features of lysosomes and phagosomes, so-called phagolysosomes (PLs), are also present. (×21,000). B: This micrograph shows a mast cell with few homogeneous electrodense granules and abundant mitochondria. Insert: Details of the electrodense granules with clearer sectors of vacuolar appearance are shown. (×6000; Insert, ×12,000).
Fig. 5.
Mast cell granules. A: One contains a typical laminar structure (large arrow) and coarse granular material. Fine regular streaks are seen between the parallel lamellae. Three granules (short arrows) show curved matrices of lamellae and coarse granular material. (×60,000). B: Granules with coarse granular content (G1), fine granular content (G2) and curved lamellae (G3). (×60,000). C: Mast cells with granules with uniform electrodense content (×30,000). D: Mastocyte with granules with uniform coarse granular material delimited by typical curved lamellae (×60,000).
Fig. 6.
Eosinophilic granular cells with abundant homogeneous electrodense granules (×21,000).
Fig. 7.
A: Eosinophilic granular cell with abundant granules (G). Vacuoles surrounded by electrodense material (arrows) (×18,000) are observed. Insert: Detail of homogeneous electrodense granules (×30,000). B: In some eosinophilic granular cells, abundant compactly arranged mitochondria were observed (M). Lipofuscin (Li) is observed surrounding the vacuolar structure, nucleus (N), and lipid globule (L). (×22,000). Insert: Granule with inhomogeneous electrodense material with a thin membrane (arrow). (×18,000).
4. Discussion
Mast cells (MCLs) are responsible for both immediate and delayed hypersensitivity phenomena in mammals and humans. This indicates that MCLs are related to allergic reactions and anaphylaxis [18,19,20,21]. For the most part, MCLs are distributed in connective tissue, often surrounding blood vessels [22,23,24,25]. The functions of MCLs are closely related to the inflammatory response, and the cells participate directly and indirectly in the immune response through, among other things, the release of a series of biologically active mediators and vasoactive peptides. MCLs are also considered to be antigen-presenting cells [26]. These characteristics allow mast cells to act as the first line of defense against various antigenic stimuli and to respond to changes in their environment by communicating with a variety of other cells involved in physiological and immunological responses [27,28,29,30]. Fish mast cells have similarities to human and mammalian MCLs; however, some functions of these cells are not yet fully understood. Some authors state that the importance of MCLs in fish biology seems to have been underestimated [31]. Other authors indicate that MCLs have often been confused with other cells, such as eosinophils [32]. In this sense, some authors affirm that EGCs and MCLs are the same cells, while other authors clearly differentiate these cells [33,34]. In this work, we propose that MCLs and EGCs, although they are homologous, are two different cell populations. We based this on the different staining affinities of the cell types, especially the intensity of the metachromaticity with dyes such as Giemsa. Immunohistochemistry indicated MCLs were positive for immunolabeling with anti-CD117 (c-kit), while EGCs gave negative results. In our laboratory we use several monoclonal antibodies used in humans to mark different populations of lymphocytes in fish and to diagnose various pathologies, especially neoplasms in these animals [35,36,37,38,39]. On the other hand, reactivity has been demonstrated in zebrafish to the antibody c-kit (CD117) [40].
On the other hand, with the ultrastructural analysis of both cell types, clear differences could be established. There were several differences between MCLs and EGCs regarding their staining affinities. Some authors have compared different stains, especially for MCLs, such as toluidine blue, astra blue, Alcian blue-pyronin Y and May-Grunwald Giemsa stains, concluding that the latter is more suitable for MCLs [41]. As we mentioned earlier, we used Giemsa staining to observe both cell types in tissues. In MCLs, the granules were small with partial metachromacy. In EGCs, the granules were abundant with marked metachromacy. Both cell types have demonstrated their ability to produce histamine, which is why these cells are related to allergic processes, hypersensitivity and anaphylaxis [42,43,20].
In summary, we believe that in the case of O. mykiss, MCLs and EGCs appear to constitute two different populations. This is because we found differences between these cells in terms of their cytomorphological type by light microscopy and their metachromaticity with Giemsa staining. Ultrastructural differences and the expression of CD117 (c-kit) were only observed in MCLs.
Declaration of Competing Interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This study was supported by research funds from MCT/CNPq - Project #301245/2016–09 MCT/CNPq/CT- Agronegocio/MPA Public Notice 036/2009 Project #308013/2009–3, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Ministério da Pesca e Aquicultura (MPA).
References
- 1.da Silva E.Z., Jamur M.C., Oliver C. Mast cell function: a new vision of an old cell. J. Histochem. Cytochem. 2014;62(10):698–738. doi: 10.1369/0022155414545334. OctEpub 2014 Jul 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Combs J.W. Maturation of rat mast cells. an electron microscope study. J. Cell Biol. 1966;31(3):563–575. doi: 10.1083/jcb.31.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kitamura Y., Shimada M., Hatanaka K., Miyano Y. Development of mast cells from grafted bone marrow cells in irradiated mice. Nature. 1977;268:442–443. doi: 10.1038/268442a0. [DOI] [PubMed] [Google Scholar]
- 4.Ellis A.E. Eosinophilic granular cells (EGC) and histamine responses to Aeromonas salmonicida toxins in rainbow trout. Dev. Comp. Immunol. Spring. 1985;9(2):251–260. doi: 10.1016/0145-305x(85)90116-8. [DOI] [PubMed] [Google Scholar]
- 5.Mulero I., Sepulcre M.P., Meseguer J., García-Ayala A., Mulero V. Histamine is stored in mast cells of most evolutionarily advanced fish and regulates the fish inflammatory response. Proc. Natl. Acad. Sci. U. S. A. 2007;104(49):19434–19439. doi: 10.1073/pnas.0704535104. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romano L.A., Pedrosa V. Effect of the Dexamethasone and the Difenihidramiana on the Degranulation of the Eosinophilic Granular Cell of Tilapia. Asian J. Res. Zool. 2021;4:20–26. [Google Scholar]
- 7.Lanz H., Tsutsumi V., Aréchiga H. Morphological and biochemical characterization of Procambarus clarki blood cells. Dev. Comp. Immunol. 1993;17(5):389–397. doi: 10.1016/0145-305x(93)90030-t. [DOI] [PubMed] [Google Scholar]
- 8.Kadota K., Walter S., Claveria F.G., Igarashi I., Taylor D., Fujisaki K. Morphological and populational characteristics of hemocytes of Ornithodoros moubata nymphs during the ecdysial phase. J. Med. Entomol. 2003;40(6):770–776. doi: 10.1603/0022-2585-40.6.770. [DOI] [PubMed] [Google Scholar]
- 9.Allender M.C., Fry M.M. Amphibian hematology. Vet. Clin. North Am. Exot. Anim. Pract. 2008;11(3):463–480. doi: 10.1016/j.cvex.2008.03.006. vi. [DOI] [PubMed] [Google Scholar]
- 10.Baccari G.C., Pinelli C., Santillo A., Minucci S., Rastogi R.K. Mast cells in nonmammalian vertebrates: an overview. Int Rev Cell Mol Biol. 2011;290:1–53. doi: 10.1016/B978-0-12-386037-8.00006-5. [DOI] [PubMed] [Google Scholar]
- 11.Födinger M., Fritsch G., Winkler K., Emminger W., Mitterbauer G., Gadner H., Valent P., Mannhalter C. Origin of human mast cells: development from transplanted hematopoietic stem cells after allogeneic bone marrow transplantation. Blood. 1994;84(9):2954–2959. Nov 1. [PubMed] [Google Scholar]
- 12.Hallgren J., Gurish M.F. Mast cell progenitor trafficking and maturation. Adv. Exp. Med. Biol. 2011;716:14–28. doi: 10.1007/978-1-4419-9533-9_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dahlin J.S., Hallgren J. Mast cell progenitors: origin, development and migration to tissues. Mol. Immunol. 2015;63(1):9–17. doi: 10.1016/j.molimm.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 14.Luna G.L. McGraw-Hill Book Co; New York: 1968. Manual of histologic staining methods of the Armed Forces Institute of Pathology, Tercera Ed; p. 258. [Google Scholar]
- 15.Hsu S.M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC an unlabeled antibody (PAP) procedures. J. Histochem. Cytochem. 1981;29:577–585. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- 16.Mills B. In: Laboratory Methods in Histotechnology. Prophet E.B., Mills B., Arrington J.B., Sobin L.H., editors. American Registry of Pathology; Washington, D.C.: 1992. Immunohistochemistry; pp. 247–255. [Google Scholar]
- 18.Bergeron J.A., Singer M. Metachromasy: an experimental and theoretical reevaluation. J. Biophys. Biochem. Cytol. 1958;4(4):433–457. doi: 10.1083/jcb.4.4.433. Jul 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaplan A.P., Hunt K.J., Sobotka A.K. Human anaphylaxis: a study of mediator systems. Clin. Rev. 1977;25:361. [Google Scholar]
- 19.Bradding P., Saito H. In: Middleton's Allergy: Principles and Practice. Ed 8. Franklin Adkinson N. Jr., Bochner Bruce B.S., editors. 2014. Biology of mast cells and their mediators; pp. 228–251. [Google Scholar]
- 20.Lieberman P., Garvey L.H. Mast cells and anaphylaxis. Curr. Allergy Asthma Rep. 2016;16(3):20. doi: 10.1007/s11882-016-0598-5. [DOI] [PubMed] [Google Scholar]
- 21.Bahri R., Custovic A., Korosec P., Tsoumani M., Barron M., Wu J., Sayers R., Weimann A., Ruiz-Garcia M., Patel N., Robb A., Shamji M.H., Fontanella S., Silar M., Mills E.N.C., Simpson A., Turner P.J., Bulfone-Paus S. Mast cell activation test in the diagnosis of allergic disease and anaphylaxis. J. Allergy Clin. Immunol. 2018;142(2) doi: 10.1016/j.jaci.2018.01.043. Aug485-496.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ranieri G., Passantino L., Patruno R., Passantino G., Jirillo F., Catino A., Mattioli V., Gadaleta C., Ribatti D. The dog mast cell tumour as a model to study the relationship between angiogenesis, mast cell density and tumour malignancy. Oncol. Rep. 2003;10(5):1189–1193. Sep-Oct. [PubMed] [Google Scholar]
- 23.Abid A., Malone M.A., Curci K. Mastocytosis. Prim. Care. 2016;43(3):505–518. doi: 10.1016/j.pop.2016.04.007. Sep. [DOI] [PubMed] [Google Scholar]
- 24.Chen S., Noordenbos T., Blijdorp I., van Mens L., Ambarus C.A., Vogels E., A Te Velde, Alsina M., Cañete J.D., Yeremenko N., Baeten D. Histologic evidence that mast cells contribute to local tissue inflammation in peripheral spondyloarthritis by regulating interleukin-17A content. Rheumatology (Oxford) 2019;58(4):617–627. doi: 10.1093/rheumatology/key331. Apr 1. [DOI] [PubMed] [Google Scholar]
- 25.Valent P., Akin C., Nedoszytko B., Bonadonna P., Hartmann K., Niedoszytko M., Brockow K., Siebenhaar F., Triggiani M., Arock M., Romantowski J., Górska A., Schwartz L.B., Metcalfe D.D. Diagnosis, classification and management of mast cell activation syndromes (MCAS) in the era of personalized medicine. Int. J. Mol. Sci. 2020;21(23):9030. doi: 10.3390/ijms21239030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galli S.J., Gaudenzio N. Human mast cells as antigen-presenting cells: when is this role important in vivo? J. Allergy Clin. Immunol. 2018;141(1):92–93. doi: 10.1016/j.jaci.2017.05.029. [DOI] [PubMed] [Google Scholar]
- 27.da Silva E.Z., Jamur M.C., Oliver C. Mast cell function: a new vision of an old cell. J. Histochem. Cytochem. 2014;62(10):698–738. doi: 10.1369/0022155414545334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mukai K., Tsai M., Saito H., Galli S.J. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol. Rev. 2018;282(1):121–150. doi: 10.1111/imr.12634. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dudeck A., Köberle M., Goldmann O., Meyer N., Dudeck J., Lemmens S., Rohde M., Roldán N.G., Dietze-Schwonberg K., Orinska Z., Medina E., Hendrix S., Metz M., Zenclussen A.C., von Stebut E., Biedermann T. Mast cells as protectors of health. J. Allergy Clin. Immunol. 2019;144(4S):S4–S18. doi: 10.1016/j.jaci.2018.10.054. Oct. [DOI] [PubMed] [Google Scholar]
- 30.Komi D.Elieh E.Ali A., Shafaghat F., Kovanen P.T., Meri S. Mast cells and complement system: ancient interactions between components of innate immunity. Allergy. 2020;75(11):2818–2828. doi: 10.1111/all.14413. Epub 2020 Jun 15. [DOI] [PubMed] [Google Scholar]
- 31.Sfacteria A., Brines M., Blank U. The mast cell plays a central role in the immune system of teleost fish. Mol. Immunol. 2015;63(1):3–8. doi: 10.1016/j.molimm.2014.02.007. Epub 2014 Mar 7. [DOI] [PubMed] [Google Scholar]
- 32.Holland J.W., Rowley A.F. Studies on the eosinophilic granule cells in the gills of the rainbow trout, Oncorhynchus mykiss. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1998;120(2):321–328. doi: 10.1016/s0742-8413(98)10016-6. [DOI] [PubMed] [Google Scholar]
- 33.Ellis A.E. The leucocytes of fish: a review. J. Fish Biol. 1977;11:453–491. [Google Scholar]
- 34.Reite O.B. Mast cells/eosinophilic granule cells of teleostean fish: a review focusing on staining properties and functional responses. Fish Shellfish Immunol. 1998;8:489–513. [Google Scholar]
- 35.Romano L.A., Klosterhoff M., Fabiane F., Pereira E.G., Pereira M.A., Sampaio L.A., Tesser M.B. Neoplasia of the Sertoli cells in wild carp, Cyprinus carpio: optical, immunohistochemical and ultrastructural study. Bull. Eur. Assoc. Fish Pathol. 2013;33:84. [Google Scholar]
- 36.Romano L.A., Klosterhoff M., Führ F., Pereira M.A., Pedrosa V.F. Multiple neurofibromas of the heart in wild carp, Cyprinus carpio: optical, immunohistochemical and ultrastructural study. Bull. Eur. Assoc. Fish Pathol. 2015;34:201–207. [Google Scholar]
- 37.Batista C.R., Figueiredo M.A., Almeida D.V., Romano L.A., Marins L.F. Impairment of the immune system in GH-overexpressing transgenic zebrafish (Danio rerio) Fish Shellfish Immunol. 2014;36:519–524. doi: 10.1016/j.fsi.2013.12.022. [DOI] [PubMed] [Google Scholar]
- 38.Batista C.R., Figueiredo M.A., Almeida D.V., Romano L.A., Marins L.F. Effects of somatotrophic axis (GH/GHR) double transgenesis on structural and molecular aspects of the zebrafish immune system. Fish Shellfish Immunol. 2015;45:725–732. doi: 10.1016/j.fsi.2015.05.034. [DOI] [PubMed] [Google Scholar]
- 39.Klosterhoff M.C., Pereira Júnior J., Rodrigues R.V., Gusmão E.P., Sampaio L.A., Tesser M.B., Romano L.A. Ontogenic development of kidney, thymus and spleen and phenotypic expression of CD3 and CD4 receptors on the lymphocytes of cobia (Rachycentron canadum) Anais da Academia Brasileira de Ciências (Online) 2015;87:2111–2121. doi: 10.1590/0001-3765201520140623. 2015. [DOI] [PubMed] [Google Scholar]
- 40.Ball E.R., Matsuda M.M., Dye L., Hoffmann V., Zerfas P.M., Szarek E., Rich A., Chitnis A.B., Stratakis C.A. Ultra-structural identification of interstitial cells of Cajal in the zebrafish Danio rerio. Cell Tissue Res. 2012;349(2):483–491. doi: 10.1007/s00441-012-1434-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mutsaddi S., Kotrashetti V.S., Nayak R.S., Pattanshetty S.M. Comparison of histochemical staining techniques for detecting mast cells in oral lesions. Biotech. Histochem. 2019;94(6):459–468. doi: 10.1080/10520295.2019.1597986. [DOI] [PubMed] [Google Scholar]
- 42.Jurd R.D. Hypersensitivity in fishes: a review. J. Fish Biol. 1987;31:1–7. [Google Scholar]
- 43.Yang R., Lao Q.C., Yu H.P., Zhang Y., Liu H.C., Luan L., Sun H.M., Li C.Q. Tween-80 and impurity induce anaphylactoid reaction in zebrafish. J. Appl. Toxicol. 2015;35:295–301. doi: 10.1002/jat.3069. [DOI] [PubMed] [Google Scholar]