Highlights
-
•
Feed supplemented with a blend of Guava, Bitter leaf, and Neem ethanolic extracts can improve the haematological variables of Nile tilapia.
-
•
Significant increase disease resistance to co-infections of Streptococcus agalactaie and Aeromonas jandaie is possible in Nile tilapia with feed supplemented with a blend of Guava, Bitter leaf, and Neem ethanolic extracts.
-
•
Prolong feeding of Nile tilapia with a blend of Guava, Bitter leaf, and Neem ethanolic extracts could damage their liver leading to poor health and death.
Keywords: Guava, Bitter leaf, Neem, Tilapia, Aquaculture, Herbs
Abstract
Given the intense interest in the use of herbal extracts to improve fish growth, fish health, and disease resistance in fish in culture systems, in this study, we examined the effects of a blend of Guava, Bitter and Neem leaf extracts (GBNL) (i.e., 1:1:1 for GL, BL, and NL respectively) at different inclusion (i.e. 0 GBNL gkg−1, 1 GBNL gkg−1, 3 GBNL gkg−1, 5 GBNL gkg−1, 7 GBNL gkg−1 and 10 GBNL gkg−1) levels on growth, haematology, immunity, liver toxicity and resistance to bacterial co-infections in Nile tilapia. After 8 weeks of feeding, Nile tilapia fed 3 GBNL gkg−1 diets showed significant effects in improving weight gain compared to those fed the control diet. GBNL fed fish showed improved health of fish by stimulating significant increases in levels of White blood cells, Red blood cells, Haemoglobin, and Haematocrit in relation to those fed the control diet. Also, the applications of deferent GBNL levels in Nile tilapia diets showed the potential to upregulate the expression of the immune-related genes heat shock protein 70, chicken type lysozymes, and Beta-defensin, with significant effects shown in fish fed 5GBNL gkg−1 diets in comparison to the control. The results also indicate that GBNL supplementation can decrease mortalities to co-infection of Streptococcus agalactiae and Aeromonas jandaie in Nile tilapia with the lowest mortalities of 13.65% and relative per cent survival of 82.57 % in fish fed 5GBNL gkg−1. Despite the potential of GBNL applications in Nile tilapia, findings of this study indicate fish fed the different concentrations of GBNL, particularly with 7 GBNL gkg−1 can promote the leaching of the liver enzymes: alanine transaminase, aspartate aminotransferase, and alkaline phosphate into the bloodstream which is suggestive of potential liver damage in Nile tilapia. Histological examinations of a cross-section of the liver tissues of fish fed GBNL showed various injuries including hydropic changes, pyknosis nuclei, erythrocytes congestion and vacuolation with the severest seen in those fed 7 GBNL gkg−1. Taking all of the above into consideration, 5GBNL gkg−1 application could improve the health and disease resistance of Nile tilapia; however, prolong use thus after 8 weeks of administration could be injurious to fish liver health.
Introduction
Nile tilapia, Oreochromis niloticus also known as Tilapia is an important commercial aquaculture fish cultured in many parts of the world. Tilapia production has gained prominence because it is highly acceptable in the market and is easy to culture [1]. As of 2018, Tilapia production reached about 6.532 million tons and is envisaged to reach 7.3 million tons by 2030 [2]. Despite the huge increase in production potential, Tilapia production has been hampered by a surge in the occurrence and proliferation of pathogenic infections [3]. Bacteria seem the topmost among the pathogens that plague Tilapia production and matters have been worsened by the occurrence of co-infections particularly, Streptococcal and Aeromonas infections [4,2].
Over the years the aquaculture industry has battled pathogenic infections through many prominent strategies, for instance, the use of antibiotics, probiotics, prebiotics, and chemotherapeutics. In recent times, there has been the need to shift to other treatment regimens that may be more environmentally friendly, readily available, cost-effective, and may have other additional benefits [5]. The addition of herbal additives in fish feeds has been purported to be biodegradable, improve feed palatability, enhance growth, boost immunity and increase stress and disease resistance in animals [6]. In fish, there have been reports of positive effects of herbal supplements on growth, immune response, and disease resistance [7,8]. This has been attributed to their antibacterial, antifungal, antioxidant, antiviral, antiparasitic and immunosuppressive properties [9]. Herbs such as Guava (Psidium guajava) leaves have lots of veterinary applications, particularly in the management of diarrhoea in livestock [10]. Bitter (Vernonia amygdalina) leaf is used in the treatment of amoebic dysentery and gastrointestinal disorders in humans [11]. The leaves of Neem (Azadirachta indica) have been used in treating microbial and parasitic infections in livestock [12]. Despite the good qualities of Guava leaves (GL), Bitter leaves (BL), and Neem leaves (NL), to the best of our knowledge, research on many herbal supplements are based on the use of individual species [13]. However, greater benefits could be derived from them by combining compatible species to explore the possibility of their synergistic effects [14]. Concerning disease resistance tests, research on how herbal supplemented fed fish respond to co-infections is still rudimentary.
Given the increasing interest in the use of herbal feed additives to improve the growth and survival of fish in culture systems, in this study, we examined the effects of a blend of GL, BL, and NL (GBNL) (i.e. 1:1:1 for GL, BL, and NL respectively) at different inclusion (i.e. 0 GBNL gkg−1, 1 GBNL gkg−1, 3 GBNL gkg−1, 5 GBNL gkg−1, 7 GBNL gkg−1, and 10 GBNL gkg−1) levels on growth, haematology, gene expression, liver toxicity, and resistance to Streptococcus agalactiae and Aeromonas jandaie co-infections in Tilapia. It is hoped that the findings of this study will offer avenues to enable stakeholders such as fish farmers and aquaculture drug and feed manufacturers to utilize these herbal extracts in achieving the production of more resilient fish in a healthier environment and increasing food production to contribute to food security.
Materials and methods
Preparation of fish feed
Succulent GL, BL, and NL samples were collected from the environs of the UDS-Nyankpala campus and leaf extracts were obtained using procedures with slight modification as have been described in previous reports [15,16,17]. Briefly, an electronic scale (Shinko Denshi scale model: RJ-620) was used to weigh 0.5 kg of either GL or BL or NL into a blender containing 1 litre of absolute ethanol (99%). The leaves and ethanol were blended, and stored overnight and the solution was sieved out using 93, 65, and 23-micron mesh to separate liquid from the fibrous leaf materials. The filtrate (i.e. sieved leaf extracts) was heated in a water bath at 100°C for 30 min to evaporate the ethanol and water and to concentrate the crude extract which was then put into zip lock bags and stored at 4°C in the refrigerator until use. To prepare the feed, extracts of GL, BL and NL were mixed in the ratio 1:1:1 respectively (i.e. designated as GBNL) out of which 1 g, 3 g, 5 g, 7 g, and 10 g were weighed and each added to a kilogram of powdered commercial feed (i.e Koudijs Tilapia of composition: Crude protein (30 %), Crude fat (6 %). crude fibre (5 %), Moisture (10 %), Ash (10 %), phosphorus (1 %), calcium (1 %)). The feed were designated 1 GBNL gkg−1, 3 GBNL gkg−1, 5 GBNL gkg−1, 7 GBNL gkg−1, and 10 GBNL gkg−1. 200 ml of distilled water was added to the mixture and moulded using a manual animal feed pelletizing machine into 2 mm pellets. The feed was designated 1 GBNL gkg−1, 3 GBNL gkg−1, 5 GBNL gkg−1, 7 GBNL gkg−1, and 10 GBNL gkg−1 . Also, to a kilogram of commercial feed, 200 ml of distilled water was added and similarly, moulded into pellets for use as a control diet designated as 0 GBNL gkg−1. Mounded pellets were dried under shade, bagged, stored in a cool room and used until the experiment was terminated.
Experimental set-up
Tilapia without externally observed abnormalities of an average weight of 66.66 ± 1.3 g, were obtained from a commercial fish farm in Tamale Metropolis, Northern Region, Ghana. The fish were distributed into eighteen (18) circular concrete tanks (30 fish per tank) of dimension dimensions 90 cm in diameter and 60 cm in depth containing 3800 litres of water and allowed to stabilize within two weeks. At the start of the experiment, the fish were assigned at random in triplicates into six (6) groups each for the experimental diets prepared. The experiment lasted eight (8) weeks for the feeding trial and another two (2) weeks for a bacterial challenge experiment. Fish were fed at a rate of 2% of body weight daily in two equal rations with only the commercial administered during the stabilization period and the prepared feed (test diets) given during the experimental period. Feed rations were adjusted biweekly after 24 h starvation and bulk weighing of the biomass per treatment replicate. Water was constantly aerated (BOYU air compressor) and the quality was monitored using a multiparameter water meter (Bante 900). 80% of the cultured water was renewed once every 4 weeks.
Sample measurement/collection
Growth measurement
Parameters that were assessed as indices for growth included the: initial and final weight, weight gain, feed conversion ratio (FCR), hepatosomatic index (HSI), viscerosomatic index (VSI) and condition factor (K) as have been previously described [18].
Blood and tissue sample collection
After 8 weeks of experimental feeding, blood samples from test fish were collected as previously described [18]. Whole blood was taken using a 2 mL disposable syringe and discharged into two different tubes (i.e., one for haematological analysis and the other for liver toxicity test) each containing EDTA to prevent clotting. The blood was then sent to a Laboratory at the Tamale Teaching Hospital for determination of haematological parameters including white blood cells (WBC), red blood cells (RBC), haemoglobin (HGB), and haematocrits (HCT) and plasma constituents (i.e., Total Protein (TP), Albumin (ALB), gamma-glutamyl transferase (y-GT), Total bilirubin (T-BIL) and Direct bilirubin (D-BIL) using a haematological analyzer (Urit 5250). A part of the extracted blood was analyzed using a Roche Automated Chemical Analyzer to determine the levels of aspartate aminotransferase (AST), alanine transaminase (ALT), and alkaline phosphate (ALP). Liver tissues from the same fish were collected in two portions. One portion was put into 1.5 ml Eppendorf tubes containing 1 ml of RNA later and stored at 4°C for use in detecting the expression of growth hormone (GH) and insulin-like growth factor (IGF-1) as growth-related genes and the immune genes: beta-defensin (β-defensin), C-type lysozyme and heat shock proteins (HSP) 70. Beta-actin (β-actin), a housekeeping gene was used as an internal control gene. Primers of genes used in this study are listed in Table 1. The other portion of the liver was fixed in buffered formalin for histological studies of the effects of treatments on liver integrity.
Table 1.
Primers of genes used.
| Gene name | Primer sequence (5ˈ-3ˈ) | Source |
|---|---|---|
| β-actin (housekeeping gene) | F-AACAACCACACACCACACATTTC R-TGTCTCCTTCATCGTTCCAGTTT |
GenBank: EF206801.1 |
| HSP70 | F-ACCCAGACCTTCACCACCTA R- GTCCTTGGTCATGGCTCTCT |
[19] |
| β-defensin | F-ATGTAGAAAGGTTTGCCTCCCA R-ACAGCCCAGAGGTCCAAAGAAC |
GenBank: KJ577575.1 |
| C-type lysozyme | F- AAGGGAAGCAGCAGCAGTTGTG R-CGTCCATGCCGTTAGCCTTGAG |
[20] |
| GH | F- AGCAACGTCAGCTCAACAAA R- CGATCGGGCTGATGATGTA |
[21] |
| IGF-1 | F- GGGAAGGAACAAATGGACAA R- TTACAGTGAACCATTCCACAGG |
[21] |
Where: Growth hormone = GH, Insulin-like growth factor = IGF-1, Beta-actin= β-actin, Beta-defensin = β-defensin, Chicken type lysozyme = C-type lysozyme and Heat shock proteins 70 = HSP70
Gene expression study
RNA isolation
Total RNA derived from Tilapia liver tissues were extracted using TRIzol0020 reagent (Transgen, China) according to the manufacturer's protocol. The quality of total RNA was measured spectrophotometrically (NanoDrop 2000, Thermo Scientific) and by electrophoresis on 1% agarose gel. First-strand cDNA from RNA of the best quality (absorbance 260/280 > 1.8 and 260/230 > 1.8) was synthesised using One-Step gDNA removal and cDNA Synthesis SuperMix kit (Transgen, China).
RT-qPCR
The qRT-PCR assay was carried out using the AriaMx real-time PCR System (Agilent Technologies). The amplification was carried out in a 20 μl reaction volume containing 10 μl tip-mix (Transgen, China), 0.4 μl sense and 0.4 μl anti-sense primers, 1 μl undiluted cDNA, and 8.2 μl double distilled water. The thermal profile for qPCR was 94°C for 5 min followed by 45 cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 30 s. Polymerase Chain Reaction (PCR) efficiency was determined according to Livak and Schmittgen [19] and the relative expression of the target genes was analyzed using the 2-△△CT method [20]. The specific primers used for qRT-PCR are listed in Table 2.
Table 2.
Growth performance and feed utilization of Nile Tilapia fed test diets.
| Diets | Initial weight (g) | Final weight (g) | Weight gain (g) | VSI | HSI | FCR | K | SR(%) |
|---|---|---|---|---|---|---|---|---|
| 0 GBNL gkg−1 | 68.36 ± 2.02a | 84.65 ± 3.17a | 16.28 ± 1.16b | 11.79 ± 0.17a | 4.52 ± 0.38a | 4.26 ± 0.68a | 1.85 ± 0.04a | 90a |
| 1 GBNL gkg−1 | 69.47 ± 1.79a | 98.95 ± 4.91a | 29.48 ± 3.12ab | 9.92 ± 0.30a | 4.32 ± 0.34a | 3.49 ± 1.87a | 2.24 ± 0.08a | 92a |
| 3 GBNL gkg−1 | 62.14 ± 2.29a | 99.22 ± 4.53a | 37.07 ± 2.24a | 9.31± 0.43a | 4.07 ± 0.38a | 1.79 ± 0.51a | 2.33 ± 0.10a | 90a |
| 5 GBNL gkg−1 | 67.18 ± 2.45a | 97.20 ± 5.65a | 30.02 ± 3.20ab | 7.97 ± 0.59a | 3.27 ± 0.50a | 2.37 ± 0.57a | 2.14 ± 0.03a | 90a |
| 7 GBNL gkg−1 | 66.10 ± 1.85a | 91.56 ± 3.51a | 25.46 ± 1.66ab | 9.29 ± 0.71a | 3.88 ± 0.24a | 2.66 ± 0.58a | 2.12 ± 0.06a | 94a |
| 10 GBNL gkg−1 | 65.14 ± 2.45 | 88.31 ± 4.25a | 23.17 ± 1.81ab | 8.72 ± 0.42a | 3.35 ± 0.29a | 2.86 ± 0.69a | 2.15 ± 0.06a | 92a |
Where, VSI = viscerosomatic index; HSI = hepatosomatic index; FCR = feed conversion ratio; K = condition factor.SR= survival rate Means ± SE (Turkeys’ HSD test, n = 3) with different superscript letters in the same column denote
Histological examination
Fixed liver samples of fish in buffered formalin were grossed by resecting to measure about 2.2 cm and embedded in a cassette. Grossed tissues were then processed using Leica TP1020 automated processor. Tissues were put in increasing concentrations of ethanol (i.e., 70%, 80%, 90% and 100%) and clearing was done using 2 changes of xylene followed by impregnation with molten paraffin wax (melting point 55-57 °C) and embedding done using SLEEMPS/P1 to form tissue blocks. Sections of the tissue block were cut to about 4 μm using SLEE microtome and thin sections transferred onto frosted slides, stained with hematoxylin and eosin, dried and mounted using DPX mounter. Prepared slides were then viewed under a Leica LAS EZ microscope and photographed using an ICC50 HD camera.
Challenge test
After 8 weeks of the feeding trial, Tilapia from the respective treatments were infected with Aeromonas jandaie and Streptococcus agalactiae suspension. Bacteria were cultured, purified and resuspended in physiologically buffered saline (PBS) to make concentrations of 1.0 × 105 CFUml−1 (Manuscript in production; DOI: 10.1002/aah.10165) and 1.0 × 109 CFUml−1 [18] for Aeromonas jandaie and Streptococcus agalactiae respectively and then mixed to make one bacterial suspension. All test groups were injected intraperitoneally with 0.2 ml of the mixed bacterial suspension and observed for mortalities for 14 days. Dead fish were examined for clinical signs and bacteria re-isolated to confirm the cause of death. Cumulative mortality and survival were computed using the formula:
Statistical analysis
Using SPSS (IBM SPSS STATISTICS, 16.0 package, IBM Corporation, New York, USA) for Windows version 10 (SPSS, Chicago, USA), between the experimental and control groups, growth parameters, haematological indices, liver toxicity parameters, and expression of growth and immune-related genes were analyzed using one-way analysis of variance (ANOVA). Differences in means were further analyzed using Tukey HSD and presented as means ± standard error (SE).
Results
Growth parameters
Fish fed GBNL supplemented diets were found to have higher weight gains, lower hepatosomatic and viscerosomatic indexes, improved (lower) feed conversion ratio, and condition factor in comparison to those fed the control diet. Except for fish fed 3 GBNL gkg−1 all other GBNL fed fish did not show significant improvement in the growth and feed utilization variables compared to those fed the control diet (P > 0.05, Table 2).
Expression of growth-related genes
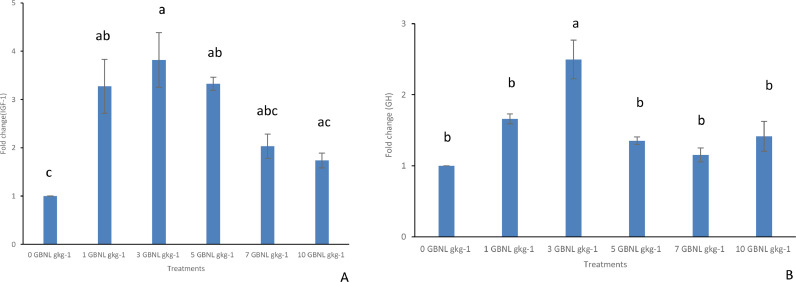
Illustrated in Fig. 1 is the effect of control and GBNL fed diets on the expression of Insulin Growth Factor (IGF-1) and Growth Hormone (GH). Fish fed 1, 3, and 5 GBNL gkg−1 diets significantly upregulated the expression of the IGF-1 gene (Fig. 1A) in comparison to those fed the control. However, a significant difference was found only in fish fed 3 GBNL gkg−1 compared to the other fish groups in the expression of the GH gene (Fig. 1 B) (P < 0.05).
Fig. 1.
Comparison (mean ± SE, Turkeys’ HSD test, n = 3) of the expression of Insulin growth factor IGF-1 (graph A), and growth hormone [GH] (graph B) genes in the head kidney of Nile Tilapia fed with control (0GBNL gkg−1) and GBNL supplemented diets (1 GBNL gkg−1, 3GBNL gkg−1, 5 GBNL gkg−1, 7 GBNL gkg−1 and 10 GBNL gkg−1).
Haematological profiles of Tilapia
As shown in Fig. 2, it was found that the levels of White Blood Cells [WBC] (Fig. 2A), Red Blood Cells [RBC] (Fig. 2B), Haemoglobin [HB] (Fig. 2C), and percentage Haematocrit [HCT] (Fig. 2D) were significantly increased in the blood of fish fed different supplemental levels of GBNL in comparison to those fed the control diet. No significant differences in haematological variables were observed among the GBNL fed fish (P > 0.05).
Fig. 2.
Comparison (mean ± SE, Turkeys’ HSD test, n == 3) of White blood cells [WBC] (graph A), Red blood cells [RBC] (graph B), and Hemoglobin [HB] (graph C) and Hemotocit [HCT] (graph D) of Nile Tilapia fed with control (0GBNL gkg−1) and GBNL supplemented diets (1 GBNL gkg−1, 3GBNL gkg−1, 5 GBNL gkg−1, 7 GBNL gkg−1 and 10 GBNL gkg−1).
Expression of immune-related genes
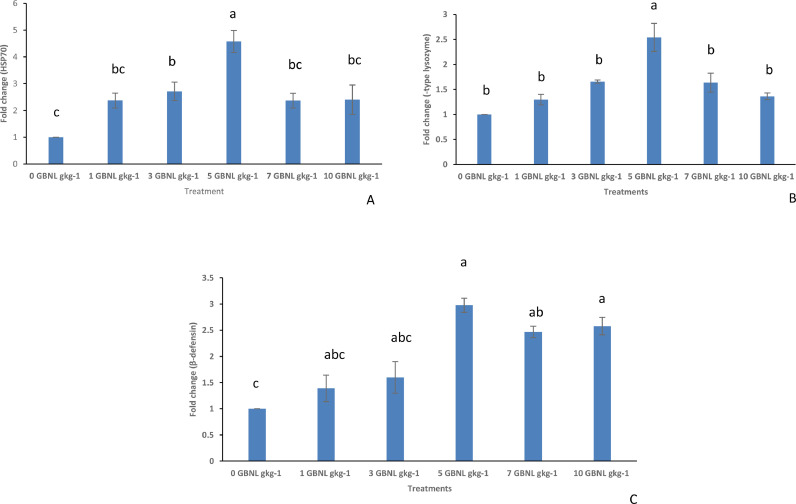
Inclusion of GBNL in diets of Nile tilapia was found to upregulate the expression of the immune-regulated genes; heat shock protein [HSP70] (Fig. 3A), C-type lysozymes (Fig. 3B) and Beta-defensin (Fig. 3C) relative to the fish fed the control diet. Generally, the results indicate that the significant upregulation of expression of these genes was noticeable in the 5 GBNL gkg−1 fed fish group in comparison to the other groups. It was realized that 1, 3, 7, and 10 GBNL gkg−1 fed fish stimulated the upregulation of the immune genes studied in comparison to the control, however, the pattern was not consistent.
Fig. 3.
: Comparison (mean ± SE, Turkeys’ HSD test, n == 3) of the expression of immune related genes; Heat shock protein (graph A), c-type lysozymes (graph B), and Beta-defensin (graph C) in the headkidney of Nile Tilapia fed with control (0GBNL gkg−1) and GBNL supplemented diets (1 GBNL gkg−1, 3GBNL gkg−1, 5 GBNL gkg−1, 7 GBNL gkg−1 and 10 GBNL gkg−1).
Plasma components
Measured values of the total protein (TP), albumin (ALB), globulin (GLO), and total-bilirubin (T-bill) showed an increasing trend with increasing levels of GBNL in diets of fish in comparison to those fed the control diet (Table 3). Among the GBNL fed fish, TP, ALB, and T-bill were consistently highest in fish fed 5 GBNL gkg−1 and those fed 7 GBNL gkg−1 were lowest.
Table 3.
Effects of GBNL and control fed Nile Tilapia on plasma components.
| Treatments | TP | ALB | GLO | T-bill |
|---|---|---|---|---|
| 0 GBNL gkg−1 | 47.00 ± 1.01c | 14 ± 0.38d | 32.5 ± 1.06c | 1.85 ± 0.04c |
| 1 GBNL gkg−1 | 53.10 ± 4.97bc | 23.3 ± 1.85b | 33.63 ± 3.34c | 6.97 ± 0.98c |
| 3 GBNL gkg−1 | 54.30 ±3.20 bc | 24.33 ± 0.61b | 56.46 ± 1.64a | 8.62 ± 1.03b |
| 5 GBNL gkg−1 | 78.33 ± 4.03a | 28.8 ± 2.43a | 58.67 ± 0.98ab | 13.42 ± 1.07ab |
| 7 GBNL gkg−1 | 52.56 ± 3.55bc | 16.27 ± 0.03d | 32.97 ± 1.78c | 5.19 ± 0.91c |
| 10 GBNL gkg−1 | 65.5 ± 4.05b | 19.87 ± 1.01c | 49.03 ± 3.03b | 11.05 ± 0.49b |
TP = Total protein, ALB = Albumin, GLO = Globulin, T-bill = Total Bilirubin Means ± SE with different superscript letters in the same column denote statistically difference (Turkey HSD test, P < 0.05).
Challenge test
The cumulative mortality (%) of GBNL and control fed Nile tilapia following co-infections with the bacteria, Aeromonas jandaie and Streptococcus agalactiae after 14 days of observation is shown in Fig. 4. Among the treated fish, those fed 5 GBNL gkg−1 (13.65%) recorded the lowest cumulative mortality whilst that fed 1 GBNL gkg−1 (31.06%) was found to have the highest mortality. That notwithstanding, there were no significant differences in cumulative mortalities among GBNL fed groups (P > 0.05). In contrast, it was observed that fish fed GBNL diets showed significantly lower cumulative mortality compared to those fed the control (P < 0.05) diet. Relative present survival computed showed the order 0.00 %, 60.35%, 68.08 %, 70.99 %, 75.63 %, and 82.57 % for 0 GBNL gkg−1, 1 GBNL gkg−1, 3 GBNL gkg−1, 7 GBNL gkg−1, 10 GBNL gkg−1, and 5 GBNL gkg−1 respectively. These results suggest that significantly higher survival against bacterial infection in Nile tilapia can be archived when fed with 5 GBNL gkg−1 compared to the other GBNL treatments and the control.
Fig. 4.
Cumulative mortality (%) of Nile Tilapia, Oreochromis niloticus fed different doses of with control (0GBNL gkg−1) and GBNL supplemented diets (1 GBNL gkg−1, 3GBNL gkg−1, 5 GBNL gkg−1, 7 GBNL gkg−1, and 10 GBNL gkg−1) after 14 days post-challenge with Aeromonas jandaie and Streptococcus agalactiae. Each line graph represents the mean ± SE of three biological replicates (n == 3).
Liver health test
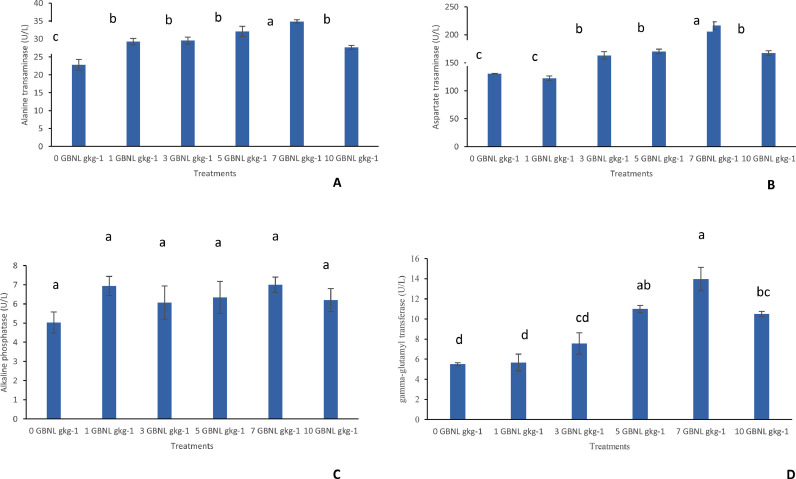
The ALT, AST, ALP, and y-GT are shown in Fig. 5. It was observed that fish fed different levels of GBNL diets had increased the levels of ALT, AST, ALP, and y-GT compared to the fish fed the control diet. Increments were most significant in the levels of these enzymes which seem to have risen from 1 GBNL gkg−1 reached a peak at 7 GBNL gkg−1 and began to decrease at 10 GBNL gkg−1.
Fig. 5.
Comparison (mean ± SE, Turkeys’ HSD test, n == 3) of the enzymes levels of Alanine transaminase (graph A), Aspartate transaminase (graph B), Alkaline phosphatase (graph C) and Gamma-glutamyltransferase (graph D) in whole blood of Nile Tilapia fed with control (0GBNL gkg−1) and GBNL supplemented diets (1 GBNL gkg−1, 3GBNL gkg−1, 5 GBNL gkg−1, 7 GBNL gkg−1 and 10 GBNL gkg−1).
Histology of liver
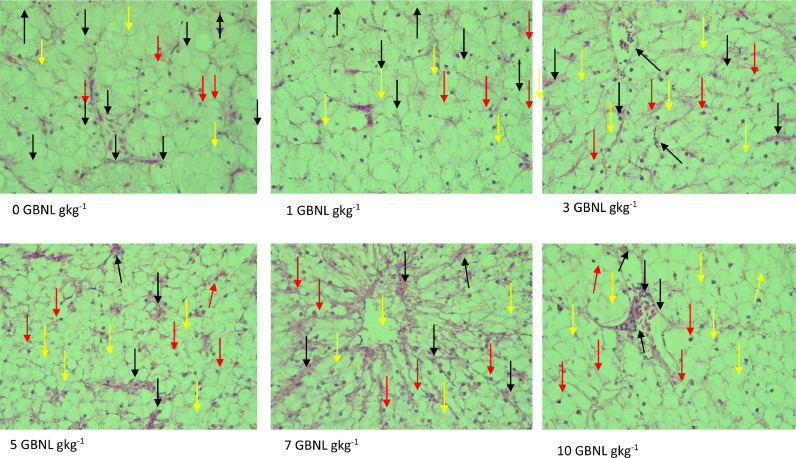
Photomicrography of GNBL and the control fed fish of liver tissues are shown in Fig. 6. Typically, histological changes observed in the livers of test fish included: hydropic changes, erythrocytes infiltration into the sinusoids, and pyknotic nuclei. These malformations, particularly with erythrocyte infiltration into the sinusoids, seem to increase in severity with increasing GBNL levels reaching their peak in 7 GNBL gkg−1 and seem to have declined slightly in 10 GNBL gkg−1 compared to the control.
Fig. 6.
Black arrows (erythrocyte infiltration/congesstion of sinusoids), Red arrows (pyknosis of nuclei), Yellow arrow (hydropic change), Purple (Vacuolation). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Discussions
Studies on the application of a blend of medicinal herbs and their extracts in sustainable aquaculture have been shown to markedly improve growth performance in cultured fish due to synergistic effects and improve feed utilization [22]. The present investigation revealed that GBNL supplementation in the diets of Nile tilapia could contribute to increased weight gain and condition factor and lower feed conversion ratio in Nile tilapia. The observed significant increase in weight gain in fish fed 3 GBNL gkg−1 diets can be attributed to the significant upregulation of the growth hormone (GH) and insulin-like growth factor, I (IGF-I) genes which play key roles in regulating fish growth [5]. Reasons, why the other GBNL treatments did not significantly improve growth, might be attributed to insufficient upregulation in the expression of growth-related genes such as IGF-1 and GH genes and slightly higher FCRs [23]. Improvements in growth performance and feed utilization have been reported in Nile tilapia [24,25,26], European eel [27] and Common Carp [28], fed a mixture of herbal extracts and in Nile tilapia [18] fed a mixture of herbal extracts with probiotic, bacillus. In addition, it was found that the GBNL application did not present any adverse effects on fish survival compared to those in the control group. Similar results have been reported in Nile tilapia fed Basella alba leaf ethanol extract, Tribulus terrestris seed ethanol extract, Mucuna pruriens seed methanol extract, and Asparagus racemosus root methanol extract [29].
Fish health is now increasingly monitored using haematological variables such as red blood cells (RBC), white blood cells(WBC), haemoglobin (Hb), and haematocrit (Hct) [30]. WBC in blood help with immune responses in an organism; Hb plays a critical role in metabolism; RBC determines the status of oxygen delivery to tissues in the organism; and haematocrit (Ht) determines the volume of RBC [31]. In this experiment, we observed that fish fed the control diet showed normality of haematological variables reported in cultured Nile tilapia [32]. This suggests the experimental conditions did not impart adversely on the normal physiology of Nile tilapia. Our results indicate that fish fed GBNL can improve the health of fish by stimulating significant increases in levels of WBC, RBC, Hb, and Hct in relation to those fed the control diet. It is believed that the presence of metabolites such as flavonoids, tannins, polyphenols and other bioactive compounds in GBNL [33,34,35] accounts for the triggered immunostimulation [36]. It was noticed that increased levels of haematological variables in Nile tilapia by the application of GBNL did not differ in a dose range between 1 and 10 gkg−1. The dynamics of different application levels of GBNL are far from being understood and may merit further investigation.
In the present investigation, it has been found that fish fed GBNL has significant improvement in TP, ALB, GLO, and T-bill compared to the control implying an improvement in the immune response in fish [37] explaining the low cumulative mortalities observed in GBNL fed fish after challenge with pathogens. Generally, our findings suggest that for optimal performance, the 5 GBNL gkg−1 application would be the best choice because it showed better performance compared to the others and the control. Similarly, the Common carp [28], and the African catfish [38] fed a blend of herbal extracts were reported to have showed significant improvement in the above-mentioned biochemical indices.
Knowledge of the expression of immune gene-related in fish is a vital tool to clarify information on the mode of action of the application of herbal supplements in aquaculture [5]. Upregulation in the expression of HSP70 [39], Chicken type-lysozymes (c-type lysozymes) [40] and Beta-defensins [41] improves the antioxidant ability of cells, innate immune systems and modulation of immune activities following administration of immunostimulants. Our findings suggest that the application of GBNL has the potential to upregulate the expression of HSP70, c-type lysozymes and Beta-defensin in Nile tilapia, with significant effects with the dose application of 5gkg−1 in which the expression of immune-regulated genes seem to have reached their peak and declined afterwards. Significant upregulation of these immune-related genes perhaps supported GBNL fed fish to have improved resistance to pathogens used in the challenge test hence could explain the low cumulative mortalities recorded. In line with other results, significant increases in the expression of lysozymes following exposure to Azadirachta indica, and Zingiber officinale among others on Catfish, Pangasianodon hypophthalmus leukocytes have been reported [42]. Also, a mixture of A.membranaceus, A. sinensis, and C. hupehensis was found to induce the expression of HSP 70 in Nile tilapia [43] and the application of Acanthopanax senticosus have been reported to modulate the expression of lysozyme and Beta-defensin in mice [44] and ultimately low communicative mortalities after pathogen challenge tests.
The efficiency of herbs fed to fish to increase their resistance to microbial infection could be evaluated in an artificial infection test with a target pathogen [45]. The results indicate that GBNL supplementation can decrease mortalities and increase the RPS up to 82.57 % (i.e. with 5 GBNL gkg−1 feed supplementation) to co-infection of Streptococcus agalactiae and Aeromonas jandaie in Nile tilapia. This could be a consequence of the increased synthesis of haematological variables and expression of immune-related genes in fish exposed to GBNL feed as have been explained earlier [46]. Also, synergies of bioactive compounds in a blend of herbal extracts can promote the stimulation of the secretion of immunological substances which can inhibit the growth of harmful pathogens and promote health benefits to the host [26]. In other reports, herbal extracts used singly or in combinations have been reported to decrease mortality in fish against many pathogens [24,7,47].
AST, ALT, ALP, and Gamma-glutamyltransferase (GGT) enzymes are haematological variables clinically measured as biomarkers for liver health [48]. AST helps metabolize amino acids; ALT helps convert proteins into energy for the liver cells; ALP is important for breaking down proteins; GGT breaks down and changes moving proteins. The findings of the study indicate fish fed the different concentrations of GBNL can promote the leaching of these liver enzymes into the bloodstream suggestive of potential liver damage in Nile tilapia. The levels of these liver enzymes increase with increasing concentrations reaching a peak at 7 g GBNL gkg-1 and then begins to decline. Higher levels of these enzymes in the blood examined were further supported by the histopathological results in the study where generally more pathological conditions were observed in a cross-section of the liver tissues examined. The rise in the levels of these liver enzymes might be due to increased metabolism to mitigate the induced stress or insufficient detoxification of GBNL by the fish liver. Despite the potential damage due to the elevation of liver enzymes in the bloodstream of Nile tilapia, mortality was null in all GBNL treatments. A possible explanation could be drawn from [49] who explained that the toxic effects of herbs on fish become lesser after a certain period of exposure when the fish gains tolerance to the concentrations administered. Nonetheless, GBNL use in fish culture should be done with caution, mindful of the fact that prolonged use thus after 8 weeks of administration could be injurious to fish health. Few investigations have reported elevated levels of liver enzymes, for instance, ALT, AST, and ALP spiked up in Indian Major Carp, Cirrhinus mrigala when fed with herbals leave extract [50].
Conclusion
These research findings have shown that the application of GBNL extracts in Nile tilapia culture can improve weight gain significantly with the inclusion of 3 gkg−1. Application of 1, 3, 5, 7, and 10 gkg−1 GBNL can significantly increase haematological variables and biochemical indices and resistance to diseases such as co-infections of Streptococcus agalactiae and Aeromonas jandaie in Nile tilapia but with the best results obtainable with the application of 5 gkg−1 of GBNL. It has been found that the application of GBNL can be injurious to the liver in Nile tilapia after 8 weeks of administration through increase the leaking of liver enzymes. Therefore, the level of these liver enzymes could be used as biomarkers of GBNL leaf extracts application to determine its toxicity to Nile tilapia in the field of environmental biomonitoring.
Authors’ contributions
E.D. Abarike conceived the idea and designed the experiment, collected and analyzed data and drafted the manuscript. A. Ampofo-Yeboah, edited and proofread the manuscript. S.O. Dandi assisted in laboratory and field experiments.
Animal rights
All fish were handled in accordance with the U.K animal act, 1986 and associated guidelines, EU Directive 2010/63/EU for animal experiments. Hormonal sex reversed Nile tilapia were used in the experiment.
Compliance with ethical standards Submission declaration and verification
This article to be considered for publication has not been published previously and is not under consideration for release elsewhere.
Funding
The study was supported by the International Foundation for Science of grant number I2-A-6542-1
Data availability statement
Data will be available on request from the corresponding author
Declaration of Competing Interest
The authors declare there is no conflict of interest.
Acknowledgments
The authors wish to thank International Foundation for Science for supporting us with funds to carry out this study. We appreciate the effort of the Dean, Faculty of Biosciences, UDS Prof Elliot H. Alhassan and the Head, Department of Fisheries and Aquatic Resources Management, UDS Dr. Daniel N. Akongyuure for facilitating the release of funds for project execution. We are also indebted to Dr. Abass Abdul-Karim, the Head, Public Health Laboratory, Tamale Teaching Hospital for providing us with laboratory space for gene expression analysis. Also, we are grateful to Associate Professor, Edmund Muonir Der, the Head, Department of Pathology, UDS for assisting in histological analysis of tissues.
References
- 1.Abarike E.D., Obodai E.A., Attipoe F.Y.K. Growth and economic performance of fingerlings of Oreochromis niloticus fed on different non-conventional feeds in out-door hapas at Akosombo in Ghana. Afr. J. Agric. 2013;8:3384–3391. doi: 10.5897/AJAR12.593. [DOI] [Google Scholar]
- 2.Monir M.S., Yusoff S.B.M., Zulperi Z.B.M., Hassim H.B.A., Mohamad A., Ngoo M.S.B.M.H., Ina-Salwany M.Y. Haemato-immunological responses and effectiveness of feed-based bivalent vaccine against Streptococcus iniae and Aeromonas hydrophila infections in hybrid red tilapia (Oreochromis mossambicus × Oreochromis niloticus) BMC Vet. Res. 2020;16:1–14. doi: 10.1186/s12917-020-02443-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dioguardi M., Guardiola F.A., Vazzana M., Cuesta A., Esteban M.A., Cammarata M. Vitamin D3 affects the innate immune status of European sea bass (Dicentrarchus labrax L.) Fish Physiol. Biochem. 2017;43:1161–1174. doi: 10.1007/s10695-017-0362-3. [DOI] [PubMed] [Google Scholar]
- 4.Basri L., Nor M.R., Salleh A., Yasin I.M.S., Saad M.Z., Rahaman N.Y.A., Barkham T., Noor Mohammad, Amal A. Co-infections of Tilapia Lake Virus, Aeromonas hydrophila and Streptococcus agalactiae in farmed Red Hybrid Tilapia. Animals. 2020;10 doi: 10.3390/ani10112141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmadifar E., Fallah H.P., Yousefi M., Mahmoud A.O.Dawood, Seyed Hossein Hoseinifar H.A., Yilmaz S., Paolucci M., Doan Van H. The gene regulatory roles of herbal extracts on the growth,immune system, and reproduction of fish. Animal. 2021;11:1–24. doi: 10.3390/ani11082167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doan H.Van, Hoseinifar S.H., Jaturasitha S., Dawood M.A.O., Harikrishnan R. The effects of berberine powder supplementation on growth performance, skin mucus immune response, serum immunity, and disease resistance of Nile tilapia (Oreochromis niloticus) fingerlings. Aquaculture. 2020;520 doi: 10.1016/j.aquaculture.2020.734927. [DOI] [Google Scholar]
- 7.Gabriel N.N. Review on the progress in the role of herbal extracts in Tilapia culture. Cogent Food Agric. 2019;5 doi: 10.1080/23311932.2019.1619651. [DOI] [Google Scholar]
- 8.Van Hai N. The use of medicinal plants as immunostimulants in aquaculture: a review. Aquaculture. 2015 doi: 10.1016/j.aquaculture.2015.03.014. [DOI] [Google Scholar]
- 9.Yilmaz E., Taşbozan O., Erbaş C. Potential of medicinal herbal products to be used in aquaculture. Int. J. Sci. Eng. Res. 2018;9:16–23. [Google Scholar]
- 10.Daswani P.G., Gholkar M.S., Birdi T.J. Psidium guajava : a single plant for multiple health problems of the rural Indian population. Pharmacogn. Rev. 2017;11:167–174. doi: 10.4103/phrev.phrev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farombi E.O., Owoeye O. Antioxidative and chemopreventive properties of Vernonia amygdalina and Garcinia biflavonoid. Int. J. Environ. Res. Public Health. 2011;8:2533–2555. doi: 10.3390/ijerph8062533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tibebu A., Haile G., Kebede A. Review on medicinal value and other application of Neem tree: senior seminar on animal health. Biomed. Nurs. 2018;4:62–69. doi: 10.7537/marsbnj040118.10. [DOI] [Google Scholar]
- 13.Kuebutornye F.K.A., Abarike E.D. The contribution of medicinal plants to tilapia aquaculture: a review. Aquac. Int. 2020;28 doi: 10.1007/s10499-020-00506-3. [DOI] [Google Scholar]
- 14.Cerezuela R., Guardiola F.A., González P., Meseguer J., Esteban M.Á. Effects of dietary Bacillus subtilis, Tetraselmis chuii, and Phaeodactylum tricornutum, singularly or in combination, on the immune response and disease resistance of sea bream (Sparus aurata L.) Fish Shellfish Immunol. 2012;33:342–349. doi: 10.1016/j.fsi.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Banerjee S., Kim M.L., Shariff M., Khatoon H., Yusoff M.F. Antibacterial Activity of Neen (Azadirachta indica) leaves on Vibro spp. isolated from cultured Shrimps. Assiian J. Anim. Vet. Adv. 2013;8:355–361. doi: 10.3923/ajava.2013.355.361. [DOI] [Google Scholar]
- 16.Biswas B., Rogers K., McLaughlin F., Daniels D., Yadav A. Antimicrobial activities of leaf extracts of Guava (Psidium guajava L.) on two gram-negative and gram-positive bacteria. Int. J. Microbiol. 2013;2013 doi: 10.1155/2013/746165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olowolafe T., Mo O. Toxicity of aqueous extracts of bitter leaf (Vernonia amygdalina) on haematological profile of African catfish (Clarias gariepinus) juveniles. Int. J. Fish. Aquat. Stud. 2018;6:596–600. [Google Scholar]
- 18.Abarike E.D., Jian J., Tang J., Cai J., Yu H., Lihua C., Jun L. Influence of traditional Chinese medicine and Bacillus species (TCMBS) on growth, immune response and disease resistance in Nile tilapia, Oreochromis niloticus. Aquac. Res. 2018;49:2366–2375. doi: 10.1111/are.13691. [DOI] [Google Scholar]
- 19.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ÄÄCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Schmittgen T.D., Livak K.J. Analyzing real-time PCR data by the comparative C T method. Nat. Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 21.Saiyad Musthafa M., Asgari S.M., Kurian A., Elumalai P., Jawahar Ali A.R., Paray B.A., Al-Sadoon M.K. Protective efficacy of Mucuna pruriens (L.) seed meal enriched diet on growth performance, innate immunity, and disease resistance in Oreochromis mossambicus against Aeromonas hydrophila. Fish Shellfish Immunol. 2018;75:374–380. doi: 10.1016/j.fsi.2018.02.031. [DOI] [PubMed] [Google Scholar]
- 22.Magouz F.I., Shehab El-Din M.T., Amer A.A., Gewaily M.S., El-Dahdoh W.A., Dawood M.A.O. A blend of herbal essential oils enhanced the growth performance, blood bio-immunology traits, and intestinal health of Nile tilapia (Oreochromis niloticus) Ann. Anim. Sci. 2022;22:751–761. doi: 10.2478/aoas-2021-0066. [DOI] [Google Scholar]
- 23.Aanyu M., Betancor M.B., Monroig O. Effects of dietary limonene and thymol on the growth and nutritional physiology of Nile tilapia (Oreochromis niloticus) Aquaculture. 2018;488:217–226. doi: 10.1016/j.aquaculture.2018.01.036. [DOI] [Google Scholar]
- 24.Abarike E.D., Jian J., Tang J., Cai J., Yu H., Chen L. Traditional Chinese Medicine enhances growth, immune response, and resistance to Streptococcus agalactiae in Nile tilapia. J. Aquat. Anim. Health. 2019;31:46–55. doi: 10.1002/aah.10049. [DOI] [PubMed] [Google Scholar]
- 25.Hassan A.A.M., Yacout M.H., Khalel M.S., Hafsa S.H.A., Ibrahim M.A.R., Mocuta D.N., Turek Rahoveanu A., Dediu L. Effects of some herbal plant supplements on growth performance and the immune response in Nile tilapia (Oreochromis niloticus). “Agriculture Life, Life Agric. Conf. Proc. 2018;1:134–141. doi: 10.2478/alife-2018-0020. [DOI] [Google Scholar]
- 26.Poolsawat L., Yu Y., Li X., Zhen X., Yao W., Wang P., Luo C., Leng X. Efficacy of phytogenic extracts on growth performance and health of tilapia (Oreochromis niloticus × Oreochromis aureus) Aquac. Fish. 2022;7:411–419. doi: 10.1016/j.aaf.2020.08.009. [DOI] [Google Scholar]
- 27.Huang Z., Lu J., Ye Y., Xu A., Li Z. Effects of dietary Chinese herbal medicines mixture on growth performance, digestive enzyme activity and serum biochemical parameters of European eel, Anguilla anguilla. Aquac. Rep. 2020;18 doi: 10.1016/j.aqrep.2020.100510. [DOI] [Google Scholar]
- 28.Raissy M., Ghafarifarsani H., Hoseinifar S.H., El-Haroun E.R., Shahbazi Naserabad S., Van Doan H. The effect of dietary combined herbs extracts (oak acorn, coriander, and common mallow) on growth, digestive enzymes, antioxidant and immune response, and resistance against Aeromonas hydrophila infection in common carp, Cyprinus carpio. Aquaculture. 2022;546 doi: 10.1016/j.aquaculture.2021.737287. [DOI] [Google Scholar]
- 29.Ghosal I., Mukherjee D., Chakraborty S.B. The effects of four plant extract on growth, sex reversal, immunological and haemato-biochemical parameters in Nile tilapia, Oreochromis niloticus (Linnaeus, 1758) Aquac. Res. 2021;52:559–576. doi: 10.1111/are.14914. [DOI] [Google Scholar]
- 30.Maftuch M. Haematological Analysis of Nile tilapia (Oreochromis niloticus) and Striped Catfish (Pangasius hypophthalmus) using hematology analyzer tool software at Fish Breeding Center Jojogan, Tuban, East Java. Res. J. Life Sci. 2018;5:107–115. doi: 10.21776/ub.rjls.2018.005.02.4. [DOI] [Google Scholar]
- 31.Esmaeili M. Blood performance: a new formula for fish growth and health. Biology. 2021;10:1–17. doi: 10.3390/biology10121236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hrubec T.C., Cardinale J.L., Smith S.A. Haematology and plasma chemistry reference intervals for cultured Tilapia (Oreochromis Hybrid) Vet. Clin. Pathol. 2000;29:7–12. doi: 10.1111/j.1939-165X.2000.tb00389.x. [DOI] [PubMed] [Google Scholar]
- 33.Nagano M.S., Batalini C. Phytochemical screening, antioxidant activity and potential toxicity of Azadirachta indica A. Juss (neem) leave. Rev. Colomb. Ciencias Quim. 2021;50:29–47. doi: 10.15446/rcciquifa.v50n1.95447. [DOI] [Google Scholar]
- 34.Naseer S., Hussain S., Naeem N., Pervaiz M., Rahman M. The phytochemistry and medicinal value of Psidium guajava (Guava) Clin. Phytosci. 2018;4:1–8. [Google Scholar]
- 35.Ugbogu E.A., Emmanuel O., Dike E.D., Agi G.O., Ugbogu O.C., Ibe C., Iweala E.J. The phytochemistry, ethnobotanical, and pharmacological potentials of the medicinal plant Vernonia amygdalina L. (bitter Leaf) Clin. Complement. Med. Pharmacol. 2021;1 doi: 10.1016/j.ccmp.2021.100006. [DOI] [Google Scholar]
- 36.Syawal H., Kurniawan R., Effendi I., Austin B. Fermented medicinal herbs improve the haematological and physiological profile of Striped catfish (Pangasianodon hypophthalmus) F1000Research. 2021;466 doi: 10.12688/f1000research.52640.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oniovosa U., Aina O., Alarape S., Babalola O., Adeyemo O. Effects of neem leaves aqueous extract on organ histology, haematological parameters and biochemical indices in catfish. Alex. J. Vet. Sci. 2017;54:17. doi: 10.5455/ajvs.256015. [DOI] [Google Scholar]
- 38.Fawole F.J., Yisa R.O., Jayeoba O.O., Adeshina I., Ahmed A.O., Emikpe B.O. Effect of Dietary polyherbal mixture on growth performance, haemato-immunological indices, antioxidant responses, and intestinal morphometry of African Catfish, Clarias gariepinus. Aquac. Nutr. 2022;2022:1–11. doi: 10.1155/2022/5502796. [DOI] [Google Scholar]
- 39.Daneghian S., Amani R., Hosseini S.A., Ghandil P., Jafari A., Malehi A.S. Effect of herbal antioxidant‑rich formula on the improvement of the antioxidant defence system and heat shock protein‑70 expression in recreational female athletes: a randomized controlled trial. J. Res. Med. Sci. 2019;24 doi: 10.4103/jrms.JRMS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou C., Lin H., Huang Z., Wang J., Wang Y., Yu W. Molecular characterization of G-type lysozyme from juvenile golden pompano Trachinotus ovatus and the regulation of its activity and expression by dietary sodium butyrate levels. Aquac. Rep. 2019;14 doi: 10.1016/j.aqrep.2019.100198. [DOI] [Google Scholar]
- 41.Barroso C., Carvalho P., Gonçalves J.F.M., Rodrigues P.N.S., Neves J.V. Antimicrobial peptides: Identification of two beta-defensins in a teleost fish, the European sea bass (Dicentrarchus labrax) Pharmaceuticals. 2021;14:1–16. doi: 10.3390/ph14060566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nhu T.Q., Bich Hang B.T., Vinikas A., Bach L.T., Buu Hue B.T., Thanh Huong D.T., Quetin-Leclercq J., Scippo M.L., Phuong N.T., Kestemont P. Screening of immuno-modulatory potential of different herbal plant extracts using striped catfish (Pangasianodon hypophthalmus) leukocyte-based in vitro tests. Fish Shellfish Immunol. 2019;93:296–307. doi: 10.1016/j.fsi.2019.07.064. [DOI] [PubMed] [Google Scholar]
- 43.Abarike E.D., Jian J., Tang J., Cai J., Sakyi E.M., Kuebutornye F.K.A. A mixture of Chinese herbs and a commercial probiotic Bacillus species improves hemato-immunological, stress, and antioxidant parameters, and expression of HSP70 and HIF-1α mRNA to hypoxia, cold, and heat stress in Nile tilapia, Oreochromis niloticus. Aquac. Rep. 2020;18 doi: 10.1016/j.aqrep.2020.100438. [DOI] [Google Scholar]
- 44.Zhang Y.Q., Zhang Y.L., Liu Z.K. Effects of Acanthopanax senticosus supplementation on innate immunity and changes of related immune factors in healthy mice. Innate Immun. 2021;27:461–469. doi: 10.1177/1753425920955200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Awad E., Austin D., Lyndon A., Awaad A. The possible effect of hala extract (Pandanus tectorius) on immune status, anti-tumour and resistance to Yersinia ruckeri infection in rainbow trout (Oncorhynchus mykiss) Fish Shellfish Immunol. 2019;87:620–626. doi: 10.1016/j.fsi.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 46.Tiamiyu A.M., Olatoye I.O., Olayemi O.A., Ekundayo T.C., Adedeji O.B., Okocha R.C. Medicinal plant feed additives enhanced survivability and growth performance of Clarias gariepinus (African Catfish) against bacterial infection. Microbiol. Res. 2021;12:744–752. doi: 10.3390/microbiolres12040054. [DOI] [Google Scholar]
- 47.Stratev D., Zhelyazkov G., Noundou X.S., Krause R.W.M. Beneficial effects of medicinal plants in fish diseases. Aquac. Int. 2018;26:289–308. doi: 10.1007/s10499-017-0219-x. [DOI] [Google Scholar]
- 48.Rastiannasab A., Afsharmanesh S., Rahimi R., Sharifian I. Alternations in the liver enzymatic activity of Common carp, Cyprinus carpio in response to parasites, Dactylogyrus spp. and Gyrodactylus spp. J. Parasit. Dis. 2016;40:1146–1149. doi: 10.1007/s12639-014-0638-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Doctolero J.S., Estrella E.S., Vera Cruz E.M. Influence of Water Immersion using Guava (Psidium guajava), Lemon-Grass (Cymbopogon citratus) and Horse Radish (Moringa oleifera) aqueous leaves extracts on the nursing of Nile tilapia (Oreochromis niloticus) Int. J. Zool. Anim. Biol. 2021;4:1–8. doi: 10.23880/izab-16000279. [DOI] [Google Scholar]
- 50.Saravanan M., Ramesh M., Malarvizhi A., Petkam R. Toxicity of neem leaf extracts (Azadirachta indica A. Juss) on some haematological, ionoregulatory, biochemical and enzymological parameters of Indian Major carp, Cirrhinus mrigala. J. Trop. For. Environ. 2011;1 doi: 10.31357/jtfe.v1i1.80. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be available on request from the corresponding author