Abstract
Hematopoiesis refers to the phenomenon that hematopoietic stem cells (HSCs) continuously form and produce blood cells with multiple functions. In crustacean, the hematopoietic process produces a variety of hemocytes that form the core and basis of cellular and humoral immunity, which is crucial for crustacean to maintain their lives and protect themselves against microbial infection. The expression of many factors, in the form of transcription factors and humoral factors, are altered during hematopoiesis, which are involved in the regulation of hematopoiesis. Meanwhile, there are also factors that, although not directly involved in the HSCs differentiation or hemocytes production and release, play an essential role in maintaining cellular homeostasis. In this review, we summarize current knowledge on the hematopoietic lineage of crustacean, with a particular focus on the molecular regulation of hematopoiesis, including transcriptional regulation, humoral factors involved in the differentiation of HSCs and the maintenance of hematopoietic homeostasis, which contributes to a systematic understanding of the crustacean hematopoiesis.
Keywords: Crustacean, Hematopoiesis, Hematopoietic homeostasis, Hemocyte
1. Introduction
Hematopoiesis refers to the dynamic and complex process whereby hematopoietic stem cells (HSCs) in hematopoietic tissues form and release different types of blood cells [1]. Generally, vertebrate hematopoiesis occurs in two successive waves, primitive and definitive, that differ in cell types produced and anatomic location. The primitive wave occurs transiently and produces only nucleated red blood cells, whereas the definitive wave lasts the lifetime of the organism and produces HSCs capable of producing all bloodlines [2]. As for invertebrates with body cavities, blood cells are usually called hemocytes, and unlike vertebrates, invertebrates lack red blood cells with the ability to carry oxygen and lymphocytes involved in adaptive immune responses, thus its hematopoiesis is relatively less complex and can be used as a simplified model to study the hematopoietic mechanism and the involvement of hematopoiesis in the regulation of innate immunity. Among invertebrates, crustaceans have longer lifespan compared to short-lived insects, and their survival depends on the continuous production of hemocytes. Therefore, crustaceans are now gradually being used to study hematopoiesis for adult invertebrate, while insect such as Drosophila usually acts as a model for studying embryonic and larval hematopoiesis.
As an important group of invertebrates that lack adaptive immunity, crustaceans often rely on cellular and humoral immunity, such as phagocytosis and release of antimicrobial substances mediated by hemocytes, to defense pathogenic infection [3]. During this process, hemocytes are consumed in large quantities, so the rapid and continuous synthesis of new hemocytes is required during infection. Meanwhile, the production of hemocytes is also necessary for physiological homeostasis and life activity in crustacean. For example, the signal crayfish, Pacifastacus leniusculus, can live up to 20 years, of which the hematopoietic stem cells continuously renew themselves and generate new hemocytes in their specific hematopoietic tissues, providing an ideal model for studying hematopoiesis in invertebrates with long life span [4]. Furthermore, the earliest and most in-depth research on the hematopoiesis of crustacean was originated from the exploration of P. leniusculus, which research progress, as well as the research on the hematopoiesis of other crustaceans, has been discussed in details by Söderhäll [5]. Meanwhile, studies on the regulatory mechanism of crustacean hematopoiesis have also been focused on the decapods dominated by P. leniusculus, and some key factors have been summarized also in other articles [4,6]. In this review, we will summarize the current knowledge on the hematopoietic lineage of crustacean, as well as the regulatory mechanisms of hematopoietic differentiation and its homeostasis maintenance in crustaceans, in which the related factors of the species involved are shown in Table 1. We hope that summary of these recent findings will be helpful for further elucidating the mechanisms of hematopoietic regulation in crustaceans and their potential role in innate immunity.
Table 1.
Factors involved in the regulation of crustacean hematopoiesis.
| Function | Factor(s) | Name | Key findings | Specie(s) | Reference(s) |
|---|---|---|---|---|---|
| Transcriptional regulation of hematopoiesis | Runx | PlRunt | Enhanced expression of Runx stimulates the differentiation of Hpt cells to SGCs and GCs. | P. leniusculus | Söderhäll et al. [23] |
| GATA-like | EsGLP | EsGLP is involved in the production and differentiation of HSCs and prohematocytes. | E. sinensis | Li et al. [31] | |
| Humoral factors regulating the differentiation and migration of Hpt cells and the production and release of hemocytes | Transglutaminase | TGase | TGase maintains the undifferentiated state of Hpt by maintaining cross-linking activity with CP. | P. leniusculus | Lin et al. [35]; Junkunlo et al. [33, 36] |
| Reactive oxygen species | ROS | ROS provide important signals that control proliferation and differentiation and promote the release of new hemocytes by affecting TGase activity. |
E. sinensis; P. leniusculus |
Junkunlo et al. [37]; Jia et al. [39] | |
| PDGF/VEGF-like receptors | Pl_PVR1 | PVR controls Hpt cells migration by regulating TGase activity. | P. leniusculus | Junkunlo et al. [25] | |
| N-terminal proPO peptide | ProPO peptide | The N-terminal proPO peptide can induce Hpt cells proliferation, differentiation and migration, especially differentiation to GCs by activating ROS signaling. | P. leniusculus | Sirikharin et al. [43] | |
| Astakine | Ast1 | Ast1 stimulates Hpt cells proliferation and induces differentiation of SGC lineages by reducing TGase activity and directly binding to the cell membrane of Hpt cells. | P. leniusculus | Lin et al. [46]; Sirikharin et al. [49, 50]; Lin et al. [51] |
|
| Ast2 | Ast2 induces the differentiation of Hpt cells to mature GCs. | Lin et al. [46] | |||
| EsAst | EsAst increases ROS levels in Hpt and induces hemocytes generation. | E. sinensis | Jia et al. [47] | ||
| β-thymosin | Pl-β-thymosin 1 | Pl-β-thymosin 1 promotes the migration of Hpt cells, which can be blocked by Ast1. | P. leniusculus | Saelee et al. [52] | |
| Pl-β-thymosin 2 | Pl-β-thymosin 2 and Ast1 act synergistically in promoting Hpt cells migration. | ||||
| Crustin | Crustin Pm4 | Crustin Pm4 and STG Ι act as unconventional ribonucleoproteins to down-regulate astakine protein expression by regulating astakine translation. | P. monodon | Chang et al. [53] | |
| Shrimp transglutaminase Ι | STG Ι | P. monodon | |||
| Serotonin (5-hydroxytryptamine) | 5-HT | 5-HT stimulats crayfish hemocytes to release Ast1, thereby affecting the release of new hemocytes into the circulatory system. | P. leniusculus | Noonin [57] | |
| Humoral factors maintaining hematopoietic homeostasis | Crustacean hematopoietic factor | CHF | Hematopoietic factor CHF expression can be induced by Ast1 and prevent Hpt cells apoptosis. | P. leniusculus | Lin et al. [58]; Noonin et al. [38] |
| Homeostasis associated protein | PmHHAP | PmHHAP is upregulated after viral infection to prevent rapid apoptosis to maintain circulating hemocytes levels. | P. monodon | Prapavorarat et al. [59] | |
| Laminin receptor | PvLamr | PvLamr knockdown results in a significant decrease in the total number of hemocytes, especially HCs. | P. (L.) vannamei | Charoensapsri et al. [6] | |
| Apoptotic protein inhibitors | LvIAP1 | LvIAP1 regulates hemocyte homeostasis via caspase-regulated spontaneous apoptosis of shrimp hemocytes. | P. (L.) vannamei | Leu-et al. [60] | |
| ClipSP2 | PmClipSP2 | PmClipSP2 knockdown allows severe disruption of hemocytes. | P. monodon | Amparyup et al. [44] |
2. Hemocytes in crustaceans
Hemocyte in the circulating hemolymph is the most important component of the innate immune system that can directly participate in cellular responses, such as recognition, phagocytosis, coagulation, and encapsulation in crustacean. Hemocyte is free-moving in the circulating hemolymph, where they perform immune defense and other physiological roles. Particularly, homeostasis of hemocytes in the circulating hemolymph is essential to maintain a stable and effective immune system in crustaceans.
Generally, hemocytes are divided into three sub-populations in crustacean, including granular cells (GCs), semi-granular cells (SGCs) and hyaline cells (HCs), which have been classified mainly based on morphological criteria, such as cell size, nuclear/cytoplasmic (N/C) ratio, and the number of intracellular granules [5]. GCs have been classified by their biggest cell size, the lowest N/C ratios, and filling with larger cytoplasmic granules, while HCs cells are the smallest cells, the highest N/C ratios, and having very few or no cytoplasmic granules. The SGCs have been characterized by their middle cell size between GCs and HCs, with smaller N/C ratios and more cytoplasmic granules, and a few large refractile granules in the cytoplasm. The distribution of hemocytes in the three subpopulations differs somewhat among species of crustacean. In signal crayfish and penaeid shrimp, GCs were found to be the predominant type of hemocytes, accounting for more than half of all hemocytes, while HCs had the lowest percentage and were not even found in some species [7], [8], [9]. As for crabs, HCs are the main types of hemocytes. In the male Chinese mitten crab Eriocheir sinensis, HCs accounted for 59.8%, while other types of hemocyte include SGCs and GCs comprised approximately 21.1% and 19.2%, respectively [10]. However, morphological characteristics of the crustacean hemocyte types are more subjective, which severely hampers the accurate classification and functional identification of hemocytes. Some new methods, such as the identification of stable and reliable biomarkers, will effectively facilitate the study of hemocyte classification. Recently, the single-cell RNA-seq (scRNA-seq) analysis was applied to classify hemocytes into 6 types (Hem 1–6) based on transcriptional profiles in Marsupenaeus japonicus [11]. In comparison, scRNA-seq provides detailed and advanced insights into hemocytes classification due to its advantage of pinpointing individual cells, but this method has not been widely used in crustaceans and the six groups of hemocytes have not been well correlated with morphologically classified hemocytes.
As mentioned above, crustacean hemocytes play a key role in immobilizing or destroying invasive pathogens. The different subpopulations of hemocytes in diverse species of crustacean exhibit variable immune activities. SGCs are reported to be phagocytes, which participate in particle encapsulation, melanization, and coagulation. GCs are the main repositories for the proPO system and are responsible for the storage and release of antimicrobial peptides (AMPs) and various proteinase inhibitors [12]. However, all three subpopulations of hemocytes are capable of mediating phagocytosis in crustacean, which has been debated [5]. Further investigations are necessary, for example, the immune function of different subpopulations of hemocytes to different bacteria or viruses.
3. Hematopoiesis in crustaceans
Hematopoiesis is a complex process, by which different types of mature hemocytes differentiate from hematopoietic stem cells and release to the circulating hemolymph. Because of the fact crustaceans have an open circulation and mature hemocytes have a certain lifespan, hemocytes need to be generated continuously and proportionally under normal physiological conditions. In addition, crustaceans live in an environment with many microbes. When animals are infected, a large number of hemocytes are recruited to remove the pathogens that result in a loss in a short period of time [5]. Meanwhile, crustaceans are aggressive and often cause hemocytes loss due to physical injury. The above events need to be supplemented by new hemocytes produced by hematopoietic tissue. But what about the process of hematopoiesis in crustaceans? Studies on hematopoiesis are essential for the normal life activities of crustaceans. In recent years, studies on hematopoiesis have mainly focused on decapod crustaceans, especially freshwater crayfish, prawns, crabs, and lobsters.
3.1. Hematopoietic tissue and hematopoietic progress
It is generally accepted that crustacean hemocytes arise throughout the life of the animal in a special hemocyte-forming tissue called hematopoietic tissue (Hpt) [4]. In decapod crustacean, Hpt is located on the dorsal part of the cardiac stomach, with a structure of thin sheets of highly packed cells. Hpt is composed of a series of ovoid lobules which are preliminary classified according to their morphological characteristics. The anterior proliferation center (APC) is located just anterior and ventral to the Hpt, and the cells in the APC and the Hpt have a different morphology. More than 50% of the cells in the APC contain nuclei with loose euchromatin, whereas most of the cells in the dorsal Hpt contain condensed heterochromatin [13].
Intriguingly, there is also more than one type of cell in Hpt cells. The Hpt cells of the signal crayfish P. leniusculus consist of at least five types of cells. Both type 1 and type 2 cells are the main proliferating cells and have the ability to differentiate into SGC and GC. Type 1 cells can develop into type 2 cells approximately accounted for 8%, closely arranged on the dorsal side of the lobule. Type 2 cells account for about 30%, loosely attached, adjacent to the type 1 cell layer distributed on the inside of the lobule. Type 3, 4 and 5 cells do not have the ability of cell division. Type 3 and type 4 cells contain granules similar to GC, which can be categorized into precursors of GCs. Type 3 cells have the highest accounting for 55%. Type 5 cells contain many small cytoplasmic granules, which can act as a precursor of SGCs. When the young hemocytes become mature, they are released from Hpt to circulation [4]. Yet only four cell types were identified in the Hpt cells in the black tiger shrimp Penaeus monodon. The type 1 cells are the precursor cells that give rise to type 2 and type 3 cells, with a large or a small granular hemocyte, respectively. The type 4 cells are typical features of interstitial cells. The nucleus of the type 1 cells has dispersed chromatin, while that of type 2 and 3 cells has predominantly randomly distributed. The precursor cells are located towards the exterior of the lobules, and maturing young hemocytes towards the inner part. Type 1 and type 4 cells are observed only in the Hpt, never in the circulatory system. The type 1–3 cells of the Hpt cells can proliferate [14]. As in most decapod crustacean, Hpt is a thin tissue covering the dorsal surface of the foregut in the American lobster Homarus americanus, of which the Hpt cells were roughly divided into two types: one type is hyaline cells lineage and the other type is the granulosa cell lineage. The lobules contain 6 to 40 Hpt cells, of which approximately 90% constitute stages in granulocyte maturation and 10% are intermediates in hyaline cell maturation [15]. Although different researchers have different criteria for the classification of Hpt cells, there is a similar pattern in the tissue form of cells in the hematopoietic lobules of decapod crustacean include in P. leniusculus, P. monodon and H. americanus. However, as with the question in cell classification, previous studies have classified the types of Hpt cells in crustacean based on morphological characteristics. A recent study suggested that almost all circulating hemocytes belonged to the GC lineage by tracking newly generated hemocytes and transplanted cells in red claw crayfish, Cherax quadricarinatus, in which the authors argued that SGCs and GCs might represent hemocytes at different developmental stages, rather than two fully differentiated cells [16]. Hence, more researches still need to better identify the characteristics of subpopulations of Hpt cells in crustaceans. Meanwhile, there is no direct evidence for the relationship between these cells and their relationship with hemocytes, which still need further addressing.
In summary, Hpt cells are usually considered to include the progenitor cells of SGC and GC in crustaceans, which can differentiate into precursors of SGC and GC, and then the precursors of SGC and GC further develop into SGC and GC [3]. In the meantime, the differentiation pathway of HCs is not as clear as GCs and SGCs in crustaceans. The percentage of immature GCs and SGCs are also unknown in hemolymph, and how these cells develop further is still unclear. Therefore, the differentiation pathway of crustacean hemocytes needs more investigations.
3.2. Models and methods of hematopoiesis
The establishment of hematopoietic models and methods plays an essential role in the study of the hematopoietic system. Söderhäll et al. have established a culturing method of Hpt cells of crayfish in vitro [17]. The Hpt was isolated from P. leniusculus and cultured in a modified L15 medium. The medium supplemented with plasma devoid of hemocyanin, the cells could be cultured for 8 to 12 weeks, while without any supplement, the cells remained viable for at least 3 weeks. Primary cultured Hpt cells have been developed as a powerful model system to study hematopoiesis. Subsequently, Liu et al. reported that an effectiveness and specificity dsRNA transfection system based on histone H2A, particularly for the cell cultures of crustacean such as crayfish and shrimp [18]. Later, a finding provided a method for highly efficient DNA transfection system based on electroporation in primary cultured crayfish cells [19]. Afterward, a method to direct differentiation of Hpt cells into mature hemocytes in crustacean hematopoiesis. Crayfish muscle extract (ME) was able to direct GC differentiation from Hpt cells in vitro [20]. Recently, allograft experiments have also been applied to explore the spectrum of hematopoietic differentiation. Purified Hpt cells and SGC/GC are labeled with different markers and then treated in suspension, followed by injection of the cell suspension into the ventral blood cavity of the second abdominal segment. This allows direct observation of the involvement of labeled cells in the process of circulation and differentiation [16]. Additionally, the Hpt cell culture was also performed in red claw crayfish [21], which was latterly shown to be suitable for the assemble and propagation of progeny white spot syndrome virus in vitro, but not with the crayfish hemocyte that is solely suitable for viral gene transcription and protein synthesis [22]. Taken together, these models and methods are valuable and enable research on fundamental biological questions such as cell lineage differentiation processes as well as immune studies in crustaceans.
4. Omics analysis of the hematopoietic lineages
To systematically understand the hematopoiesis, for example, to trace the potential key factors involved in Hpt differentiation into hemocytes and further to circulation, and to screen for biomarkers that differentiate stem cells and different hemocyte types, the researchers have performed a range of transcriptome and proteome analysis.
The earliest transcriptome data reported were determined by Söderhäll et al. on hematopoietic tissue from P. leniusculus in 2003 [23], followed by transcriptome sequencing of three other tissues of this species (brain + nerve, hemocyte and Hpt). These transcriptome databases are now used to analyze the different stages of the hematopoietic process by quantitative global proteomics approaches and to possibly find marker proteins to enable genealogical tracing, for example during microbial infection [24]. Lately, the scRNA-seq was employed to uncover the transcriptional profiles of several thousand hemocytes of M. japonicus. In addition to proposing a universal classification of shrimp hemocytes into six groups, this study also summarized markers for each cell type, as well as cell growth factors that might play a key role in hemocyte differentiation. Specifically, transglutaminase (TGase), the cell proliferation-related genes, and G2/M state-related genes were found to be strongly expressed in Hem 1, the oligopotent or initial state hemocyte type. However, many of these characteristically expressed markers are currently unannotated, and analysis of these unknown gene signatures may help reveal the mechanisms of shrimp hemocyte proliferation and differentiation [11]. Whereas differential expression of growth-related genes also exists on specific hemocyte clusters, suggesting that these genes may play roles in the differentiation and maturation of HSCs. Several of these factors, such as astakine, the crustacean hematopoietic factor-like protein CHF-like, and PDGF/VEGF-related factors, have been identified to be involved in the above mentioned process [6,25]. Overall, these findings refine the sequencing of hemocyte taxa to more precisely target the factors that play a role in certain hemocyte clusters, which provides a great reference for future functional resolution of these factors. In terms of proteomics, two-dimensional gel electrophoresis followed by mass spectrometry analysis was also used to identify proteins associated with the development of different hemocyte types in P. leniusculus in an earlier study. Within the hemocyte lineages, a two-domain Kazal proteinase inhibitor (KPI) and a superoxide dismutase (SOD) were found to be specific for SGC and GC, respectively, while the proliferation cell nuclear antigen (PCNA) was specific for Hpt cells. Hence, this study identified three different proteins/transcripts as indicators or markers of hematopoietic cell proliferation and specific differentiation into SGC or GC, which provide aids for subsequent studies on the differentiation of precursor cells into different types of hemocytes. However, due to the lack of available antibodies, only SOD was experimentally verified by Western blot to be present only in GC [26]. Using this method, along with the upgrade of detection technology, a recent study has detected 21 differentially expressed proteins between GCs and HCs in Fenneropenaeus chinensis, of which 13 were highly expressed in HCs, 4 were uniquely expressed in GCs, and 4 were significantly highly expressed in GCs. Among the identified proteins, the high expression of Ras-related nuclear protein in GC and the high expression of RuvB-like protein 2 and TAR RNA-binding protein 2 in HC were also verified by qPCR [27]. Meanwhile, the shotgun proteomics methods and their variants currently are powerful to handle a complete proteome analysis. By using this strategy, a study analyzed the complete proteome from the APC of the hematopoietic tissue, via the remaining parts of the Hpt to the mature SGCs and GCs. The results showed that STAT and pseudouridylate synthase 7 exhibited specificity for Hpt cells to some extent. As for SGC, in addition to the previously identified KPI, a hitherto uncharacterized VEGF-like protein, a low-density lipoprotein receptor, a C-type lectin, dual oxidase and a thrombospondin, and coagulins detected only in SGC fractions also demonstrated the characteristics of a potential protein marker. In GC, in addition to SOD mentioned above, a new serine protease and an uncharacterized peroxidase family protein have been identified, but the strongest protein specific to GC was the Crustin3 [24]. These findings together facilitate a better understanding of the functions of hemocyte subpopulations in crustaceans.
Taken together, these studies above provide several putative biomarkers of differentiation as well as cell-specific proteins in the crustacean hematopoietic spectrum, some of which have been applied in practical studies. However, up to now, there have not been universal, specific, and highly recognized biomarkers for different crustacean hemocytes because of the lack of available antibodies to re-validate these selected biomarkers. On the other hand, as a high-throughput sequencing technique commonly used in quantitative proteomics in recent years, iTRAQ proteomics analysis has been widely studied in the screening of differential proteins in the crustacean immune process, but these studies have not yet focused on hematopoiesis. Therefore, the future application of this technique or related methods in crustacean hematopoiesis may have great potential value for future research.
5. Molecular regulation of hematopoiesis in crustaceans
With the deepening understanding of the hematopoietic process in crustaceans, researchers have been investigating the molecular mechanisms of the formation, differentiation and release of hemocytes. Due to the technical limitations, most studies in the 20th century only included morphological analysis. However, along with the technological progress, such as the maturity of Hpt in vitro culture technology and the establishment of RNA interference technology, not only the further determination and integration of omics data but also some key molecules in the hematopoiesis process have been identified and further studied. In the following section, we will focus on how the transcriptional regulators, such as Runx protein homologs and GATA factors, and some humoral factors, such as TGase, TGase upstream regulators, elements in prophenoloxidase (proPO) system, astakines, and astakine-related factors, play complex and interrelated roles in hematopoiesis in crustaceans, which are involved in the hematopoietic processes, including Hpt cells proliferation, differentiation and migration, hemocytes formation, production and release, as well as the maintenance of hematopoietic homeostasis.
5.1. Transcriptional regulation of hematopoiesis
Stimulation with microbial polysaccharides or repeated bleeding leads to the need for new hemocytes, thereby stimulating cell division in Hpt and the release of immature and mature hemocytes. One rapid response to this stimulus is the induced expression of certain transcription factors, which in turn affect the hematopoietic process by regulating the transcriptional level of the organism [5]. And it's generally accepted that some common elements of transcriptional regulation are shared in the development of hematopoietic cells across taxonomic groups [4,5]. Detailed studies on hematopoietic transcription factors associated with hematopoiesis in D. melanogaster or other insects have been thoroughly reviewed [28,29], and it is believed that Runx protein homologs and GATA factors are essential for the survival of hemocytes and hemocyte precursors, and a similar hypothesis has also been deduced in P. leniusculus and E. sinensis.
Runx protein homologs are closely associated with the differentiation of cells that express the proPO gene. In D. melanogaster, Lozenge (LZ), a homolog of Runx, is important for crystal cell development, while in signal crayfish, Runx expression is a precondition for the eventual differentiation of SGC and GC. PlRunt was identified in P. leniusculus and its mRNA expression was found to be rapidly induced in Hpt cells in response to stimulation with microbial polysaccharides, thus may be a marker of differentiation within Hpt [23]. In addition, Srp, a homolog of GATA in D. melanogaster, acts with LZ on the promoter region to promote its transcriptional expression [30]. In crustacean, a GATA-like protein (named EsGLP) was characterized from E. sinensis. Bloodletting stimulation and interference assays showed that EsGLP expression was upregulated by blood loss and was involved in the production and differentiation of HSCs and prohemocytes, which were essential for hematopoiesis and probably also the survival of E. sinensis [31]. What's more, the specialization of plasma cells requires the involvement of two glial cell missing (Gcm and Gcm2) transcription factors, which then become mature phagocytes in response to ecdysteroid stimulation in D. melanogaster [30]. Yet, in crustacean, no hematopoietic transcriptional regulatory function-related Gcm and Gcm-like have been identified so far, although Gcm was expressed in the brain of P. leniusculus. The absence of hematopoietic functions of Gcm suggests that perhaps plasma cell-like cell types are not present in crustaceans [32]. Therefore, although the research on transcriptional regulation in crustacean started very early, there are still many deficiencies and a large number of unsolved mysteries. Worse still, the hematopoietic characteristics of D. melanogaster and crustacean have big differences, for example, the hematopoiesis of flies only occurs in the embryo and larvae stage, while crustacean can continuously produce new hemocytes. Thus, crustaceans are likely having new transcriptional regulatory mechanisms which are totally different from D. melanogaster, and it still needs further exploration and verification.
5.2. Maintaining of ECM stability by TGase to regulate Hpt cells differentiation
TGase is a Ca2+ dependent cross-linked enzyme that is involved in a variety of cellular activities, including adhesion, migration, survival, apoptosis and extracellular matrix (ECM) organization. In mammals, dysfunction of TGase has been found to play a key role in the development of many diseases, such as Alzheimer's disease, Parkinson's disease and Huntington's disease [33]. In vertebrates, the non-enzyme and enzyme function of tissue transglutaminase (TG2) have been reported, and its extracellular activity includes not only the cross-linking of ECM proteins but also the non-enzymatic regulation of ECM [34]. Among invertebrates, TGase was first identified in horseshoe crab Tachypleus tridentatus and then reported in many other species, such as crayfish P. leniusculus, shrimp Penaeus (Litopenaeus) vannamei and fruit fly D. melanogaster. The earliest studies of invertebrate TGase were limited to the involvement in coagulation or clot formation, which shares the same function with human factor XIIIA, but at that time its association with hematopoiesis was not found [34].
Notably is that studies related to the involvement of TGase in the regulation of hematopoiesis in crustaceans began in 2008. A study on the homeostasis maintenance of hemocytes of P. leniusculus determined the mRNA level and enzyme activity of TGase in different cell types. Its mRNA level and enzyme activity were very high in Hpt cells, low in HCs, and very low in GCs. In cultured Hpt cells, high enzyme activity was found in the cells at the center of Hpt, while the TGase activity of the cells migrated from the tissue was very low. The RNAi assay of TGase resulted in cell morphological changing to fusiform shape and cell diffusing, but had no effect on Hpt proliferation. TGase seemed to play a novel role in preventing hematopoietic stem cells from differentiating and migrating to hemolymph. Another evidence is the presence of GATA motif in the TGase gene of crayfish, highlighting the potential role of TGase in the regulation of hematopoiesis, as it occurs in transcriptional regulation in mammals [35]. For this function, Junkunlo et al. conducted further verification by transglutaminase inhibition. In that study, the reversible inhibitor cystamine was found to be able to reduce the TGase activity of Hpt in a concentration-dependent manner, which stimulated the differentiation of hematopoietic progenitor cells in Hpt and then promoted the release of these cells into the peripheral circulation. Moreover, compared with the newly generated cells (labeled by BrdU) showing no increase in Hpt, BrdU positive cells were more likely to be increased in circulation when TGase was inhibited. Further study suggested that newly formed Hpt cells were also primarily involved in differentiation into the circulation when TGase activity was inhibited [33]. Interestingly, the way of TGase maintaining the undifferentiated state of Hpt is related to clotting protein (CP), which is part of ECM in Hpt. Hematopoiesis in crustacean is rigorously regulated by a microenvironment consisting of several cell types and multicomponent ECM. ECM not only plays a role in supporting the hematopoietic niche but is also involved in binding to different growth factors and cytokines, while CP is secreted during Hpt cell culture. The formation of filamentous networks of CP was observed in Hpt cell culture, and a larger amount of CP protein was detected on the surface of undifferentiated cells (round) compared with the migrated cells (fusiform). Furthermore, the co-localization of CP protein and TGase activity was observed on filamentous networks on cell surfaces and between cells, which in turn induces the cross-linking activity of CP [36]. Thus, maintenance of cross-linking activity with CP may be the most important way for TGase to maintain Hpt stability.
5.3. TGase activity regulated by ROS and PVR
TGase plays a key role in hematopoiesis, but the regulation of hematopoiesis is not only a single factor that acts, as there must be the stimulation of upstream signals to regulate TGase activity. So far, astakines, reactive oxygen species (ROS) and PDGF/VEGF-like receptors (PVR) have been found to play such functions. Since astakine and astakine-related factors are involved in the regulation of hematopoiesis through a variety of ways, we will summarize them in more details later. Here, we focus on how ROS and PVF/PVR signaling pathways are involved in crustacean hematopoiesis through the regulation of TGase activity.
ROS, as the metabolic product of cellular respiration, must be maintained at a harmless level to reduce the risk of oxidative stress. Though high ROS levels can cause greater damage to cells, low/moderate ROS levels may be necessary for regulating hematopoietic function. In mammals, the hematopoietic stem cell niche retains a certain level of ROS to maintain hematopoietic stem cell function. In addition, it has been shown that increased ROS levels induce differentiation of hematopoietic stem cells in Drosophila and mammals [37]. In crayfish, high levels of ROS can be detected in the APC where active proliferating cells are located. Moreover, LPS stimulation could significantly reduce the number of circulating hemocytes, and the ROS level in the APC region was higher, followed by differentiation and release of new hemocytes, all of which suggested that ROS signal played a conservative role in the regulation of hematopoiesis [38]. In crabs, ROS levels were measured by flow cytometry with relative fluorescence intensity and it showed that ROS levels in Hpt cells were stronger than in hemocytes [39]. In 2016, Junkunlo et al. showed that ROS modulated hematopoiesis by affecting TGase activity in crayfish. In the study, the antioxidant N-Acetyl-L-cysteine (NAC) was used as ROS scavenger, and it was determined that ROS production in APC containing less differentiated cells could be most effectively reduced after NAC treatment. Then, when the crayfish were stimulated with LPS to produce hemocytes, the circulating hemocytes of the animals that were injected with NAC recovered significantly more slowly than those that were given LPS alone. After NAC treatment of in vitro APC cells, compared with the control, statistical analysis showed that the cell in the migrated state (fusiform) was significantly decreased [37]. In E. sinensis, NAC also exhibited an inhibition of Hpt cell proliferation and a reduction in the total number of circulating hemocytes [39]. As for how ROS affects cell proliferation, the researchers found that the extracellular TGase activity was higher under NPC treatment in both APC and reHpt tissue (the tissue portion of Hpt removed APC), and the TGase activity in APC was higher than that in reHpt, which was consistent with the results of previous studies [35,37]. And the latter also suggested that the extracellular TGase played a role in preventing cell migration out of Hpt. In addition, antibodies against the ϵ-(γ-glutamyl)-lysine isopeptide were used to detect the protein cross-linking activity of TGase, but no statistically significant difference was found between the TGase-knockout cells and the control cells, but overall, the inhibitory effect of ROS signaling is to some extent by affecting the extracellular TGase activity of hematopoietic precursor cells, thus slowing the release of new hemocytes into the hemolymph. Taken together, ROS has been demonstrated to provide important signals to regulate TGase activity, thus controlling differentiation and promoting the release of new hemocytes into the lymph in crustaceans [37].
PVR is a homolog of mammalian platelet derived growth factor receptor/vascular endothelial growth factor receptor (PDGFR/VEGFR) found in both Drosophila and crustaceans. In mammals, the interaction between PDGF and its target cell surface PDGFR transmits signals that have been shown to drive cellular responses including proliferation, survival, migration, and deposition of ECM and tissue remodeling factors [40]. In Drosophila, PDGF/VEGF-like factors (PVF) and their receptors (PVR) are responsible for guiding cell migration and are involved in hemocyte production. PDGFRs are important and evolutionarily conserved determinants of hematopoietic cell development and proliferation among vertebrates and invertebrates [41]. In crustacean, Junkunlo et al. found that Pl_PVR1 played an important role in controlling the behavior of progenitor cells during crayfish hematopoiesis, especially cell migration, which was achieved by regulating TGase activity. In the study, the multi-target TK inhibitor sunitinib malate was used to inhibit downstream PVR signaling. When PVR signaling was inhibited, TGase activity was enhanced, accompanied by an increase in cell surface area and a dose-dependent increase in the number of spreading cells (fusiform) with the inhibitor. Meanwhile, the increased immunoreactivity for β-tubulin and elongation of β-tubulin filaments were observed after PVR inhibitor treatment, so the migration phenomenon was further verified [25]. Regretfully, Hpt cell differentiation and hemocyte numbers were not measured in this study, so it was unable to determine whether PVR signaling would have effect on the TGase regulation of cell differentiation.
Collectively, both ROS and PVR, as upstream signals of TGase, can be recruited in hematopoiesis, with ROS influencing the proliferation and differentiation of Hpt cells and PVR acting as a regulator of Hpt cells migration. However, these studies are not sufficient. The relationship between PVR and ROS is not clear, and whether they regulate TGase activity directly or through some downstream factors cannot be confirmed, and these questions need to be explored by further studies.
5.4. The proPO system plays dual roles in both hematopoiesis and immunity
In crustacean, the proPO-activating system is generally considered to serve an important role as a non-self-recognition system that participates in the innate immune responses through accompanying with the cellular responses via hemocyte attraction and inducing phagocytosis, melanization, cytotoxic reactant production, particle encapsulation, and the formation of nodules and capsules [42]. However, it is gradually discovered that the proPO system may also play its specific role in crustacean hematopoiesis.
As we know, activation of the proPO system is controlled by a multistep pathway. Recognition of pathogen-associated molecular patterns by pattern recognition receptors is followed by activation of a series of serine proteases (SPs), which eventually culminates in the proteolytic cleavage of the proPO zymogen to the active PO enzyme [42]. In this process, a by-product of the cleavage process of proPO zymogen, an N-terminal peptide, shows an important role in hematopoiesis and is highly correlated with ROS levels in the APC [43]. In addition, ClipSP2, a member of the serine protease family, exhibits a role in the maintenance of hematopoiesis homeostasis [44]. Sirikharin et al. studied the function of the N-terminal proPO peptide in signal crayfish by producing bioactive recombinant proPO peptides in Escherichia coli. After injection of the peptides in vivo, new hemocytes were produced and interestingly proPO peptides significantly increased the percentage of cyclic GCs compared to SGCs. It was speculated that proPO peptides produced after activation of the proPO system were mainly involved in the recruitment of GCs. And then, to investigate the effect of proPO peptides on the release of hemocytes from Hpt, the binding rate of BrdU was measured after injection of the peptide. The results showed that the presence of N-terminal proPO peptides induced the synthesis of DNA and the release of immature hemocytes from Hpt into the hemolymph. Taken together, proPO peptide induces cell differentiation and possibly cell migration [43]. However, the mechanisms involved still needs further study, although stimulation of ROS signaling may be one of the important ways and the only way found so far for proPO peptide to exert its hematopoietic function.
In addition to regulating the production and release of hemocytes, the maintenance of hemocyte homeostasis is also important for hematopoiesis, and ClipSP2 was proposed to play such a role. A significant decrease in PO activity was observed in the hemolymph by dsRNA-mediated gene knockdown of PmClipSP2 in P. monodon. Meanwhile, injecting LPS into the gene knocked-down shrimps resulted in a significant reduction in circulating hemocytes count, and further study showed that the decrease was mainly due to severe disruption of hemocytes, including cell destruction, uncontrolled specialization, and eventually bursting, which was observed by scanning electron microscopy [44]. Interestingly, PmClipSP2 also plays a role in shrimp immunity, mainly in the activation of the proPO cascade through the binding of the C-terminal SP domain to microbes [45]. Thus, ClipSP2 exhibits a dual role in regulating hematopoiesis and innate immunity in crustaceans.
In summary, the proPO system, a widely recognized innate immune system in crustaceans, is also involved in hematopoiesis, reflecting the possibility that the proPO system may play its unique function in hematopoiesis following infection-induced blood loss, thus linking the immune response and hematopoiesis in crustaceans. However, current studies on the involvement of the proPO system in hematopoiesis are limited to only two factors, and more studies are needed to establish the relationship between hematopoiesis regulation and immune regulation.
5.5. Hematopoiesis regulated by astakines and astakine-related factors
In contrast to the conserved role of transcriptional regulatory factors, there are also some humoral regulators which are not so conserved, such as astakine, one of the most critical cytokines in crustaceans. Astakine is involved in hematopoietic effects in many ways, either by inhibiting TGase activity to promote Hpt cells differentiation or by using β-subunit of ATP synthase as an acceptor to attach to the surface of Hpt cells, in which thymosin β4 (Tβ4) is also involved. Meanwhile, astakine, as a key hematopoietic factor, is regulated by some factors, such as Crustin Pm4, transglutaminase Ι (STG Ι), and Serotonin (5-hydroxytryptamine, 5-HT). And astakine is also involved in the maintenance of hematopoietic homeostasis by regulating the expression of some factors, such as crustacean hematopoietic factor (CHF). Although laminin receptor (Lamr), hemocyte homeostasis-associated protein (HHAP), and inhibitor of apoptosis protein (IAP) have not been found to be directly related to astakine, but they might perform unique functions in hematopoietic homeostasis needing further elucidation.
Homologous to the structure of vertebrate prokineticin, astakine was firstly identified from Hpt in P. leniusculus, which contains a conserved domain with 10 cysteines showing the conserved spacing [17]. Astakine1 (Ast1) and Astakine2 (Ast2), two members of the astakine family, were identified in signal crayfish in 2005 and 2010, respectively, with Ast2 showing high similarity to shrimp and other arthropods [46]. So far, astakine has been found in not only crustaceans, but also other arthropods, like scorpions, spiders, ticks, as well as in hemipteran, hymenopteran, and blattodean insects. But surprisingly, it is not found in dipterans such as D. melanogaster, reflecting the non-conservativeness of astakine among invertebrates [46]. Among crustaceans, studies on astakines have been not only conducted in P. leniusculus, but also in E. sinensis, Scylla paramamosain, L. vannamei and Procambarus clarkii. However, studies in some of these species have focused more on immunity, and the relationship between astakines and hematopoiesis has been established mainly in P. leniusculus and partly in E. sinensis [47,48].
In crayfish, Ast1 was initially identified by its ability to induce Hpt cells proliferation in vitro, followed by cDNA cloning from crayfish hemocytes. Such cytokines are most abundant in SGC and are mainly secreted from SGC among three subtypes of hemocytes [4]. A follow-up study showed that Ast1 was present in plasma as a high molecular weight complex (HMW), which was identified by Western blot of P. leniusculus hemolymphs and was named after the presence of Ast1 in a polymer form of approximately 65–70 kDa in size. The formation of this HMW complex requires the participation of other proteins secreted hemocytes; moreover, it is not secreted directly by Hpt cells, but is formed under low plasma calcium concentration [49]. Further studies showed that plasma Ast1 level was elevated before the recruitment of hemocytes after blood loss in the organism, and both in vitro and in vivo experiments showed that recombinant Ast1 stimulated Hpt cell proliferation and induced differentiation of SGC lineages; in contrast, Ast2 did not induce Hpt cell proliferation but increased the number of mature GCs [50]. Additionally, similar phenomenon was found in E. sinensis, in which rEsAst treatment could induce hemocytes production and increase the level of ROS in Hpt [47].
As to how Ast1 plays a role in inducing proliferation and differentiation, a combined study on astakine and TGase provides an explanation, which was conducted to observe the change of endogenous TGase activity in crayfish by adding purified Ast1 in vitro. As a result, Ast1 was able to inhibit TGase activity and acted as a non-competitive inhibitor of TGase because the inhibition was not decreased as the substrate concentration was increased. In addition, the coagulation response was impaired in the presence of Ast1, and the reduction of ε (γ-glutamyl) -lysine bond formed by TGase-mediated CP cross-linking could be detected by Western blot [50]. Ast1, in addition to acting indirectly by regulating TGase activity, also binds directly to the cell membrane of Hpt cells. To identify the receptor, the binding site of astakine on the surface of Hpt cells was characterized by affinity cross-linking technique. The results revealed that the β-subunit of F1ATP synthase was cross-linked to Ast1 in a 1:1 ratio. Follow-up experiments further verified that Ast1 was bound to the surface of Hpt cells due to cross-linking with the β-subunit F1ATP synthase on the surface of type II Hpt cells, and this action inhibited the formation of extracellular ATP [51]. ATP synthase is a well-known mitochondrial enzyme complex often present on the surface of highly proliferating cells and various cancer cell lines, and its involvement in hematopoiesis in crustaceans was further characterized in P. leniusculus, mainly in relation to Thymosin β4 (Tβ4). Thymosins are well known for their actin-binding activity. Thymosin β4 has been associated with biological activities in tissue repair and cell migration via interaction with ATP synthase in vertebrates. Pl-β-thymosin 1 and 2 identified from signal crayfish could bind to the β-subunit of ATP synthase; however, no direct interaction was found between Ast1 and the two P1-β-thymosins, suggesting that these proteins bind to different sites on the β subunit of F1 ATP synthase. The recombinant Pl-β-thymosin 1 was able to increase extracellular ATP synthesis in Hpt cells and block the effect exerted by Ast1, while Pl-β-thymosin 2 did not have this function. This in turn led the different performance of these two factors in hematopoietic function. Pl-β-thymosin 1 promoted the migration of Hpt cells, which can be blocked by Ast1; meanwhile the ROS activity of APC was decreased. In contrast, Pl-β-thymosin 2 and Ast1 acted synergistically in promoting cell migration and caused a significant induction of ROS with a significant but transient effect on circulating hemocytes numbers, especially SGCs. In addition, the increased plasma Ast1 could induce Pl-β-thymosins production and secretion, but due to the lack of specific antibodies, it could not be determined whether both thymosins were increased. Overall, there is a synergistic effect of Ast1 and Pl-β-thymosin 2 in regulating crayfish hematopoiesis, and the different effects of Pl-β-thymosin 1 reflect the complex interaction of these proteins involved in regulating hematopoietic stem cell proliferation and differentiation [52].
As a regulator for hematopoiesis, astakine is also regulated by other factors at various levels. At the transcriptional level, like other hematopoietic factors, astakine is regulated by universal transcriptional regulators, and no specific transcriptional regulators have been identified, while at the translational level, Crustin Pm4 and STG Ι serve as ribonucleoproteins down-regulated astakine-mediated hematopoiesis. In addition, 5-HT promotes astakine secretion and thus Hpt cells proliferation. In general, the translational control mechanisms are modulated via the interaction of RNA binding proteins or microRNA at 3′-UTR of mRNA. Chang et al. constructed various deletion mutants of the astakine 3′-UTR in order to find the localization of the blocker and enhancer on the black tiger shrimp astakine (Ast1) transcript, and identified 3′-UTR242–483 to act as a blocker. Subsequently, shrimp STG Ι and Crustin Pm4 were screened to associate with 3′-UTR242–483 by the electrophoresis mobility shift assay and RNA pull-down assay [53]. Crustin is one of the most important AMPs found in decapod crustaceans, and its antimicrobial and antiviral functions have been discussed in detail in several reviews [54,55]. Crustin Pm4 is classified as a member of the Crustin family but its antibacterial activity remains unknown. STG Ι, on the other hand, is a member of the TGase family identified in P. monodon, but STG Ι showed no clotting activity [53]. Further functional studies showed that gene knockdown of Crustin Pm4 and STG Ι was associated with the increased astakine protein expression, but had no effect on astakine mRNA expression. Once the translation of astakine mRNA was not repressed, the increasingly secreted astakine then promoted the production of hemocytes from Hpt [53]. In short, Crustin Pm4 and STG Ι interact with the astakine transcript at 3′-UTR242–483 and act as unconventional ribonucleoproteins to down-regulate astakine protein expression by regulating astakine translation but not transcription. 5-HT, on the other hand, is involved in the regulation of crustaceans as a neurotransmitter. 5-HT is a conserved monoamine neurotransmitter that plays an important role in vertebrate brain functions such as the regulation of sleep and appetite. Although its role as a neurotransmitter in the central nervous system is well-known, it also plays a role in regulating the function of many peripheral systems, such as vertebrate gastrointestinal function, coagulation, hematopoiesis, and the immune system [56]. In signal crayfish, the increase in the number of circulating hemocytes was mediated by the injection of 5-HT, the phenomenon that occurs mainly when 5-HT promoted the secretion of Ast1 in a concentration-dependent manner, and Ast1 in turn regulated hematopoiesis [57]. Therefore, 5-HT is involved in crustacean hematopoiesis as a key neuromodulatory factor, and it is hypothesized that it may play a unique function as an upstream neural signal during circadian-influenced hematopoiesis, but this hypothesis needs further confirmation.
When crustaceans require more blood, astakine is needed not only to increase hemocytes through continued proliferation and differentiation of Hpt cells, but also to reduce apoptosis of Hpt cells and hemocytes to achieve cellular homeostasis. When Hpt cells are cultured in the absence of Ast1, these cytokine-deficient cell cultures died due to apoptosis. Thus, Ast1 is an important regulator between cell proliferation/differentiation and apoptosis. It is in signal crayfish that CHF was identified by suppression subtractive hybridization (SSH) assay as an important factor that can inhibit apoptosis of Hpt cells, whose expression was regulated by Ast1 [58]. When CHF was silenced, though Hpt cell differentiation was unaltered, the number of apoptotic cells was increased and the number of Hpt cells was significantly decreased, while further studies showed that CHF targeting was important for the survival of SGC precursors [38,58]. This phenomenon was demonstrated by localization of CHF transcripts, which showed that CHF was restricted to SGC and Hpt cells and that cells of the GC lineage did not express this transcript [38]. Besides, the CHF-like protein (PvCHF-like) was also identified in P. vannamei, both this protein and the CHF of P. leniusculus contained an insulin growth factor binding protein structural domain, but they differed in their conserved structural domains [6]. In P. vannamei, yeast two-hybrid assays showed that PvCHF-like bind to Lamr and that PvLamr knockdown also reduced the expression of PvCHF-like, whose gene knockdown eventually led to a significant reduction in total hemocytes counts, especially in the number of HCs. However, the fate of the missing HCs has not been determined. And it is also unlikely that this phenomenon was caused by an inhibition of Hpt cell proliferation or an increase in apoptosis, but probably more of an effect on hemocytes apoptosis [6]. Another protein that binds to PvLamr in a manner similar to CHF-like protein is HHAP. HHAP was originally identified in P. monodon as a highly responsive gene in monocytes in the early and late stages of WSSV infection by SSH screening. PmHHAP played a direct role in hemocyte persistence, and PmHHAP knockdown was followed by a decrease in the level of circulating hemocytes. It could be observed that some hemocytes became abnormal and started to deform, lysis and division into apoptotic bodies. Therefore, the upregulation of PmHHAP after viral infection is a mechanism to maintain circulating hemocytes by preventing rapid apoptosis [59]. However, there are no further studies on how HHAP acts through binding to Lamr and how it relates to CHF and astakine. In P. monodon and P. vannamei, another factor involved in both immune and hemocytes homeostasis was identified: IAP. IAPs, as anti-apoptotic regulators, can inhibit the activity of caspase, which is the main executor of the apoptotic process and plays an important role in the process of apoptosis in many species. The first crustacean IAP gene was cloned from P. monodon, and the identification of the function of PmIAP was mainly related to the antiviral effect against WSSV. Subsequently, three LvIAP homologues were identified in L. vannamei: LvIAP1, LvIAP2, and LvSurvivin. Among the three, LvIAP1 is crucial for shrimp survival, and the main way it works is by inducing hemocyte apoptosis. In the case of LvIAP1 knockdown, it was observed that hemocytes showed characteristic apoptotic morphological changes and high levels of effecter caspase activity, along with undergoing DNA fragmentation into oligonucleosome steps. Thus, LvIAP1 regulates hemocyte homeostasis via caspase-regulated spontaneous apoptosis of shrimp hemocytes [60]. Taken together, astakines and some factors related to astakine are involved in hematopoiesis in a dual manner. In addition to these factors, there are also some other factors that are not directly related to astakine, but they also create important conditions for the proper functioning of hematopoiesis in the form of maintaining hematopoietic homeostasis. However, the association among these factors and between these factors and astakine needs to be further confirmed.
6. Conclusion and future perspective
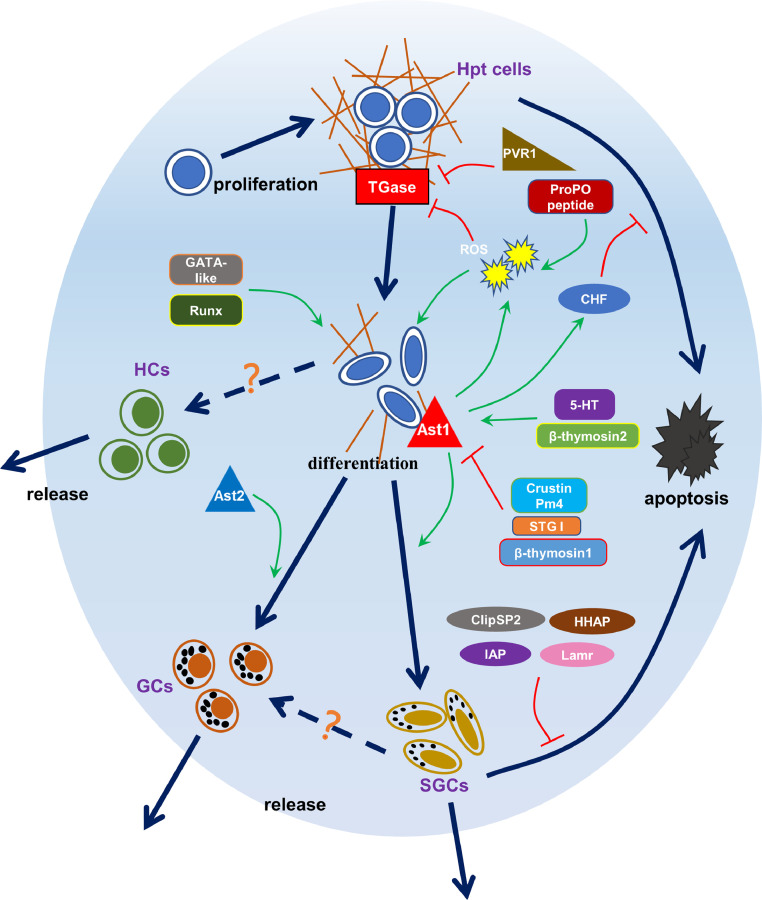
To date, several studies have explored the regulatory mechanisms of hematopoiesis in crustacean and the relevant information is reflected in the table and figure (Table 1, Fig. 1). In conclusion, the regulation of hematopoiesis includes transcriptional regulation, which provides an active transcriptional environment for hematopoiesis. The most direct regulator of hematopoiesis is TGase activity, which maintains the undifferentiated state of Hpt cells by maintaining the ECM. Astakines, PVR, and ROS act as upstream signals to reduce TGase activity and promote Hpt cell differentiation and migration. The proPO system, as a key innate immune system, interestingly exhibits both immune and hematopoietic regulation. Astakines, in addition to TGase, also binds directly to the surface of Hpt cells for hematopoietic effects, and at the same time, astakine is regulated by a variety of factors, including those involved in the maintenance of hematopoietic homeostasis. It should be noted that this review mainly summarizes the regulatory mechanisms of hematopoiesis in crustaceans through a holistic view. However, it cannot be denied that there are also differences among crustaceans, which require more species-specific studies to be conducted. Nevertheless, there are still many shortcomings and imperfections for the present studies under the overall framework. Certain omics data are available, but the mining and analysis of these data are not sufficient, and the exploration of detailed mechanisms of all screened factors has not been achieved. In addition, the current studies are limited to the independent action of several factors and some simple synergistic or antagonistic studies, so the complete systemic protein interaction network and more detailed and precise studies will hopefully be constructed in the future. More importantly, the invention and application of new observation methods which can directly monitoring the whole process of hematopoiesis are also wanted. The resolution of these issues will further contribute to the comprehensive elucidation of the regulatory mechanisms of hematopoiesis in crustacean and provide further insight into innate immunity taking crustaceans as models.
Fig. 1.
The multiple factors involved in hematopoiesis in crustaceans.
Acknowledgement
This work was supported by grants from the National Key Research and Development Program of China (2018YFD0900502), the National Natural Science Foundation of China (U2005210), and the Fundamental Research Funds for the Central Universities of China (20720180123, 20720200120).
References
- 1.Gautam D.K., Chimata A.V., Gutti R.K., Paddibhatla I. Comparative hematopoiesis and signal transduction in model organisms. J. Cell Physiol. 2021;236(8):5592–5619. doi: 10.1002/jcp.30287. [DOI] [PubMed] [Google Scholar]
- 2.Galloway J.L., Zon L.I. Ontogeny of hematopoiesis: examining the emergence of hematopoietic cells in the vertebrate embryo. Curr. Top. Dev. Biol. 2003;53:139–158. doi: 10.1016/s0070-2153(03)53004-6. [DOI] [PubMed] [Google Scholar]
- 3.Liu S., Zheng S.C., Li Y.L., Li J., Liu H.P. Hemocyte-mediated phagocytosis in crustaceans. Front. Immunol. 2020;11:268. doi: 10.3389/fimmu.2020.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin X., Söderhäll I. Crustacean hematopoiesis and the astakine cytokines. Blood. 2011;117(24):6417–6424. doi: 10.1182/blood-2010-11-320614. [DOI] [PubMed] [Google Scholar]
- 5.Söderhäll I. Crustacean hematopoiesis. Dev. Comp. Immunol. 2016;58:129–141. doi: 10.1016/j.dci.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 6.Charoensapsri W., Sangsuriya P., Lertwimol T., Gangnonngiw W., Phiwsaiya K., Senapin S. Laminin receptor protein is implicated in hemocyte homeostasis for the whiteleg shrimp Penaeus (Litopenaeus) vannamei. Dev. Comp. Immunol. 2015;51(1):39–47. doi: 10.1016/j.dci.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 7.Li F., Chang X., Xu L., Yang F. Different roles of crayfish hemocytes in the uptake of foreign particles. Fish Shellfish Immunol. 2018;77:112–119. doi: 10.1016/j.fsi.2018.03.029. [DOI] [PubMed] [Google Scholar]
- 8.Ding Z.F., Du J., Ou J.T., Li W.J., Wu T., Xiu Y.J., Meng Q.G., Ren Q., Gu W., Xue H., Tang J.Q., Wang W. Classification of circulating hemocytes from the red swamp crayfish Procambarus clarkii and their susceptibility to the novel pathogen Spiroplasma eriocheiris in vitro. Aquaculture. 2012;356:371–380. doi: 10.1016/j.aquaculture.2012.04.042. [DOI] [Google Scholar]
- 9.Koiwai K., Alenton R.R.R., Shiomi R., Nozaki R., Kondo H., Hirono I. Two hemocyte sub-populations of kuruma shrimp Marsupenaeus japonicus. Mol. Immunol. 2017;85:1–8. doi: 10.1016/j.molimm.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 10.Lv S.J., Xu J.H., Zhao J., Yin N., Lu B.J., Li S., Chen Y.Y., Xu H.S. Classification and phagocytosis of circulating haemocytes in Chinese mitten crab (Eriocheir sinensis) and the effect of extrinsic stimulation on circulating haemocytes in vivo. Fish Shellfish Immunol. 2014;39(2):415–422. doi: 10.1016/j.fsi.2014.05.036. [DOI] [PubMed] [Google Scholar]
- 11.Koiwai K., Koyama T., Tsuda S., Toyoda A., Kikuchi K., Suzuki H., Kawano R. Single-cell RNA-seq analysis reveals penaeid shrimp hemocyte subpopulations and cell differentiation process. Elife. 2021;10 doi: 10.7554/eLife. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sricharoen S., Kim J.J., Tunkijjanukij S., Söderhäll I. Exocytosis and proteomic analysis of the vesicle content of granular hemocytes from a crayfish. Dev. Comp. Immunol. 2005;29(12):1017–1031. doi: 10.1016/j.dci.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 13.da Silva P.G.C., Benton J.L., Sandeman D.C., Beltz B.S. Adult neurogenesis in the crayfish brain: the hematopoietic anterior proliferation center has direct access to the brain and stem cell niche. Stem Cells Dev. 2013;22(7):1027–1041. doi: 10.1089/scd.2012.0583. [DOI] [PubMed] [Google Scholar]
- 14.van de Braak C.B.T., Botterblom M.H.A., Liu W., Taverne N., van der Knaap W.P.W., Rombout J.H.W.M. The role of the haematopoietic tissue in haemocyte production and maturation in the black tiger shrimp (Penaeus monodon) Fish Shellfish Immunol. 2002;12(3):253–272. doi: 10.1006/fsim.2001.0369. [DOI] [PubMed] [Google Scholar]
- 15.Martin G.G., Hose J.E., Choi M., Provost R., Omori G., Mckrell N., Lam G. Organization of hematopoietic-tissue in the intermolt lobster, Homarus-Americanus. J. Morphol. 1993;216(1):65–78. doi: 10.1002/jmor.1052160108. [DOI] [PubMed] [Google Scholar]
- 16.Li F., Zheng Z., Li H., Fu R., Xu L., Yang F. Crayfish hemocytes develop along the granular cell lineage. Sci. Rep. 2021;11(1):13099. doi: 10.1038/s41598-021-92473-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Söderhäll I., Kim Y.A., Jiravanichpaisal P., Lee S.Y., Söderhäll K. An ancient role for a prokineticin domain in invertebrate hematopoiesis. J. Immunol. 2005;174(10):6153–6160. doi: 10.4049/jimmunol.174.10.6153. [DOI] [PubMed] [Google Scholar]
- 18.Liu H.P., Söderhäll I. Histone H2A as a transfection agent in crayfish hematopoietic tissue cells. Dev. Comp. Immunol. 2007;31(4):340–346. doi: 10.1016/j.dci.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Shi H., Ruan L.W., Söderhäll I., Söderhäll K., Xu X. Transfection of crayfish hematopoietic tissue cells. Dev. Comp. Immunol. 2018;88:70–76. doi: 10.1016/j.dci.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Li F., Xu L.M., Hui X., Huang W.Z., Yang F. Directed differentiation of granular cells from crayfish hematopoietic tissue cells. Fish Shellfish Immunol. 2019;88:28–35. doi: 10.1016/j.fsi.2019.02.054. [DOI] [PubMed] [Google Scholar]
- 21.Liu H.P., Chen R.Y., Zhang Q.X., Peng H., Wang K.J. Differential gene expression profile from haematopoietic tissue stem cells of red claw crayfish, Cherax quadricarinatus, in response to WSSV infection. Dev. Comp. Immunol. 2011;35(7):716–724. doi: 10.1016/j.dci.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 22.Wu J., Li F., Huang J., Xu L., Yang F. Crayfish hematopoietic tissue cells but not hemocytes are permissive for white spot syndrome virus replication. Fish Shellfish Immunol. 2015;43(1):67–74. doi: 10.1016/j.fsi.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 23.Söderhäll I., Bangyeekhun E., Mayo S., Söderhäll K. Hemocyte production and maturation in an invertebrate animal; proliferation and gene expression in hematopoietic stem cells of Pacifastacus leniusculus. Dev. Comp. Immunol. 2003;27(8):661–672. doi: 10.1016/S0145-305x(03)00039-9. [DOI] [PubMed] [Google Scholar]
- 24.Söderhäll I., Junkunlo K. A comparative global proteomic analysis of the hematopoietic lineages in the crustacean Pacifastacus leniusculus. Dev. Comp. Immunol. 2019;92:170–178. doi: 10.1016/j.dci.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 25.Junkunlo K., Söderhäll K., Noonin C., Söderhäll I. PDGF/VEGF-related receptor affects transglutaminase activity to control cell migration during crustacean hematopoiesis. Stem Cells Dev. 2017;26(20):1449–1459. doi: 10.1089/scd.2017.0086. [DOI] [PubMed] [Google Scholar]
- 26.Wu C., Söderhäll I., Kim Y.A., Liu H., Söderhäll K. Hemocyte-lineage marker proteins in a crustacean, the freshwater crayfish, Pacifastacus leniusculus. Proteomics. 2008;8(20):4226–4235. doi: 10.1002/pmic.200800177. [DOI] [PubMed] [Google Scholar]
- 27.Zhu L., Chang Y., Xing J., Tang X., Sheng X., Zhan W. Comparative proteomic analysis between two haemocyte subpopulations in shrimp Fenneropenaeus chinensis. Fish Shellfish Immunol. 2018;72:325–333. doi: 10.1016/j.fsi.2017.09.074. [DOI] [PubMed] [Google Scholar]
- 28.Hillyer J.F. Insect immunology and hematopoiesis. Dev. Comp. Immunol. 2016;58:102–118. doi: 10.1016/j.dci.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waltzer L., Gobert V., Osman D., Haenlin M. Transcription factor interplay during Drosophila haematopoiesis. Int. J. Dev. Biol. 2010;54(6–7):1107–1115. doi: 10.1387/ijdb.093054lw. [DOI] [PubMed] [Google Scholar]
- 30.Crozatier M., Meister M. Drosophila haematopoiesis. Cell Microbiol. 2007;9(5):1117–1126. doi: 10.1111/j.1462-5822.2007.00930.x. [DOI] [PubMed] [Google Scholar]
- 31.Li Y., Jia Z., Yi Q., Song X., Liu Y., Jia Y., Wang L., Song L. A novel GATA-like zinc finger transcription factor involving in hematopoiesis of Eriocheir sinensis. Fish Shellfish Immunol. 2018;74:363–371. doi: 10.1016/j.fsi.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 32.Junkunlo K., Söderhäll K., Söderhäll I. A transcription factor glial cell missing (Gcm) in the freshwater crayfish Pacifastacus leniusculus. Dev. Comp. Immunol. 2020;113 doi: 10.1016/j.dci.2020.103782. [DOI] [PubMed] [Google Scholar]
- 33.Junkunlo K., Söderhäll K., Söderhäll I. Transglutaminase inhibition stimulates hematopoiesis and reduces aggressive behavior of crayfish, Pacifastacus leniusculus. J. Biol. Chem. 2019;294(2):708–715. doi: 10.1074/jbc.RA118.005489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Belkin A.M. Extracellular TG2: emerging functions and regulation. FEBS J. 2011;278(24):4704–4716. doi: 10.1111/j.1742-4658.2011.08346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin X., Söderhäll K., Söderhäll I. Transglutaminase activity in the hematopoietic tissue of a crustacean, Pacifastacus leniusculus, importance in hemocyte homeostasis. BMC Immunol. 2008;9:58. doi: 10.1186/1471-2172-9-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Junkunlo K., Söderhäll K., Söderhäll I. Clotting protein - An extracellular matrix (ECM) protein involved in crustacean hematopoiesis. Dev. Comp. Immunol. 2018;78:132–140. doi: 10.1016/j.dci.2017.09.017. [DOI] [PubMed] [Google Scholar]
- 37.Junkunlo K., Söderhäll K., Söderhäll I., Noonin C. Reactive oxygen species affect transglutaminase activity and regulate hematopoiesis in a crustacean. J. Biol. Chem. 2016;291(34):17593–17601. doi: 10.1074/jbc.M116.741348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noonin C., Lin X., Jiravanichpaisal P., Söderhäll K., Söderhäll I. Invertebrate hematopoiesis: an anterior proliferation center as a link between the hematopoietic tissue and the brain. Stem Cells Dev. 2012;21(17):3173–3186. doi: 10.1089/scd.2012.0077. [DOI] [PubMed] [Google Scholar]
- 39.Jia Z., Wang M., Wang X., Wang L., Qiu L., Song L. Transcriptome sequencing reveals the involvement of reactive oxygen species in the hematopoiesis from Chinese mitten crab Eriocheir sinensis. Dev. Comp. Immunol. 2018;82:94–103. doi: 10.1016/j.dci.2017.12.030. [DOI] [PubMed] [Google Scholar]
- 40.Pascual-Anaya J., Albuixech-Crespo B., Somorjai I.M., Carmona R., Oisi Y., Alvarez S., Kuratani S., Munoz-Chapuli R., Garcia-Fernandez J. The evolutionary origins of chordate hematopoiesis and vertebrate endothelia. Dev. Biol. 2013;375(2):182–192. doi: 10.1016/j.ydbio.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 41.Parsons B., Foley E. The Drosophila platelet-derived growth factor and vascular endothelial growth factor-receptor related (Pvr) protein ligands Pvf2 and Pvf3 control hemocyte viability and invasive migration. J. Biol. Chem. 2013;288(28):20173–20183. doi: 10.1074/jbc.M113.483818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amparyup P., Charoensapsri W., Tassanakajon A. Prophenoloxidase system and its role in shrimp immune responses against major pathogens. Fish Shellfish Immunol. 2013;34(4):990–1001. doi: 10.1016/j.fsi.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 43.Sirikharin R., Söderhäll K., Söderhäll I. The N-terminal peptide generated after activation of prophenoloxidase affects crayfish hematopoiesis. Dev. Comp. Immunol. 2020;108 doi: 10.1016/j.dci.2020.103687. [DOI] [PubMed] [Google Scholar]
- 44.Amparyup P., Promrungreang K., Charoensapsri W., Sutthangkul J., Tassanakajon A. A serine proteinase PmClipSP2 contributes to prophenoloxidase system and plays a protective role in shrimp defense by scavenging lipopolysaccharide. Dev .Comp. Immunol. 2013;41(4):597–607. doi: 10.1016/j.dci.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 45.Khorattanakulchai N., Amparyup P., Tassanakajon A. Binding of PmClipSP2 to microbial cell wall components and activation of the proPO-activating system in the black tiger shrimp Penaeus monodon. Dev. Comp. Immunol. 2017;77:38–45. doi: 10.1016/j.dci.2017.07.016. [DOI] [PubMed] [Google Scholar]
- 46.Lin X., Novotny M., Söderhäll K., Söderhäll I. Ancient cytokines, the role of astakines as hematopoietic growth factors. J. Biol. Chem. 2010;285(37):28577–28586. doi: 10.1074/jbc.M110.138560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jia Z., Wang M., Wang X., Xu J., Wang L., Zhang H., Song L., Prokineticin A. (PK)-like cytokine from Chinese mitten crab Eriocheir sinensis promotes the production of hemocytes via reactive oxygen species. Fish Shellfish Immunol. 2018;77:419–428. doi: 10.1016/j.fsi.2018.03.059. [DOI] [PubMed] [Google Scholar]
- 48.Liang G.F., Liang Y., Xue Q., Lu J.F., Cheng J.J., Huang J. Astakine LvAST binds to the beta subunit of F1-ATP synthase and likely plays a role in white shrimp Litopeneaus vannamei defense against white spot syndrome virus. Fish Shellfish Immunol. 2015;43(1):75–81. doi: 10.1016/j.fsi.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 49.Sirikharin R., Noonin C., Junkunlo K., Söderhäll K., Söderhäll I. Astakine1 forms protein complex in plasma. Fish Shellfish Immunol. 2019;94:66–71. doi: 10.1016/j.fsi.2019.08.063. [DOI] [PubMed] [Google Scholar]
- 50.Sirikharin R., Junkunlo K., Söderhäll K., Söderhäll I. Role of astakine1 in regulating transglutaminase activity. Dev. Comp. Immunol. 2017;76:77–82. doi: 10.1016/j.dci.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 51.Lin X., Kim Y.A., Lee B.L., Söderhäll K., Söderhäll I. Identification and properties of a receptor for the invertebrate cytokine astakine, involved in hematopoiesis. Exp. Cell Res. 2009;315(7):1171–1180. doi: 10.1016/j.yexcr.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 52.Saelee N., Noonin C., Nupan B., Junkunlo K., Phongdara A., Lin X., Söderhäll K., Söderhäll I. beta-thymosins and hemocyte homeostasis in a crustacean. PLoS ONE. 2013;8(4):e60974. doi: 10.1371/journal.pone.0060974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chang Y.T., Lin C.Y., Tsai C.Y., Siva V.S., Chu C.Y., Tsai H.J., Song Y.L. Correction: the new face of the old molecules: crustin Pm4 and transglutaminase type I serving as RNPs down-regulate astakine-mediated hematopoiesis. PLoS ONE. 2017;12(7) doi: 10.1371/journal.pone.0182405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zanjani N.T., Miranda-Saksena M., Cunningham A.L., Dehghani F. Antimicrobial peptides of marine crustaceans: the potential and challenges of developing therapeutic agents. Curr. Med. Chem. 2018;25(19):2245–2259. doi: 10.2174/0929867324666171106155936. [DOI] [PubMed] [Google Scholar]
- 55.Li C., Wang S., He J. The Two NF-kappaB pathways regulating bacterial and WSSV infection of shrimp. Front. Immunol. 2019;10:1785. doi: 10.3389/fimmu.2019.01785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fouquet G., Coman T., Hermine O., Cote F. Serotonin, hematopoiesis and stem cells. Pharmacol. Res. 2019;140:67–74. doi: 10.1016/j.phrs.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 57.Noonin C. Involvement of Serotonin in crayfish hematopoiesis. Dev. Comp. Immunol. 2018;86:189–195. doi: 10.1016/j.dci.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 58.Lin X., Söderhäll K., Söderhäll I. Invertebrate hematopoiesis: an astakine-dependent novel hematopoietic factor. J. Immunol. 2011;186(4):2073–2079. doi: 10.4049/jimmunol.1001229. [DOI] [PubMed] [Google Scholar]
- 59.Prapavorarat A., Vatanavicharn T., Söderhäll K., Tassanakajon A. A novel viral responsive protein is involved in hemocyte homeostasis in the black tiger shrimp, Penaeus monodon. J. Biol. Chem. 2010;285(28):21467–21477. doi: 10.1074/jbc.M110.130526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leu J.H., Chen Y.C., Chen L.L., Chen K.Y., Huang H.T., Ho J.M., Lo C.F. Litopenaeus vannamei inhibitor of apoptosis protein 1 (LvIAP1) is essential for shrimp survival. Dev. Comp. Immunol. 2012;38(1):78–87. doi: 10.1016/j.dci.2012.04.006. [DOI] [PubMed] [Google Scholar]