Highlights
-
•
Mugil liza is a species with potential production in aquaculture.
-
•
It is a spice that suffers from various diseases.
-
•
Knowing your immune system is important for this activity.
-
•
Electron microscopy unlike optical microscopy allows to study in detail the different types of cells that compose it.
-
•
Electron microscopy allows evaluating thymus histiogenesis .
The fine structural characteristics of the normal thymus have been described in many animal species [1], [2], [3]. Additionally, the thymus in humans has been described in detail both in normal and pathological conditions [4], [5], [6].
Stannius identified the thymus in teleost fish in 1850. Since then, this gland has been described in many teleosts belonging to approximately 28 different families [7]. Hamxar examined the greatest part of this literature in 1909, and since then, the thymus has been studied as a fundamental organ of the fish immune system [8].
Several studies of the histology and physiology of the thymus of fish have been carried out; however, studies of the ultrastructure of the thymus of these animals are not frequent [9], [10], [11].
Mullets, especially the Mugil liza in the south of Brazil, have several attributes that make them suitable for aquaculture, such as hardiness and easy feeding, because they readily accept rations and exhibit a wide tolerance to salinity and temperature variations [12].
We carried out a systematic study of the ultrastructure of the thymus in Mugil liza, a species with potential value in aquaculture.
Ten mullets (M. liza) were caught at Cassino Beach (Rio Grande – RS, Brazil; 32°170 S-52°100 W) and transferred to the Laboratory of Immunology and Pathology of Aquatic Organisms of the Universidade Federal do Rio Grande – FURG. The mean weight of the fish was 0.46 g ± 0.01 g, and the approximate age was 10 days. The fish were euthanized with a bath in M-222 at 100 ppm (Western Chemical, USA) and were subsequently dissected, and the pharyngeal region of the branchial chamber was removed; in smaller fish, the cephalic region was cut and used in its entirety. Fish tissue was fixed in 10% buffered formalin, and after fixation, the tissues were dehydrated through ascending concentrations of ethanol, diaphanized in xylol and embedded in paraffin. Then, the samples were cut in a microtome (Leica RM2245) with a thickness of 30 mM. The tissue was stained with haematoxylin and eosin.
Small fragments of tissue were cut into 1 mm blocks and immediately fixed in phosphate buffered glutaraldehyde (pH 6.9 at 4 °C), washed in Millonig's solution, and postfixed in 1% osmium tetroxide; the tissue blocks were then dehydrated in a graded series of ethanol-acetone, immersed in propylene oxide, and embedded in Durcupan ACNI (Fluka Chemie A.G., Switzerland). Thin sections were cut with an LKB ultramicrotome and double stained with uranyl acetate and lead citrate before examination with a Jeol JEM-8T electron microscope (Jeol, Tokyo, Japan).
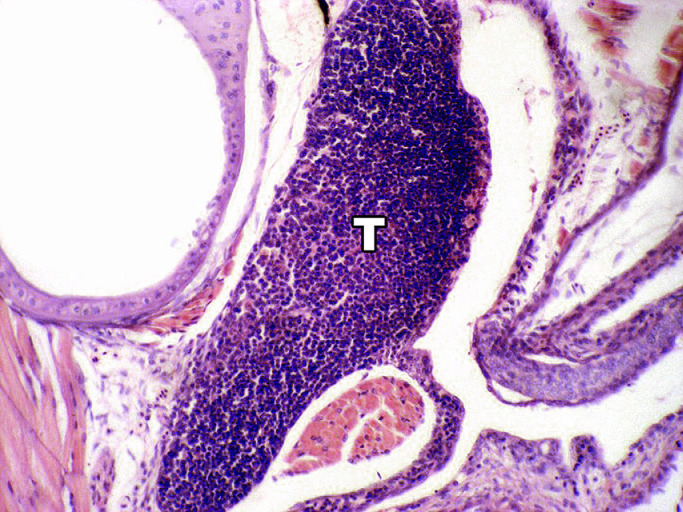
Optical microscopy showed that the thymus is elongated, extending over the branchial chamber epithelium, and it is a bilateral organ. We did not observe differentiation between the cortex and medulla in any of the fish. The thymic tissue was composed of lymphocytes, epithelial cells and other mesenchymal cells (Fig. 1).
Fig. 1.
Mugil liza thymus: Lymphoid tissue is observed without identification of the cortex and marrow. The mass of thymic cells detaches from the epithelium (T). H-E, X 40.
With electron microscopy of the thymus of M. liza, we observed three basic cell types in the thymus, namely, cells of the lymphocytic series (small, medium, and large lymphocytes), epithelial cells (characterized by complex cytoplasmic inclusions, desmosomes, tonofibrils and surface specializations such as cilia and microvilli), and macrophages (phagocytes). On the basis of this substructural organization, the relationship of this cell with other types of thymus cells could not be established with certainty. These cells were similar to "blast cells" and may be related to the regeneration of lymphoid elements. Therefore, the substructure of these cells was very similar to that of thymic lymphocytes, except for the presence of a greater number of cytoplasmic ribosomes, rough endoplasmic reticulum, large, often multiple nucleoli, and a cell centre containing a Golgi complex
Among the lymphoid cells, cells that could be identified as epithelial in nature were observed due to the presence of desmosomes and collections of tonofibrils. In several sections, a clear identification of epithelial cells was not possible because the substructural features of the normal thymic epithelium (complex cytoplasmic inclusions, tonofibrillar material, desmosomes) were not present. However, it should be noted that the two types of cells, those definitely identifiable as epithelial and those that cannot be identified as such with certainty, had an identical nuclear and cytoplasmic organization. In addition to the cells described above, some less differentiated cells were present that contained a greater amount of peripherally marginalized nuclear chromatin and clustered in an identical pattern to that of the large thymus lymphocytes. The cytoplasm of these cells contained many ribosomes both in free form and associated with the membrane, greater than that found in epithelial cells. We also observed numerous macrophages containing degenerating lymphocytes and cellular debris. Some small lymphocyte-like cells were observed around the blood vessels. These cells were characterized in addition to their small size because they have a condensed nuclear mass that often appears rectangular or square and a relatively indistinct cytoplasm.
Irregular and nodular collections of epithelial cells were observed in some areas, within which macrophages and occasionally some lymphocytes were found. Epithelial cells were more easily identified because cytoplasmic inclusions, tonofibrils, and desmosomes were more frequently observed (Fig. 2,Fig. 3, Fig. 4). The observations presented here confirm the lymphoepithelial nature of the thymus. With a light microscope, it is impossible to specifically define whether certain cells of the thymus are lymphoid or epithelial in nature, as can be done with an electron microscope. Therefore, we used the term “epithelial” to refer to these large cells (20–30 µm in diameter) with a large ovoid nucleus, finely dispersed chromatin, and abundant pale staining cytoplasm. It seems, therefore, that there are functionally two cell populations in the thymus, one (containing epithelial elements) that may direct or regulate the regeneration of lymphoid elements and, consequently, the maintenance of the lymphocyte mass and a second population composed of lymphocytes.
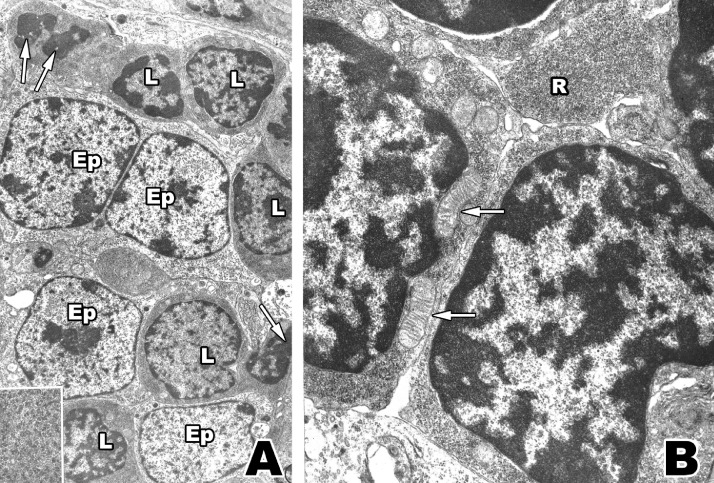
Fig. 2.
A: Electron micrograph where lymphocytes (L) are observed in various stages of differentiation within an epithelial network (Ep). Lymphocyte nuclei sectioned in a tangential plane often appear pyknotic and square or rectangular in shape (arrows). A high concentration of ribosomes is observed, mainly as fairly large polysomal aggregates (inset, lower left), within all lymphocytes. X 10,000; inset X 68,000. B: Portion of four well-differentiated lymphocytes with a densely packed nuclear chromatin (C) pattern, mitochondria (arrows) and a large number of ribosomes (R). X 21,500.
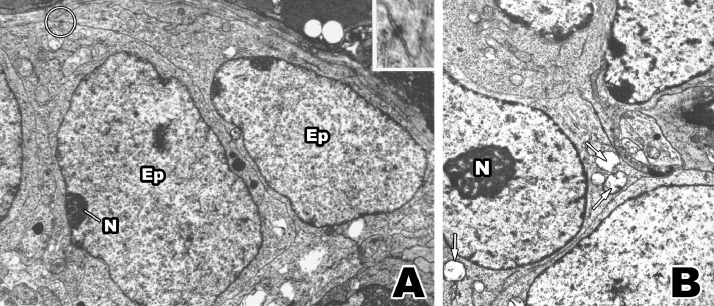
Fig. 3.
A: Epithelial cells (Ep) showing finely dispersed nuclear chromatin, small nucleoli (N) and the absence of vacuolar and granular cytoplasmic inclusions. A single desmosome (circle and box at the top tight) identifies these cells as epithelial. X 8,500. B: Immature epithelial cells which contains small vacuolar inclusions (arrowheads) and large nucleolus (N). X 11,200.
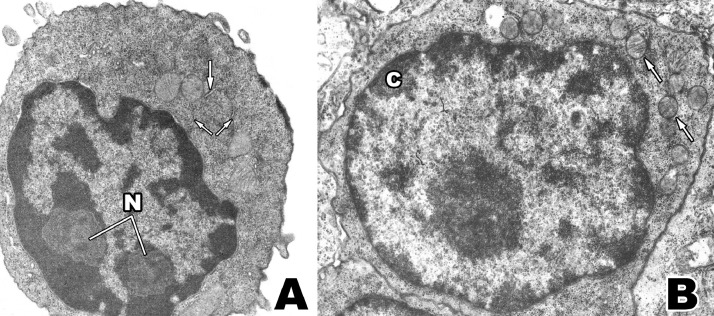
Fig. 4.
A: Small "lymphocyte-like" cell, containing coarsely clumped nuclear chromatin, prominent nucleoli (N), and a high concentration of cytoplasmic ribosomes, some of which are arranged along short segments of endoplasmic reticulum (arrows). X 20,200. B: A large thymus lymphocyte with nuclear chromatin pattern and cytoplasmic organization identical to that of. X 15,000.
These differences between epithelial and lymphoid cells are also evident in the human thymus and are reflected in the divergent appearance of thymomas [13]. Epithelial cells within Hassall's corpuscles may have a marked hairy surface configuration [14]. A recent paper demonstrated the similarities and differences of the human thymus and the teleost fish thymus; the medulla and cortex were not observed, but as in the thymus of Humans and other mammals, there are populations of lymphocytes with CD3 and CD4 receptors. [15].
In several animal species, gland-like spaces have been described in which villous epithelial cells connected by desmosomes are arranged around the lumen of the gland [16].
Well-differentiated small and medium lymphocytes were observed with ultracytology similar to that of normal intact large lymphocytes. The size of the different lymphocytes does not appear to have functional importance. We do not have information on the role of these lymphocytes; it seems that large lymphocytes are related to lymphoid regeneration in cases of necrosis due to some type of injury [17]. We found small cells similar to lymphocytes, and the ultracytology was similar to that of lymphocytes; we do not know their functional nature. These cells are similar to those observed in lymphohaematopoietic tissues. There is some evidence to suggest that these cells likely originate from the central lymphohaematopoietic organs, the pronephros (anterior kidney), and possibly the spleen [18,19].
The understanding that the epithelial-reticular and lymphocyte lines are quite histogenetically separated, but functionally related, avoids much confusion in interpreting the origin of the epithelium and lymphocytes, and a low level can be found ultrastructurally in the normal thymus from other cells. These include mast cells, myoid cells, and macrophages [20]. Macrophages are easily distinguished from epithelial cells because they lack desmosomes and tonofilaments but contain many lysosomes and cytoplasmic vesicles. Some authors have referred to thymic macrophages as mesenchymal reticular cells to emphasize their distinction from epithelial cells.
The thymus differs from other important lymphoid organs in that the reticular framework is epithelial-reticular in nature [21]. This structure may be difficult to see in light microscope slides but is quite evident on electron microscope examination. The basic architecture of the thymus consists of epithelial cells with very elongated processes, among which is the lymphoid component. Therefore, the epithelial cell of the thymus has a function similar to that of the cells of the mesenchymal reticulum of the mammalian lymph node. The term epithelial-reticular cell is acceptable because it denotes the tendency of these cells to have long processes and indicates their epithelial nature.
Declaration of Competing Interest
The authors declare that there are no conflicts of interest.
Acknowledgements
This study was supported by research funds from MCT/CNPq - Project #301245/2016-09 MCT/CNPq/CT- Agronegocio/MPA Public Notice 036/2009 Project #308013/2009-3, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Ministério da Pesca e Aquicultura (MPA).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.fsirep.2020.100005.
Appendix. Supplementary materials
References
- 1.Gorgollón P., Ottone-Anaya M. Fine structure of canine thymus. Acta Anat (Basel) 1978;100(1):136–152. doi: 10.1159/000144893. [DOI] [PubMed] [Google Scholar]
- 2.Bigaj J., Płytycz B. Cytoarchitecture of the thymus gland of the adult frog (Rana temporaria) Folia Histochem. Cytobiol. 1984;22(1):63–69. [PubMed] [Google Scholar]
- 3.Chan A.S. Ultrastructure of epithelial cells of the chick embryo thymus. Acta Anat (Basel) 1994;150(2):96–103. doi: 10.1159/000147608. [DOI] [PubMed] [Google Scholar]
- 4.Mikušová R., Mešťanová V., Polák Š., Varga I. What do we know about the structure of human thymic Hassall's corpuscles? A histochemical, immunohistochemical, and electron microscopic study. Ann. Anat. 2017;211:140–148. doi: 10.1016/j.aanat.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Avilova O., Shyian D., Marakushin D., Erokhina V., Gargin V. Ultrastructural changes in the organs of the immune system under the influence of xenobiotics. Georgian Med. News. 2018;279:132–137. [PubMed] [Google Scholar]
- 6.Erokhina V., Avilova O. Ultramicroscopic changes of rats parathyroid glands and thymus after single administration of cyclophosphamide at the different periods of observation. Wiad Lek. 2019;72(3):362–367. [PubMed] [Google Scholar]
- 7.Stannius H. 1850. Ueber eine der Thymus entsprechende Drüse bci Knochcn- fischen. Muller's Arch; pp. 501–507. [Google Scholar]
- 8.Hamxar J.A. Zur Kenntnis der Teleostierthymus. Arch. F. Mikr. Anat. 1909;73:1–68. [Google Scholar]
- 9.Zapata A. Lymphoid organs of teleost fish. I. Ultrastructure of the thymus of Rutilus rutilus. Dev Comp Immunol. 1981;5(3):427–436. doi: 10.1016/s0145-305x(81)80055-9. [DOI] [PubMed] [Google Scholar]
- 10.Kissa K., Murayama E., Zapata A., Cortés A., Perret E., Machu C., Herbomel P. Live imaging of emerging hematopoietic stem cells and early thymus colonization. Blood. 2008;111(3):1147–1156. doi: 10.1182/blood-2007-07-099499. [DOI] [PubMed] [Google Scholar]
- 11.Cao J., Chen Q., Lu M., Hu X., Wang M. Histology and ultrastructure of the thymus during development in tilapia, Oreochromis niloticus. J Anat. 2017;230(5):720–733. doi: 10.1111/joa.12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miranda-Filho K.C., Tesser M.B., Sampaio L.A., Godinho H.M., B. Baldisserotto e L.C. Gomes (Org.) Espécies nativas para a piscicultura no Brasil. UFSM; Santa Maria: 2010. Tainha; pp. 541–552. [Google Scholar]
- 13.Romano L.A., Marozzi A.V. Epithelio-reticular cell thymoma in carp Cyprinus Carpio L: a case with ultrastructural study. J. Fish Dis. (Print) 2004;27:369–373. doi: 10.1111/j.1365-2761.2004.00552.x. [DOI] [PubMed] [Google Scholar]
- 14.B. Bodey, H.E. Kaiser, Development of Hassall's bodies of the thymus in humans and other vertebrates (especially mammals) under physiological and pathological conditions: immunocytochemical, electronmicroscopic and in vitro observations, In Vivo 11(1) (1997) 61-85. [PubMed]
- 15.Klosterhoff M.C., Medeiros A.F.F., Guerreiro A., Pedrosa V.F., Romano L.A. Comparative histology of the human and teleost fish thymus. Braz. J. of Develop. 2020;6(9):68460–68481. doi: 10.34117/bjdv6n9-331. [DOI] [Google Scholar]
- 16.Liu Y., Zhang S., Jiang G., Yang D., Lian J., Yang Y. The development of the lymphoid organs of flounder, Paralichthys olivaceus, from hatching to 13 months. Fish Shellfish Immunol. 2004;16:621–632. doi: 10.1016/j.fsi.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Kendall M.D., Blackett N.M. Ultrastructural studies on the thymus gland after the administration of phenylhydrazine to bank voles (Clethrionomys glareolus) Cell Tissue Res. 1984;232:201–219. doi: 10.1007/BF00222384. [DOI] [PubMed] [Google Scholar]
- 18.Kendall M.D. Hemopoiesis in the thymus. Dev. Immunol. 1995;4:157–168. doi: 10.1155/1995/69454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klosterhoff M.C., Pereira Junior J., Rodrigues R.V., Gusmão E.P., Sampaio L.A., Tesser M.B., Romano L.A. Ontogenic development of kidney, thymus and spleen and phenotypic expression of CD3 and CD4 receptors on the lymphocytes of cobia (Rachycentron canadum) Anais da Academia Brasileira de Ciências (Online) 2015;87:2111–2121. doi: 10.1590/0001-3765201520140623. [DOI] [PubMed] [Google Scholar]
- 20.Crivellato E., Nico B., Battistig M., Beltrami C.A., Ribatti D. The thymus is a site of mast cell development in chicken embryos. Anat Embryol (Berl) 2005;209(3):243–249. doi: 10.1007/s00429-004-0439-5. [DOI] [PubMed] [Google Scholar]
- 21.Haynes B.F. The human thymic microenvironment. Adv. Immunol. 1984;36:87–142. doi: 10.1016/s0065-2776(08)60900-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.