Abstract
A feeding trial was conducted to investigate the effects of dietary yeast culture (YC) on health status in digestive tract of juvenile Pacific white shrimp Litopenaeus Vannamei. Shrimps (initial weight: 3.33 ± 0.06 g) were fed with graded levels of dietary YC (control, 0.3%, 0.5% and 1.0%). Results of the present study showed that villus height and the ratio between villus height and crypt depth in the digestive tract of juvenile shrimp was significantly increased by dietary 0.5% and 1.0%YC (P < 0.05). Besides, dietary 0.5% and 1.0%YC significantly activities of phenoloxidase (PO), lysozyme (LZ), acid phosphatase (ACP) and alkaline phosphatase (AKP) (P < 0.05), significantly up-regulated mRNA levels of prophenoloxidase (propo), lysozyme (lz), anti-lipopolysaccharide factor (alf), crustin and penaienadin (P < 0.05) and down-regulated mRNA levels of caspase-1, nuclear factor κB p65 (nf-κbp65) myeloid differentiation primary response protein (myd88) and toll like receptor (tlr) in the digestive tract of juvenile shrimp (P < 0.05). Compared with the control, dietary 0.5%YC increased Chao1 index in the digestive tract of juvenile shrimp. In addition, compared with the control, dietary 0.5% and 1.0%YC significantly increased relative abundance of Lactobacillus (P < 0.05). It can be concluded that dietary YC made positive contribution to health status in digestive tract of juvenile shrimp through improving morphology and microbiota, enhancing immune function, and inhibiting inflammation of digestive tract.
Keywords: Juvenile shrimp, Yeast culture, Immune function, Inflammation, Digestive tract microbiota
1. Introduction
As one of the most important organs of aquatic animals, digestive tract of aquatic animals is facing more challenges with the continuous development of intensive breeding models. Digestive tract is continuously and directly exposed to anti-nutritional factors, microbiotas, pathogens and some other unsafe substances from feed and environment, so it is of great importance to maintain healthy of digestive tract, thereby promoting the growth and development of aquatic animals [1,2]. Over the years, probiotics and prebiotics have been used to improve digestive tract health of aquatic animals [3]. But in the recent years, Yeast culture (YC) with better stability, and richer and more direct functionality, has drawn more attention [4], [5], [6]. YC contains nutrients including protein, lipid, and, B-vitamins, β-glucan, chitin, nucleic acid, mannan oligosaccharides (MOS) and so on [7,8]. And what is worth noting that the protective effects of YC on health status has been observed in some fish and shrimp [9,10].
Healthy digestive tract is needed for growth and development of aquatic animals, and its health status is influenced by antibacterial substances, antibacterial peptides and inflammatory response [11], [12], [13]. In aquatic animals, lysozyme (LZ), acid phosphatase (ACP) and alkaline phosphatase (AKP) are all important immune enzymes, protecting cells from pathogen damage [14,15] and antibacterial peptide is also essential in protecting cells from damage [16]. Inflammatory response during injury and infection is an essential part of immune function [17]. According to previous study in fish, during an inflammatory process, TLRs (Toll-like receptors) triggered cell signaling cascades through myeloid differentiation primary response protein MyD88 (MyD88)-interleukin 1 receptor associated kinase 4 complex, and induced the subsequent nuclear localization of nuclear factor κB (NF-κB), finally induced the expression of pro-inflammatory cytokines and other inflammatory molecular such as caspase-1 [18], [19], [20]. In addition to what mentioned above, the microbiota is also vital to health status in digestive tract of aquatic animals [13]. Some of beneficial microbiota is helpful to digestion, absorption and utilization of nutrient, resistance to pathogens and regulation of immune system [21].
Pacific white shrimp Litopenaeus Vannamei is one of the most important and profitable aquaculture species in China, which accounts for about 85 % of total shrimp production [22]. Previous study has shown that dietary YC could increase growth performance, enhance immune function in serum and improve microbiota composition in digestive tract of Pacific white shrimp [9]. But up to now, there is no study investigating the effects of dietary YC on antibacterial substances, antibacterial peptides, and inflammatory response in digestive tract of shrimp and in the early stage of farming, the shrimp is fragile [23]. Hence, focusing on digestive tract of juvenile shrimp, the present study investigated whether dietary YC could improve its development, as well as antibacterial substances, antibacterial peptides, inflammatory response, and microbiota composition in a short time. Findings from the present study will provide theoretical basis for the application of YC in shrimp diet in the future.
2. Materials and methods
All animal care and handling procedures in present study were approved by the Animal Care Committee of Ocean University of China.
2.1. Experimental diets
Four isonitrogenous and isolipid experimental diets were formulated by supplementing YC at 0.0 %, 0.3 %, 0.5 % and 1.0 % respectively. The YC derived from Saccharomyces cerevisiae (corn and bran as fermentation substrates) was provided by GBW Biotechnology Group (Qingdao, Shandong, China). Fish meal, shrimp meal, squid offal meal, soybean meal and peanut meal were used as the main protein sources. Fish oil, soybean oil and soyabean lecithin were used as the main lipid sources. High gluten flour and α-starch was used as the main carbohydrate source. Ingredients and proximate compositions of the experimental diets are shown in Table S1. All ingredients were grounded into fine powder and passed through a 100-mesh sieve. After that, fish oil and soybean oil were mixed into soybean meal and peanut meal, and then the other ingredients were gradually added and mixed. Adding 15 % of the water into the premix, then the premix was granulated into 1.0 mm diameter particles. Finally, the diets were dried at 55°C for 12 h and stored at -20°C until used.
2.2. Feeding trial
Feeding trial was conducted in the temperature controllable re-circulating seawater aquaculture system of GBW Biotechnology Group (Qingdao, Shandong, China). A total of 1152 juvenile shrimps (initial body weight: 3.33 ± 0.06 g) were collected from a fishery company in Qingdao, China. Before the feeding trial, shrimps were acclimatized to the experimental environment for 10 days. Then shrimps were randomly assigned to 32 sea cages (36 shrimps per cage). Each diet was fed to eight cages three times a day at 8:30, 12:30 and 16:30. Uneaten diets and feces were removed before feeding. During the 30-day feeding trial, the water temperature was set at 27 °C, the pH value was about 8, the salinity was about 20‰ and the dissolved oxygen was above 8 mg/L.
2.3. Sample collection
At the end of the feeding trial, all shrimps were fasted for 24 hours. After that, shrimps were removed from tanks, and then weighted and counted to determine the weight gain rate (WGR), and survival rate (SR). After shrimps were anaesthetized using 5 % ethyl alcohol, the digestive tract of shrimps was collected, then washed with cold saline (0.86 % NaCl) and finally frozen in liquid nitrogen.
2.4. Proximate composition
The proximate composition of diets and the whole body of shrimps were determined according to the standard methods [24]. Samples were dried to constant weight to determine moisture content under 105°C. Crude protein content was determined by measuring nitrogen (N × 6.25) according to the Kjeldahl method (2300-Auto-analyzer, FOSS, Hillerød, Denmark). Crude lipid contents were determined by ether extraction following the Soxhlet method (36,680-analyzer, BUCHI, Flawil, Switzerland).
2.5. Immune enzyme activities
The activities of total phenoloxidase (PO), lysozyme (LZ), acid phosphatase (ACP) and alkaline phosphatase (AKP) in digestive tract of shrimps were determined using the commercial assay kit (Nanjing Jiancheng Bioengineering Institute, China).
2.6. Real-time polymerase chain reaction (PCR) analysis
Total RNA samples were isolated from the digestive tract using RNAiso Plus Kit (9109, Takara Biotech, China). Agarose gel electrophoresis was used to assess the quality of RNA. A PrimeScripte RT reagent Kit (RR047A; Takara Biotech, China) was used to get single-stranded cDNA from total RNA by reverse transcription. Primers are listed in Table 1. Real-time PCR assays were carried out in a quantitative thermal cycler (CFX96, Bio-Rad, USA) in a final volume of 15 μL containing 7.5 μL 2 × SYBR Green Realtime PCR Master Mix (Q711–02, Vazyme Biotech, China), 0.3 μL each of primers (10 μmol/L) and 1 μL of cDNA mix. Real-time PCR temperature profile was 95°C for 30 s followed by 40 cycles of 10 s at 95°C, 30 s at 58°C. Then, to confirm the specificity and purity of all PCR products, melt curve analysis was carried out: 95°C for 15 s, 60°C for 1 min and 95°C for 1 s. Fluorescent data were acquired during each annealing phase. β-actin was used as an endogenous reference. Results of gene expression were analyzed using the 2−ΔΔCT method according to Livak and Schmittgen [25]. Control group was chosen as normalized gene expression, which was set at 1.
Table 1.
Real-time PCR primer sequences.
| Target gene | Primer sequence Forward | Primer sequence Reverse | Accession number |
|---|---|---|---|
| propo | TACATGCACCAGCAAATTATCG | AGTTTGGGGAAGTAGCCGTC | AY242387 |
| lz | GTTCCGATCTGATGTCCGATG | AAGCCACCCAGGCAGAATAG | AY170126.2 |
| alf | CACCGTCAAACCTTACATCAAG | GTTAGTTCAGCCACCGCTTAG | AY859500.1 |
| crustin | ATTCTGTGCGGCCTCTTTAC | ATCGGTCGTTCTTCAGATGG | AF430076 |
| penaiedin | GTTGACGGAGAACACGATGAA | CATTCCACAAGCCAGAGTAAGA | AY260151.2 |
| myd88 | GCTGTTCCACCGCCATTT | GCATCATAGTGCTGTAGTCCAAGA | JX073566 |
| tlr | TGAGAGATGCCCACTGCCTG | CGCTTGAAGGTTTGTGAGGGAG | DQ923424 |
| β-actin | CGCGACCTCACAGACTACCT | CTCGTAGGACTTCTCCAGCG | AF300705 |
| Target gene | Primer sequence Forward | Primer sequence Reverse | Gene ID |
| caspase-1 | AAGTGTCCATCCTTGGCAGG | CGAAACCCCACTGGACGTAA | 113,823,293 |
| nf-κbp65 | TGGCTGGTGGCAAAGAGATT | TCACCGAAGGCTTCCCATTC | 113,829,101 |
propo, Prophenoloxidase; lz, Lysozyme; alf, Anti-lipopolysaccharide factor; nf-κbp65, Nuclear factor κb p65; myd88, Myeloid differentiation primary response protein; tlr, Toll like receptor.
2.7. Digestive tract microbiota profiling
The bacterial DNA was extracted from all samples using the MN NucleoSpin 96 Soi DNA Isolation Kit (MN-MACHEREY-NAGEL, Germany). The hypervariable region V3-V4 of the bacterial 16S rRNA gene was amplified by an ABI 9902 thermal cycler (Applied Biosystems, USA). Raw reads were firstly filtered by Trimmomatic v0.33 [26], then the primer sequences were identified and removed by cutadapt 1.9.1 [27], which finally generated high-quality reads without primer sequences. Based on overlapping sequences, high-quality reads were assembled by FLASH v1.2.7 [28]. Chimeric sequences were identified and removed by UCHIME v4.2, generating effective reads. Operational taxonomic units (OTUs) with 97 % similarity cutoff were clustered using USEARCH 10.0 [29]. Alpha diversity analysis including Chao1 index, Shannon index, Simpson and ACE were computed with QIIME 1.7.0. The dissimilarity between microbial communities was assessed by unweighted-UniFrac distance based principal coordinate analysis (PCoA). The statistical significance of the separation among groups was identified by the linear discriminant analysis effect size (LEfSe) method.
2.8. Calculations and statistical analysis
The weight gain rate (WGR, %) and feed conversion ratio (FCR) were calculated as follows:
Statistical analysis was performed using SPSS 25.0 (SPSS, Chicago, IL, USA) and Microsoft Excel 2010. All the results are represented with the means ± SD. All data were analyzed by one-way analysis of variance to determine whether dietary lipid levels significantly (P < 0.05) affected the observed responses. Duncan's multiple range test was used when significant differences (P < 0.05) were found.
3. Results
3.1. Growth performance and proximate analysis of juvenile shrimp
As shown in Table 2, compared with control, dietary 1.0 % YC increased WGR and decreased FCR of juvenile shrimp. As shown in Table 4, dietary YC levels had no significant influences on contents of dry matter and crude lipid. Compared with the control, 0.5 % and 1.0 % of dietary YC significantly increased crude lipid and crude ash levels (P < 0.05).
Table 2.
Proximate analysis of Pacific white shrimp L. Vannamei (dry matter %).
| Dietary YC levels | ||||
|---|---|---|---|---|
| Control | 0.3 % | 0.5 % | 1 % | |
| Dry matter (%) | 22.42±1.85 | 22.62±0.68 | 23.19±1.38 | 23.23±1.70 |
| Crude protein (%) | 74.25±0.39a | 74.74±0.10ab | 75.77±0.82b | 75.79±0.44b |
| Crude lipid (%) | 6.01±0.09 | 5.98±0.13 | 6.04±0.23 | 5.99±0.06 |
| Crude ash (%) | 13.53±0.06a | 13.98±0.02c | 13.75±0.12b | 13.85±0.01bc |
All values were presented as the mean ± SE (n = 8). Means in the same row sharing with different superscript letter were significantly different (P < 0.05) as determined by Duncan's test.
Table 4.
Effects of dietary YC on immune enzymes activities in digestive tract of Pacific white shrimp L. Vannamei.
| Dietary YC levels | ||||
|---|---|---|---|---|
| Control | 0.3 % | 0.5 % | 1 % | |
| PO | 2.30±0.14a | 2.22±0.15a | 2.56±0.09b | 2.55±0.13b |
| LZ | 21.02±1.93a | 21.24±1.18a | 25.23±1.08b | 25.78±0.78b |
| ACP | 3.16±0.20a | 3.37±0.17b | 3.98±0.15c | 3.96±0.14c |
| AKP | 4.46±0.24a | 4.53±0.25a | 5.18±0.30b | 5.41±0.22b |
All values were presented as the mean ± SE (n = 8). Means in the same row sharing with different superscript letter were significantly different (P < 0.05) as determined by Duncan's test. PO, phenoloxidase; LZ, lysozyme; ACP, acid phosphatase; AKP, alkaline phosphatase.
3.2. Histologic morphology of digestive tract
As shown in Table 3 and Fig. 1, with the increasing of dietary YC level, the development of digestive tract gradually improved. Compared with control, 0.5 % and 1.0 % dietary YC levels significantly increased villus height, decreased crypt depth and increased the ratio between them in the digestive tract of juvenile shrimp (P < 0.05).
Table 3.
Effects of dietary YC on villus height, crypt depth and the ratio between in digestive tract of Pacific white shrimp L. Vannamei.
| Dietary YC levels | ||||
|---|---|---|---|---|
| Control | 0.3 % | 0.5 % | 1 % | |
| Villus height (μm) | 29.13±5.32a | 28.99±3.65a | 44.99±3.93b | 59.56±6.62c |
| Crypt depth (μm) | 28.65±6.14c | 20.76±1.62ab | 18.89±2.03a | 23.96±3.93b |
| Ratio | 1.04±0.23a | 1.40±0.21b | 2.42±0.40c | 2.53±0.41c |
All values were presented as the mean ± SE (n = 8). Means in the same row sharing with different superscript letter were significantly different (P < 0.05) as determined by Duncan's test.
Fig. 1.
Effects of dietary YC levels on villus height and crypt depth in the digestive tract of Pacific white shrimp L. Vannamei.
3.3. Immune enzyme activities and gene expression of immune related parameters
As shown in Table 4, compared with control and 0.3 % dietary YC level, 0.5 % and 1.0 % dietary YC levels significantly increased activities of PO, LZ, ACP and AKP in the digestive tract of juvenile shrimp (P < 0.05).
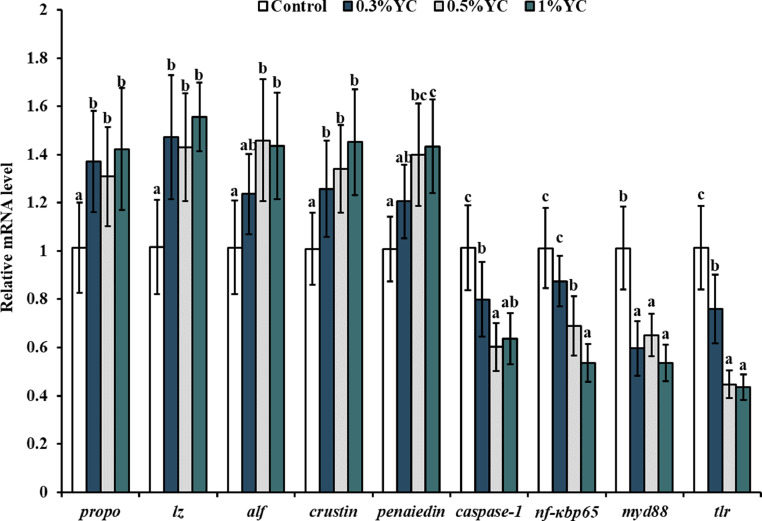
As shown in Fig. 2, with the supplementation of dietary YC, mRNA levels of propo, lz and crustin in control were significantly lower than the other three groups in the digestive tract of juvenile shrimp (P < 0.05), while the mRNA level of myd88 was significantly higher than the other three groups (P < 0.05). Besides, compared with control, 0.5 % and 1.0 % of dietary YC levels significantly up-regulated the mRNA level of alf and penaiedin (P < 0.05), while significantly down-regulated mRNA level of caspase-1, nf-κbp65 and tlr in the digestive tract of juvenile shrimps (P < 0.05).
Fig. 2.
Effects of dietary YC levels on gene expression of antibacterial substances, antibacterial peptides and inflammation parameters in the digestive tract of L. Vannamei. All values were presented as the mean ± SE (n = 8). Means with different superscript letter were significantly different (P < 0.05) as determined by Duncan's test.
3.4. Digestive tract microbiota
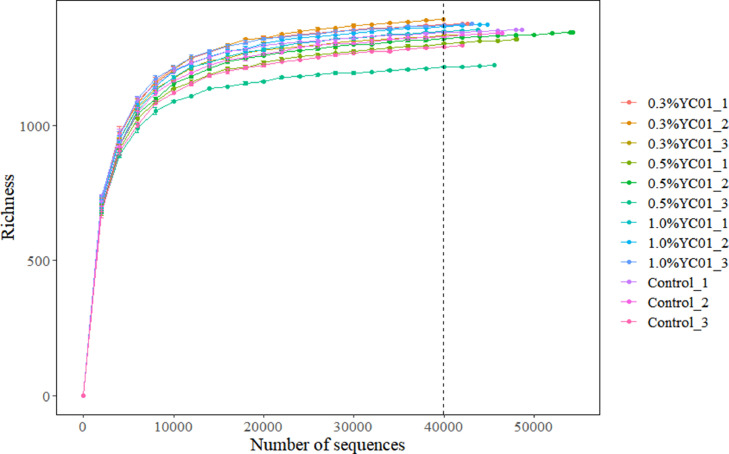
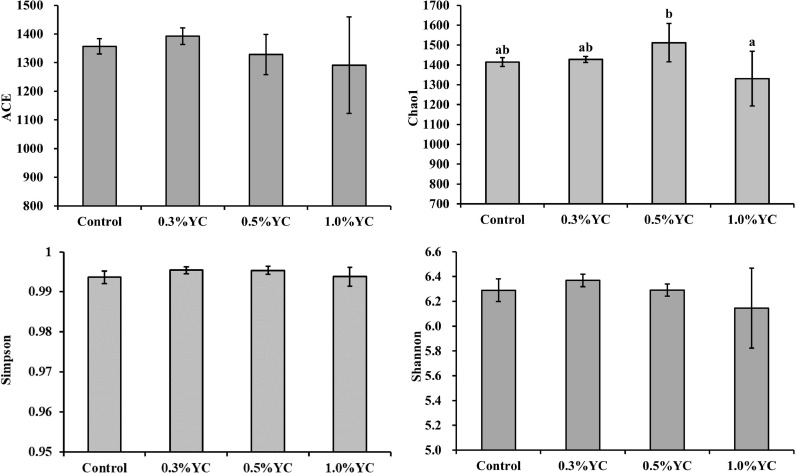
As shown in Fig. 3, the rarefaction analysis tended to approach a plateau, which indicated adequate sequencing efforts for all the samples. The impact of dietary YC on α-diversity was shown in Fig. 4. Dietary 0.5 % YC increased Chao1 index in the digestive tract of juvenile shrimp, but dietary YC had no effects on ACE, Simpson and Shannon indexes. As shown in Fig. 5 and Table 5, according to the unweighted unifrac-based principal coordinate analysis (PCoA) and Adonis analysis, there was clear clustering of microbial samples between the control and, 0.5% and 1.0% YC groups, respectively.
Fig. 3.
Rarefaction curves of observed OTUs for all the digestive tract microbiota samples. Each line represents one sample.
Fig. 4.
Effects of dietary YC on ACE, Chao1, Simpson and Shannon indexes of microbiota in the digestive tract of Pacific white shrimp L. Vannamei. All values were presented as the mean ± SE (n = 3). Means with different superscript letter were significantly different (P < 0.05) as determined by Duncan's test.
Fig. 5.
Unweighted unifrac-based principal coordinate analysis (PCoA) analysis in the digestive tract of Pacific white shrimp L. Vannamei. Small dots represent individual samples.
Table 5.
Dissimilarity between microbial communities assessed by Bray-curtis distance-based Adonis.
| Group1 | Group2 | F.Model | R2 | P.Value |
|---|---|---|---|---|
| Control | 0.3%YC | 1.1 | 0.23 | 0.8 |
| Control | 0.5%YC | 1.9 | 0.3 | 0.01 |
| Control | 1.0%YC | 1.8 | 0.31 | 0.04 |
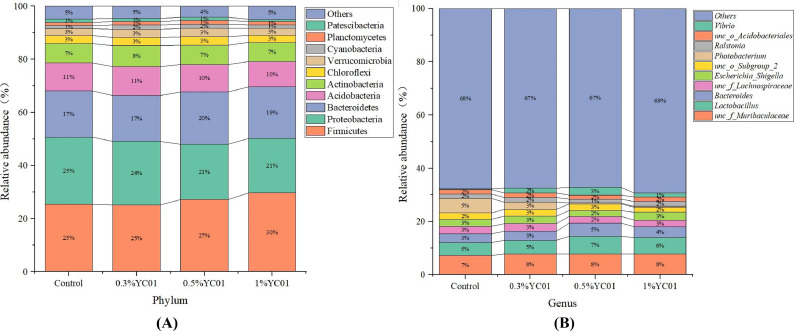
As shown in Fig. 6, at phylum level, top 10 bacteria in abundance were Firmicutes, Proteobacteria, Bacteroidetes, Acidobacteria, Actinobacteria, Chloroflexi, Verrucomicrobiar, Cyanobacteria, Planctomycetes, and Patescibacteria. At genus level, top 10 bacteria in abundance were Muribaculaceael, Lactobacillus, Bacteroides, Lachnospiraceae, Escherichia Shigella, unc_o_Subgroup2, Photobacterium, Vibrio, Ralstonia and Acidobacteriales.
Fig. 6.
Effects of dietary YC on bacterial composition in the digestive tract of Pacific white shrimp L. Vannamei. A, top 10 most abundant taxa at phylum level; B, top 10 most abundant taxa at genus level.
As shown in Fig. 7, LEfSe analysis was used to identify differentially abundant taxa between the control group and the treatment groups (Fig. 4). Compared with the control, dietary 0.5 % YC significantly increased the relative abundance of Lactobacillus and Bacteroides, and dietary 1.0 % YC significantly increased the relative abundance of Lactobacillus.
Fig. 7.
LEfSe analysis of intestinal microbiota communities of Pacific white shrimp L. Vannamei fed the experimental diets. Histogram of LEfSe analysis showed differentially microbiota abundant taxa (LDA score > 3) in the digestive tract of Pacific white shrimp fed with control and YC diets. The length of the column is proportional to the taxa abundance. A) Control VS 0.3 % YC; B) Control VS 0.5 % YC; C) Control VS 1.0 % YC.
4. Discussion
The present study showed that compared with the control, dietary YC increased WGR, and decreased FCR of shrimp but not significantly. In previous study, dietary YC significantly increased the growth performance of L. vannamei [9]. The discrepancies might be related to different initial size of shrimp, different culture system and nutrient composition in the diets. There were also similar results that dietary YC did not show increased growth performance of hybrid tilapia Oreochromis niloticus ♀ × O. aureus ♂ [30]. Generally, all the data above indicated a positive effect of YC on growth performance of juvenile shrimp.
Analysis of whole-body composition could reflect the nutritional status and the composition of diet [31]. In the present study, dietary YC increased crude protein content of juvenile shrimp. This might be attributed to the protein content in YC. Also, dietary YC might improve protein utilization rate, but it requires further investigation. Similar result has already been found in the study of Abdel-Tawwab et al. [32]. These data indicated that dietary YC could improve nutritional status of juvenile shrimp.
Morphology and structures of digestive tract could reflect the health status of aquatic animals [33]. According to previous study, increased villus height and villus height:crypt depth ratio are usually correlated with increased absorption efficiency of nutrients, and healthier digestive tracts [34,35]. The present study showed a positive effect of dietary YC on villus height and villus height:crypt depth ratio. Similar results were reported in the study of Islam et al. [36] that dietary probiotic yeast improved villus height of Nile tilapia Oreochromis niloticus. The improved morphology in the digestive tract of juvenile shrimp might be related to MOS and nucleotide. It has been reported that dietary MOS and nucleotide could improve morphology in the digestive tract of shrimp [37,38]. All the data above indicated that dietary YC could positively altered digestive tract morphology of juvenile shrimp.
Health status of digestive tract is also influenced by its immune function [39]. As a species of crustacean, shrimp lack an adaptive immune system [40], which makes the innate immune responses more important defending against pathogenic attack. PO, LZ, ACP and AKP are vital components in innate immune responses [41]. The prophenoloxidase (proPO) system is active in innate immune system and cleavage of the inactive proPO lead to the release of active PO, which produces the melanin and toxic reactive intermediates against invading pathogens [42]. LZ is effective in destroying the cell walls of bacteria [43]. Besides, ACP and AKP are also considered to be important immune enzymes in defending against pathogene [44]. In the present study, dietary YC increased activities of PO, LZ, ACP and AKP, and up-regulated mRNA levels of propo and lz in the digestive tract of juvenile shrimp. Similar results have been reported in Bu, Lian [10]’s study that dietary 10 % YC improved plasma ACP, AKP, and serum LZM activities of Ussuri catfish (Pseudobagrus ussuriensis). The possible reason for the results could be related to the β-glucan contents in YC, which has been observed to play an important role in immune enhancement in Pacific white shrimp [45] and sea cucumber Apostichopus japonicus [46]. All the data above indicated that dietary YC could improve health status in digestive tract of juvenile shrimp through increased enzymes activities and up-regulated gene expression of immune substance.
In addition to immune substances, antibacterial peptides are also essential in innate immune system, which have broad-spectrum activity and could elicit multiple responses on immune modulation [47]. It has been reported that anti-lipopolysaccharide factor (ALF) exhibit strong antibacterial activity against Gram-negative bacteria [48] while crustin is considered to have an antibacterial effect on Gram-positive bacteria in shrimp [49]. What's more, penaiedin have strong activity against Gram-positive bacteria, fungi, and also moderate activity against Gram-negative bacteria [50]. In the present study, dietary YC up-regulated mRNA levels of alf, crustin and penaiedin in digestive tract of juvenile shrimp. Similar results were observed that marine yeast Candida aquaetextoris S527 could up-regulated the expression of crustin in the hemolymph of black tiger shrimp Penaeus monodon [51]. All the data above indicated that dietary YC could improve digestive tract health status through up-regulating the gene expression of antibacterial.
Study revealed that the development of inflammation will induce damage to digestive tract of aquatic animals [52]. In the process of inflammation, TLRs are key pattern recognition receptors with central roles in induction of inflammatory cytokines [53]. The activation of TLRs could recruit MyD88 and then activate downstream signaling molecules, finally leading to finally induce the nuclear translocation of NF-κB [53,54], which could lead to the activation of caspase-1 and induce inflammation [55,56]. In the present study, dietary YC down-regulated mRNA levels of tlr, myd88, nf-κbp65 and caspase-1 in digestive tract of juvenile shrimp. Similar results were found in Ussuri catfish Pseudobagrus ussuriensis that dietary YC down-regulated mRNA levels of tlr2, myd88 and nf-κbp65 in liver [10]. The anti-inflammatory effects of dietary YC might be related to β-glucan and vitamin contents Previous study showed that dietary β-glucan and vitamins play crucial roles in suppressing inflammation in aquatic animals [57], [58], [59], [60]. Taken together, dietary YC showed a protective effect on digestive tract of juvenile shrimp through suppressing inflammation, improving its health status.
As mentioned above, some microbiotas are helpful to digestion, absorption and utilization of nutrient, and the regulation of immune system, whose composition has an important impact on intestinal health [61]. Some studies indicated that the predominant bacterial phyla in shrimp digestive tract were Firmicutes, Proteobacteria and Bacteroidetes [13,62,63]. In the present study, Firmicutes, Proteobacteria and Bacteroidetes were the predominant bacterial phyla in the digestive tract of juvenile shrimp in all treatments, while at genus level, the bacterial composition of all the treatment were dominated by Muribaculaceael, Lactobacillus and Bacteroides. In the study of Ayiku et al. [9], the predominant bacterial at phylum level in the digestive tract of shrimp were Proteobacteria, Bacteroidetes and Actinobacteria, while the predominant bacterial at genus level were Vibrio, Motilimonas and Pseudoalteromonas. Results at phylum were similar with the present study, but different at genus level. Possible reason could be related to different life stage, diet composition, rearing conditions, and sequencing method, but it requires further investigation. According to previous study, Bacteroides species mutualize with the host and participate in a series of metabolic processes including carbohydrate metabolism, polysaccharides utilization and immune enhancement [64], [65], [66]. Besides, Lactobacillus is generally accepted as beneficial species [47]. It has been reported that some species of the Lactobacillus in aquaculture organisms can improve morphology of digestive tract [67], which also plays an important role in immune enhancement [68]. Varies studies have observed that dietary Lactobacillus could increase growth performance and enhance immune function of L. vannamei [69], Red sea bream Pagrus major [70] and Nile tilapia Oreochromis mossambicus [71]. In the present study, compared with the control, dietary YC significantly increased relative abundance of Bacteroides and Lactobacillus. Similar results has already been observed in hybrid grouper ♀Epinephelus fuscoguttatus × ♂E. lanceolatus that dietary YC increased relative abundance of Lactobacillus in the intestine [72]. The increased relative abundance of Bacteroides and Lactobacillus might be responsible to the improved digestive tract morphology and enhanced immune function. However, compared with the control, dietary 1.0 % YC increased relative abundance of Vibrio, and the specific reason requires further investigation. All the data above indicated that dietary YC could improve health status in digestive tract of juvenile shrimp through improving microbiota.
5. Conclusion
Results of the present study showed that dietary YC improved nutritional status of juvenile Pacific white shrimp, and increased health status of digestive tract through enhancing immune function and improving morphology and microbiota.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declaration of Competing Interest
The authors declare that there are no conflicts of interest.
Acknowledgements
This study was funded by the National Key Research and Development Program of China (2020YFD0900201, 2019YFD0900403, 2017YFC1600906) and National Natural Science Foundation of China (41976095).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.fsirep.2022.100065.
Appendix. Supplementary materials
References
- 1.Rungrassamee W., Klanchui A., Maibunkaew S., Karoonuthaisiri N. Bacterial dynamics in intestines of the black tiger shrimp and the Pacific white shrimp during Vibrio harveyi exposure. J. Invertebr. Pathol. 2016;133:12–19. doi: 10.1016/j.jip.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Duan Y., Liu Q., Wang Y., Zhang J., Xiong D. Impairment of the intestine barrier function in Litopenaeus vannamei exposed to ammonia and nitrite stress. Fish Shellfish Immunol. 2018;78:279–288. doi: 10.1016/j.fsi.2018.04.050. [DOI] [PubMed] [Google Scholar]
- 3.Amenyogbe E., Chen G., Wang Z., Huang J., Huang B., Li H. The exploitation of probiotics, prebiotics and synbiotics in aquaculture: present study, limitations and future directions.: a review. Aquaculture International. 2020:1–25. [Google Scholar]
- 4.Shen Y., Piao X., Kim S., Wang L., Liu P., Yoon I., et al. Effects of yeast culture supplementation on growth performance, intestinal health, and immune response of nursery pigs. J. Anim. Sci. 2009;87:2614–2624. doi: 10.2527/jas.2008-1512. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J.-C., Chen P., Zhang C., Khalil M.M., Zhang N.-Y., Qi D.-S., et al. Yeast culture promotes the production of aged laying hens by improving intestinal digestive enzyme activities and the intestinal health status. Poult. Sci. 2020;99:2026–2032. doi: 10.1016/j.psj.2019.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xv Z., Zhong Y., Wei Y., Zhang T., Zhou W., Jiang Y., et al. Yeast culture supplementation alters the performance and health status of juvenile largemouth bass (Micropterus salmoides) fed a high-plant protein diet. Aquacult. Nutr. 2021;27:2637–2650. [Google Scholar]
- 7.Pongpet J., Ponchunchoovong S., Payooha K. Partial replacement of fishmeal by brewer’s yeast (Saccharomyces cerevisiae) in the diets of Thai Panga (Pangasianodon hypophthalmus × Pangasius bocourti) Aquacult. Nutr. 2016;22:575–585. [Google Scholar]
- 8.Miles R., Bootwalla S. Direct-fed microbials in animal production, Direct-fed microbials in animal production A review. 1991:117–132. [Google Scholar]
- 9.Ayiku S., Shen J.-f., Tan B.-p., Dong X.-h., Liu H.-y. Effects of dietary yeast culture on shrimp growth, immune response, intestinal health and disease resistance against Vibrio harveyi. Fish Shellfish Immunol. 2020;102:286–295. doi: 10.1016/j.fsi.2020.04.036. [DOI] [PubMed] [Google Scholar]
- 10.Bu X., Lian X., Wang Y., Luo C., Tao S., Liao Y., et al. Dietary yeast culture modulates immune response related to TLR2-MyD88-NF-kβ signaling pathway, antioxidant capability and disease resistance against Aeromonas hydrophila for Ussuri catfish (Pseudobagrus ussuriensis) Fish Shellfish Immunol. 2019;84:711–718. doi: 10.1016/j.fsi.2018.10.049. [DOI] [PubMed] [Google Scholar]
- 11.López Nadal A., Ikeda-Ohtsubo W., Sipkema D., Peggs D., McGurk C., Forlenza M., et al. Feed, microbiota, and gut immunity: using the zebrafish model to understand fish health. Front. Immunol. 2020;11:114. doi: 10.3389/fimmu.2020.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huyben D., Vidakovic A., Sundh H., Sundell K., Kiessling A., Lundh T. Haematological and intestinal health parameters of rainbow trout are influenced by dietary live yeast and increased water temperature. Fish Shellfish Immunol. 2019;89:525–536. doi: 10.1016/j.fsi.2019.04.047. [DOI] [PubMed] [Google Scholar]
- 13.Rajeev R., Adithya K., Kiran G.S., Selvin J. Healthy microbiome: a key to successful and sustainable shrimp aquaculture. Reviews in Aquaculture. 2021;13:238–258. [Google Scholar]
- 14.Long L., Zhang H., Ni Q., Liu H., Wu F., Wang X. Effects of stocking density on growth, stress, and immune responses of juvenile Chinese sturgeon (Acipenser sinensis) in a recirculating aquaculture system. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019;219:25–34. doi: 10.1016/j.cbpc.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Wang C.Y., Li Z.B., Sun Y.Z., Chen Q., Li W.J., Huang Y.C., et al. Effects of Chinese herbal medicines mixture on growth performance digestive enzyme activity immune response of juvenile Japanese seabass, Lateolabrax japonicus. Aquacult. Nutr. 2018;24:683–693. [Google Scholar]
- 16.Dong X.-Q., Zhang D.-M., Chen Y.-K., Wang Q.-J., Yang Y.-Y. Effects of antimicrobial peptides (AMPs) on blood biochemical parameters, antioxidase activity, and immune function in the common carp (Cyprinus carpio) Fish Shellfish Immunol. 2015;47:429–434. doi: 10.1016/j.fsi.2015.09.030. [DOI] [PubMed] [Google Scholar]
- 17.Grimble R. Nutritional modulation of immune function. Proc. Nutr. Soc. 2001;60:389–397. doi: 10.1079/pns2001102. [DOI] [PubMed] [Google Scholar]
- 18.Rauta P.R., Samanta M., Dash H.R., Nayak B., Das S. Toll-like receptors (TLRs) in aquatic animals: signaling pathways, expressions and immune responses. Immunol. Lett. 2014;158:14–24. doi: 10.1016/j.imlet.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 19.Shan S., Liu R., Jiang L., Zhu Y., Li H., Xing W., et al. Carp toll-like receptor 8 (Tlr8): an intracellular Tlr that recruits TIRAP as adaptor and activates AP-1 pathway in immune response. Fish Shellfish Immunol. 2018;82:41–49. doi: 10.1016/j.fsi.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Kim S.-J., Kim M.-C., Lee B.-J., Park D.-H., Hong S.-H., Um J.-Y. Anti-inflammatory activity of chrysophanol through the suppression of NF-κB/caspase-1 activation in vitro and in vivo. Molecules. 2010;15:6436–6451. doi: 10.3390/molecules15096436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang A.R., Ran C., Ringø E., Zhou Z.G. Progress in fish gastrointestinal microbiota research. Reviews in Aquaculture. 2018;10:626–640. [Google Scholar]
- 22.Li F., Xiang J. Recent advances in researches on the innate immunity of shrimp in China. Developmental & Comparative Immunology. 2013;39:11–26. doi: 10.1016/j.dci.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 23.Kiran R.P., Rajendran K., Jung S., Oh M. Experimental susceptibility of different life-stages of the giant freshwater prawn, Macrobrachium rosenbergii (de Man), to white spot syndrome virus (WSSV) J. Fish Dis. 2002;25:201–207. [Google Scholar]
- 24.Association of Analytical Chemists. Official methods of analysis of AOAC International. AOAC Washington; 1995.
- 25.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego, Calif) 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Bolger A.M., Lohse M., Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet journal. 2011;17:10–12. [Google Scholar]
- 28.Magoč T., Salzberg S.L. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27:2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edgar R.C. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods. 2013;10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- 30.He S., Zhou Z., Liu Y., Shi P., Yao B., Ringø E., et al. Effects of dietary Saccharomyces cerevisiae fermentation product (DVAQUA®) on growth performance, intestinal autochthonous bacterial community and non-specific immunity of hybrid tilapia (Oreochromis niloticus♀ × O. aureus♂) cultured in cages. Aquaculture. 2009;294:99–107. [Google Scholar]
- 31.Mohd Khan Y., Khan M.A. Effects of dietary cyanocobalamin on growth performance, non-specific immune response, antioxidant capacity, haematological parameters, body composition and liver cyanocobalamin concentration of fingerling major carp, Catla catla (Hamilton) Aquacult. Nutr. 2021;27:604–614. [Google Scholar]
- 32.Abdel-Tawwab M., Abdel-Rahman A.M., Ismael N.E. Evaluation of commercial live bakers’ yeast, Saccharomyces cerevisiae as a growth and immunity promoter for Fry Nile tilapia, Oreochromis niloticus (L.) challenged in situ with Aeromonas hydrophila. Aquaculture. 2008;280:185–189. [Google Scholar]
- 33.Tan X., Sun Z., Zhou C., Huang Z., Tan L., Xun P., et al. Effects of dietary dandelion extract on intestinal morphology, antioxidant status, immune function and physical barrier function of juvenile golden pompano Trachinotus ovatus. Fish Shellfish Immunol. 2018;73:197–206. doi: 10.1016/j.fsi.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 34.de Araújo E.P., de Carvalho P.L.P.F., de Freitas J.M.A., da Silva R.L., Rocha M.K.H.R., Teixeira C.P., et al. Dietary spray-dried plasma enhances the growth performance, villus: crypt ratio and cold-induced stress resistance in Nile tilapia (Oreochromis niloticus) Aquaculture. 2017;479:675–681. [Google Scholar]
- 35.Abdel-Tawwab M., Shukry M., Farrag F.A., El-Shafai N.M., Dawood M.A., Abdel-Latif H.M. Dietary sodium butyrate nanoparticles enhanced growth, digestive enzyme activities, intestinal histomorphometry, and transcription of growth-related genes in Nile tilapia juveniles. Aquaculture. 2021;536 [Google Scholar]
- 36.Islam S.M., Rohani M.F., Shahjahan M. Probiotic yeast enhances growth performance of Nile tilapia (Oreochromis niloticus) through morphological modifications of intestine. Aquaculture Reports. 2021;21 [Google Scholar]
- 37.Zhang J., Liu Y., Tian L., Yang H., Liang G., Xu D. Effects of dietary mannan oligosaccharide on growth performance, gut morphology and stress tolerance of juvenile Pacific white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2012;33:1027–1032. doi: 10.1016/j.fsi.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 38.Xiong J., Jin M., Yuan Y., Luo J.X., Lu Y., Zhou Q.C., et al. Dietary nucleotide-rich yeast supplementation improves growth, innate immunity and intestinal morphology of Pacific white shrimp (Litopenaeus vannamei) Aquacult. Nutr. 2018;24:1425–1435. [Google Scholar]
- 39.Guo Y.-L., Jiang W.-D., Wu P., Liu Y., Zhou X.-Q., Kuang S.-Y., et al. The decreased growth performance and impaired immune function and structural integrity by dietary iron deficiency or excess are associated with TOR, NF-κB, p38MAPK, Nrf2 and MLCK signaling in head kidney, spleen and skin of grass carp (Ctenopharyngodon idella) Fish Shellfish Immunol. 2017;65:145–168. doi: 10.1016/j.fsi.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 40.Kulkarni A., Krishnan S., Anand D., Kokkattunivarthil Uthaman S., Otta S.K., Karunasagar I., et al. Immune responses and immunoprotection in crustaceans with special reference to shrimp. Reviews in Aquaculture. 2021;13:431–459. [Google Scholar]
- 41.Li Y., Han Z., Xu W., Li X., Zhao Y., Wei H., et al. Antioxidant and immune responses of the Oriental river prawn Macrobrachium nipponense to the isopod parasite Tachaea chinensis. Fish Shellfish Immunol. 2020;101:78–87. doi: 10.1016/j.fsi.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 42.Amparyup P., Charoensapsri W., Tassanakajon A. Prophenoloxidase system and its role in shrimp immune responses against major pathogens. Fish Shellfish Immunol. 2013;34:990–1001. doi: 10.1016/j.fsi.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 43.Yao C.-L., Wu C.-G., Xiang J.-H., Li F., Wang Z.-Y., Han X. The lysosome and lysozyme response in Chinese shrimp Fenneropenaeus chinensis to Vibrio anguillarum and laminarin stimulation. J. Exp. Mar. Biol. Ecol. 2008;363:124–129. [Google Scholar]
- 44.Yin X.-L., Li Z.-J., Yang K., Lin H.-Z., Guo Z.-X. Effect of guava leaves on growth and the non-specific immune response of Penaeus monodon. Fish Shellfish Immunol. 2014;40:190–196. doi: 10.1016/j.fsi.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 45.Li H., Xu C., Zhou L., Dong Y., Su Y., Wang X., et al. Beneficial effects of dietary β-glucan on growth and health status of Pacific white shrimp Litopenaeus vannamei at low salinity. Fish Shellfish Immunol. 2019;91:315–324. doi: 10.1016/j.fsi.2019.05.052. [DOI] [PubMed] [Google Scholar]
- 46.Zhao Y., Ma H., Zhang W., Ai Q., Mai K., Xu W., et al. Effects of dietary β-glucan on the growth, immune responses and resistance of sea cucumber, Apostichopus japonicus against Vibrio splendidus infection. Aquaculture. 2011;315:269–274. [Google Scholar]
- 47.Dai J., Zheng J., Ou W., Xu W., Ai Q., Zhang W., et al. The effect of dietary cecropin AD on intestinal health, immune response and disease resistance of juvenile turbot (Scophthalmus maximus L.) Fish Shellfish Immunol. 2020;100:117–125. doi: 10.1016/j.fsi.2020.02.052. [DOI] [PubMed] [Google Scholar]
- 48.Supungul P., Klinbunga S., Pichyangkura R., Hirono I., Aoki T., Tassanakajon A. Antimicrobial peptides discovered in the black tiger shrimp Penaeus monodon using the EST approach. Dis. Aquat. Organ. 2004;61:123–135. doi: 10.3354/dao061123. [DOI] [PubMed] [Google Scholar]
- 49.Chen M., Chen X.-Q., Tian L.-X., Liu Y.-J., Niu J. Improvement of growth, intestinal short-chain fatty acids, non-specific immunity and ammonia resistance in Pacific white shrimp (Litopenaeus vannamei) fed dietary water-soluble chitosan and mixed probiotics. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2020;236 doi: 10.1016/j.cbpc.2020.108791. [DOI] [PubMed] [Google Scholar]
- 50.Pourmozaffar S., Hajimoradloo A., Miandare H.K. Dietary effect of apple cider vinegar and propionic acid on immune related transcriptional responses and growth performance in white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2017;60:65–71. doi: 10.1016/j.fsi.2016.11.030. [DOI] [PubMed] [Google Scholar]
- 51.Babu D.T., Antony S.P., Joseph S.P., Bright A.R., Philip R. Marine yeast Candida aquaetextoris S527 as a potential immunostimulant in black tiger shrimp Penaeus monodon. J. Invertebr. Pathol. 2013;112:243–252. doi: 10.1016/j.jip.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 52.Xie S., Liu Y., Tian L., Niu J., Tan B. Low dietary fish meal induced endoplasmic reticulum stress and impaired phospholipids metabolism in juvenile Pacific White Shrimp, Litopenaeus vannamei. Frontiers in physiology. 2020;11:1024. doi: 10.3389/fphys.2020.01024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kawai T., Akira S. Seminars in immunology. Elsevier; 2007. TLR signaling; pp. 24–32. [Google Scholar]
- 54.Korneev K.V., Atretkhany K.-S.N., Drutskaya M.S., Grivennikov S.I., Kuprash D.V., Nedospasov S.A. TLR-signaling and proinflammatory cytokines as drivers of tumorigenesis. Cytokine. 2017;89:127–135. doi: 10.1016/j.cyto.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 55.Jearaphunt M., Noonin C., Jiravanichpaisal P., Nakamura S., Tassanakajon A., Söderhäll I., et al. Caspase-1-like regulation of the proPO-system and role of ppA and caspase-1-like cleaved peptides from proPO in innate immunity. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yina S., Zhongjie C., Chenghua L., Weiwei Z., Xuelin Z., Ming G. A novel caspase-1 mediates inflammatory responses and pyroptosis in sea cucumber Apostichopus japonicus. Aquaculture. 2019;513 [Google Scholar]
- 57.Dawood M.A., Abdo S.E., Gewaily M.S., Moustafa E.M., SaadAllah M.S., AbdEl-Kader M.F., et al. The influence of dietary β-glucan on immune, transcriptomic, inflammatory and histopathology disorders caused by deltamethrin toxicity in Nile tilapia (Oreochromis niloticus) Fish Shellfish Immunol. 2020;98:301–311. doi: 10.1016/j.fsi.2020.01.035. [DOI] [PubMed] [Google Scholar]
- 58.Qiang J., Wasipe A., He J., Tao Y.-F., Xu P., Bao J.-W., et al. Dietary vitamin E deficiency inhibits fat metabolism, antioxidant capacity, and immune regulation of inflammatory response in genetically improved farmed tilapia (GIFT, Oreochromis niloticus) fingerlings following Streptococcus iniae infection. Fish Shellfish Immunol. 2019;92:395–404. doi: 10.1016/j.fsi.2019.06.026. [DOI] [PubMed] [Google Scholar]
- 59.Trichet V.V. Nutrition and immunity: an update. Aquaculture research. 2010;41:356–372. [Google Scholar]
- 60.Liu S., Wang X., Bu X., Zhang C., Qiao F., Qin C., et al. Influences of dietary vitamin D3 on growth, antioxidant capacity, immunity and molting of Chinese mitten crab (Eriocheir sinensis) larvae. J. Steroid Biochem. Mol. Biol. 2021;210 doi: 10.1016/j.jsbmb.2021.105862. [DOI] [PubMed] [Google Scholar]
- 61.Tarnecki A.M., Burgos F.A., Ray C.L., Arias C.R. Fish intestinal microbiome: diversity and symbiosis unravelled by metagenomics. J. Appl. Microbiol. 2017;123:2–17. doi: 10.1111/jam.13415. [DOI] [PubMed] [Google Scholar]
- 62.Landsman A., St-Pierre B., Rosales-Leija M., Brown M., Gibbons W. Impact of aquaculture practices on intestinal bacterial profiles of pacific whiteleg shrimp Litopenaeus vannamei. Microorganisms. 2019;7:93. doi: 10.3390/microorganisms7040093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vinay T., Patil P., Aravind R., PS S.A., Baskaran V., Balasubramanian C. Microbial community composition associated with early developmental stages of the Indian white shrimp. Penaeus indicus. 2021 doi: 10.1007/s00438-022-01865-7. [DOI] [PubMed] [Google Scholar]
- 64.Rhee K.-J., Sethupathi P., Driks A., Lanning D.K., Knight K.L. Role of commensal bacteria in development of gut-associated lymphoid tissues and preimmune antibody repertoire. J. Immunol. 2004;172:1118–1124. doi: 10.4049/jimmunol.172.2.1118. [DOI] [PubMed] [Google Scholar]
- 65.Ravcheev D.A., Godzik A., Osterman A.L., Rodionov D.A. Polysaccharides utilization in human gut bacterium Bacteroides thetaiotaomicron: comparative genomics reconstruction of metabolic and regulatory networks. BMC genomics. 2013;14:1–17. doi: 10.1186/1471-2164-14-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jin Z., Fang Z., Pei Z., Wang H., Zhu J., Lee Y.-k., et al. A low molecular weight brown algae Laminaria japonica glycan modulation of gut microbiota and body weight in mice. Food & function. 2021 doi: 10.1039/d1fo03024h. [DOI] [PubMed] [Google Scholar]
- 67.Suzer C., Çoban D., Kamaci H.O., Saka Ş., Firat K., Otgucuoğlu Ö., et al. Lactobacillus spp. bacteria as probiotics in gilthead sea bream (Sparus aurata, L.) larvae: effects on growth performance and digestive enzyme activities. Aquaculture. 2008;280:140–145. [Google Scholar]
- 68.Apata D. Growth performance, nutrient digestibility and immune response of broiler chicks fed diets supplemented with a culture of Lactobacillus bulgaricus. J. Sci. Food Agric. 2008;88:1253–1258. [Google Scholar]
- 69.Zheng X., Duan Y., Dong H., Zhang J. Effects of dietary Lactobacillus plantarum in different treatments on growth performance and immune gene expression of white shrimp Litopenaeus vannamei under normal condition and stress of acute low salinity. Fish Shellfish Immunol. 2017;62:195–201. doi: 10.1016/j.fsi.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 70.Dawood M.A., Koshio S., Ishikawa M., Yokoyama S. Effects of heat killed Lactobacillus plantarum (LP20) supplemental diets on growth performance, stress resistance and immune response of red sea bream, Pagrus major. Aquaculture. 2015;442:29–36. [Google Scholar]
- 71.Van Doan H., Hoseinifar S.H., Naraballobh W., Paolucci M., Wongmaneeprateep S., Charoenwattanasak S., et al. Dietary inclusion of watermelon rind powder and Lactobacillus plantarum: Effects on Nile tilapia’s growth, skin mucus and serum immunities, and disease resistance. Fish Shellfish Immunol. 2021;116:107–114. doi: 10.1016/j.fsi.2021.07.003. [DOI] [PubMed] [Google Scholar]
- 72.Wang Q., Ayiku S., Liu H., Tan B., Dong X., Chi S., et al. Effects of dietary ESTAQUA® yeast culture supplementation on growth, immunity, intestinal microbiota and disease-resistance against Vibrio harveyi in hybrid grouper (♀ Epinephelus fuscoguttatus × ♂ E. lanceolatus) Aquaculture Reports. 2022;22 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.