Abstract
Introduction
Long COVID (LC), also known as post-COVID-19 syndrome, refers to symptoms persisting 12 weeks after COVID-19 infection. It affects up to one in seven people contracting the illness and causes a wide range of symptoms, including fatigue, breathlessness, palpitations, dizziness, pain and brain fog. Many of these symptoms can be linked to dysautonomia or dysregulation of the autonomic nervous system after SARS-CoV2 infection. This study aims to test the feasibility and estimate the efficacy, of the heart rate variability biofeedback (HRV-B) technique via a standardised slow diaphragmatic breathing programme in individuals with LC.
Methods and analysis
30 adult LC patients with symptoms of palpitations or dizziness and an abnormal NASA Lean Test will be selected from a specialist Long COVID rehabilitation service. They will undergo a 4-week HRV-B intervention using a Polar chest strap device linked to the Elite HRV phone application while undertaking the breathing exercise technique for two 10 min periods everyday for at least 5 days a week. Quantitative data will be gathered during the study period using: HRV data from the chest strap and wrist-worn Fitbit, the modified COVID-19 Yorkshire Rehabilitation Scale, Composite Autonomic Symptom Score, WHO Disability Assessment Schedule and EQ-5D-5L health-related quality of life measures. Qualitative feedback on user experience and feasibility of using the technology in a home setting will also be gathered. Standard statistical tests for correlation and significant difference will be used to analyse the quantitate data.
Ethics and dissemination
The study has received ethical approval from Health Research Authority (HRA) Leicester South Research Ethics Committee (21/EM/0271). Dissemination plans include academic and lay publications.
Trial registration number
Keywords: COVID-19, Health informatics, REHABILITATION MEDICINE, CARDIOLOGY
STRENGTHS AND LIMITATIONS OF THIS STUDY.
To our knowledge, this is the first study of heart rate variability biofeedback (HRV-B) in long COVID and will provide new information regarding the feasibility of the technology-based intervention in this condition.
The estimation of efficacy will determine the scope and sample size for a larger controlled trial in the condition that currently has no definitive treatments.
The study will provide preliminary evidence on the correlation between long COVID symptoms and dysautonomia.
The limitation of this study is the small sample size of 30 participants, which might not give an accurate estimate of efficacy.
HRV-B is a technology-based intervention; therefore, its take-up could be limited in those with a lack of experience in using digital technology in daily life, particularly those from less privileged backgrounds.
Introduction
Post-COVID-19 syndrome or Long COVID (LC) refers to persistent symptoms 12 weeks after SARS-COV2 infection and can include symptoms of physical fatigue, cognitive fatigue or ‘brain fog’, breathlessness, pain and psychological distress.1–3 An estimated 1.4 million people are reported to be affected by LC in the UK alone.4 The condition can be highly debilitating for some, particularly middle-aged individuals who were previously functioning at a high level and in demanding vocational roles.5 Many will experience significant disruption to employment, social and caregiving roles and participation in society.
Many LC symptoms such as palpitations, dizziness, fatigue, pain and breathlessness can be explained by the theory of dysautonomia.6 7 This is a state of episodic dysregulation in the autonomic nervous system (ANS) with sympathetic overdrive and reduced parasympathetic activity. Dysautonomia plays a significant role in the symptomology of many long-term conditions including multiple sclerosis, Parkinson’s disease, diabetes mellitus, fibromyalgia, chronic fatigue syndrome and migraine.8
One way of estimating and measuring autonomic function is through heart rate variability (HRV), as cardiac rate and rhythm are controlled largely by the ANS. The parasympathetic nervous system chiefly activates a slowing of heart rate through the vagus nerve, and the sympathetic response acts through the activation of β-adrenergic receptors.9 HRV can be measured either in the time domain or frequency domain. HRV represents a measure of the variation in time between heartbeats (captured on an Electrocardiogram (ECG) strip as a time interval between the R waves of the QRS complexes). A low HRV is associated with sympathetic nervous system activation, also described as a state of ‘fight or flight’. Higher HRV correspond with parasympathetic nervous system activation and is believed to reflect a state of rest and recovery. Lower HRV has been observed to be associated with fatigue and pain symptoms of chronic fatigue syndrome/myalgic encephalomyelitis and fibromyalgia10–12 as well as other chronic physical and mental health pathologies including asthma, anxiety and stress.10–14
HRV biofeedback
When physiological parameters such as HRV are monitored in real time with self-regulation techniques such as breathing exercises applied to influence the parameters, this is known as biofeedback.15 16 In this study, for monitoring and modulating the HRV, we are using breathing techniques to encourage the predominance of parasympathetic nervous activity through vagus nerve activation. To the best of our knowledge, there have not yet been any studies of HRV biofeedback (HRV-B) in LC. However, HRV-B using breathing techniques has been tested in other clinical conditions such as asthma,13 depression17 and fibromyalgia.12 A normal respiratory rate is between 12 bpm and 20 bpm.18 The optimal breathing frequency to produce maximal increase in HRV varies for each individual but on average is between 5.5 bpm and 6 bpm and is known as resonant breathing.13 18 19 Resonant breathing helps to restore autonomic balance due to baroreflex gain and vagal activation.13 18–20
There are several means of assessing HRV but most commonly these include the use of either wearable devices such as smartwatches or chest straps, or through small attachable Holter ECG units. These are non-invasive and readily available, although reliability differs between devices and platforms. Many commercial HRV devices are associated with smartphone app technology, which can be readily downloaded and made available to participants for monitoring. Of the consumer grade devices available to monitor HRV the Polar H10 chest strap is felt to be the most reliable and remains accurate even during high-intensity activity.21 The Polar H10 can be linked with the Elite HRV app, which provides real-time feedback on HRV and the user’s response to breathing techniques. The combination of Polar H10 chest strap and Elite HRV app has been effectively used to harness real-time physiological data, for example, in athletes.22 In contrast, many wrist-worn devices such as Fitbit return a measure of HRV only while the user is asleep due to motion and other interference sources, meaning real-time HRV-B is not possible.
The aim of this study is to determine the feasibility and impact of a structured HRV-B regime incorporating diaphragmatic breathing exercise, on LC symptoms. We wish to test the acceptability and compliance of the intervention and estimate effect on symptoms using standardised validated measures of LC and dysautonomia.
Aims and objectives
The aim of this study is: to assess the feasibility of a 4-week HRV-B-structured breathing programme in individuals with LC.
The objectives include:
Does breathing exercises through HRV-B increase HRV among participants with LC?
Are consumer-grade monitors appropriate technology to use for HRV-B in the domiciliary setting?
Does regular HRV-B have any effect on LC symptoms?
Methods
Study design
This is a phase 2 uncontrolled open-label feasibility study of a home technology-based HRV-B in 30 individuals with LC. Potential participants will be identified through the Leeds COVID-19 Rehabilitation Service, based at Leeds Community Healthcare NHS Trust. The study period will be 6 weeks for each participant. The study start date is 24 January 2022, and the anticipated end date is 31 March 2024.
Eligibility criteria
The inclusion criteria are
Age ≥18 years.
Confirmed LC diagnosis as per the National Institute for Health and Care Excellence (NICE) criteria for post-COVID syndrome.1
Self-rating of at least ‘moderate’ or ’severe’ on dysautonomia questions of palpitations or dizziness on the COVID-19 Yorkshire Rehabilitation Scale (C19-YRSm).23
-
Abnormal NASA Lean Test (NLT).24–26
HR increase in 30 bpm or ≥120 bpm.
Blood pressure (BP) decrease in 20 mm Hg systolic or 10 mm Hg diastolic in the first 3 min of standing.
NLT is an accepted measure of cardiovascular instability and is conducted at initial assessment clinic for all LC service users in the Leeds COVID-19 rehabilitation service. The patient lies down for 2–5 min prior to the test with HR and BP taken each minute to calculate average supine values. They then stand with heels 6 inches from a wall and lean back against it with HR and BP taken each minute for 10 min. Abnormal results (as described above) are demonstrated through orthostatic hypotension or tachycardia on standing which are hallmarks of dysautonomia and, therefore, objectively quantifiable. The participants who have dysautonomia symptoms but do not meet the mentioned thresholds will not be included in this feasibility study but will be potential recruits for future larger scale studies using the same intervention.
The exclusion criteria are
Unable to use the wearable or smartphone app technology.
Cognitive difficulties or mental health disorders causing inability to consent.
Any cardiac arrhythmias that are being planned for further investigations and specialist management in the Cardiology service.
Any unstable cardiorespiratory disease that needs further medical interventions (except asthma management).
Equipment and technology
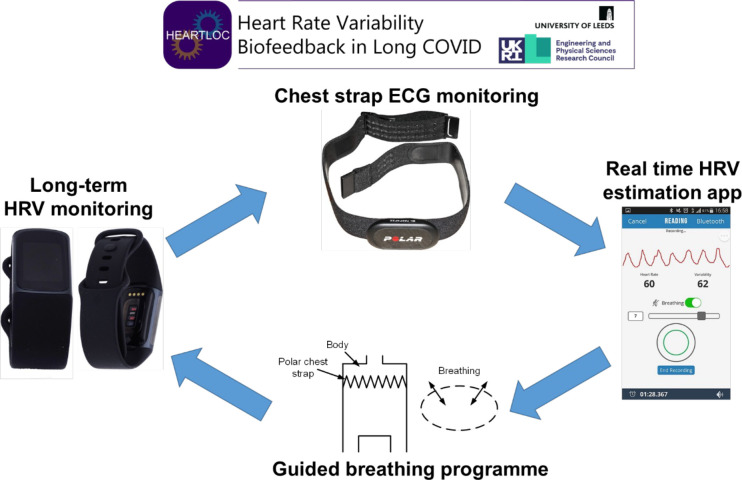
To collect medium-term HRV data, participants will wear a Fitbit Charge 5 smartwatch for a total of 6 weeks. The HRV-B itself will be conducted using a Polar H10 chest strap for 10 min two times per day. This connects via Bluetooth to the Elite HRV smartphone app, which is downloaded to participants’ phones. Participants will aim to increase their HRV score as displayed in Elite HRV in real time using a diaphragmatic breathing technique (figure 1). Omron M2 blood pressure monitor (endorsed by the British Hypertension Society) will be used to conduct NLT in clinic and the adapted Autonomic Profile (aAP).27
Figure 1.
Heart rate variability biofeedback (HRV-B) using a breathing technique and chest strap for real-time HRV monitoring. Polar H10 picture from Wikimedia commons, reprinted under CC BY-SA 3.0 license. EliteHRV screenshot from Wikimedia commons, reprinted under CC BY-SA 4.0 license.
Study phases
The study will be carried out in the following three phases:
Pre-HRV-B phase.
HRV-B phase.
Post-HRV-B phase.
Pre-HRV-B phase
The participant will either be invited to a research clinic or visited at their home by a member of the research team (first appointment A1). They would have already received the participant information sheet (PIS) at screening and would have had more than 24 hours to read and understand the content of the PIS. Written consent will be signed by the participant and the researcher during this first visit. Devices and baseline outcome measures used in this stage are:
Fitbit charge five device and the Fitbit smartphone application: The participant will be requested to have the Fitbit device on most of the time during the 6-week period. The application records HRV at night along with other measures of sleep (sleep stages, HR) and daytime activity (such as step count).
C19-YRSm: the COVID-19 Yorkshire Rehabilitation Scale (C19YRS) is the literature’s first condition-specific patient recorded outcome measure, which has been validated in the LC population.28 29 The modified scale provides a symptom severity score (out of 30), functional disability score (out of 15), other symptoms score (out of 25) and overall health score (out of 10).23 The participant will complete C19YRSm (online supplemental file 1) at weekly intervals to monitor the impact of the intervention on LC symptoms. They will also have weekly telephone reviews with study researchers for troubleshooting and to ensure maximal compliance with the study.
Composite Autonomic Symptom Score (COMPASS): the COMPASS 31 will be completed by the participant at the initial visit and again 6 weeks later at the end of the study. Autonomic symptoms are scored for different domains including orthostatic intolerance, vasomotor, secretomotor, gastrointestinal, bladder and pupillomotor. Total scores for each domain are multiplied by a set weighting and then added together to provide a score out of 100 representing severity of autonomic symptoms. A higher score represents greater severity.30
aAP: this is an autonomic profile test developed by St Mary’s Hospital and the National Hospital for Neurology and Neurosurgery and later adapted for domiciliary use during the COVID-19 pandemic.27 Participants are asked to monitor their heart rate and BP on lying, and at 3 min of standing at various intervals over 24 hours, including after waking, after eating breakfast/lunch/dinner, before and after 5 min of exercise, and before bed (online supplemental file 2). Abnormal results are calculated using the same criteria for heart rate and BP differences as the NLT (HR increase >30/min or BP drop >20 mm Hg).
WHO Disability Assessment Schedule (WHODAS): this is validated generic measure of functioning and disability. The 36-item scale captures six domains of life (cognition, mobility, self-care, getting along, life activities and participation) with a summary score ranging from 0 (no disability) to 100 (full disability)31 32
EQ-5D-5L: The EQ-5D-5L instrument, provided by the EuroQol Group, is one of widely used quality of life measures, consists of five items covering: mobility, self-care, usual activities, pain/discomfort and anxiety/depression.33 The item scores can be converted into a total index score by applying health preference weights elicited from a general population. This index score can also be used in economic evaluations to assess the cost-effectiveness of health interventions.34
bmjopen-2022-066044supp001.pdf (125.9KB, pdf)
bmjopen-2022-066044supp002.pdf (147.6KB, pdf)
The A1 appointment will last approximately 2 hours and may be longer for those with cognitive fatigue or ‘brain fog’. If felt necessary, it will be divided into two 1-hour visits to reduce cognitive fatigue.
HRV-B phase
One week after the A1 appointment, the participant will be either invited to attend a research clinic or visited at home by a researcher (second appointment A2) to commence the HRV-B study phase. This involves:
Polar H10 chest strap and Elite HRVB application: The participant will be familiarised with the technology and introduced to a paced breathing regimen via a one-to-one demonstration. They will be instructed to perform the breathing technique using the application at least two times a day, 10 min per session, for a period of 4 weeks. The chest strap device will record HRV for the duration of the session, and the data get recorded in the application. While this phase is ongoing, participants will continue to wear the Fitbit Charge 5 device for the duration of this phase.
Fitbit charge 5 device and the Fitbit smartphone application.
C19-YRSm.
Post-HRV-B phase
The participant will be asked to stop the HRV-B intervention after completing 4 weeks of the treatment. They will be asked to continue using the Fitbit device for another week when not doing the intervention. They will then either be invited to a research clinic or be visited at home by a study researcher. At this appointment (A3), the participant will complete:
C19 YRSm: the C19-YRSm will be completed by the patient every week for a total of 6 weeks. There will be a total of 7 C19-YRS documents completed.
Fitbit charge 5 device and the Fitbit smartphone application.
NLT and aAP.
COMPASS 31.
WHODAS.
EQ-5D-5L.
During the A3 appointment, the Polar H10 strap and the Fitbit device will be retrieved.
The participants will be invited to complete a further C19-YRS, by email or postal 4 weeks after completion aAP for 24 hours and to email or post the results to the study researcher.
Outcome measures
The primary outcome measure is the C19YRSm, a self-reported patient-reported outcome measure to assess LC symptom severity, functional disability and overall health status.
Secondary outcome measures include:
Heart rate measures from chest strap:
7-day average HRV score out of 100—quantified by the Elite HRV app via the root mean square of successive differences between normal heartbeats (rMSSD). A natural log (ln) is applied to this figure and then expanded to generate a 1 to 100 score.
Mean R–R interval.
Heart rate.
rMSSD.
SDNN (SD of Normal to Normal intervals).
Total power.
Low-frequency power (LF).
High-frequency power (HF).
LF:HF ratio.
Fitbit data:
Sleep staging data.
Resting heart rate.
Daily activity levels, for example, step count and exercise type and duration.
Patient-reported outcome measures:
NLT and aAP.
COMPASS 31.
WHODAS.
EQ-5D-5L.
During our final interaction with participants in the study, we will ask them the following questions to assess the feasibility of the study:
‘How did you find using the technology?’
‘How did you find the breathing intervention?’
‘Have you noticed any change in your symptoms?’
Their opinions and suggestions will be recorded as quotes in their participant files. However, we are not planning to undertake a formal qualitative analysis of responses as it is not one of the main objectives of this study.
A summary of the schedule for the completion of outcome measures is shown in table 1.
Table 1.
Outcome measures summary schedule
| Initial assessment Clinic | Pre HRV-B phase (1 week) | HRV-B phase (4 weeks) | Post HRV-B phase (1 week) | |
| Autonomic screening (NLT) | √ | √ | ||
| Autonomic function (COMPASS 31) | √ | √ | ||
| Home autonomic test (aAP) | √ | √ | ||
| Fitbit wrist strap HRV, sleep data | √ | √ daily | √ | |
| Polar H10 chest strap HRV data | √ daily | |||
| LC-specific PROM C19-YRSm | √ | √ weekly | √ | |
| Daily function (WHODAS) | √ | √ | ||
| Quality of life (EQ5D-5L) | √ | √ |
aAP, adapted Autonomic Profile; COMPASS 31, Composite Autonomic Symptom Score; C19-YRSm, modified COVID-19 Yorkshire Rehabilitation Scale; EQ-5D-5L, EuroQol 5-Dimension 5-Level; HRV-B, heart rate variability biofeedback; LC, long COVID; NLT, NASA Lean Test; WHODAS, WHO Disability Assessment Schedule.
Sample size
A formal sample size calculation is not required for a feasibility study as it does not mimic a definitive randomised trial and aim is not to measure effect size.35 A sample size of 30 is the average sample size across feasibility studies and is accepted as reasonable size to assess the acceptability and suitability of the intervention.36
Statistical analysis
Quantitative data from standardised questionnaires will be scored as per standard procedures. Data downloaded from the wearable devices will be extracted, cleaned and summarised using specific software packages, including Matlab and Python. Quantitative data will be analysed with simple descriptive statistics. The presence and magnitude of pre and postintervention differences will be examined using repeated paired sample t tests (with Bonferroni adjustment for multiple comparisons), and the effect size will be explored using both Analysis of Variance (ANOVA) partial η2, and Cohen’s d. Additional exploratory analyses may also be performed to fully analyse the data set produced, guided by the findings of the descriptive statistics.
Patient and public involvement
Members of the Patient Advisory Group (PAG) with lived experience of LC have been involved in the design, development and delivery of the project. Members of the PAG attended proposal research planning meetings and shared their experiences on symptoms of dysautonomia, which helped shaped the research question, design and outcome measures of this study. Members of this group have contacts with wider patient community groups and helped disseminate information about the study. The PAG meets quarterly with the research team to review progress, ensure the research continues to answer relevant issues and that findings can inform LC care. The group will be involved in the dissemination of research findings and writing lay summary reports that will be shared with the participants.
Ethics and dissemination
The study has received ethical approval from Health Research Authority (HRA) Leicester South Research Ethics Committee (21/EM/0271). Informed consent will be obtained from all participants. Potential participants will have a minimum of 24 hours to review the PIS and discuss queries with the researcher prior to signing the written consent. General Data Protection Regulation (GDPR) rules will be strictly followed for all data gathered during the study. All data will be fully anonymised as soon as practical. All devices used are (Conformité Européenne (CE) marked and are being used for their intended purposes. There is potential for minor skin irritation from wearing the Fitbit and Polar H10 devices. This will be enquired about at each weekly telephone review.
For participants with cognitive fatigue or ‘brain fog’ relating to LC, the length of the appointments with the researcher (A1, A2 and A3) may be longer than normal. Supplementary written information will be provided, and if necessary, each of these appointments may be conducted in two shorter sessions to reduce information overload and possible impact on LC symptoms. Participants will be advised that they do not need to proceed with the appointments or the study if they do not want to. All appointments other than the initial NLT can occur at the participants’ homes to reduce travel and inconvenience. Participants are free to withdraw at any point in the study. They will be encouraged to give reasons for the withdrawal, but it will not be compulsory to give a reason for withdrawal.
Dissemination will include both academic publications and lay summaries in various formats. Academic outputs will include both medical and engineering literature. Policy impact will be aided by our strong existing links to NHS England and the UK Long COVID National Task Force. Dr Sivan, who leads the NIHR project Long COVID Multidisciplinary consortium for Optimising Treatments and Services across the NHS (LOCOMOTION),37 is also an advisor for the WHO—Europe on COVID-19 rehabilitation and is also involved in the WHO working party developing a core set of outcome measures for LC.
Supplementary Material
Acknowledgments
The authors would like to thank individuals with autonomic problems in long covid and healthcare professionals from the Leeds Covid Rehabilitation service who provided valuable suggestions and feedback during the iterative process of development of this protocol. We are grateful to the Patient Advisory Group for its involvement in all stages of this study.
Footnotes
Twitter: @doc_simmsy, @LeedsADRM
Contributors: MS and AC conceptualised the study. MS, AC and RJO’C were awarded EPSRC IAA pump-priming grant for the feasibility study with MS as the Principal Investigator. JC, SH, NP, DW, RT, JD, ADS, RJO’C, AC and MS contributed to the study design and obtained ethical approval. JC and MS wrote an initial draft of the paper by adapting the grant proposal and the ethics protocol. All authors approved the final manuscript. All authors will contribute to recruitment, data acquisition and analysis of the study findings. MS is the corresponding author and guarantor.
Funding: This research is supported by IAA EPSRC [Ref 112538] with University of Leeds as the sponsor organisation and the Leeds Community Healthcare NHS Trust Covid Rehabilitation service as the research site organisation.
Competing interests: Manoj Sivan is an advisor to the WHO for the Long COVID policy in Europe.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.National Institute for Health and Care Excellence (NICE), Scottish Intercollegiate Guidelines Network (SIGN), Royal College of General Practitioners (RCGP) . COVID-19 rapid guideline: managing the long- term effects of COVID-19. London: NICE, 2022. [Google Scholar]
- 2.Crook H, Raza S, Nowell J, et al. Long covid-mechanisms, risk factors, and management. BMJ 2021;374:n1648. 10.1136/bmj.n1648 [DOI] [PubMed] [Google Scholar]
- 3.World Health Organisation . Support for rehabilitation: self-management after COVID-19-related illness. Copenhagen: WHO Regional Office for Europe, 2021. [Google Scholar]
- 4.Office of National Statistics . Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK. London: ONS, 2022. [Google Scholar]
- 5.OoN S. Coronavirus and the social impacts of ‘long COVID’ on people’s lives in Great Britain, 2021. Available: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/coronavirusandthesocialimpactsoflongcovidonpeopleslivesingreatbritain/7aprilto13june2021 [Accessed 02 May 2022].
- 6.Dani M, Dirksen A, Taraborrelli P, et al. Autonomic dysfunction in 'long COVID': rationale, physiology and management strategies. Clin Med 2021;21:e63–7. 10.7861/clinmed.2020-0896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shouman K, Vanichkachorn G, Cheshire WP, et al. Autonomic dysfunction following COVID-19 infection: an early experience. Clin Auton Res 2021;31:385–94. 10.1007/s10286-021-00803-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zalewski P, Slomko J, Zawadka-Kunikowska M. Autonomic dysfunction and chronic disease. Br Med Bull 2018;128:61–74. 10.1093/bmb/ldy036 [DOI] [PubMed] [Google Scholar]
- 9.Heart rate variability . Heart rate variability. standards of measurement, physiological interpretation, and clinical use. Task force of the European Society of cardiology and the North American Society of pacing and electrophysiology. Eur Heart J 1996;17:354–81. [PubMed] [Google Scholar]
- 10.Fournié C, Chouchou F, Dalleau G, et al. Heart rate variability biofeedback in chronic disease management: a systematic review. Complement Ther Med 2021;60:102750. 10.1016/j.ctim.2021.102750 [DOI] [PubMed] [Google Scholar]
- 11.Escorihuela RM, Capdevila L, Castro JR, et al. Reduced heart rate variability predicts fatigue severity in individuals with chronic fatigue syndrome/myalgic encephalomyelitis. J Transl Med 2020;18:4. 10.1186/s12967-019-02184-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hassett AL, Radvanski DC, Vaschillo EG, et al. A pilot study of the efficacy of heart rate variability (HRV) biofeedback in patients with fibromyalgia. Appl Psychophysiol Biofeedback 2007;32:1–10. 10.1007/s10484-006-9028-0 [DOI] [PubMed] [Google Scholar]
- 13.Lehrer PM, Vaschillo E, Vaschillo B, et al. Biofeedback treatment for asthma. Chest 2004;126:352–61. 10.1378/chest.126.2.352 [DOI] [PubMed] [Google Scholar]
- 14.Goessl VC, Curtiss JE, Hofmann SG. The effect of heart rate variability biofeedback training on stress and anxiety: a meta-analysis. Psychol Med 2017;47:2578–86. 10.1017/S0033291717001003 [DOI] [PubMed] [Google Scholar]
- 15.Lehrer P, Vaschillo B, Zucker T, et al. Protocol for heart rate variability biofeedback training. Biofeedback 2013;41:98–109. 10.5298/1081-5937-41.3.08 [DOI] [Google Scholar]
- 16.Gevirtz R. The promise of heart rate variability biofeedback: evidence-based applications. Biofeedback 2013;41:110–20. 10.5298/1081-5937-41.3.01 [DOI] [Google Scholar]
- 17.Karavidas MK, Lehrer PM, Vaschillo E, et al. Preliminary results of an open label study of heart rate variability biofeedback for the treatment of major depression. Appl Psychophysiol Biofeedback 2007;32:19–30. 10.1007/s10484-006-9029-z [DOI] [PubMed] [Google Scholar]
- 18.Vaschillo EG, Vaschillo B, Lehrer PM. Characteristics of resonance in heart rate variability stimulated by biofeedback. Appl Psychophysiol Biofeedback 2006;31:129–42. 10.1007/s10484-006-9009-3 [DOI] [PubMed] [Google Scholar]
- 19.Lehrer PM, Gevirtz R. Heart rate variability biofeedback: how and why does it work? Front Psychol 2014;5:756. 10.3389/fpsyg.2014.00756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pagaduan JC, Chen Y-S, Fell JW, et al. Can heart rate variability biofeedback improve athletic performance? A systematic review. J Hum Kinet 2020;73:103–14. 10.2478/hukin-2020-0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilgen-Ammann R, Schweizer T, Wyss T. Rr interval signal quality of a heart rate monitor and an ECG Holter at rest and during exercise. Eur J Appl Physiol 2019;119:1525–32. 10.1007/s00421-019-04142-5 [DOI] [PubMed] [Google Scholar]
- 22.Flatt AA, Howells D. Effects of varying training load on heart rate variability and running performance among an Olympic rugby sevens team. J Sci Med Sport 2019;22:222–6. 10.1016/j.jsams.2018.07.014 [DOI] [PubMed] [Google Scholar]
- 23.Sivan M, Preston N, Parkin A. The modified COVID-19 Yorkshire rehabilitation scale (C19-YRSm) patient-reported outcome measure for long Covid or Post-COVID-19 syndrome. J Med Virol 2022. 10.1002/jmv.27878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bungo MW, Charles JB, Johnson PC. Cardiovascular deconditioning during space flight and the use of saline as a countermeasure to orthostatic intolerance. Aviat Space Environ Med 1985;56:985–90. [PubMed] [Google Scholar]
- 25.Lee J, Vernon SD, Jeys P, et al. Hemodynamics during the 10-minute NASA lean test: evidence of circulatory decompensation in a subset of ME/CFS patients. J Transl Med 2020;18:314. 10.1186/s12967-020-02481-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hyatt KH, Jacobson LB, Schneider VS. Comparison of 70 degrees tilt, LBNP, and passive standing as measrues of orthostatic tolerance. Aviat Space Environ Med 1975;46:801–8. [PubMed] [Google Scholar]
- 27.Sivan M, Corrado J, Mathias C. The adapted autonomic profile (aAP) home-based test for the evaluation of neuro-cardiovascular autonomic dysfunction. ACNR 2022. 10.47795/QKBU6715 [DOI] [Google Scholar]
- 28.Sivan M, Halpin S, Gees J, et al. The self-report version and digital format of the COVID-19 Yorkshire rehabilitation scale (C19-YRS) for long Covid or Post-COVID syndrome assessment and monitoring. ACNR 2021;20. 10.47795/QROO4168 [DOI] [Google Scholar]
- 29.O'Connor RJ, Preston N, Parkin A, et al. The COVID-19 Yorkshire rehabilitation scale (C19-YRS): application and psychometric analysis in a post-COVID-19 syndrome cohort. J Med Virol 2022;94:1027–34. 10.1002/jmv.27415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sletten DM, Suarez GA, Low PA, et al. Compass 31: a refined and abbreviated composite autonomic symptom score. Mayo Clin Proc 2012;87:1196–201. 10.1016/j.mayocp.2012.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garin O, Ayuso-Mateos JL, Almansa J, et al. Validation of the "World Health Organization Disability Assessment Schedule, WHODAS-2" in patients with chronic diseases. Health Qual Life Outcomes 2010;8:51. 10.1186/1477-7525-8-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ustun TB, Kostanjsek N, Chatterji S. Measuring health and disability : manual for WHO Disability Assessment Schedule (WHODAS 2.0), 2010. Available: https://www.who.int/publications/i/item/measuring-health-and-disability-manual-for-who-disability-assessment-schedule-(-whodas-2.0) [Accessed 19 June 2022].
- 33.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727–36. 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53–72. 10.1016/0168-8510(96)00822-6 [DOI] [PubMed] [Google Scholar]
- 35.Whitehead AL, Sully BGO, Campbell MJ. Pilot and feasibility studies: is there a difference from each other and from a randomised controlled trial? Contemp Clin Trials 2014;38:130–3. 10.1016/j.cct.2014.04.001 [DOI] [PubMed] [Google Scholar]
- 36.Billingham SAM, Whitehead AL, Julious SA. An audit of sample sizes for pilot and feasibility trials being undertaken in the United Kingdom registered in the United Kingdom clinical research network database. BMC Med Res Methodol 2013;13:104. 10.1186/1471-2288-13-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sivan M, Greenhalgh T, Darbyshire JL, et al. Long COvid multidisciplinary Consortium optimising treatments and servIces acrOss the NHS (locomotion): protocol for a mixed-methods study in the UK. BMJ Open 2022;12:e063505. 10.1136/bmjopen-2022-063505 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-066044supp001.pdf (125.9KB, pdf)
bmjopen-2022-066044supp002.pdf (147.6KB, pdf)