Highlights
-
•
A new macrophage cell line divided from siganus fuscescens was established and named as RFM cell.
-
•
The RFM cell exhibited sensitive responses to LPS-induced inflammation and identified as M1 type macrophage cell.
-
•
The aerial part of S. baicalensis extract and it's two effective compounds showed anti-inflammatory effect on RFF cell, indicating the potential application as an aquaculture feeding additive.
Keywords: Cell line establishment, Siganus fuscescens, Anti-inflammation, Traditional Chinese medicine, Scutellaria baicalensis
Abstract
A new cell line was isolated and characterized from the head kidney of Siganus fuscescens (rabbit fish). The new macrophagic-like cell line was named as rabbit fish macrophage (RFM), and which could be sub-cultured for over 50 cycles since the development. RFM cell line was tested for growth in different temperatures and serum concentrations: the best growing condition was optimized at 20% serum under 28 °C. In cultured RFM cells, sequencing of 18S rRNA, as well as immunostaining of cytokeratin and CD 68, confirmed the identity as macrophagic cell of S. fuscescens. Cultured RFM cells were exposed to challenge of inflammation, as triggered by LPS, showing highly sensitive responses to inflammation, including release of nitric oxide, expression of cytokine, and activation of phagocytosis. The water extract of aerial part of Scutellaria baicalensis, named as SBA, has been shown anti-inflammatory property in S. fuscescens fish. In order to extend the application of SBA in aquaculture, the extract and its effective flavonoids, i.e. baicalin and scutellarin, were applied in LPS-treated RFM cells. Application of SBA extract, baicalin or scutellarin, inhibited the expressions of LPS-induced inflammatory cytokines, i.e. IL-1β, TNF-α, as well as the signaling of transcription factor NF-κB. The results support the established RFM cell line could be an ideal in vitro model in drug screening relating to inflammation. Additionally, the notion of SBA herbal extract in fish aquaculture is supported by its efficacy against inflammation.
1. Introduction
Siganus fuscescens, a gray rabbit fish, is wildly cultured and consumed in Australia, Indonesia and China [5]. In recent years, the diseases, caused by pathogenic microbes and inflammation deriving from marine bacteria, are leading to the death of S. fuscescens, and therefore an economic lost to fishermen. The fish immune system plays an essential role in fish survival when fighting against bacterial pathogens during infections [22]. Finding drugs in stimulating the immune responses in fish aquaculture is commonly practiced today. However, in vivo experiment in fish farm is time consuming and expensive. To boost the process of drug discovery, fish-derived cell lines are valuable tools in identifying the drug targets and their mechanistic actions. Additionally, the establishment of cell line can help to practice the 3R principles by reducing the experimental fish number. Moreover, the development of characterized cell line is generally associated with research on drug screening.
Cell lines have been used as vital in vitro tools in performing various studies, including virology, toxicology, cryobiology, oncology, drug screening and so on [31]. In teleost, inflammatory macrophages play several critical roles in killing infection pathogens, e.g. to eliminate pathogen by phagocytosis [19], to produce reactive oxygen and nitrogen intermediate [2], and to restrict nutrient availability of infected pathogen [10]. In order to reveal the inflammatory response of fish, several macrophagic cell lines have been isolated from salmon (SSP‐9) (Rodriguez [20]), goldfish (GMCL) [16] and rainbow trout (RTS-11) [3]. Establishing new cell lines and their characterization could provide alternative model systems to study specific mechanisms of pathogenesis, and to gain a better understanding of bacterial infection, as well as to provide an useful model to study immunological responses in fish [20]. The head kidney is one of the most common sources of macrophages [8,11]. However, a macrophagic cell line from S. fuscescens fish has not been established, which hinders the drug screening procedure for this fish species. In accord to the need, a cell line deriving from kidney of S. fuscescens was established and characterized here.
Root of Scutellaria baicalensis Georgi. (Scutellariae Radix), a traditional Chinese medicine (TCM), has long history of usage as herbal medicine with various pharmacological activities, including anti-virus, anti-microbial and anti-inflammation [9]. The aerial parts of this plant are being disposed during production of medicinal root parts. The aerial part of S. baicalensis (SBA) contains reasonable amounts of effective flavonoids, i.e. baicalin and scutellarin [26], in comparing with that of medicinal part. These flavonoids are being considered as major active ingredients responsible for anti-microbial functions [14]. To recycle the waste materials deriving from farming of S. baicalensis, SBA extract has been proposed as a feed for aquaculture. The intake of SBA by S. fuscescens greatly improved the fish survival, as well as its inflammatory response to marine bacteria [26], [27], [28]. The effects of SBA on immune system of S. baicalensis, as well as its mechanistic action in macrophage, are unknown. Having the established macrophagic-like cell line from S. baicalensis kidney, we therefore determined the efficacy of SBA, as well as its flavonoids, in fighting against inflammation.
2. Method
2.1. Isolation and culture of macrophagic cells
The isolation process and culture medium of fish macrophage was followed by reported protocol with minor modification [27]. Two healthy S. fuscescens fish (approximately 15 g in weight) were collected from an aquaculture farm (Shenzhen, China): the fish were maintained in an aquarium equipped with sea water recirculation system. The fish were anesthetized with 2-phenoxyethanol (1:10,000) and then washed with diluted bleach (1:100), wiped with 70% ethanol, to remove surface contamination. The fish were decapitated, and the head of kidney tissue was subsequently removed and placed in HBSS medium with antibiotics (penicillin, 100 U/ mL; streptomycin, 100 μg/mL; amphotericin-B 0.01 μg/ mL) (Thermo Fisher Scientific, Waltham, MA) for washing. Then, the tissue fragments were minced into small pieces (approximately 2 mm2) using surgical scissors. Tissue pieces were put into DMEM (FBS free) (Thermo Fisher Scientific) with antibiotics, then 5 mL collagenase A (0.4 mg/mL; Sigma-Aldrich, St Louis, MO) was added. The tissue mixture was kept in an incubator with orbital shaking at 28 ⁰C at a speed of 90 rpm for 60 min. The solution was centrifuged and washed with DMEM to remove collagenase. Then, 5 mL of 0.25% trypsin-EDTA solution were added and maintained for 20 min at 37 ⁰C. Three mL FBS (Thermo Fisher Scientific) were added for trypsin neutralization and centrifuged at 200 X g for 10 min. The cell pellet was resuspended in DMEM and filtered with 70 µm nylon to remove undigested tissues. The cells were placed onto top of 5 mL Ficoll-Paque solution (Ficoll PM400/sodium diatrizoate solution) in a 15 mL tube. The tube was centrifuged at 400 X g for 30 min at 18 °C. The mononuclear layer of cell was collected (∼2 mL) and resuspended with 6 mL 1X PBS and centrifuged at 300 X g for 10 min at 18 °C. Cell pellets were suspended in 4 mL DMEM medium and seeded into 25 cm2 flasks having 3 mL of DMEM (supplemented with 20% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin, amphotericin-B 0.01 μg/mL; pH 7.4) in an incubator at 28 °C with 5% CO2. Afterwards, the medium was replaced with fresh medium by half, every 2–3 days. The subculture was carried out at 1:2 split subsequently by trypsinization when primary cell cultures grew to 90–100% of confluence. The experimental procedures were reviewed and approved by Animal Ethics Committee at the University (HKUST).
2.2. DNA extraction and PCR analysis
Total genomic DNA was extracted from cultured RFM (at passage 30), or fin tissues of S. fuscescens [27], using microElute Genomic DNA kit (Omega Bio-tek, Norcross, GA). The quantitation and quality of isolated DNA was tested by NanoDrop™ 2000 spectrophotometers (Thermo Fisher Scientific). Fragments of S. fuscescens 18S ribosomal RNA (∼200 bp) were amplified using primers 3′-CCA TTG GTT CAT TCG GAG TA-5′ and 3′ TTG GAT GGT TTA GTG AGG TC-5′. The PCR products were sequenced, and the obtained sequences were aligned against known S. fuscescens 18S rRNA sequences from NCBI database (GenBank Accession No. AB276986.1). For genes that specifically expressed in macrophage, the primers were as followed: 3′- AAA GCG CTG TGT TTG AGC TG-5′ (S) and 3′-CCT TTG TCA GGT CCA CCT CC-5′ (AS) for CD11b (XM_009306409.3); 3′-CAC AGG TCT ACA CAC AGG AGC-5′ (S) and 3′-CTT GGT GAA ATT CAA CTC TTT AC-5′ (AS) for CD18 (BI476115.1); 3′-CAA GTG CCA GAC GGG AAA CT-5′ (S) and 3′-CGA GAG CTG TTA TCT GCG TT-5′ (AS) for CD32, (MG993323.1); 3′-AGC GGC ACA TTC ATC AGA CA-5′ (S): 3′-TCT GGG TCA GTC TGG TCC TT-5′ (AS) for CSFR (NM_001114480.1); 3′-TTG CCC TTG GAT TCT GGG TT-5′ (S) and 3′-GCC GTG TTT ACG TTG GT-5′ (AS) for F4/80 (XM_021478071.1); S: 3′-GTG TTG AAT CAG TGT TGA ATG TAG A −5′ (S) and 3′-TTC CCA TCA AAC AGC GTC CA-5′ (AS) MHC-II (AF114830.1).
2.3. Growth curves
The effects of serum concentration and culture temperature on cell growth were determined, as previously described with modification [27]. RFM cells at a density of 3 × 103 cells per well were seeded onto 96-well plates or 2 × 104 cells per well were seeded onto 35-mm dishes and incubated at different temperatures (28 °C and 37 °C) in DMEM, or DMEM/F-12, containing different FBS (10 to 30%). After cultured for 24 h, the non-adherent cells were removed by PBS washing. The macrophagic cells were cultured over 10 days, and the medium was changed every 2 days. Every day, viability assay by MTT was applied, and the value of OD570nm was used to measure the relative cell number. The cell number in 35 mm dishes was counted by a hemocytometer, every day as well.
2.4. Cell viability assay
MTT assay was employed for revealing cell viability. In brief, RFM cells were seeded in 96-well plate. After the treatment for 1 day, the final concentration of 0.5 mg/mL of MTT (Thermo Fisher Scientific) solution was applied, the production of purple crystal was dissolved in DMSO. The optimized absorbance was set at 570 nm.
2.5. Nitric oxide production
The nitric oxide (NO), released by RFM cell, was determined by measuring nitrite concentration in culture media with NO Fluorometric Kit (BioVision # K262–200) according to the manufacturer's manual. The 85 µL of culture medium was mixed with 115 µL assay buffer in a 96-well plate. Then, 5 µL of nitrate reductase mixture and 5 µL of enzyme cofactor were added into the well. The plate was incubated at room temperature for 1 h. After the incubation, 5 µL of the enhancer was added into each well and incubated for 10 min. Fifty µL Griess reagent R1 was added into each well, followed with 50 µL of Griess reagent R2. The absorbance at 540 nm after 10 min incubation at room temperature was determined.
2.6. Hematoxylin and eosin stain
Cells were grown in a 35 mm culture dish pre-coated by poly-L-lysine for 24 h. After PBS wash, cells were fixed with 4% paraformaldehyde for 15 min. The hematoxylin and eosin stain (H&E stain) of macrophagic cell was performed by a kit from Beyotime Biotechnology Co. (Haimen, Jiangsu, China), according to the manufacturer's manual.
2.7. DNA construction and transfection
The vector, pGL4.32 [luc2P/NF-κBRE/Hygro], contains five copies of a NF-κB response element (5′-GGG AAT TTC CG-3′) that drives transcription of a luciferase reporter geneluc2P (Promega Corporation, Madison, WI), namely as pNF-κB-Luc. The vector pGL3-basic contains an interleukin-6 (IL-6) promoter sequence from seabream that drives transcription of the firefly luciferase sequence. Cultured RFM cells (at passage 30) were seeded onto a 24-well cell culture plate at a density of 6 × 104 cells per well. After being cultured for 24 h at 28 °C, RFM cells were transfected with plasmid pNF-κB-Luc, or pIL-6-Luc, by Lipofectamine™ 3000 transfection kit (Thermo Fisher Scientific). Briefly, 1 μL of P 3000™ reagent and 0.25 μg plasmid were diluted by 25 μL of Opti-MEM™ medium. Then, the diluted plasmid was added to a tube containing diluted Lipofectamine™ 3000 reagent (0.75 μL in 25 μL of Opti-MEM™ medium) and mixed well. After incubation for 10–15 min at room temperature, 50 µL mixture was added to each well. The cells were treated at 28 °C for 5 h, then the transfecting reagent was removed. The cells were pre-treated with various concentrations of drugs including, SBA, flavonoid and dexamethasone for 4 h, then challenged with an inflammatory response by lipopolysaccharide (LPS; Sigma-Aldrich), for 24 h in serum free medium. The medium was aspirated, and the cultures were washed by PBS, twice. The cells were lysed by a buffer containing 0.2% Triton X-100, 1 mM dithiothreitol (DTT) and 100 mM potassium phosphate buffer (pH 7.8) at 4 °C. Followed by centrifugation at 10,000 X g at 10 min, the supernatant was collected and used to perform luciferase assay (Tropix, Bedford, MA). The luminescent reaction was quantified in a Tropix TR717TM Microplate Luminometer, and the activity was expressed as absorbance (up to 595 nm) per mg of protein.
2.8. Quantitative real-time PCR
In order to reveal the inflammation response in cultured RFM cells, the expression levels of inflammation cytokines, i.e. IL-1β, IL-6, TNF-α, IL-4, IL-10, induced by LPS were measured by real-time PCR. For anti-inflammatory test, the cells were pre-treated with various concentrations of drugs, including SBA, flavonoid and dexamethasone, for 4 h, then the cells were challenged with LPS for another 24 h in serum free medium. Total RNA from the treated cultures was isolated by RNAzol reagent (Molecular Research Center, Cincinnati, OH) and then reversed transcribed into cDNAs by using MMLV (Moloney Murine Leukemia Virus) reverse transcriptase according to the manufacturer's instructions (Invitrogen). Real-time PCR was employed here by using FastStart Universal SYBR Green Master (ROX) according to the manufacturer's instructions (Roche Applied Science, Mannheim, Germany). For fish inflammatory cytokines, the primers were as followed: 5′-ACC ATC TGG CTG CGG GAA C-3′ (S) and 5′-GAA TGA GTC GTG TGG TCT GGA AG-3′ (AS) for fish IL-6 (NM_010927.3); 5′-AGC CAA TCT GGC AAG GAT CA-3′ (S) and 5′- GAT GAA CCA GTT GT-3′ (AS) for fish IL-1 β (DQ306711.1); 5′-CTT CAC GCT CAA GTC TCA G-3′ (S) and 5′-AAA GCC TGG TCC TGG TTC ACT C-3′ (AS) for fish TNF-α (XM_039680790.1); β-actin was used as an internal control, and its primer sequences were 5′-TTA TGA AGG CTA TGC CCT GCC-3′ (S) and 5′-TGA AGG AGT AGC CAC GCT CTG T-3′(AS). SYBR green signal was revealed by ABI 7500 Fast Real-Time PCR system (Applied Biosystems, Foster City, CA). Transcript levels were quantified by using ΔCt value method, where the values of target genes were normalized by β-actin in the same sample for comparison. PCR products were analyzed by gel electrophoresis and melting curve analysis, as to confirm the specific amplification.
2.9. Phagocytic assay
Cells were grown on glass coverslip pre-coated by poly-L-lysine for 24 h. Then fluorescent beads (diameter = 1 µm; Thermo Fisher Scientific, F8816) were added at the rate of 1:10 (cells: beads) and co-cultured for 2 h. Then, the floating beads was discarded, as well as the medium, and the coverslip was washed by 1X PBS for 5 times to wash away the attached beads. Cells were fixed with 4% paraformaldehyde for 15 min before immunofluorescent staining.
2.10. Reactive oxygen species (ROS) assay
Cells were grown on glass coverslip pre-coated by poly-L-lysine for 24 h. Then, dexamethasone, LPS or H2O2 was added into cell medium, separately, and co-cultured for 2 h. After the treatment, the medium was discarded, and DCFH-DA (D6883 Sigma-Aldrich) solution (10 µM) was added and cultured for 30 min in dark. Then, DCFH-DA solution was discarded, and the coverslip was washed by 1X PBS for 3 times to wash away the floating cells. Cells were fixed with 4% paraformaldehyde for 15 min. Samples were mounted with ProLong™ Gold Antifade Mountant with DAPI (Thermo Fisher Scientific). Samples were then examined by Leica SP8 confocal microscope.
2.11. Immunofluorescent staining
Cells were grown on glass coverslip pre-coated by lysine for 24 h. After PBS wash, cells were fixed with 4% paraformaldehyde for 15 min. Cells were incubated with 0.2% Triton X-100 in PBS for 10 min and then blocked by 5% BSA for 1 h. Cultures were separately stained with anti-CD68 antibody (ab955, Abcam, Cambridge, UK) 1:100 at 4 °C overnight, followed with the Alexa-Fluor 647-conjugated anti-mouse antibodies (ab150119, Abcam) or Alexa Fluor™ 555 Phalloidin for F-actin staining to view the cell morphology. Samples were mounted with ProLong™ Gold Antifade Mountant with DAPI (Thermo Fisher Scientific). Samples were then examined by Leica SP8 confocal microscope.
2.12. Statistical analysis and other assays
Protein concentration was measured by a kit from Bio-Rad (Hercules, CA). Each result was presented as the mean ± SD, calculated from 3 - 5 independent samples, with triplicated. Comparisons of the mean for untreated control cells and treated cells were analyzed using one-way analysis of variance (ANOVA) and Student's t-test. Significant values were represented as *, p < 0.05, **, p < 0.01.
3. Result
3.1. Characterization of established RFM cell line
The cell line RFM was derived from head kidney of healthy S. fuscescens fish. The culture reached full confluence in 4 days at 28 °C. The cells were sub-cultured at a split ratio of 1: 2 for every 1–2 days The staining of F-actin was applied here to clearly show cytoskeleton and membrane of the cells. The morphology of macrophage-like cells, as compared to a macrophagic cell line, RAW 264.7 (Fig. 1A& B), was recognized in first few passages of cultures. A strong immunostaining signal in the cells, incubated with anti-CD 68 antibody, was identified (Fig. 1C and Supplementary Fig. 1A), as well as for RAW 264.7 cell line, revealing RFM cell as a type of monocyte. RFM cells could be sub-cultured over 50 times without much change in cell morphology and identity. To confirm the origin of RFM cells, a 200-bp PCR product flanking 18S rRNA was amplified from RFM cells, or head kidney of S. fuscescens (Supplementary Fig. 2A&B). DNA sequencing and comparative analysis showed that the partial 18S rRNA sequence from genomic DNA of cultured RFM cells showed ∼99% identity with S. fuscescens tissue, and both showed ∼99% identity to known S. fuscescens 18S rRNA sequence from NCBI database (GenBank Accession No. AB276986.1) (Supplementary Fig. 2A&B). Thus, the identity of newly developed RFM cells was being confirmed here. Additionally, the expressions of genes specific in macrophage, including CD11b, CD18, CD32, F4/80, CSFR and MHC-II, were identified in RFM cells by PCR analyses (Supplementary Fig. 2C), supporting the identity of macrophagic-like in nature.
Fig. 1.
Morphology of cultured RFM cells isolated from rabbit fish.
(A): Morphology of RFM cells and RAW cells after H & E stained at passage 30 after culture. (B): Cells were fixed with 4% paraformaldehyde for 15 min and stained with Alexa Fluor™ 555 Phalloidin for F-actin staining. Confocal image showed the cell skeleton. Nucleus was stained with DAPI. (C): RFM cells were fixed with 4% paraformaldehyde for 15 min and stained with anti-CD68 antibody with 0.2% Triton X-100. Confocal image showed CD 68 expression in RFM cells. Nucleus was stained with DAPI. Asterisk indicates the enlarged area, n = 4.
RFM cell at 30th passage was analyzed for growth. RFM cells could only grow under the temperature of 28 °C, and which did not survive under 37 °C (Fig. 2). The growth rate of RFM cells was rather different under various concentrations of serum (from 10 to 30%) in DMEM, or DMEM/F-12, showing a maximal growth rate at 20% FBS in DMEM at 28 °C (Fig. 2). The growth was at peak after 6–7 days of culture. Serum at 10% or 30% however did not support proper growth of RFM cells in culture. Thus, 20% FBS was employed in culture at 28 °C, and which should be used within 6 days of culture. To further verify the growth curve of RFM cell, absolute cell number was counted as well (Supplementary Fig. 3).
Fig. 2.
Growth curves of RFM cells at different temperatures and serum concentrations.
RFM cells were cultured under 28 ⁰C (A) or 37 ⁰C (B) in Dulbecco's modified Eagle's medium (DMEM) or DMEM-F12 medium, supplemented with different fetal bovine serum (FBS) concentration from 10% to 30%, as indicated. MTT assay was performed every day. The cell viability was calculated by MTT assay. Data are shown in percentage of change, as compared to control group (day 0), and in Mean ± SD, where n = 4, each with triplicate samples.
3.2. The inflammatory response of RFM cell
Testing inflammatory response of cultured RFM cells, LPS was applied here to induce inflammation. Different dosages of LPS applied in cultures induced robust inflammation, and therefore the release of nitic oxide (NO) was determined. Here, the release of NO by RFM cell was dose-dependently increased by applied LPS: the induction was fully suppressed by dexamethasone, a well-known steroid to suppress inflammation serving as a control (Fig. 3A). The mRNA expressions of pro-inflammatory cytokines, i.e. IL-1β, TNF-α, were increased from 2 to 11 folds, as induced by LPS, and the induction was in a dose-dependent manner (Fig. 3B). Again, the induction was fully blocked by dexamethasone. In contrast, the expressions of anti-inflammatory cytokines, i.e. IL-4 and IL-10, showed no significant difference in LPS-induced inflammation. To demonstrate the activation of transcriptions of NF-κB and IL-6, the luciferase reporters, i.e. pNF-κB-Luc and pIL-6-Luc, were employed here in cell transfection. The transfection efficiency of RFM cell was ∼60%, as determined by a RFP control plasmid (Supplementary Fig. 1B). In transfected RFM cells, the luciferase activities, driven by pNF-κB-Luc and pIL-6-Luc, were increased 10 to 40 folds and 5 to 20 folds, respectively, when challenged with LPS (Fig. 3C). In line to mRNA expression, this induction of promoter-driven activity was in a dose-dependent manner, and which was blocked by dexamethasone. These results show that the macrophagic cell, isolated from S. fuscescens fish, is highly sensitive to inflammatory response.
Fig. 3.
The inflammatory response of RFM cells.
(A): The cell culture medium was collected, and the amount of NO, released from RFM cells, was measured after treating with different concentrations of LPS for 24 h. Dexamethasone (Dex; 40 µM) was applied with 50 µg/mL LPS as a positive control here. (B): Expression levels of pro-inflammation cytokines, IL1-β, TNF-α, and anti-inflammation cytokines, IL-4, IL-10, in cultured RFM cells were measured after treating with LPS at different doses, as indicated, for 24 h. Total mRNAs were extracted from RFM cells, as described to perform real-time PCR analysis. Dexamethasone (Dex; 40 µM) was applied with 40 µg/mL LPS as a positive control here. (C): Two luciferase reporters containing NF-κB and IL-6 promoter sequences, named pNF-κB-Luc and pIL-6-Luc, were transfected in cultured RFM. The transfection efficiency was 50 - 60%, as indicated by vector containing a fluorescence protein. The transfected cells were treated with LPS for 24 h. Dexamethasone (Dex; 40 µM) was applied with 50 µg/mL LPS as a positive control here. Values are expressed as NO concentration in medium, or the fold of change to basal reading (as 1, no LPS), in Mean ± SD, where n = 4. Statistical comparison was made with the basal group; * p < 0.05; ** p < 0.01.
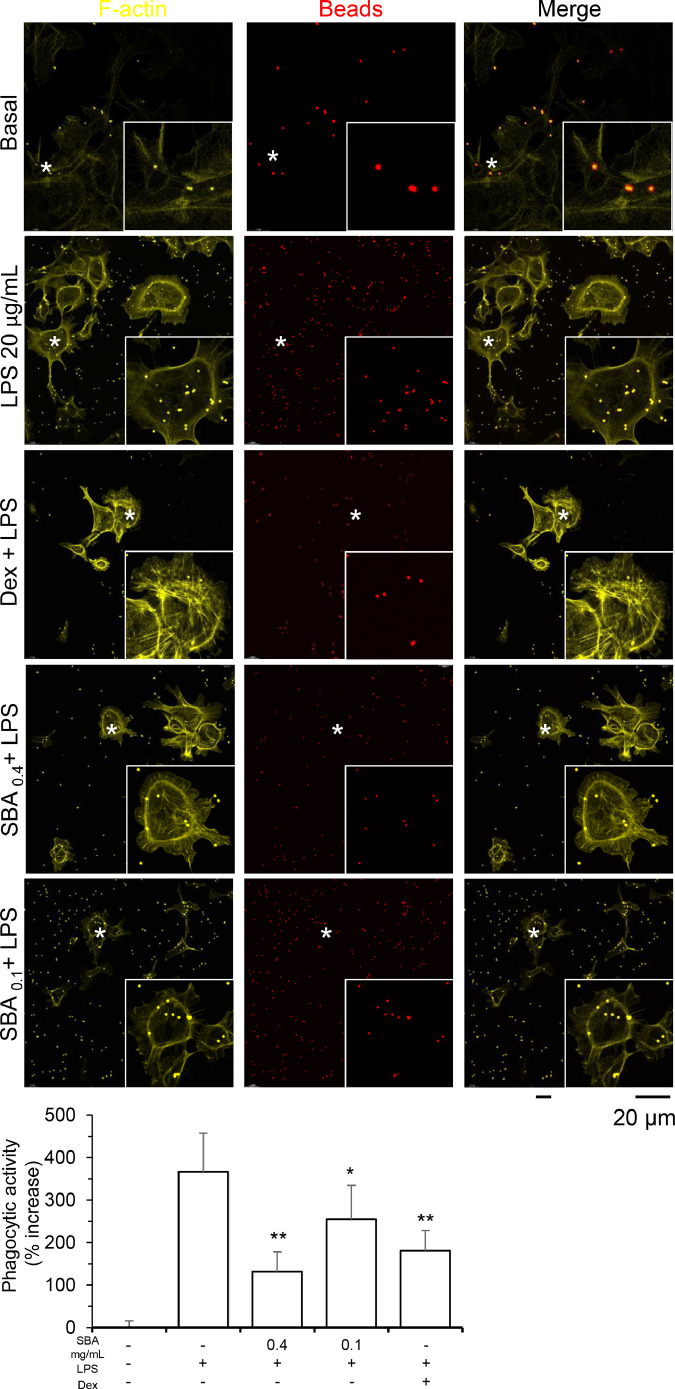
The oxidative burst activity of RFM cell was determined. During the uptake of beads, or endotoxicity triggered by LPS, the production of ROS was increased, as compared with the control group (Fig. 4). Here, H2O2 and dexamethasone were the controls for the experiments. Phagocytosis ability is an indicative property of macrophage. Here, the phagocytosis activity of RFM cell was tested by the intake of fluorescent beads. To further determine the intake of beads, the images of RFM cell and beads on Z-sack were taken and shown in Supplementary Fig. 4. The inclusion of LPS increased the number of beads being taken up, ranging from 150% to 300% of increase, in a dose-dependent manner (Fig. 5). The LPS-induced phagocytosis was blocked by dexamethasone.
Fig. 4.
Induction of ROS in cultured RFM cells.
RFM cells were seeded on cover slide for 24 h. Forty µM dexamethasone (Dex) was pre-treated the cells for 4 h, then LPS (20 μg/mL) or beads (1 µm) were included together to trigger inflammatory response. The formation ROS was detected by DCFH-DA, and H2O2 (1 mM) was used as a positive control here. Cells were fixed with 4% paraformaldehyde for 15 min. Nucleus were stained with DAPI. Asterisk indicates the enlarged area, n = 4. Representative photo is shown.
Fig. 5.
Phagocytic activity of RFM cells
RFM cells were seeded onto cover slide for 24 h. Forty µM dexamethasone (Dex; a control) was pre-treated the cells for 4 h, LPS (20∼40 µg/mL) were included to trigger inflammatory response and fluorescence beads (1 µm; red) were added at a ratio of 1:10. After 2 h, the beads were washed away by PBS. Cells were fixed with 4% paraformaldehyde for 15 min. The cell membrane was stained by F-actin and nucleus were stained with DAPI. Asterisk indicates the enlarged area. Representative photo is shown (upper panel). Values are expressed in percentage of increase, as compared to basal group (no LPS), in Mean ± SD, where n = 4, each with triplicate samples (lower panel). Statistical comparison was made with the control group; * p < 0.05; ** p < 0.01.
3.3. SBA relieves inflammation in RFM cell
In order to test the developed cell in drug screening, the water extract of aerial part of S. baicalensis (SBA) was used here. This SBA extract was proposed as a feeding for rabbit fish in anti-inflammation [27]. In cultured RFM cells, different concentrations of SBA extract were applied to test the cytotoxicity. Up to 0.4 mg/mL of SBA extract, the cultured REF cells did not show significant cell death (Supplement Fig. 5). The SBA was applied onto cultured RFM's to test the effect on phagocytosis activity. The beads up taken triggered by LPS was decreased significantly with SBA addition (Fig. 6). Dexamethasone suppressed the LPS-induced phagocytosis as well.
Fig. 6.
SBA extract reduces LPS-induced phagocytic activity.
RFM cells were seeded on cover slide for 24 h. RFM cells were pre-treated by different dosages of SBA (0.1 and 0.4 mg/mL) or 40 µM dexamethasone (Dex) for 4 h. Then, LPS (20 µg/mL) was included to trigger inflammatory response, and the fluorescence beads (1.0 µm, red) were added at a ratio of 1:10. After 2 h, the floating beads were washed away by PBS. Cells were fixed with 4% paraformaldehyde for 15 min. The cell membrane was stained by F-actin. Asterisk indicates the enlarged area. Representative photo is shown (upper panel). Values are expressed in percentage of increase, as compared to basal group (no LPS), in Mean ± SD, where n = 4, each with triplicate samples (lower panel). Statistical comparison was made with the control group; * p < 0.05; ** p < 0.01.
Here, SBA extract was applied for 4 h in cultured RFM cells before the challenged of LPS. After 24 h, the culture medium was collected, and the expressions of pro-inflammatory cytokines, i.e. IL-1β, TNF-α, and nitric oxide were measured. The treatment of SBA in LPS-treated RFM cells reduced the expressions of IL-1β and TNF-α in dose-dependent manners (Fig. 7A). Under the treatment of 0.1 to 0.2 mg/mL of SBA extract, the suppression of cytokine expression by cultured RFM cells was almost reaching maximum. Besides, we found that the LPS-induced nitric oxide production was suppressed by SBA dose-dependently (Fig. 7D). To further demonstrate potential suppression of NF-κB and IL-6, mediating by SBA extract, the transfected RFM cells with pNF-κB-Luc or pIL-6-Luc were used here. In transfected RFM cells, the activities of pNF-κB-Luc and pIL-6-Luc were robustly induced by LPS, and which were suppressed markedly by pre-treatment of SBA extract (Fig. 7A). All these results were similar to our previous research on rabbit fin cells [27]. Dexamethasone served as a positive control suppressing the LPS-induced mRNA level to completion. The maximal suppression, triggered by SBA, was as good as that of dexamethasone.
Fig. 7.
The anti-inflammatory effects of SBA, baicalin and scutellarin.
The cultured RFM cells were seeded and cultured for 24 h. Two luciferase reporter plasmids contain NF-κB and IL-6 promoter sequences, named pNF-κB-Luc and pIL-6-Luc, were transfected in cultured RFM. Then, different dosages of (A) SBA (0.2, 0.3, 0.4 mg/mL), (B) baicalin (0.5, 0.75, 1.0 µM), (C) scutellarin (4.5, 6.75, 9.0 µM) or dexamethasone (Dex; 40 µM), as indicated, was added into cultures separately and treated for ∼4 h. After treating with LPS (20 µg/mL) for 24 h. The luciferase activity in RFM cell were calculated as described. Total mRNAs were extracted from RFM cells as described to perform real-time PCR analysis, and the expression levels of pro-inflammation cytokines, IL1-β and TNF-α, in cultured RFM cells were measured. (D) Cultured RFM cells were seeded and cultured for 24 h. Different dosages of SBA, baicalin, scutellarin or dexamethasone (Dex), as in (A), were added onto culture for ∼4 h. After treating with LPS (20 µg/mL) for 24 h, the medium was collected for nitric oxide (NO) measurement. Values are expressed as the fold of increase to basal reading (as 1, no LPS), or NO amount, in Mean ± SD, where n = 4. Statistical comparison was made with the basal group; * p < 0.05; ** p < 0.01.
3.4. Scutellarin and baicalin relieve inflammation
The chemical standardization of SBA was verified by HPLC fingerprints, having scutellarin and baicalin as indicative markers (Supplementary Fig. 5). In addition, the amounts of scutellarin and baicalin were measured in SBA extract by HPLC. Scutellarin and baicalin were calculated as 1.083% and 0.123%, respectively, in SBA extract. Here, 0.4 mg/mL SBA equaled to 9 µM scutellarin and 1 µM baicalin. The amount of scutellarin and baicalin applied onto RFM cells was calculated as SBA concentration. In cultured RFM cells, different concentrations of scutellarin and baicalin were applied to test the cytotoxicity. Up to 10 µM scutellarin and baicalin, the cultured REF cells did not show significant cell death (Supplementary Fig. 5). Similar to SBA, scutellarin and baicalin was applied for 4 h before the challenged of LPS. After 24 h of treatment, the production of nitric oxide was measured. According to the real-time PCR result, the pre-treatment with the two flavonoids in the cultures, dose-dependently, suppressed the LPS-induced expressions of mRNAs encoding IL-1β, and TNF-α (Fig. 7B&C). Dexamethasone served as a positive control. In transfected RFM cells, the enhancements of pNF-κB-Luc and pIL-6-Luc, induced by LPS, were suppressed markedly by pre-treatment of scutellarin and baicalin (Fig. 7B&C). Additionally, the LPS-induced nitric oxide production was suppressed by these two flavonoids, dose-dependently (Fig. 7D). However, we found that the anti-inflammation effects of these two flavonoids were not as good as SBA extract under the same concentration (Fig. 7). These results indicated that synergistic effects between baicalin and scutellarin could be happened.
4. Discussion
A macrophagic-like cell line derived from S. fuscescens fish was established and characterized here: this cell line was named as RFM. The cultured RFM cell was able to sub-culture for over 50th passages under the optimized condition. The identity of RFM cell was confirmed by immunohistochemical staining and genomic sequencing. Cultured RFM cells showed maximal growth rate in DMEM medium having 20% FBS, The DMEM medium used here was slightly different with other established fish macrophagic cells. For example, Leibovitz-15 with 20% fetal calf serum [20] and NMGFL-15 with 2.5% heat-inactivated carp serum and 5% bovine calf serum [18] have been used. The best growth temperature of RFM was 28 °C, this temperature restriction was also applied to other macrophagic cell lines isolated from marine fish (Sheldon [24]). In addition, the inflammatory response of RFM cell was illustrated by applied LPS, as well as the anti-inflammatory effect triggered by dexamethasone. Dexamethasone is a synthetic glucocorticoid with anti-inflammatory properties, which has been widely used in clinical practice as a common anti-inflammatory drug [33]. Here, the application of dexamethasone was to suppress the inflammatory response, as a positive control.
Macrophage activates innate and adaptive immunity during the host facing infective agents [12]. Macrophage is acting as scavengers to engulf and destroy pathogenic microbes, infected matters or altered cells, and which alerts the immune system through secretion of cytokines. In addition, macrophage contributes to wound healing and tissue repair [7]. In cultured RFM cells, applied LPS triggered production of nitric oxide, similar to macrophagic cell lines from other fish [15,29]. In parallel, the expressions of pro-inflammation cytokines, i.e. IL-1β, TNF-α, as well as transcription of NF-κB-mediated genes, were induced under LPS challenge, as reported in fish macrophages [3,6,17]. Moreover, cultured RFM cells showed strong phagocytic activity against exogenic matters, and the production of ROS was increased during phagocytosis. Secretion of cytokines and phagocytosis are considered as classical response of macrophage to pathogenic bacteria [21,23].
Traditional Chinese medicine (TCM) provides an abundant resource for drug and/or food development. Because of low toxicity and inexpensive cost, Chinese herbal extracts have been applied in fish feedings to strength their immunity [4]. Scutellaria Radix (the dried root of S. baicalensis) has been used as a Chinese medicine for centuries because of its multiple pharmacological activities [13]. Scutellarin and baicalin are considered as main functional flavonoids in Scutellaria Radix [25], as well as in aerial part of this plant, i.e. SBA, which is a waste product during production of Scutellaria Radix. Baicalin is known to have a protective effect against LPS-induced inflammatory injury and plays an anti-inflammatory role by inhibiting the activation of NF-κB [32]. On the other hand, scutellarin was reported with anti-inflammation effect by inhibiting release of pro-inflammatory cytokines, TNF-α, and IL-6, and promoting activation of JAK2/STAT signaling leading to reduction of oxidative stress [1]. However, all these previous researchers were mainly focusing on mammalian cells. Here, we have shown, for the first time, the anti-inflammatory functions of these two flavonoids in a fish macrophagic cell line. Noticeable, the anti-inflammatory property of baicalin or scutellarin was worse than that of SBA under the same condition. These results indicated that synergy could be happened among baicalin, or scutellarin, with components within SBA. Indeed, this synergistic mechanism has been proposed for baicalin and scutellarin in activating kinase signaling [30]. Thus, the current result supports the application of SBA in aquaculture as feeds. This notion is supported by anti-inflammatory efficacy of SBA in cultured RFM cells, and this effect could be triggered, at least partly, by scutellarin and baicalin within SBA extract.
5. Conclusion
A macrophagic cell line from S. fuscescens (rabbit fish), designated as RFM, was isolated and characterized. The cells exhibited sensitive responses to inflammation. The established RFM cell line can be an excellent in vitro model in drug/feeding screening in fish biology. The water extract of aerial part of S. baicalensis, as well as its flavonoids, i.e. scutellarin and baicalin, dose-dependently showed anti-inflammation effect in cultured RFM cells. For an economic reason, the aerial part of S. baicalensis is the waste product of Scutellaria Radix production.
Declaration of Competing Interest
The authors declare that there are no conflicts of interest.
All authors have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Acknowledgments
This work is supported by Sustainable Fisheries Development Fund of Hong Kong (SFDF-0041), Dr. Lau Wah Shem Fund (PD18SC01), Hong Kong RGC Theme-based Research Scheme (T13–605/18-W); Hong Kong Innovation Technology Fund (UIM/340, UIM/385, ITS/500/18FP; TCPD/17–9); TUYF19SC02, and HMRF18SC06, Shenzhen Science and Technology Innovation Committee (ZDSYS20170728143 2317; JCYJ20170413173747440; JCYJ20180306174 903174), China Post-doctoral Science Foundation (2019M653087), Zhongshan Municipal Bureau of Science and Technology (ZSST20SC03), Guangzhou Science and Technology Committee Research Grant (GZSTI16SC02; GZSTI17SC02) .
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.fsirep.2021.100036.
Appendix. Supplementary materials
Reference
- 1.Akter R., Afrose A., Rahman M., Chowdhury R., Nirzhor S.S.R., Khan R.I., Kabir M. A comprehensive analysis into the therapeutic application of natural products as sirt6 modulators in Alzheimer's disease, aging, cancer, inflammation, and diabetes. Int. J. Mol. Sci. 2021;22(8):4180. doi: 10.3390/ijms22084180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barreda D.R., Neumann N.F., Belosevic M. Flow cytometric analysis of PKH26-labeled goldfish kidney-derived macrophages. Develop. Compar. Immunol. 2000;24(4):395–406. doi: 10.1016/s0145-305x(99)00059-2. [DOI] [PubMed] [Google Scholar]
- 3.Castro R., Zou J., Secombes C.J., Martin S.A. Cortisol modulates the induction of inflammatory gene expression in a rainbow trout macrophage cell line. Fish Shellfish Immunol. 2011;30(1):215–223. doi: 10.1016/j.fsi.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 4.Choi W.M., Mo W.Y., Wu S.C., Mak N.K., Bian Z.X., Nie X.P., Wong M.H. Effects of traditional Chinese medicines (TCM) on the immune response of grass carp (Ctenopharyngodon idellus) Aquacult. Int. 2014;22(2):361–377. [Google Scholar]
- 5.Duray M. Biology and culture of siganids: aquaculture department. Southeast Asian Fisheries. 1998 [Google Scholar]
- 6.Fierro-Castro C., Barrioluengo L., López-Fierro P., Razquin B., Villena A. Fish cell cultures as in vitro models of inflammatory responses elicited by immunostimulants. Expression of regulatory genes of the innate immune response. Fish Shellfish Immunol. 2013;35(3):979–987. doi: 10.1016/j.fsi.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 7.Galli G., Saleh M. Immunometabolism of macrophages in bacterial infections. Front. Cell Infect. Microbiol. 2021;10(903) doi: 10.3389/fcimb.2020.607650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.GarduÑo R.A., Kay W.W. In: Biochemistry and Molecular Biology of Fishes. Hochachka P.W., Mommsen T.P., editors. Elsevier; 1994. CHAPTER 28 - Isolation and culture of head kidney macrophages; pp. 327–339. 3: [Google Scholar]
- 9.Gong G., Wang H., Kong X., Duan R., Dong T.T., Tsim K.W. Flavonoids are identified from the extract of Scutellariae Radix to suppress inflammatory-induced angiogenic responses in cultured RAW 264.7 macrophages. Sci. Rep. 2018;8(1):1–11. doi: 10.1038/s41598-018-35817-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grayfer L., Hodgkinson J.W., Belosevic M. Antimicrobial responses of teleost phagocytes and innate immune evasion strategies of intracellular bacteria. Develop. Compar. Immunol. 2014;43(2):223–242. doi: 10.1016/j.dci.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Joerink M., Ribeiro C.M., Stet R.J., Hermsen T., Savelkoul H.F., Wiegertjes G.F. Head kidney-derived macrophages of common carp (Cyprinus carpio L.) show plasticity and functional polarization upon differential stimulation. J. Immunol. 2006;177(1):61–69. doi: 10.4049/jimmunol.177.1.61. [DOI] [PubMed] [Google Scholar]
- 12.Lavin Y., Mortha A., Rahman A., Merad M. Regulation of macrophage development and function in peripheral tissues. Nat. Rev. Immunol. 2015;15(12):731–744. doi: 10.1038/nri3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li C., Lin G., Zuo Z. Pharmacological effects and pharmacokinetics properties of Radix Scutellariae and its bioactive flavones. Biopharm. Drug Dispos. 2011;32(8):427–445. doi: 10.1002/bdd.771. [DOI] [PubMed] [Google Scholar]
- 14.Liu G., Rajesh N., Wang X., Zhang M., Wu Q., Li S., Chen B., Yao S. Identification of flavonoids in the stems and leaves of Scutellaria baicalensis Georgi. J. Chromatogr. B. 2011;879(13–14):1023–1028. doi: 10.1016/j.jchromb.2011.02.050. [DOI] [PubMed] [Google Scholar]
- 15.Macmicking J., Xie Q.W., Nathan C. Nitric oxide and macrophage function. Ann. Rev. Immunol. 1997;15(1):323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 16.Neumann N.F., Stafford J.L., Belosevic M. Biochemical and functional characterisation of macrophage stimulating factors secreted by mitogen-induced goldfish kidney leucocytes. Fish Shellfish Immunol. 2000;10(2):167–186. doi: 10.1006/fsim.1999.0236. [DOI] [PubMed] [Google Scholar]
- 17.Newton K., Dixit V.M. Signaling in innate immunity and inflammation. Cold Spring Harb. Perspect. Biol. 2012;4(3) doi: 10.1101/cshperspect.a006049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ribeiro C.M., Pontes M.J., Bird S., Chadzinska M., Scheer M., Verburg-Van Kemenade B.L., Savelkoul H.F., Wiegertjes G.F. Trypanosomiasis-induced Th17-like immune responses in carp. PLoS ONE. 2010;5(9):e13012. doi: 10.1371/journal.pone.0013012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rieger A.M., Hall B.E., Barreda D.R. Macrophage activation differentially modulates particle binding, phagocytosis and downstream antimicrobial mechanisms. Develop. Compar. Immunol. 2010;34(11):1144–1159. doi: 10.1016/j.dci.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Saint-Jean Rodriguez, S González, C Monrás, M Romero, A Ballesteros, N Enríquez, Perez-Prieto S. Establishment and characterization of a new cell line (SSP-9) derived from Atlantic salmon Salmo salar that expresses type I ifn. J. Fish Biol. 2014;85(5):1526–1545. doi: 10.1111/jfb.12503. R. [DOI] [PubMed] [Google Scholar]
- 21.Saqib U., Sarkar S., Suk K., Mohammad O., Baig M.S., Savai R. Phytochemicals as modulators of M1-M2 macrophages in inflammation. Oncotarget. 2018;9(25):17937–17950. doi: 10.18632/oncotarget.24788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Semple S.L., Dixon B. Salmonid antibacterial immunity: an aquaculture perspective. Biology (Basel) 2020;9(10) doi: 10.3390/biology9100331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharp G.J.E., Secombes C.J. The role of reactive oxygen species in the killing of the bacterial fish pathogen Aeromonas salmonicida by rainbow trout macrophages. Fish Shellfish Immunol. 1993;3(2):119–129. [Google Scholar]
- 24.Jr Sheldon, M W., Blazer V.S. Influence of dietary lipid and temperature on bactericidal activity of channel catfish macrophages. J. Aquat. Anim. Health. 1991;3(2):87–93. [Google Scholar]
- 25.Wang Z.L., Wang S., Kuang Y., Hu Z.M., Qiao X., Ye M. A comprehensive review on phytochemistry, pharmacology, and flavonoid biosynthesis of Scutellaria baicalensis. Pharm Biol. 2018;56(1):465–484. doi: 10.1080/13880209.2018.1492620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xia Y.T., Chan G.K.L., Wang H.Y., Dong T.T.X., Duan R., Hu W.H., Qin Q.W., Wang W.X., Tsim K.W.-K. The anti-bacterial effects of aerial parts of Scutellaria baicalensis: potential application as an additive in aquaculture feedings. Aquaculture. 2020;526 [Google Scholar]
- 27.Xia Y.T., Hu W.H., Wu Q.Y., Dong T.T.X., Duan R., Xiao J., Li S.P., Qin Q.W., Wang W.X., Tsim K.W.K. The herbal extract deriving from aerial parts of Scutellaria baicalensis shows anti-inflammation and anti-hypoxia responses in cultured fin cells from rabbit fish. Fish Shellfish Immunol. 2020;106:71–78. doi: 10.1016/j.fsi.2020.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xia Y.T., Cheng E.H.C., Zheng B.Z.Y., Wu Q.Y., Dong T.T.X., Duan R., Qin Q.-.W., Wang W.X., Tsim K.W.K. Feeding containing the aerial part of Scutellaria baicalensis promotes the growth and nutritive value of rabbit fish Siganus fuscescens. Food Sci. Nutr. 2021;9:4827–4838. doi: 10.1002/fsn3.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiang Y., Rice C.D. Expression of fish iNOS is increased by pro-inflammatory signals and xenobiotics. Mar. Environ. Res. 2000;50(1):466–467. [Google Scholar]
- 30.Yang L.L., Xiao N., Liu J., Liu K., Liu B., Li P., Qi L.W. Differential regulation of baicalin and scutellarin on AMPK and Akt in promoting adipose cell glucose disposal. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2017;1863(2):598–606. doi: 10.1016/j.bbadis.2016.11.024. [DOI] [PubMed] [Google Scholar]
- 31.Yashwanth B.S., Goswami M., Kooloth Valappil R., Thakuria D., Chaudhari A. Characterization of a new cell line from ornamental fish Amphiprion ocellaris (Cuvier, 1830) and its susceptibility to nervous necrosis virus. Sci. Rep. 2020;10:20051. doi: 10.1038/s41598-020-76807-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yin B., Li W., Qin H., Yun J., Sun X. The use of Chinese skullcap (Scutellaria baicalensis) and its extracts for sustainable animal production. Animals. 2021;11(4):1039. doi: 10.3390/ani11041039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang J., Qin X., Zhu Y., Zhang S., Zhang X., Lu H. Mechanism of dexamethasone in the context of Toxoplasma gondii infection. Parasitology. 2017;144(11):1551–1559. doi: 10.1017/S0031182017001111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.