Abstract
Introduction
People treated with haemodialysis are at increased risk for all-cause and cardiovascular mortality. Plasma magnesium concentration has been inversely associated with these risks. Therefore, plasma magnesium may be a new modifiable risk factor and an increase of dialysate magnesium concentration may be an easy, safe and effective way to increase plasma magnesium concentrations. Detailed information on modulating dialysate magnesium concentrations is limited in literature. Primary objective of this study is to determine the safety and feasibility to increase plasma magnesium concentrations in people treated with haemodialysis by means of sequentially increasing concentration of magnesium in the dialysate.
Methods and analysis
In this randomised double-blinded standard of care controlled trial, 53 persons treated with haemodialysis will be randomly allocated 2:1 to either a stepwise individually titrated increase of dialysate magnesium concentration from 0.50 to 0.75 to 1.00 mmol/L during 8 weeks, or a standard dialysate magnesium concentration of 0.50 mmol/L. Other study measurements include dietary records, questionnaires, ECG, Holter registration and pulse wave velocity. The primary endpoint is predialysis plasma magnesium after the long interdialytic interval at the end of week 8. In addition, the predictive effect of dialysate magnesium concentration and other baseline parameters and dialysis characteristics on plasma magnesium concentration will be explored using linear mixed models. Safety endpoint is defined by the occurrence of hypermagnesemia above 1.25 mmol/L, or bradycardia or prolonged QTc interval detected on the ECG.
Ethics and dissemination
The study is conducted in accordance with the declaration of Helsinki as revised in 2013 and was approved by the Ethical Committee of the VU University Medical Centre. The results of the study will be disseminated by publication in peer-reviewed scientific journals and presentation at national or international conferences in the field of interest.
Trial registration number
NTR6568/NL6393.
Keywords: dialysis, nephrology, nephrology, end stage renal failure
Strengths and limitations of this study.
Blinding and randomisation prevents bias occurring from differences in life-style between groups, and enables objective collection and processing of data.
Dialysate concentrations will be individually titrated based on individual plasma magnesium concentrations by an independent physician.
Both the effects of dialysate magnesium concentrations on plasma magnesium concentrations will be determined and also the factors that are predictive for these effects.
Major limitation is that the study will not provide information on clinical outcomes including cardiovascular events and mortality, due to limited study duration.
Introduction
People with chronic kidney disease (CKD) including those treated with dialysis are at increased risk for all-cause and cardiovascular mortality.1 This increased risk persists after adjustment for traditional cardiovascular risk factors, indicating that other kidney specific factors contribute to the cardiovascular risk.1 Recently, lower magnesium has been identified as a potential novel risk factor.2 Magnesium is involved in many physiological functions, including energy metabolism and regulation of transmembrane transport of ions and consequently, it is essential for muscle function, cardiac rhythm and vascular tone.3 In a meta-analysis of studies in people with CKD including those on dialysis, we showed that plasma magnesium concentration is inversely associated with all-cause and cardiovascular mortality, and that this not only applies for normal compared with low magnesium, but also revealed protective effects of magnesium above as compared with within the reference range (generally 0.70–1.05 mmol/L).4 Magnesium concentration is also inversely associated with the risk for sudden death and with arrhythmia in people treated with haemodialysis.5–7 In the general population, serum magnesium has been inversely associated with frequent or complex premature complexes, which predict prognosis including all-cause mortality in the general population.8 9 Moreover, serum magnesium was inversely associated with pulse wave velocity (PWV) in people treated with maintenance haemodialysis,10 and in a short randomised cross-over study in people treated with haemodialysis, higher dialysate magnesium concentration compared with standard dialysate magnesium concentration decreased PWV.11 PWV is a marker of vascular stiffness and a strong predictor of cardiovascular outcome in people with CKD stage 4-5D.12 In most studies that included multiple categories of plasma magnesium concentration in people with CKD including those treated with dialysis, there was a monotonic inverse association between magnesium and all-cause mortality.4 We previously showed that a commonly used dialysate magnesium concentration of 0.50 mmol/L in haemodialysis, usually induces a decline of magnesium towards magnesium concentrations in the lower range of normal.13 Therefore, an increase of dialysate magnesium concentration, may be an easy, safe and effective way to increase plasma magnesium concentrations in people treated with haemodialysis, without the need of oral supplementation. The results of one observational study suggest that there may be an optimal concentration of plasma magnesium in-between 1.15 and 1.27 mmol/L, with an increasing risk for mortality if magnesium values exceed this range.14 Although these findings were not confirmed by other studies that included magnesium values in this high range, this requires to take into account safety when increasing plasma magnesium concentrations.6 15 16 In a previous 4 weeks trial by Bressendorf et al, increasing dialysate magnesium concentration from 0.50 to 1.00 mmol/L resulted in a 0.4 mmol/L increase of plasma magnesium concentration (95% CI 0.3 to 0.5).17 Here, we describe a randomised standard-of-care controlled trial of stepwise increment of dialysate magnesium concentration in people treated with haemodialysis. Primary objective is to determine the feasibility to increase plasma magnesium concentrations in individuals treated with haemodialysis by means of sequentially increasing concentration of magnesium in the dialysate. Secondary objectives are to determine safety of using higher dialysate magnesium concentrations, the effect of dialysate magnesium on plasma magnesium concentration and to define which parameters are predictive for the increment of plasma magnesium concentration by increasing dialysate magnesium. We will also explore the effects of using higher dialysate magnesium on cardiac rhythm and PWV.
Methods
Trial design
In this randomised, double-blind, standard of care (SOC) controlled multicentre trial, individuals treated with haemodialysis will be randomly allocated to either a stepwise increase of dialysate magnesium concentration from 0.50 to 1.00 mmol/L, or continue on a standard dialysate magnesium concentration of 0.50 mmol/L. The protocol was written in accordance with the Standard Protocol Items: Recommendation for Interventional Trials (SPIRIT) and originally prospectively registered at www.trialregister.nl, which is now included in the International Clinical Trial Registry Platform (ICTRP) and can be accessed via https://trialsearch.who.int.18 The first participant was randomised in April 2018 and ending of the study is expected at the end of December 2022.
Characteristics of participants and recruitment
Adult persons treated with haemodialysis on a 3-times weekly dialysis schedule will be enrolled in the study. The inclusion and exclusion criteria are listed in box 1. Participants will be recruited from multiple centres in the Netherlands, including Amsterdam University Medical Centre location VU Medical Centre, Amsterdam; Diapriva dialysis centre, Amsterdam; Niercentrum aan de Amstel, Amstelveen and Spaarne Gasthuis, Hoofddorp. Participants need to provide written informed consent prior to enrollment.
Box 1. Inclusion and exclusion criteria.
Inclusion criteria
Age ≥18 years.
Haemodialysis with regular three times weekly dialysis schedule.
Haemodialysis since at least 3 months.
Standard dialysate magnesium concentration 0.50 mmol/L.
Providing informed consent.
Predialysis plasma magnesium concentration ≤1.00 mmol/L after the long intradialytic interval.
Exclusion criteria
Intravenous magnesium supplementation (including total parenteral nutrition) in the last 2 weeks.
Expected cessation of dialysis treatment within 3 months after inclusion or expected permanent or temporary dialysis centre switch to a centre not participating in the trial within 3 months after inclusion.
Prolongation of QTc interval: male >450 ms or female >460 ms on baseline ECG.
Bradycardia: heart rate below 60 beats per minute on baseline ECG.
Chronic arrythmia or cardiac conduction disorder other than atrial fibrillation or ventricular extrasystole that poses the patient at risk at the discretion of the treating physician.
Change of proton pump inhibitor prescription in the last 2 weeks.
In order to be eligible to participate in this study, a subject must meet all of the inclusion criteria. A potential subject who meets any of the exclusion criteria will be excluded from participation in this study.
Intervention
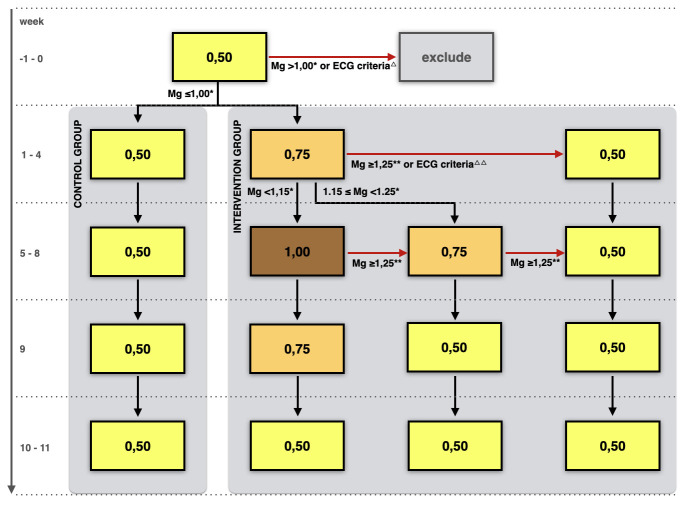
In the intervention group, dialysate magnesium is increased stepwise, from 0.50 mmol/L at baseline to 0.75 mmol/L during weeks 1–4, and to 1.00 mmol/L during weeks 5–8. The participant proceeds to the next increment step of dialysate magnesium concentration after week 4 only if predialysis plasma magnesium after the long interdialytic interval is below 1.15 mmol/L in week 4. Otherwise, the dialysate magnesium concentration of 0.75 mmol/L is continued in weeks 5–8. After week 8, dialysate magnesium will be gradually reduced with 0.25 mmol/L in week 9 and thereafter return to the standard dialysate magnesium concentration of 0.50 mmol/L in week 10.
Participants in the control group are treated with a standard dialysate magnesium concentration of 0.50 mmol/L (see figure 1).
Figure 1.
Flow chart of the study intervention. Numbers within frames represent dialysate magnesium concentrations in mmol/L. All other magnesium concentrations represent plasma magnesium concentrations, also in mmol/L. Mg, magnesium; Δ, ECG criteria at baseline: bradycardia defined as heartrate <60 bpm or prolonged QTc interval >450 ms in male or >460 ms in female; ΔΔ, ECG criteria in week 4: bradycardia with heart rate <50 bpm or prolonged QTc interval >450 ms in male or >460 ms in female; *predialysis plasma Mg concentration after the long interdialytic interval; **plasma Mg concentration at any time point (predialysis and postdialysis measurements included).
The dialysate contains a potassium concentration of 2 or 3 mmol/L, as determined in routine care by the treating physician based on individual needs. For the respective magnesium concentrations, six dialysis concentrates are used in weeks 1–9 (Haemodialysis A-concentrate, D761, D987, D907, D283, D961 and D908, MTN Neubrandenburg GmbH, Neubrandenburg, Germany). In the mineral composition of these concentrates, besides potassium based on individual needs, only the amount of magnesium chloride is different. Calcium concentration in these dialysates is 1.25 mmol/L and the acidifier is acetate.
Study procedures and participant time line
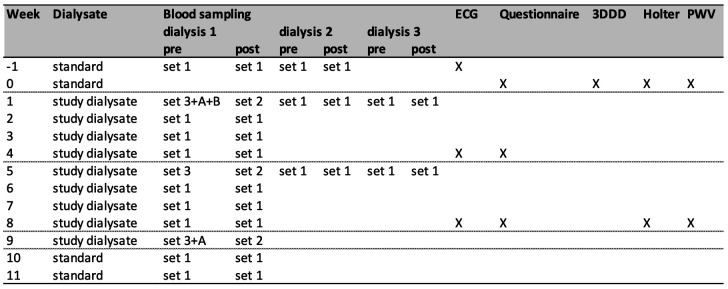
The study procedures and participant time line are shown in figure 2. After informed consent is provided by participants that meet inclusion and exclusion criteria, blood sampling and ECG are performed at baseline. Eligible persons meeting the criteria for plasma magnesium and ECG, as provided in box 1, are allocated to the either SOC or incremental magnesium dialysate. During the trial, blood sampling will be performed before and after the dialysis sessions following the long interdialytic interval weekly, and every dialysis session in week 1 and 5 to measure plasma magnesium concentration. In addition, in weeks 1, 5 and 9, blood is collected for measurements of potassium, bicarbonate, calcium, albumin, phosphate, parathyroid hormone, haemoglobin and CRP. For laboratory measurements, standard methods of the local laboratory are used. Participants record dietary intake for 3 days at baseline. From this record, dietary magnesium intake is extracted using the Dutch Food Composition Database (NEVO) by using the calculator on the website of the Dutch Nutrition Center.19 20 A questionnaire regarding the presence of subjective symptoms that can be associated with hypermagnesemia is completed at baseline, weeks 4 and 8. This is a 7-point yes or no questionnaire to ask if a participant experienced the following symptoms in the last week: nausea, vomiting, dizziness, drowsiness, reduced muscle strength, itching and leg cramps and at baseline, also a question about chronic diarrhoea and over-the-counter use of magnesium supplements is included. An ECG is repeated before dialysis in weeks 4 and 8 to determine heart rhythm, frequency and QTc interval. In addition, at baseline and in week 8, participants undergo continuous heart rhythm monitoring and pulse wave velocity (PWV) measurements. Heart rhythm monitoring is performed using a Holter recorder (Fysiologic, Amsterdam, The Netherlands) for 48 hours including one dialysis sessions and one interdialytic period. Carotid-femoral PWV is measured using the Sphygmocor tonometer (Atcor Medical Pty, Software V.9.0, Sydney, Australia) preceding the midweek dialysis sessions. Participants are requested to avoid coffee, tea and smoking for 4 hours, and alcohol for 12 hours preceding the measurements, as is recommended by the manufacturer of the device. The patient is placed in supine position, in a quiet environment at room temperature. After attachment of ECG electrodes and bedrest for at least 5 min, blood pressure is measured with an automated Omron device at least three times with a few minutes in-between, and until no substantial change occurs. Then, the last blood pressure measurement is recorded. The carotid to femoral artery distance is measured directly and multiplied by 0.8 to estimate the difference between cardiac-carotid and cardiac-femoral distance as recommended by expert consensus.21 The pulse wave is recorded using the tonometer at the carotid and femoral site and then PWV is calculated automatically by the Sphygmocor software from entered blood pressure, distance and ECG and tonometer recordings. Measurements are performed at least twice or until two measurements are of appropriate quality. A measurement is considered of sufficient quality based on the criteria set by the manufacturer: adequate shape of detected signal of ECG and pulse wave, difference of heart rate ≤5 bpm between carotid and femoral measurement, ECG R-tops and pulse wave beginning are correctly identified by the software, and SD of the ECG to carotid and of ECG to femoral time are both <6%. PWV measurement is not performed in participants with irregular heart rhythm, pacemaker rhythm, atrial fibrillation or flutter, heart frequency below 40 or above 160 bpm, second or third degree atrioventricular block, severe aortic valve stenosis or instable carotid plaque, as contraindicated by the manufacturer. Furthermore, persons’ characteristics are recorded at baseline and characteristics of the dialysis are recorded at baseline and weekly for the time of dialysis after the long interdialytic interval and for every dialysis during the first and fifth weeks of intervention. Recorded dialysis characteristics include modality (haemodialysis or haemodiafiltration), vascular access (catheter, fistula or graft), estimation of dialysis efficiency (Kt/Vurea per session according to Daugirdas’ formula), treatment time per session, blood flow, dialysate flow and ultrafiltration volume. Medication use and dosage is recorded at baseline and at week 8. During the trial, all participants receive three times weekly haemodialysis sessions according to their regular schedule. Changes of dialysis schedule during the study will be avoided as much as possible if there is no medical indication to change the scheme. Also, changes in prescription of proton pump inhibitors and magnesium-containing supplements, laxatives and phosphate binders will be avoided if clinically allowed.
Figure 2.
Study procedures. Dialysis 1, dialysis 2 and dialysis 3, first, second and third dialysis sessions after the long interdialytic interval of the week respectively; pre, predialysis; post, postdialysis; blood sampling set 1, lithium heparin gel tube for plasma magnesium measurement; set 2, lithium heparin gel tube for plasma magnesium and potassium measurement; set 3, lithium heparin gel tube for plasma magnesium, potassium, calcium, albumin, phosphate, bicarbonate, CRP and 2x EDTA tube for haemoglobin and PTH; set A, serum tube for biobanking in participants who provided additional informed consent; set B, 2x EDTA tube for biobanking in participants who provided additional informed consent; ECG, predialysis ECG; Questionnaire, 7-point yes or no questionnaire to ask if a participant experienced the following symptoms in the last week: nausea, vomiting, dizziness, drowsiness and reduced muscle strength, itching and leg cramps, in addition at baseline a question about chronic diarrhoea and over-the-counter use of magnesium supplements is included; 3DDD, 3 days dietary diary including one dialysis weekday, one non-dialysis weekday, one non-dialysis weekend day; Holter, 48-hours ECG registration including one dialysis session and one interdialytic interval; PWV, pulse wave velocity measurement before the mid-week dialysis session. CRP, C-reactive protein; PTH, parathyroid hormone.
Safety monitoring
If participants in the intervention group reach a plasma magnesium of 1.25 mmol/L or above, as noted by an unblinded independent nephrologist not involved in the trial (see below), at any time point during follow-up in weeks 1 to 8, dialysate magnesium concentration will be reduced to the previous level in the next week. If plasma magnesium remains 1.25 mmol/L or above in the next week in intervention phase 2 (weeks 5–8), dialysate magnesium is further reduced one step (to 0.50 mmol/L). If a participant develops bradycardia with heart rate below 50 beats per minute (bpm), or prolonged QTc interval (>450 ms in male or >460 ms in female), noticed on ECG in week 4, dialysate magnesium concentration is also reduced to the previous step (figure 1). Plasma magnesium above 1.25 mmol/L, bradycardia<50 bpm and prolonged QTc will be recorded as adverse event. Serious adverse events will be recorded and reported to the primary investigator and medical ethical committee.
Main endpoints
Primary endpoint is predialysis plasma magnesium concentration after the long interdialytic interval at the end of week 8, in the intervention group compared with the control group. The change of predialysis plasma magnesium concentration after the long interdialytic interval from baseline to the end of week 8 will also be determined in the intervention group compared with the control group.
Secondary endpoints
Postdialysis plasma magnesium concentrations after the dialysis sessions following the long interdialytic interval will also be determined. Safety endpoint is the safety of using higher magnesium concentrations in the dialysate, as indicated by the incidence of respectively hypermagnesemia (>1.25 mmol/L) at any timepoint, or bradycardia (defined as heart rate below 60 bpm) or prolonged duration of QTc interval (>450 ms in male or >460 ms in female) identified on the ECG in week 4 or 8. Other explorative endpoints include the change of PWV from baseline to week 8; and the number of complex premature ventricular complexes (complex PVCs), premature atrial complexes (PACs), and heart rate variability as detected with Holter ECG monitoring. Complex PVCs are defined as PVCs that are multiform, repetitive or have a frequency of >30/h.8 Furthermore, the following outcomes will be recorded: subjective symptoms that can be associated with hypermagnesemia determined from self-reporting in questionnaires in weeks 4 and 8; hospitalisation; mortality and cardiovascular events that lead to hospitalisation or mortality including arrhythmia, cardiac arrest, acute coronary syndrome, cerebrovascular accident and haemorrhage from ruptured aneurysms of the abdominal aorta.
Blinding and randomisation
Participants who fulfil all screening criteria will be randomly allocated 2:1 in tranches of 6 to either the intervention group or the control group by the pharmacy according to a computer-generated random list. Participants, treating physicians, nurses and researchers are blinded to treatment allocation. Dialysate cannisters are relabelled by the pharmacy for the individual participant per individual study week. Labels include information on participant name, date of birth, study week and dialysate potassium concentration, but no further information on dialysate composition. For the first study week, the pharmacy chooses the appropriate dialysate concentrate based on treatment allocation and the in routine individual care determined potassium concentration. From week 2 on, one independent nephrologist who is not blinded for treatment allocation, weekly decides on the dialysate magnesium concentration, after review of plasma magnesium concentrations according to the algorithm shown in figure 1. The pharmacy then relabels the dialysate as prescribed by the independent nephrologist.
Sample size calculation
We previously performed a study to determine plasma magnesium concentrations and variability in people receiving 3-times weekly haemodialysis treatment with a standard dialysate magnesium concentration of 0.50 mmol/L.13 That study showed a mean predialysis plasma magnesium concentration of 0.88±0.14 mmol/L.13 After excluding predialysis magnesium levels above 1.00 mmol/L from the analysis, mean predialysis plasma magnesium level was 0.83 mmol/L in that study population. Based on these results, we expect a mean plasma magnesium concentration of 0.83±0.14 mmol/L in the control group. Based on the results from the CONTRAST cohort analysis, in which plasma magnesium was associated with all-cause and cardiovascular mortality, we consider an increase of plasma magnesium concentration to 0.96 mmol/L in the intervention group relevant, which is a 0.13 mmol/L rise.5 The required sample size calculated for two independent groups, based on the values just mentioned, a power of 0.80, probability of 0.05 and 2:1 randomisation, would be 28 in the intervention and 14 in the control group. To account for an estimated drop-out of 20%, the required sample size is 53 participants in total: 35 in the intervention and 18 in the control group.
Statistical analysis
Continuous variables will be expressed as mean and SD for normally distributed variables or median and IQR (Q1–Q3) for non-parametric distributed variables. Categorical variables will be presented as number and percentage. The primary endpoint, predialysis plasma magnesium after the long interdialytic interval at the end of week 8 will be compared between the intervention and control group, using univariable analysis with an unpaired Student’s t-test if variables are normally distributed (if necessary after logarithmic transformation) and with Mann-Whitney U test if variables are non-normally distributed. The analysis is performed as intention to treat, including all participants who are still in study follow-up at week 8. The change of plasma magnesium from baseline to week 8 is first analysed within each group using a paired Student’s t-test, and then the difference from baseline to week 8 (delta) is compared between the groups using linear mixed models. The predictive effect of dialysate magnesium concentration and other baseline parameters and dialysis characteristics on plasma magnesium concentration will be explored in two separate analyses (for predialysis and postdialysis concentration) using linear mixed models. In addition, we will explore which parameters are predictive for the increment of plasma magnesium concentration from baseline to week 8 after sequentially increasing dialysate magnesium concentration, using linear mixed models. For secondary endpoints, univariable analysis for within-group changes and between-group differences will be performed using respectively paired and unpaired Student’s t-test or Mann-Whitney U tests for continuous variables, and chi-quadrate or Fisher’s exact test for dichotomous variables. Linear mixed models will be used for multivariable analysis of secondary endpoints with repeated measures.
Data management
Data are recorded in electronic case report forms using the webbased software Castor EDC (Amsterdam, The Netherlands). All data are stored in coded form. The identification key is stored at the local study site only. Randomisation codes are stored at the pharmacy. The randomisation code will not be broken until follow-up of all participants is completed.
Ethics and dissemination
The study is conducted in accordance with the declaration of Helsinki as revised in 2013 and was approved by the Ethical Committee of the VU University Medical Center (registration number 2017.408, NL62679.029.17).
The results of this study will be offered for publication in international peer-reviewed journals. In addition, the results can be presented at national and international conferences and meetings in the field.
Patient and public involvement
This study protocol was reviewed and approved by the Dutch Kidney Foundation (DKF). A patient panel of the Dutch Renal Patients Association is involved in the review of research protocols submitted to the DKF. Investigators will communicate results to participants once the final results become available. The results will also be shared with patient organisations.
Discussion
This study aims to determine feasibility, safety and predictive parameters for the effect of using dialysate with higher magnesium concentration to increase plasma magnesium concentrations in people treated with haemodialysis. In addition, this study will explore effects of using higher dialysate magnesium on cardiac rhythm and pulse wave velocity.
Relation with previous studies
In a previous study, we demonstrated that a commonly used dialysate magnesium concentration of 0.50 mmol/L generally induces a decline of plasma magnesium concentrations towards concentrations in the lower range of normal.13 Detailed information in literature on the effects of increasing dialysate magnesium concentration on predialysis and postdialysis plasma magnesium concentrations and safety is sparse. Two other studies showed that a dialysate magnesium concentration of 0.75 mmol/L generally resulted in a relatively stable plasma magnesium concentration, with a mean predialysis concentration of 1.2 mmol/L and mean postdialysis concentrations of 1.1 up to 1.2 mmol/L.22 23 In another trial, a 4-weeks single-step increment of dialysate magnesium concentration from 0.50 to 1.00 mmol/L resulted in a 0.4 mmol/L increase (95% CI 0.3 to 0.5) of predialysis plasma magnesium concentration and a mean predialysis plasma Mg concentration of 1.4±0.2 mmol/L.17 That study did not perform ECG nor Holter monitoring. As outlined in the introduction, an observational study in people treated with haemodialysis found an inverse association between plasma magnesium concentrations and arrhythmia.7 Moreover, in a short randomised cross-over study in people treated with haemodialysis, a dialysate magnesium concentration of 0.75 mmol/L compared with 0.50 mmol/L decreased pulse wave velocity.11
Strengths and limitations
The strengths of this study protocol are the blinding and randomisation. Although the primary outcome is an objective outcome measure, blinding and randomisation are essential to prevent bias occurring from changes in life styles including dietary magnesium intake. In addition, it is of relevance for objective collection and processing of data from questionnaires, pulse wave velocity measurements, ECG and reporting of SAEs. Another strength of this protocol is that dialysate magnesium concentration will be individually titrated based on individual plasma magnesium concentrations. In addition, not only the effect of dialysate magnesium will be determined but also other factors that are predictive for this effect will be determined. The major limitation of this protocol is that the study will not provide information on clinical outcomes including cardiovascular events and mortality, due to a limited duration of the study.
Potential impact
The study described in this protocol may provide relevant information on the effect of dialysate magnesium on plasma magnesium concentrations, the strategy for titration of dialysate magnesium based on individual needs, the safety of increasing plasma magnesium concentrations and about factors that are predictive for the effect of dialysate magnesium on plasma magnesium concentration. This information may enable to safely increase plasma magnesium concentrations by individualised dialysate magnesium concentrations. In addition, this study will provide explorative data about the effects of increased dialysate magnesium concentration on intermediate cardiovascular outcomes including cardiac rhythm and PWV. The information provided by this study may pave the way to larger long-term randomised controlled trials on the effects of increasing plasma magnesium concentrations on clinical outcomes including all-cause and cardiovascular mortality in people treated with haemodialysis. If plasma magnesium indeed improves clinically relevant outcomes, and can be safely increased by means of individualised increasing dialysate magnesium concentrations, potentially large health benefits may be achieved if magnesium is increased slightly above the reference range by an increase of dialysate magnesium concentration. If so, the cost-effect ratio is likely low, as raising magnesium concentration is an inexpensive intervention. In addition, it would be an easy intervention that needs no additional patient effort and no oral supplementation would be needed in these persons that already experience a high pill burden. Therefore, the study described in this protocol may provide information of high relevance to patients, clinicians and healthcare providers and may eventually help to decrease morbidity and mortality in people treated with haemodialysis.
Safety considerations
Plasma magnesium concentrations are expected to rise up to values above the reference range by the increase of dialysate magnesium concentration. However, clinical symptoms of hypermagnesemia, typically are not observed before plasma magnesium concentrations exceed 2.0 mmol/L, which is high above the target concentrations in this study. Reported symptoms of hypermagnesemia (if plasma concentrations are above 2.0 mmol/L) include lethargy, drowsiness, flushing, nausea and vomiting, and diminished deep tendon reflexes.24 In even more severe hypermagnesemia (plasma concentrations above 3.0 mmol/L) also somnolence, loss of deep tendon reflexes, hypotension and ECG changes can occur, and in extreme hypermagnesemia (above 5.0 mmol/L), complete heart block, cardiac arrest, apnoea, paralysis and coma have been reported.24 As a result of haemodialysis inherent techniques, the increment of (free) plasma magnesium is restricted by its concentration in the dialysate (maximally 1.00 mmol/L in this study), so overshoot to symptomatic concentrations is virtually impossible. The risk for severe or symptomatic hypermagnesemia is further minimised by intensive monitoring. As dialysate magnesium concentration is not further increased after a plasma magnesium concentration of 1.15 mmol/L is reached, and is reduced if plasma concentrations of 1.25 mmol/L are reached at any time point, severe or symptomatic hypermagnesemia will be prevented. These maximal target concentrations are set taking into account an observational study in people treated with haemodialysis that suggested an optimal magnesium concentration in-between 1.15 and 1.27 mmol/L and increased risk for mortality if magnesium exceeds 1.27 mmol/L.14 Furthermore, for safety reasons, individuals with bradycardia or a prolonged QTc interval on the ECG at baseline will be excluded from participation, and in individuals with bradycardia with a heart rate below 50 bpm or a prolonged QTc interval identified on the ECG in week 4, dialysate magnesium will be reduced. Based on these careful methods, the risk for individuals participating in this study is low. Considering the limited burden and risks associated with this study and a possible highly relevant contribution to future improvement of treatment and prognosis in people treated with haemodialysis, the potential benefits outweigh the burden and possible risks.
Trial status
The trial is currently ongoing. The first participant was randomised on 4 April 2018 and up till now, 43 of 53 participants have been randomised.
Supplementary Material
Footnotes
Contributors: NHJL, MGV and JGJH conceived the study. NHJL, MGV and CED designed the study. NHJL wrote the manuscript and MGV, JGJH and CED revised the manuscript. Each author approved the final version of the manuscript.
Funding: This work was supported by the Dutch Kidney Foundation (PhD grant no. 15OP02) and the PPP Allowance made available by Top Sector Life Sciences & Health to The Dutch Kidney Foundation to stimulate public–private partnerships (grant no. LSHM17034-HSGF).
Competing interests: MGV received research grants from Vifor, Amgen, Fresenius, and acted as consultant for Medice, Astra Zeneca, Vifor, Amgen, Fresenius, Otsuma, Kyowa Kirin.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet 2013;382:339–52. 10.1016/S0140-6736(13)60595-4 [DOI] [PubMed] [Google Scholar]
- 2.Leenders NHJ, Vervloet MG. Magnesium: a magic bullet for cardiovascular disease in chronic kidney disease? Nutrients 2019;11:455. 10.3390/nu11020455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jahnen-Dechent W, Ketteler M. Magnesium basics. Clin Kidney J 2012;5:i3–14. 10.1093/ndtplus/sfr163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leenders NHJ, Vermeulen EA, van Ballegooijen AJ, et al. The association between circulating magnesium and clinically relevant outcomes in patients with chronic kidney disease: A systematic review and meta-analysis. Clin Nutr 2021;40:3133–47. 10.1016/j.clnu.2020.12.015 [DOI] [PubMed] [Google Scholar]
- 5.de Roij van Zuijdewijn CLM, Grooteman MPC, Bots ML, et al. Serum magnesium and sudden death in European hemodialysis patients. PLoS One 2015;10:e0143104. 10.1371/journal.pone.0143104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lacson E, Wang W, Ma L, et al. Serum magnesium and mortality in hemodialysis patients in the United States: a cohort study. Am J Kidney Dis 2015;66:1056–66. 10.1053/j.ajkd.2015.06.014 [DOI] [PubMed] [Google Scholar]
- 7.Tumlin JA, Roy-Chaudhury P, Koplan BA, et al. Relationship between dialytic parameters and reviewer confirmed arrhythmias in hemodialysis patients in the monitoring in dialysis study. BMC Nephrol 2019;20:80. 10.1186/s12882-019-1212-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsuji H, Venditti FJ, Evans JC, et al. The associations of levels of serum potassium and magnesium with ventricular premature complexes (the Framingham heart study). Am J Cardiol 1994;74:232–5. 10.1016/0002-9149(94)90362-X [DOI] [PubMed] [Google Scholar]
- 9.Bikkina M, Larson MG, Levy D. Prognostic implications of asymptomatic ventricular arrhythmias: the Framingham heart study. Ann Intern Med 1992;117:990–6. 10.7326/0003-4819-117-12-990 [DOI] [PubMed] [Google Scholar]
- 10.Yorifuji M, Kuragano T, Kawada S, et al. Factors associated with serum magnesium and vascular stiffness in maintenance hemodialysis patients. Hemodial Int 2018;22:342–50. 10.1111/hdi.12625 [DOI] [PubMed] [Google Scholar]
- 11.Del Giorno R, Lavorato Hadjeres S, Stefanelli K, et al. Consequences of supraphysiological dialysate magnesium on arterial stiffness, hemodynamic profile, and endothelial function in hemodialysis: a randomized crossover study followed by a Non-Controlled follow-up phase. Adv Ther 2020;37:4848–65. 10.1007/s12325-020-01505-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zoungas S, Cameron JD, Kerr PG, et al. Association of carotid intima-medial thickness and indices of arterial stiffness with cardiovascular disease outcomes in CKD. Am J Kidney Dis 2007;50:622–30. 10.1053/j.ajkd.2007.07.012 [DOI] [PubMed] [Google Scholar]
- 13.Leenders NHJ, van Ittersum FJ, Hoekstra T, et al. Routine hemodialysis induces a decline in plasma magnesium concentration in most patients: a prospective observational cohort study. Sci Rep 2018;8:10256. 10.1038/s41598-018-28629-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakaguchi Y, Fujii N, Shoji T, et al. Hypomagnesemia is a significant predictor of cardiovascular and non-cardiovascular mortality in patients undergoing hemodialysis. Kidney Int 2014;85:174–81. 10.1038/ki.2013.327 [DOI] [PubMed] [Google Scholar]
- 15.Kurita N, Akizawa T, Fukagawa M, et al. Contribution of dysregulated serum magnesium to mortality in hemodialysis patients with secondary hyperparathyroidism: a 3-year cohort study. Clin Kidney J 2015;8:744–52. 10.1093/ckj/sfv097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selim GN, Spasovski G, Tozija L, et al. Hypomagnesemia and cause-specific mortality in hemodialysis patients: 5-year follow-up analysis. Int J Artif Organs 2017;40:542–9. 10.5301/ijao.5000611 [DOI] [PubMed] [Google Scholar]
- 17.Bressendorff I, Hansen D, Schou M, et al. The effect of increasing dialysate magnesium on serum calcification propensity in subjects with end stage kidney disease: a randomized, controlled clinical trial. Clin J Am Soc Nephrol 2018;13:1373–80. 10.2215/CJN.13921217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan A-W, Tetzlaff JM, Gøtzsche PC, et al. Spirit 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ 2013;346:e7586. 10.1136/bmj.e7586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.RIVM Rijksinstituut voor Volksgezondheid en Milieu . Dutch food composition database NEVO-online version, 2019. Available: https://nevo-online.rivm.nl
- 20.Voedingscentrum . Mijn Eetmeter. Available: https://www.voedingscentrum.nl/
- 21.Van Bortel LM, Laurent S, Boutouyrie P, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens 2012;30:445–8. 10.1097/HJH.0b013e32834fa8b0 [DOI] [PubMed] [Google Scholar]
- 22.Kyriazis J, Kalogeropoulou K, Bilirakis L, et al. Dialysate magnesium level and blood pressure. Kidney Int 2004;66:1221–31. 10.1111/j.1523-1755.2004.00875.x [DOI] [PubMed] [Google Scholar]
- 23.Truttmann AC, Faraone R, Von Vigier RO, et al. Maintenance hemodialysis and circulating ionized magnesium. Nephron 2002;92:616–21. 10.1159/000064109 [DOI] [PubMed] [Google Scholar]
- 24.Topf JM, Murray PT. Hypomagnesemia and hypermagnesemia. Rev Endocr Metab Disord 2003;4:195–206. 10.1023/A:1022950321817 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.