Abstract
RNA interference (RNAi) is a conservative and important functional pathway in eukaryocyte. It regulates the expression of genes that are engaged in a variety of cellular physiological functions. Among the functions of RNAi, its antiviral function have attracted many attentions.The RNAi pathway molecules are able to recognize virus-related dsRNA and degrade it, therefore killing the virus. More importantly, RNAi could mediate systemic antiviral responses, transmit from cell to cell, and systemic RNA interference defective 1 (SID1) was thought to play an important role in this process. In the present study, a SID1 gene (LvSID1) of Litopenaeus vannamei was cloned. LvSID1 could locate to both plasma membrane and endoplasmic reticulum. Result of real-time RT-PCR assay showed that it was highly expressed in shrimp gills. Besides, it was shown that over-expressed LvSID1 in Sf9 cells could significant enchane RNAi efficiency. It was found that the expression of LvSID1was regulated by white spot syndrome virus (WSSV), and knockdown expression of LvSID1 increased the cumulative mortality of WSSV infection shrimp. These results suggested that LvSID1 likely to played a role in L. vannamei systemic RNAi, and was involved in WSSV resistence.
Keywords: Litopenaeus vannamei, RNA interference, Systemic RNA interference defective 1, White spot syndrome virus
1. Introduction
RNA interference (RNAi) is a phenomenon that specific mRNA degradation induced by double-stranded RNA (dsRNA) [1]. It is a mechanism left over from biological evolution that regulates gene expression post RNA transcription [2]. In cytoplasm, nuclease Dicer cuts dsRNA into a number of small segments RNA (about 21∼23 bp) with a specific length and structure, namely siRNA [3]. Studies showed that siRNA has similar structural features in organisms: a double-stranded RNA (dsRNA) about 21∼23 bp long with 5′ single phosphate and 3′ hydroxyl end, and a single chain protrusion of 2∼3 nt at the 3′end of the complementary double chain [3]. Then siRNA, endoenzyme, exoenzyme and other factors formed a RNA-induced silencing complex (RISC), which specifically bound to the homologous region of exogenous gene expressed mRNA, cutting mRNA and causes specific gene silencing [4]. And RNAi lead to exert its systemic effect requires other factors, such as systemic RNA interference defective 1 (SID1) [5]. The SID1 is a transmembrane protein, which can transport the dsRNA out of the cells, then these dsRNA is absorbed by other surrounding cells. By this way, SID1 could mediate RNAi from cell to cell [5].
RNAi is an important part of the immune response to viruses and other exotic genetic substances [6, 7]. Long before RNAi pathway was fully understood, it is known that induced gene silence in the plant can spread systematically throughout the plant and can be transferred from anvil to spikes by grafting [8]. This phenomenon was later considered to be a feature of the plant adaptive immune system, thus triggering the overall immune response of the plant after a local infection with the virus [9]. Accordingly, many plant viruses evolve complex mechanisms to suppress RNAi. This includes a viral protein that can bind to double-stranded RNA fragments using a single-stranded RNA. Some plant genomes also express endogenous siRNA as a response to specific types of bacterial infections [10]. These effects may be part of a general response to pathogens, controlling the infection by downgrading any metabolic processes in the host benefiting the pathogen.
Although animal usually express less variants of Dicer enzymes than plant, RNAi also triggers antiviral reactions in animal [7, 11, 12]. In flies, RNAi is essencial for antiviral innate immunity [7]. In fact, much knowledge of the composition and function of the invertebrate RNAi pathway came from the studies of Drosophila melanogaster. Besides, it was showd that the expression of Argonaute was increased upon virus infection, and the over-expressing proteins associated with RNAi allows the nematode to enhance the resistence to virus [13]. So it was believed that RNAi has similar immune functions in nematodes. There were also evidences that a functional antiviral RNAi pathway in mammalian cells [12]. In addition to antiviral functions, RNAi also to play an important role in animal development, especially in the early development of the nervous system [14].
Before shrimp RNAi pathway was fully uncovered, application of RNAi in shrimp antiviral had been carried out [15, 16]. And the results showed that RNAi treatment could depress viruses infection in shrimp. As report, dsRNA-treated shirmp primary cells were resistanted to yellow head virus (YHV) infection [17]; dsRNA injection could provid resistance to WSSV and taura syndrome virus [15, 18]. Yodmuang et al. reported that the antiviral immunity function induced by dsRNA in shrimp is dose-dependent, and its efficacy could last for 5 day (d) [19]. And injected dsRNA that corresponding to sequences of WSSV vp28, vp281 or protein kinas 3d before viral infection, shrimp survival reached 100%, 53% or 93%, respectively. Consecutive injections of vp28-siRNA, at 0, 24, and 48 h post WSSV infection were reported to completely eliminate the virus in Penaeus japonicus [16]. Tirasophon et al. reported that injected with specific dsRNA within 12 h post YHV infection, could completely inhibited viral replication. There were also researchers developed an anti-TSV Penaeus monodon that expresses the antisense RNA of TSV gene, and it was found that these transgenic shrimp had a certain resistance to TSV [20]. These results suggested that RNAi could not only be used as a precaution, but also as a remedy for shrimp virual disease. As far as it is currently known, in pathway composition, shrimp RNAi pathway has high similarity to Drosophila RNAi pathway [21]. Whether of Litopenaeus vannamei SID1 (LvSID1) participates in the antiviral immune response remains poorly studied.
2. Materials and methods
2.1. Bioinformatics analysis
The SID1 protein sequences of L. vannamei and other species in the GenBank were collected and analyzed via the BLAST program (http://www.ncbi.nlm.nih.gov/BLAST/). Multiple sequences alignment was performed by the ClustalW program. A neighbor-joining phylogenetic tree was constructed using the deduced amino acid sequences of SID1 proteins by employing MEGA 6.0 software. Bootstrap sampling was reiterated 5, 000 times. Protein domains were predicted using the SMART program (http://www.smart.emblheidelberg.de/).
2.2. Subcellular localization assay
The eGFP-fused protein expression vectors of eGFP-fused LvSID1 was constructed by inserting the LvSID1 DNA fragment into the recombinant vectors of pACB-eGFP. Drosophila S2 cells were seeded onto cover slips treated with poly-L-lysine in 24-well plates. 24 h later, cells were transfected with the eGFP-LvSID1 vector. At 48 h post transfection, cells on the cover slips were washed twice with PBS and stained with Hoechst 33,258 solution (Beyotime, China). The treated cells were observed under a Leica laser scanning confocal microscope. LvSID1 expression vectors was constructed by inserting the LvSID1 DNA fragment into the recombinant vectors of pIZ-V5/His (primers lised in table 1).
Table 1.
Summary of primers used in this study.
| Primers | Sequence (5′−3′) |
|---|---|
| For genes expressiona | |
| pIZ/pACB-LvSID1-EcoR Ⅴ-F | CGGGATATCATGGCCCGAAGTACATCCC |
| pIZ/pACB-LvSID1-Xba I-R | CCTCTAGACCTGAAAACTGGAATCTTCTCTCGAG |
| pIZ-eGFP-KpnI-F | CGGGGTACCATGGTGAGCAAGGGCGAGGAGCTG |
| pIZ-eGFP-EcoRI-R | CCGGAATTCCTTGTACAGCTCGTCCATGCCGAG |
| For real-time RT-PCR | |
| QPCR-LvSID1-F | TGTGAGTGACCTGGCAAGAAA |
| QPCR-LvSID1-R | AATGACTGGGACGGCATAGAA |
| QPCR-LvEF1a-F | GCTGATTGCGCCGTACTCAT |
| QPCR-LvEF1a-R | TCACGGGTCTGTCCGTTCTT |
| For WSSV copy determined | |
| QPCR-IE1-F | TGTTTTCTGTATGTAATGCGTGTAGGT |
| QPCR-IE1-R | CCCACTCCATGGCCTTCA |
| For dsRNA templates amplification | |
| DsRNA-LvSID1–471-T7-F | GGATCCTAATACGACTCACTATAGGACCCAGACATCAACGCCAGT |
| DsRNA-LvSID1–471-R | GGCAAAGCAGCAAGTAAACCA |
| DsRNA-LvSID1–471-F | ACCCAGACATCAACGCCAGT |
| DsRNA-LvSID1–471-T7-R | GGATCCTAATACGACTCACTATAGGGGCAAAGCAGCAAGTAAACCA |
| DsRNA-Luc-514-T7-F | GGATCCTAATACGACTCACTATAGGCGAGGTGGACATCACTTACGC |
| DsRNA-Luc-514-R | TCTCACGCAGGCAGTTCTATG |
| DsRNA-Luc-514-F | CGAGGTGGACATCACTTACGC |
| DsRNA-Luc-514-T7-R | GGATCCTAATACGACTCACTATAGGTCTCACGCAGGCAGTTCTATG |
Nucleotides in bold indicate restriction sites introduced for clonin.
2.3. Synthesis of double-stranded RNA
The DNA templates of LvSID1 double strand RNA (designated dsLvSID1) were amplified using the primer pairs DsRNA-dsLvSID1–471-T7-F/DsRNA-dsLvSID1–471-R and DsRNA-dsLvSID1–471-F/DsRNA-dsLvSID1–471-T7-R; and the dsRNA specially for Renilla lucife (Luc) mRNA as the contol dsRNA, its DNA templates amplified using the primer pairs DsRNA-dsLuc-514-T7-F/DsRNA-dsLuc-514-R and DsRNA-dsLuc-514-F/DsRNA-dsLuc-514-T7-R. The PCR products were used as RNA templates, and then transcribed and purified in vitro using the RiboMAXTM Large-Scale RNA Production System-T7 (Promega, USA), following the manufacturer's protocols. DsLvSID1 or dsLuc fragments were 471 bp or 514 bp in length, respectively. DNA templates for eGFP dsRNA (dseGFP) were prepared as previously described [22]. Then determined the dsRNA concentration and diluted it properly, and stored in −80 °C refrigerator. The dsRNA transfection was carried out using Lipofectamine 2000 reagent according to the instruction (Invitrogen, USA) .
2.4. Preparation of Sf9 cells that stably expressing eGFP
The pIZ-eGFP vector was constructed by inserting the ORF of eGFP into the pIZ-V5/His-vector (Invitrogen, USA). Sf9 cells were transfected with pIZ-eGFP and pIZ-V5/His-vector (as negative control) using Lipofectamine 2000 reagent (Invitrogen, USA). Following selection with 300 μg/mL Zeocin, the stable eGFP-expressing cells and the pcDNA-tranfected Sf9 control cells were isolated into single colonies, termed Sf9-eGFP. eGFP expression in the stably transfected cell lines was identified by RT-PCR using a pair of eGFP specific primers. pIZ-V5/His-or pIZ-LvSID1 plus dsLuc or dseGFP co-transfected Sf9 cells. 48 h later, the cells were observed under a Leica laser scanning confocal microscope.
2.5. Pathogenic challenge and preparation of templates for real-time RT-PCR assays
Healthy L. vannamei (∼7 g) were obtrained from a shrimp farm of Haiou island, Guangzhou City, Guangdong Province, China. The shrimps were acclimated in a recirculating water tank system filled with air-pumped seawater (∼3% salinity) at ∼28 °C. Shrimps were allowed to acclimatize for one week before experimentation. To determine the gene expression profiles of LvSID1 in tissues of L. vannamei muscle, nerve, heart, stomach, epithelium, hepatopancreas, pyloric ceca, stomach, gill, intestine and hemocytes of healthy shrimps were collected, and tissues of five shrimps were pooled together as one sample.
Total RNA was extracted using RNeasy Mini Kit (Qiagen, Germany), and was reversely transcribed into cDNA using PrimeScript RT Reagent Kit (TaKaRa, Japan). Real-time RT-PCR assays were performed with a LightCycler 480 System (Roche, Germany). The results were calculated using the 2−ΔΔCt method, after normalization to LvEF-1a (GenBank Accession No. GU136229).
2.6. Absolute qPCR of wssv copy number
The shrimps were intramuscularly injected with PBS, dsLveGFP or dsLvSID1. 48 h later, the second injection followed with either PBS or WSSV. Then, at 24 hpi and 48 hpi, the muscle samples were collected for absolute qPCR assay. And the DNA template preparation and absolute qPCR assay was performed as described before [23].
2.7. Cumulative mortality of LVSID1-knockdown shrimp following injection with WSSV
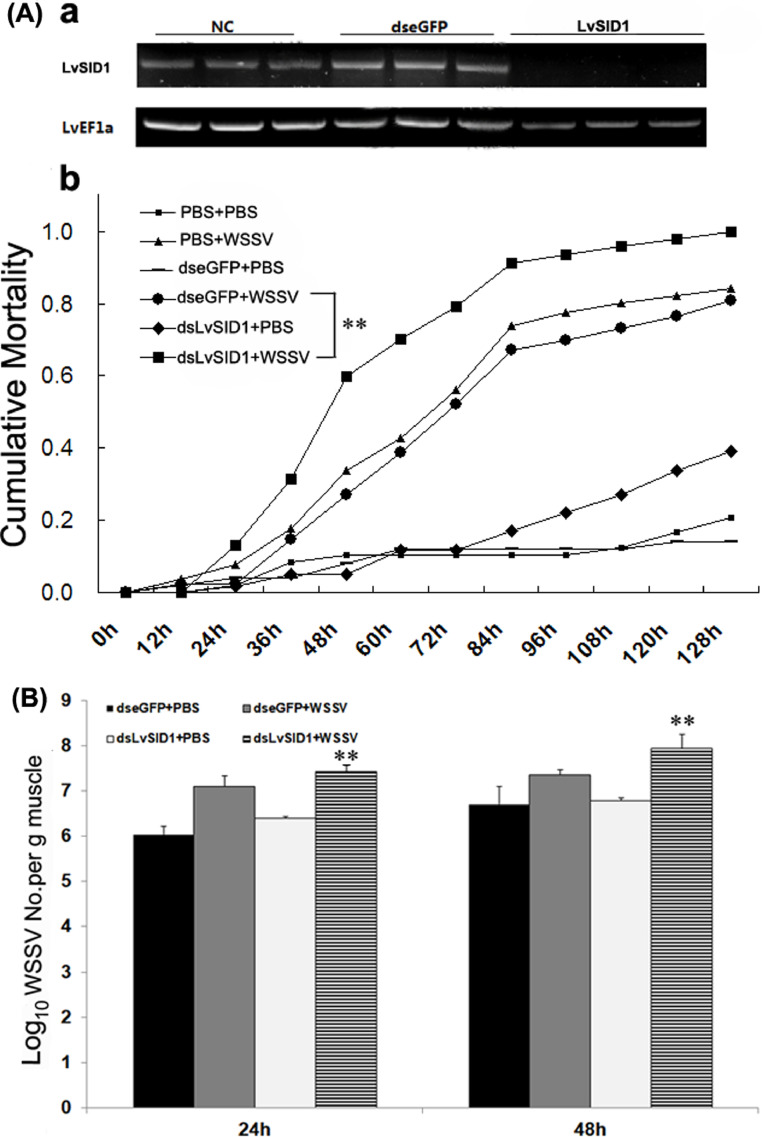
LvSID1 was downregulated by RNAi. And RT-PCR assay was performed 48 h after dsRNA injection to measure RNAi efficiency [Fig. 7A (a)]. LvEF1a was used as an internal control. To determine the cumulative mortality of LvSID1-knockdown shrimps, healthy shrimps (n = 40 per group) were injected at the second abdominal segment with 7 μg of dsLvSID1, dseGFP or PBS (per gram of body weight shrimp injected with 1 μg dsRNA, and the volume of injection was 50 μL). Approximately 48 hpi, shrimps were injected with 50 μL of WSSV inoculum as mentioned above.
Fig. 7.
Knockdown expression of LvSID1 increased cumulative mortality of WSSV-infected shrimps. [A(a)] RT-PCR analysis of LvSID1 expression with LvEF1a as internal control. [A(b)] Shrimps (n = 50) were injected intramuscularly with dseGFP (control) or dsLvSID1. At 48 h after the initial injection, shrimps were injected with WSSV or PBS. Cumulative mortality was recorded every 4 h. The mortality levels among different treatments were analyzed by Kaplan-Meier plot (log-rank Х2 test). Significant differences in L. vannamei mortality were marked with asterisks (**indicates p< 0.01). (B) The bars indicate mean values ± S.D. of the log10 WSSV copy number per 1 g muscle (n = 3). The statistical significance was calculated by Student's t-test (** indicates p < 0.01 compared with control).
3. Results and discussions
3.1. LvSID1 cloning and sequence analysis and phylogenetic analysis
The ORF of LvSID1 was 2, 757 bp, which encoded 918 aa with a molecular weight of 105 kDa. Conserved domain analysis using SMART (http://smart.embl-heidelberg.de/) indicated that SID1-RNA-chan domian at the carboxyl terminal (Fig. 1A), and a singal peptide at the amino terminal. SID1 is transmembrane protein, its signal peptide is required for their cell membrane localization, and the SID1-RNA-chan domian belong to a proteins family that could form transmembrane dsRNA-gated channels, which is important for dsRNA import in cells [24, 25].
Fig. 1.
The domains distribution of LvSID1 and phylogenetic tree of SID1s. (A) Schematic representation of the structural motifs of LvSID1; (B) NJ phylogenetic tree was constructed using the deduced amino acid sequences of SID1 proteins by employing MEGA 6.0 software. Bootstrap sampling was reiterated 5, 000 times.
To investigate the relationships among LvSID1 and its homologs, a multiple sequence alignment was conducted (Fig. 2). Both in vertebrates and invertebrates, the sequences of LvSID1 is highly conservative. LvSID1 highly similar to Armadillidium nasatum SID1, which shared 56% identities (Fig. 1B). A phylogenetic tree was generated using the neighbor-joining (NJ) method. In this phylogeny tree, SID1s and associated proteins fell into two subgroups (Fig.1B): group 1 contained 11 vertebrate SIDs (HsSID1, HgSID1, PfSID1, PcSID1, TeSID1, PtSID1, ArSID1, CmSID1, MuSID1, BbSID1 and BfSID1); group 2 included ten invertebrate SIDs (TlSID1, PmSID1, LvSID1, AnSID1, PtSID1, CsSID1, PoSID1, MeSID1, TpSID1 and FhSID1). In fact, for the distribution of the domain, SID1 is relative conservative throughout the animal kingdom. It must be noted that in some organisms, such as Drosophila melanogaster or plants, which without SID proteins, systemic RNAi effects also appear to be present in them. And in Caenorhabditis elegans, there is also a mechanism that export of RNAi from tissues does not requires the RNA channel SID1 [26].
Fig. 2.
Multiple sequence alignment of the SID1 proteins. sSID1, Homo sapiens SID1(Genbank accession No.EAW79633.1); HsSID1, Heterocephalus glaber SID1 (Genbank accession No.EHA97737.1); PfSID1, Patagioenas fasciata monilis SID1(Genbank accession No.OPJ69376.1); PcSID1, Phasianus colchicus SID1 (Genbank accession No.XP_031468896.1); TeSID1, Thamnophis elegans SID1 (Genbank accession No.XP_032069055.1); PtSID1, Pseudonaja textilis SID1 (Genbank accession No.XP_026569118.1); BbSID1, Bufo bufo SID1 (Genbank accession No.XP_040278800.1); MuSID1, Microcaecilia unicolor SID1 (Genbank accession No.XP_030059770.1); ArSID1, Acipenser ruthenus SID1(Genbank accession No.RXM98340.1); CmSID1, Callorhinchus milii SID1(Genbank accession No.XP_007902257.1); BfSID1, Branchiostoma floridae (Genbank accession No.XP_035672806.1); TlSID1,Temnothorax longispinosus SID1 (Genbank accession No.TGZ57665.1); PmSID1, Papilio machaon SID1 (Genbank accession No.KPJ13349.1); LvSID1, Litopenaeus vannamei SID1 (Genbank accession No. XP_027215373.1); AnSID1, Armadillidium nasatum SID1 (Genbank accession No.KAB7506706.1), PtSID1, Parasteatoda tepidariorum SID1(Genbank accession No.XP_015909125.1); CsSID1, Centruroides sculpturatus SID1 (Genbank accession No.XP_023227870.1); PoSID1, Plakobranchus ocellatus SID1(Genbank accession No.GFO22380.1); MeSID1, Mytilus edulis SID1 (Genbank accession No.CAG2208520.1); TpSID1, Trichinella papuae SID1 (Genbank accession No.KRZ66376.1); FhSID1, Fasciola hepatica SID1 (Genbank accession No.THD27515.1).
3.2. LvSID1 was constitutively transcribed in various tissues of L. vannamei
Real-time RT-PCR analysis indicated that LvSID1 was expressed in all examined tissues. It was extremely highly expressed in shrimp gills, which was 64.3-fold greater than that in hemocytes (Fig.3). Predicted with PSORT Ⅱ program (https://psort.hgc.jp/cgi-bin/runpsort.pl), LvSID1 was suggested that it could locate to plasma membrane and endoplasmic reticulum (Fig. 4). It had been known SID1 is a dsRNA-selective channel that could transport dsRNA between cells. So if LvSID1 is to mediate systemic RNAi, it should locate to the cell membrane. And this prediction was largely confirmed by the result of fluorescence microscopy observation that eGFP-LvSID1 over-expressed in S2 cells (Fig. 4), and suggested that it should be conserved in function, that is delivering dsRNA into the cells [5].
Fig. 3.
Expression profile of LvSID1. Total RNA extracted from different tissues were reversely transcribed into cDNAs to serve as templates. Relative expression levels of LvSID1 were normalized to LvEF1α. The results are based on three independent experiments and expressed as mean values ± S.D.
Fig. 4.
Subcellular localization of LvSID1. The location of the proteins were visualized with a Leica laser scanning confocal microscope.
3.4. Over-expression of lvsid1 in Sf9 cells improved the RNAi effect
The Sf9 cells that stable expression eGFP was obtained by plasmid transfection and Zeocin screening. Fluorescence microscopy observation revealed that expression of eGFP in LvSID1 plus dseGFP group was significantly lower than in dsLuc (NC) plus dseGFP group (Fig.5). And LvSID1 itself does not significantly reduce eGPF expression as compared to NC (Fig.5). It had been reproted that SID1 is expressed in cells sensitive to RNAi, is localized to the cell periphery, and moreover, it is required cell-autonomously for systemic RNAi [27]. And in present study, we found that LvSID1 could enhance RNAi efficiency. Combined with considering its subcellular localization, we infered that LvSID1 also played a role in shirmp systemic RNAi. While, that does not exclude the absence of any other mechanism mediating systemic RNAi in the shrimp.
Fig. 5.
LvSID1 enhanced RNAi effect in Sf9 cells. LvSID1 was overexpressed in eGFP-Sf9 cells, and the expression of were visualized with a Leica laser scanning confocal microscope.
3.5. LvSID1was induced in gills upon wssv infection
To reveal the relationship between WSSV and LvSID1, we investigated the expression of LvSID1 in the duration of WSSV infection. LvSID1 expression was measured using real-time RT-PCR assays. LvSID1 expression in L. vannamei hemocytes increased from 12∼96 hpi, and reached the peak value at 36 hpi (Fig. 6), which was about 14.4-fold of the control. Many studies had been proved that shrimp RNAi was involved in WSSV infection response [15, 16, 21]. And it also appeared that the systemic RNAi took part in this process, while it still lacked more experimental evidence. From the results of the real-time RT-PCR assay, it seems that LvSID1 was engaged in the WSSV infection response. Similar results have been reported in other studies. For example, in Siniperca chuatsi, expression of SIDT2 was induced by the infection of infection spleen and kidney necrosis virus (ISKNV) in blood cells and spleens [28].
Fig. 6.
Expression profile of LvSID1 post WSSV infection in L. vannamei gills . Total RNA extracted from different tissues were reversely transcribed into cDNAs to serve as templates. Relative expression levels of LvSID1 in gills were normalized to LvEF1α. The results are based on three independent experiments and expressed as mean values ± S.D.
3.6. Knockdown of lvsid1 increased the cumulative mortality of shrimp with wssv infection
It had been reported that SID protein was important for antiviral response. As an example, inhibition of Siniperca chuatsi SidT2 protein function promotes ISKNV infection in MFF-1 cells [28]. In this study, we tested function of LvSID1 on WSSV infection with RNAi assay. The RT-PCR assay showed that dsLvSID1 effectively down regulated LvSID1 [Fig. 7A (a)]. And LvSID1 knocked-down significantly depressed the L. vannamei cumulative mortality, compared to the control group. In dsLvSID1 plus WSSV injection group, cumulative mortality was 61.2%, 71.4%, 81.6% and 91.8% at 54, 66, 78 and 90 hpi, respectively [Fig. 7A (b)]. In shrimp of dseGFP plus WSSV injection group, cumulative mortality was 29.2%, 41.7%, 54.2% and 70.8% at 54, 66, 78 and 90 hpi, respectively (Fig. 7B). Besides, the WSSV copy number in LvSID1 knockdown group was significantly higher than that in control groups (Fig. 7B). These results comfirmed that LvSID1 played a role in WSSV infecton resistence. It should also be recognized that RNAi do not always work in viruses resistantence, some viruses could even inhibit the host RNAi in different ways. For instance, nodamura virus encodes a suppressor of RNAi termed B2, which binds to dsRNA and prevents the initiation of RNAi as well as the loading of silencing complexes [29].
Acknowledgements
This research was supported by National Natural Science Foundation of China (No. 32072967 and 31772895); and Natural Science Foundation of Guangdong Province (No. 2021A1515010741).
References
- 1.Zamore P.D., Tuschl T., Sharp P.A., Bartel D.P. RNAi: double-Stranded RNA Directs the ATP-Dependent Cleavage of mRNA at 21 to 23 Nucleotide Intervals. Cell. 2000;101:25–33. doi: 10.1016/S0092-8674(00)80620-0. [DOI] [PubMed] [Google Scholar]
- 2.He L., Hannon G.J. MicroRNAs: small RNAs with a big role in gene regulation. Nat. Rev. Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 3.Tanudji M., Machalek D., Arndt G.M., Rivory L. Competition Between siRNA Duplexes: impact of RNA-Induced Silencing Complex Loading Efficiency and Comparison Between Conventional-21bp and Dicer-Substrate siRNAs. Oligonucleotides. 2010;20:27–32. doi: 10.1089/oli.2009.0195. [DOI] [PubMed] [Google Scholar]
- 4.Rand T.A., Petersen S., Du F., Wang X. Argonaute2 Cleaves the Anti-Guide Strand of siRNA during RISC Activation. Cell. 2005;123:621–629. doi: 10.1016/j.cell.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 5.Feinberg E.H., Hunter C.P. Transport of dsRNA into cells by the transmembrane protein SID-1. Science. 2003;5639:1545–1547. doi: 10.1126/science.1087117. [DOI] [PubMed] [Google Scholar]
- 6.Judge A.D., Sood V., Shaw J.R., Fang D., McClintock K., MacLachlan I. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat. Biotechnol. 2005;23:457–462. doi: 10.1038/nbt1081. [DOI] [PubMed] [Google Scholar]
- 7.Wang X.H., Aliyari R., Li W.X., Li H.W., Kim K., Carthew R., et al. RNA interference directs innate immunity against viruses in adult Drosophila. Science. 2006;312:452–454. doi: 10.1126/science.1125694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baulcombe D. RNA silencing in plants. Biochemist. 2015;37:10–13. [Google Scholar]
- 9.Vance V., Vaucheret H. RNA silencing in plants-defense and counterdefense. Science. 2001;292:2277–2280. doi: 10.1126/science.1061334. [DOI] [PubMed] [Google Scholar]
- 10.Axtell J.MichaelM. Classification and comparison of small RNAs from plants. Ann. Rev. Plant Biol. 2013;64:137–159. doi: 10.1146/annurev-arplant-050312-120043. [DOI] [PubMed] [Google Scholar]
- 11.Lu R., Yigit E., Li W.X., Ding S.W., Schneider D.S. An RIG-I-Like RNA Helicase Mediates Antiviral RNAi Downstream of Viral siRNA Biogenesis in Caenorhabditis elegans. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeang K.T. RNAi in the regulation of mammalian viral infections. BMC Biol. 2012;10:58. doi: 10.1186/1741-7007-10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Almeida M.V., Andrade-Navarro M.A., Ketting R.F. Function and Evolution of Nematode RNAi Pathways. Noncoding RNA. 2019;5:1–25. doi: 10.3390/ncrna5010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lessel D., Zeitler D.M., Reijnders M.R.F., Kazantsev A., Nia F.H., Bartholomäus A., et al. Germline AGO2 mutations impair RNA interference and human neurological development. Nat. Communicat. 2020;11:5797. doi: 10.1038/s41467-020-19572-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu J., Fang H., Zhang X. Silencing shrimp white spot syndrome virus (WSSV) genes by siRNA. Antivir Res. 2007;73:126–131. doi: 10.1016/j.antiviral.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Kim C., Kosuke Z., Nam Y.K., Kim S., Kim K. Protection of shrimp (Penaeus chinensis) against white spot syndrome virus (WSSV) challenge by double-stranded RNA. Fish Shellfish Immunol. 2007;23:242–246. doi: 10.1016/j.fsi.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 17.Tirasophon W., Roshorm Y., Panyim S. Silencing of yellow head virus replication in penaeid shrimp cells by dsRNA. Biochemical & Biophysical Res. Communicat. 2005;334:102–107. doi: 10.1016/j.bbrc.2005.06.063. [DOI] [PubMed] [Google Scholar]
- 18.Ongvarrasopone C., Saejia P., Chanasakulniyom M., Panyim S. Inhibition of Taura syndrome virus replication in Litopenaeus vannamei through silencing the LvRab7 gene using double-stranded RNA. Arch. Virol. 2011;156:1117–1123. doi: 10.1007/s00705-011-0952-9. [DOI] [PubMed] [Google Scholar]
- 19.Supansa Yodmuang, Witoon Tirasophon, et al. YHV-protease dsRNA inhibits YHV replication in Penaeus monodon and prevents mortality. BBRC. 2006;2:351–356. doi: 10.1016/j.bbrc.2005.12.186. [DOI] [PubMed] [Google Scholar]
- 20.Lu Y., Sun P.S. Viral resistance in shrimp that express an antisense Taura syndrome virus coat protein gene - ScienceDirect. Antivir. Res. 2005;67:141–146. doi: 10.1016/j.antiviral.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Chen T., Lv M., Lu X., Xie D. Review on application of RNAi in immunization and growth of shrimp. J. Aquacul. 2018;10:28–31. [Google Scholar]
- 22.Chen Y.H., Yuan F.H., Bi H.T., Zhang Z.Z., Yue H.T., Yuan K., et al. Transcriptome analysis of the unfolded protein response in hemocytes of Litopenaeus vannamei. Fish Shellfish Immunol. 2016;54:153–163. doi: 10.1016/j.fsi.2015.10.027. [DOI] [PubMed] [Google Scholar]
- 23.Wei Q., Zhang S., Chen Y.G., Wang P.H., He J.G. Litopenaeus vannamei NF-κB is required for WSSV replication. Dev. Comp. Immunol. 2014;45:156–162. doi: 10.1016/j.dci.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 24.Whangbo J.S., Weisman A.S., Chae J., Hunter C.P. SID-1 Domains Important for dsRNA Import in Caenorhabditis elegans. G3 (Bethesda, Md) 2017;7:3887–3899. doi: 10.1534/g3.117.300308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao J., G.u X., Zhang H. SID1 transmembrane family, member 2 (Sidt2): a novel lysosomal membrane protein. BBRC. 2010;402:588–594. doi: 10.1016/j.bbrc.2010.09.133. [DOI] [PubMed] [Google Scholar]
- 26.Jose A.M., Smith J.J., Hunter C.P. Export of RNA silencing from C. elegans tissues does not require the RNA channel SID-1. PNAS. 2009;106:2283–2288. doi: 10.1073/pnas.0809760106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winston W.M., Molodowitch C., Hunter C.P. Systemic RNAi in C. elegans Requires the Putative Transmembrane Protein SID-1. Science. 2002;295:2456–2459. doi: 10.1126/science.1068836. [DOI] [PubMed] [Google Scholar]
- 28.Ren R.W., Xu X.P., Lin T., Weng S.P., Liang H., Huang M.M., et al. Cloning, characterization, and biological function analysis of the SidT2 gene from Siniperca chuatsi. Dev. Comp. Immunol. 2011;35:692–701. doi: 10.1016/j.dci.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Allen W.J., Wiley M.R., Myles K.M., Adelman Z.N., Bevan D.R. Steered molecular dynamics identifies critical residues of the Nodamura virus B2 suppressor of RNAi. J. Mol. Mod. 2014;20:1–10. doi: 10.1007/s00894-014-2092-0. [DOI] [PMC free article] [PubMed] [Google Scholar]