Abstract
Introduction
Breast cancer survivors treated with adjuvant endocrine therapy commonly experience weight gain, which has been associated with low adherence to therapy and worse breast cancer prognosis. We aim to assess whether a personalised postprandial glucose targeting diet will be beneficial for weight management as compared with the recommended Mediterranean diet in this patient population
Methods and analysis
The BREAst Cancer Personalised NuTrition study is a phase-2 randomised trial in hormone receptor positive patients with breast cancer, treated with adjuvant endocrine therapy. The study objective is to assess whether dietary intervention intended to improve postprandial glycaemic response to meals results in better weight and glycaemic control in this population as compared with the standard recommended Mediterranean diet. Consenting participants will be assigned in a single blinded fashion to either of two dietary arms (Mediterranean diet or an algorithm-based personalised diet). They will be asked to provide a stool sample for microbiome analysis and will undergo continuous glucose monitoring for 2 weeks, at the initiation and termination of the intervention period. Microbiome composition data will be used to tailor personal dietary recommendations. After randomisation and provision of dietary recommendations, participants will be asked to continuously log their diet and lifestyle activities on a designated smartphone application during the 6-month intervention period, during which they will be monthly monitored by a certified dietitian. Participants’ clinical records will be followed twice yearly for 5 years for treatment adherence, disease-free survival and recurrence.
Ethics and dissemination
The study has been approved by the ethics committee in the Sheba medical centre (file 5725-18-SMC, Ramat Gan, Israel) and the Weizmann Institutional Review Board (file 693-2, Rehovot, Israel). The findings of this study will be published in a peer reviewed publication.
Trial registration number
Keywords: nutrition & dietetics, breast tumours, microbiology
STRENGTHS AND LIMITATIONS OF THIS STUDY.
A single blinded study where patients are being assigned to one out of two dietary arms (Mediterranean diet or an algorithm-based personalised diet).
The personalised diet involves advanced machine-learning analysis of multi-omics dataset, including microbiome, continuous glucose monitoring, metabolomics features and full dietary records.
A homogeneous study population including hormone receptor positive, early-stage breast cancer survivors treated with adjuvant endocrine therapy, albeit representing mostly women from the centre of Israel.
Patients are being randomised into the two study arms and stratified by stage, treatment type, menopausal status and body mass index.
The study design includes daily use of smartphone application for logging dietary intake and lifestyle events. This may lead to exclusion of patients inaccessible to smartphone app on a daily basis.
Introduction
The majority (~75%) of patients with breast cancer are diagnosed with hormone receptor-positive (HR+) tumours and are assigned adjuvant endocrine treatment (ET) for a period of at least 5 years, which was shown to improve survival. However, adjuvant ET is associated with distressing side effects which may be long-lasting and substantially impair patients’ quality of life and adherence to treatment. These side effects include weight gain and body composition changes, which are common in breast cancer survivors and are experienced by many women during treatment and for years after diagnosis.1–3 Weight gain in this population is complex and is associated with various factors such as tumour type, menopausal status,4 prediagnosis body mass index (BMI)5 and neoadjuvant/adjuvant treatment type including chemotherapy and ET.2 6 Importantly, weight gain after breast cancer diagnosis is associated with increased risk for metabolic syndrome and cardiac disease,7 8 and was reported as a risk factor for breast cancer recurrence and shorter survival.4 9 10 Therefore, weight management strategies including diet, regular physical activity (PA) and cognitive behavioural therapy are recommended for controlling weight gain in patients with breast cancer. Previous studies showed that weight loss interventions, incorporating diet, exercise and psychosocial support, in overweight or obese breast cancer survivors appear to result in decreased body weight, BMI and waist circumference and improvement in overall quality of life.11 We chose the Mediterranean (MED) diet as a control diet because it is commonly recommended in different countries, including Israel12 and was suggested to improve metabolic health in the general population as well as within breast cancer survivors.13–15 Still, the optimal weight loss intervention method and the impact of weight loss on survival outcomes is unclear. Furthermore, the interaction between the microbiome of patients with breast cancer and dietary intervention has not been assessed.
The comprehensive role of the gut microbiome in modulating immune and metabolic health is increasingly recognised. Dysbiosis, referring to the disruption in the balance of gut bacterial communities, is associated with many conditions.16 The gut microbiome homeostasis can be influenced by internal factors, such as genetic, age related and hormonal related, as well as by external factors, such as stress, lifestyle and antibiotics.17 In addition, the microbiome is directly affected by the individual diet which in turn affects the body’s response to food.18 19 Particularly relevant to breast cancer, diet plays an important role in creating a microbiome environment involved in oestrogen metabolism.20 High oestrogen levels contribute to breast cancer risk in postmenopausal women.21 In a recent study, gut microbiome diversity was linked to weight gain22 and microbiome alterations were found to contribute to postdieting weight regain.23 In addition, it was found that the increase in breast cancer risk with increasing BMI among postmenopausal women is associated with an increase in estrogens, particularly bioavailable estradiol.24 In a previous study, we showed in an unprecedented scale of 800 people that individuals vary greatly in their glycaemic response to the same food.25 Importantly, this study emphasised the involvement of functional microbial pathways and bacterial taxa in host glucose metabolism. This unique dataset yielded an algorithm capable of accurately predicting personalised postprandial glycaemic response (PPGR) to arbitrary meals. The algorithm’s predictions are based on personal measurements, including blood tests, personal lifestyle and gut bacteria profiles. In a following study implementing a 6-month dietary intervention plan in individuals with pre-diabetes, the PPGR targeting (PPT) approach significantly improved glycaemic control and reduced PPGRs as compared with the commonly recommended MED diet.26
In this study, we seek to evaluate the clinical efficacy of the PPT diet combined with caloric restriction, compared with the MED diet, in promoting weight maintenance or weight loss and glycaemic control in HR+ early stage breast cancer survivors treated with adjuvant ET.
Methods
Study design
This study is a two-arm, parallel group, single-blinded, randomised controlled trial in early stage HR+ patients with breast cancers treated with adjuvant endocrine therapy. Eligible participants will undergo a 6-month nutrition intervention programme, which will include dietary recommendations, daily logging and monthly follow-up meetings provided by a certified dietician. On trial entry and after profiling (described below) participants will be randomly and equally assigned to the personalised PPT dietary (arm A) or to the MED-style dietary (arm B). All meetings will take place in the Breast Oncology Institute at the Sheba Medical Center. The primary objective of the study is to evaluate the efficacy of the PPT arm vs the MED arm in controlling body mass changes in the patient population during the intervention period (see summarised study endpoints in box 1). For complete Standard Protocol Items: Recommendations for Interventional Trials checklist and the full protocol, see online supplemental materials 1 and 2, respectively).
Box 1. Study endpoints.
Primary endpoint
Body weight changes defined as the net body weight gained/lost in the 6-month intervention period.
Secondary endpoint
Glycaemic response as measured by the area under the glucose curve during continuous glucose monitoring period preintervention and during the intervention.
Exploratory endpoint
Five years disease-free survival.
Microbiome and blood metabolites modulation during the diet interventions—tested using the samples taken at profiling and 6-month time points.
Adherence to algorithm-based personalised diets, compared with standard diets advised for weight control—assessed by monthly compliance questionnaire.
Hormone receptor positive patients with breast cancer adherence to hormonal treatment.
Translational studies.
bmjopen-2022-062498supp001.pdf (69.4KB, pdf)
bmjopen-2022-062498supp002.pdf (1MB, pdf)
Patient and public involvement
Research questions and outcome measures were partially based on numerous encounters of ENG in the clinic with patients with breast cancer voicing concerns regarding weight gain and optimal diet while on ET for breast cancer. Furthermore, patients are being involved in the recruitment effort by actively publishing the study recruitment information and sharing their own experience during the study, via social networks and breast cancer survivors’ groups. The patients are being followed up in the clinic for a long period of time (~10 years). Accumulating study results will be summarised periodically, transferred to the study team including treating and recruiting physicians and through them transferred to patients during clinic visits. Patients will also be informed regarding publications and specific results through the cancer institute social network and digital resources (such as the Sheba oncology web application and Sheba oncology Facebook page).
Study population
This trial will enrol breast cancer survivors treated with ET and followed at the Breast Oncology Institute at the Sheba Medical Center. Eligibility criteria (inclusion and exclusion criteria) are detailed in table 1. Potentially eligible participants will be identified and recruited to the study by the medical team during regular clinic visits or via database search and phone calls by the clinical study coordinator (SC). Information leaflets and a poster describing the study design and contact information will be available at the institute’s reception and waiting area. In addition, a video explaining the study and its aims will be shown on screens at the institute’s reception and waiting area and will be sent to potential participants (https://youtu.be/kxrqONj3KGM). All participants will assign informed consent.
Table 1.
Eligibility criteria
| Inclusion criteria | Exclusion criteria |
| Female patients | Oral antibiotics/antifungal use in the previous 1 month to profiling stage* |
| Age ≥18 and ≤80 | Known diagnosis of diabetes or the use of anti-diabetic and/or weight-loss medication |
| Diagnosis of stage 1–3 HR+ breast cancer, who underwent surgery | BMI <18.5 kg/m2 |
| At least 60 days after last non-endocrine oncology treatments (ie, definitive surgery, radiation or chemotherapy—whichever is last) if these were indicated. | Patients under another diet regimen and/or a dietitian consultation/clinical study |
| Adjuvant endocrine therapy (either Tamoxifen or Aromatase inhibitor+/-GNRH agonists) taken for at least 30 days but no more than 24 months. | Pregnancy, breast feeding |
| Willing to operate a smartphone application | HIV carriers, cushing syndrome, chronic kidney disease, acromegaly, hyperthyroidism, liver cirrhosis |
| Known diagnosis of psychiatric disorders (schizophrenia, bipolar disorder) | |
| Known diagnosis of (inflammatory bowel diseases) | |
| Patients that underwent bariatric surgery | |
| Known alcohol or substance abuse |
*Patients will be offered to join the study at a later point.
BMI, body mass index.
Study procedures and intervention
Informed Consent Form (ICF) signed (weeks −8 to −4)
Eligible participants will be invited to sign an inform consent at the oncologic clinic in Sheba medical centre (as shown in figure 1).
Figure 1.
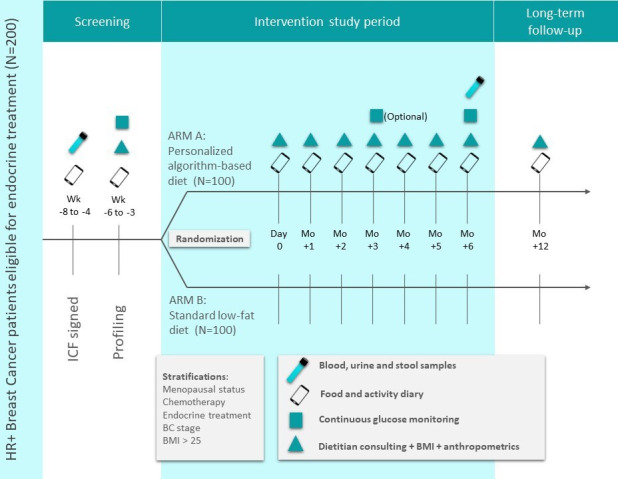
An illustration of the study design. BC, breast cancer; BMI, body mass index; HR+, hormone receptor positive; ICF, informed consent form.
Profiling stage (weeks −6 to −3)
Consenting patients will proceed to the profiling stage. During this stage they will undergo the following procedures:
Meeting with the SC and completion of questionnaires detailing relevant medical background, nutritional habits and lifestyle activities. Questionnaires will be filled online using the REDCap27 software (a secure web application for managing online surveys and clinical trials).
Participants will provide blood samples after a night fast (12 hours) for complete blood count and blood chemistry, including liver function, lipid profiling, fasting plasma glucose (FPG) and Glycated Hemoglobin (HbA1c). Luteinising hormone (LH), follicle stimulating hormone (FSH) and estradiol will be measured only in premenopausal patients. Participants will provide urine sample for estradiol derivatives for future exploratory analyses.
Anthropometrics measurements, including weight, height, waist and hip circumference will be taken at this meeting.
Stool sample: Patients will receive a designated stool kit (Genotek OMR200) to collect stool at home. The SC will instruct them how to provide the stool samples and will ask to return this kit during the following week for further processing of the microbiome data. Microbiome sequenced data are essential for the algorithm predictions, thus, stool sample is obligatory for participation in the study.
Continuous glucose monitoring (CGM) connection: Patients will be connected to a CGM (Abbott Freestyle LibrePro) for 2 weeks. The CGM kit includes a sensor affixed to the back of the arm that continuously monitors glucose levels in the interstitial fluid, translates and records blood glucose levels.
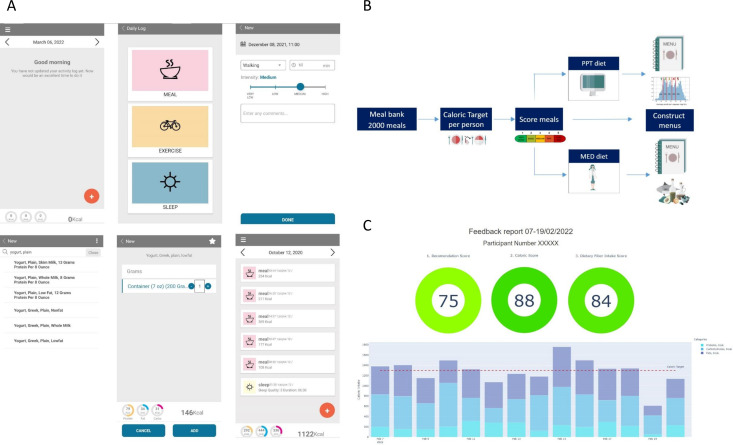
Food diary: Patients will be instructed to download the study dedicated smartphone application (‘personalised nutrition project’) for food logging. They will log in real-time their food intake, PAs, sleep duration and quality and special events. During the profiling period, patients will be asked to follow their regular dietary and lifestyle habits (see examples of logging activities in figure 2A). All participants will receive a registration code and their data will be anonymised.
Data collected during the profiling period, including microbiome, anthropometrics, blood parameters and questionnaires, will be analysed and used by the PPT algorithm to provide personal dietary recommendations for each participant.
Figure 2.
Study application and menus construction. (A) The food logging application, examples of logging activities and information available on the application. (B) Menus construction flow. (C) An example of the biweekly feedback report that will be sent to participants. PPT, Personalized Postprandial Targeting; MED, Mediterranean.
Randomisation
After completion of the profiling stage, patients will be randomly assigned to one of two arms of the study by one programmer from the trial personnel who had no contact with participants. Approximately 100 subjects will be assigned to each arm using a blinded randomisation algorithm and the following stratification factors: (1) menopausal status at study entry (post/pre); (2) received/not received chemotherapy prior to study entry; (3) ET type (tamoxifen/aromatase inhibitor); (4) breast cancer stage at diagnosis and (5) BMI above/below 27 kg/m2. Notably, we only used the stratification factors to minimise differences between groups in the allocation process and did not analyse the data according to the stratification factors. Patients and part of the study team (oncologists and SC), excluding the dietitian, will be blinded to the study arm assigned. At the end of intervention, dietary assignment was revealed, and participants were asked to continue following their respective diets for six additional months.
Recommendation meeting (day 0)
On menus construction, patients from both arms will be invited to a recommendations meeting (hereafter ‘day 0’) with the dietitians. Patients will receive general information regarding their menu and will be instructed to consume and log their meals according to it. In order to ensure accurate logging the dietitians will schedule an online follow-up 2 weeks after ‘day 0’. Anthropometrics measurements, including weight, hip and waist circumference, taken at this meeting will be used as baseline measurements.
Follow-up meetings (month +1 up to month +5)
All patients will participate in monthly follow-up meetings with a dietitian (total of six meetings) in order to evaluate their compliance to the dietary recommendations they received and provide additional advice if needed. Anthropometric measurements (weight, hip and waist circumference) will be taken at each time point. Furthermore, patients will be asked to fill a follow-up questionnaire and report any changes within their lifestyle and treatment. At the beginning of month 4 of the intervention period, patients will be offered to be reconnected to CGM for 2 weeks (optional). At the monthly meeting before the end of intervention patients will receive a stool kit, to be returned at the end of intervention meeting.
End of intervention (month +6)
At the end of the 6-month intervention period, patients will be invited to a meeting in which anthropometrics measurement will be taken, as well as urine, blood and stool samples. In addition, participants will be connected to CGM for 2 weeks (mandatory) for the third time (figure 1). When patients return CGM they will be unblinded to their assigned intervention arm by the study dietitian.
Long term follow-up (month +12)
At 12-month time point, patients will be invited to a follow-up meeting and will be asked to fill follow-up questionnaires, including a Food Frequency Questionnaire. Anthropometric measurements and a 3-day food diary on the study app will be recorded. Patients will receive the menu of the other study arm and will be offered to follow either one of the diets. Long-term clinical follow-up information will be collected from the electronic medical records twice yearly for treatment adherence, recurrence and survival calculation purposes, for a period of up to 5 years postrandomisation.
Menus construction
Before randomisation, menus will be constructed for each patient and will be adjusted for, patients’ caloric target (CT) and clinical data. The menus construction flow is presented in figure 2B.
Meal bank (list)
The menus provided to patients in this study are constructed from a meal bank that we previously generated,26 with over 2000 meals representative of the Israeli typical diet and with a variety of different food combinations. We divided the meals in the meal bank into four meal types (breakfast, lunch, dinner and snacks) and labelled them according to meal categories (dairy, meat, fish, etc) in order to generate menus according to patients’ personal preferences.
CT calculation
In order to provide the patients with diets that support their energetic needs and meet the recommendations for weight loss in people with overweight or obesity, the daily CT for each patient (in both arms) will be calculated as an average between:
Estimated energy requirements calculated with the use of the Mifflin equation for resting energy expenditure (REE), using their weight, height, age and gender, and multiplied by PA factor, based on the level of PA that the person performs on a regular basis.28
Energy expenditure assessed by Basal Metabolic Rate value measured by body composition analyzer (Tanita). The result from this equation will be divided by 0.7 (as REE represents ~70% of total energy expenditure).
Average daily caloric intake obtained from the patients' dietary records during the profiling stage, to account for the subject’s dietary habits prior to the intervention.
Furthermore, for individuals with BMI >25 kg/m2, a total of 500 Kcal will be reduced from their calculated CT, but not less than 1200 calories/day, to allow weight loss according to common recommendations for weight loss.29 30
Diets
MED-style diet
In this arm, we included meals that were scored by four external dietitians according to the MED-style dietary recommendations. Meals were binary scored as recommended (=1) or not recommended (=0) and we applied scores 1–5 to all meals, depending on how many dietitians marked the meal as recommended or not. The diet is based on recommended foods such as vegetables, fruits, legumes, whole grain products, unsaturated fats such as olive oil and nuts, fish, poultry and low-fat dairy products. Consumption of red meat, high fat dairy products, processed foods and sweet pastries, was discouraged as part of the diet. In addition, menus in this arm were designed with the following target for daily macronutrient composition: 45%–65% of calories from carbohydrates; 15%–20% from protein and 20%–35% from fat, with up to 10% from saturated fat. Menus include only meals that received scores 1 and 2. Participants will be encouraged to consult with the dietitian regarding meals that may not appear in the constructed menu.
Personalised PPT diet
In this arm, dietary recommendations will be based on the algorithm predictions of the postprandial glucose responses,25 shown to improve glycaemic control and metabolic health in healthy individuals or in individuals with pre-diabetes and diabetes.26 31 Notably, these interventions were not caloric restricted as in the current study. Among the features used to predict PPGR to meals were anthropometrics, blood tests (FPG, HbA1c% and haemoglobin), lifestyle features derived from questionnaires, microbiome (abundances of species estimated by MetaPhlAn2 and meal features (macronutrient and micronutrient composition) were used (see online supplemental table 1 for the full list). Since no events around the meal were used for prediction, trained predictor could predict response for any profiled participant to any given meal.
bmjopen-2022-062498supp003.pdf (169.9KB, pdf)
All logged meals will be scored from 1 to 5 based on a unique scoring method that we developed and tested in previous studies, and study participants will be asked to consume only meals with score 1 or 2. Importantly, the PPT diet, by definition, was not aimed to have a predetermined macronutrient distribution, in contrast to the Med-diet.
Adherence to the study recommendations
The adherence to the prescribed diets during the intervention will be evaluated by the dietitian by a close monitoring of the patients’ self-recorded dietary intake in the logging application, as well as by monthly electronic follow-up questionnaires that participants will be asked to fill out. In order to encourage dietary adherence and self-monitoring, we will generate a biweekly semiautomatic feedback reports that will include composite grades on a scale of 0–100 (from worse to best) for diet composition, calorie intake and dietary fibre intake separately, for the entire 2-week period (figure 2C).
MED-diet composition grade: indicates how well the participant sticks to the dietary recommendations based on the MED approach including Carbohydrate (as % of daily caloric intake), fats in general (as % of daily caloric intake) and specifically saturated fat intake below and above 10% of caloric intake. Dietary fibre intake per each of 1000 kCal per day will be also calculated as part of the score.
PPT-diet composition grade: indicates how well the participant sticks to predictor-based meal scores. Each meal score was assigned with a grade as follows: meal score 1=grade 100; meal score 2=80; meal score 3=50; meal score 4=25; meal score 5=0. The grades are averaged caloriewise (with food energy trimmed to be within (100 500) kcal interval) - Σ kcal(i) · grade(i). For example, if a participant eats three meals: 600 kcal of meal score 2, 1000 kcal of meal score 5 and 80 kcal of meal score 1, feedback grade would be: (500×80+500×0+100×100)/(500+500+100)=45. If too few (100 by default) calories are logged (overall), we did not compute a score.
Calories grade: indicates how well the participant sticks to the prescribed CT. When caloric intake deviates within 15% of CT the applied grade is 100; when caloric intake deviation exceeds 60% of CT the applied grade is 0; when caloric intake deviation is between 15% and 60%, a linear penalty is applied to the grade depending on the deviation.
Dietary fibre grade: indicates if participants consumed the recommended amount of dietary fibres (set to 14 g for every 1000 kcal/day for both arms) from the diet at the referred time when fibre intake in grams per day reaches the recommended amount, or higher the applied grade is 100 and when it is below the recommended amount a linear penalty is applied to the grade.
In addition to grades, feedback reports also included a list of recommended meals and non-recommended meals (by meal score) to highlight the best and worst meals consumed on that time period (as logged by the participant). The best and worst meals lists will be generated systematically and be reviewed by a dietitian from the study team.
Statistical consideration
Sample size determination
To estimate the required sample size we performed power analysis, using an unpaired t-test assuming normal distribution of the primary outcome (weight change), estimating the effect size based on the results of different studies describing controlled diet intervention aimed at weight control. Our goal is to detect a difference of at least 2 kg in net weight loss/gain (kg) between control group and experimental group. Based on the study of Shai et al,32 the SD of weight loss is 4.2 and the projected sample size with alpha of 0.05 and power of 0.8, with an estimated dropout rate of 10%, is 107 people for each arm totaling 214 individuals.
Primary, secondary and exploratory endpoint analysis
All statistical analyses will be performed by using Python V.2.7. Continuous variables will be presented as mean±SD and dichotomous/categorical variables as proportions. The normality of the distribution of continuous variables will be tested by the Kolmogorov-Smirnov test. If normality will be rejected, non-parametric tests will be used. To test the association between continuous variables with normal distribution, the Pearson correlation coefficient will be performed and to test associations between continuous variables which do not distribute normally or for ordinal variables, the Spearman correlation coefficient will be used. To compare parameters for continuous variables in two time points, the paired-samples t-test will be performed (or Wilcoxson test for non-normally distributed variables), in dichotomous/categorical variables the McNemar test will be performed. To compare continuous variables in a number of time points analysis of variance with repeated measures will be used. For comparison of dichotomous/categorical variables in the number of time points the Cochran’s Q test will be performed. P<0.05 will be considered significant.
Data acquisition, storage and analysis
All samples will be stored at the Breast Cancer Translational Research laboratory at Sheba Medical Center. The blood and urine samples will be stored at −80°C and bacterial DNA samples will be stored at −20°C. The samples will be encoded with no identifying information. Identifying details and codes will be kept in an encrypted file stored at Sheba medical centre. Encoded stool samples will be transferred to the Weizmann Institute of science. There, samples will be processed for bacterial DNA processing. All clinical data will be coded. Data will be transferred using the REDcap server and stored on Weizmann servers behind a protected firewall and be accessible only to the study team. Samples will be stored for up to 10 years. All future use of stool and blood samples will be subjected to IRB approval.
Ethics and dissemination
The study has been approved by the Sheba medical centre Institutional Review Board (IRB 5725-18) and the Weizmann institute of science Institutional Review Board. The findings of this study will be published in a peer-reviewed publication. Deidentified individual participant data and applicable supporting clinical trial documents will be available on request for 12 months after publication.
Current status
Enrolment and recruitment initiated on July 2019. To date (February 2022), 120 participants have been recruited, out of them 60 completed the 6-month intervention period including 38 participants who completed the 12-month time point.
Discussion
Dietary interventions are the first-line treatment for weight management within breast cancer survivors and have beneficial results. Yet, the ability to maintain these outcomes is questionable and require further research.11 33 34 In this trial, we aim to assess the effect of a PPT diet on weight maintenance as compared with MED-style diet in early stage HR+ patients with breast cancer, taking ET.
This study has several strengths and limitations. Advantages of this study design include a comprehensive profiling of each participant, which allow us to better understand participants’ metabolic baseline and to assess the effect of the dietary changes. In addition, the continuous food logging by the study patients using a designated smartphone app can provide us with insights on the patients’ compliance to the dietary recommendations in both arms. However, this may limit the study population to individuals who are able to work with smartphone application on a daily basis. Furthermore, the study participants are being closely followed by a dietitian from the study team who monitor their food intake and meet them on a monthly basis in order to increase compliance during the first 6 months. However, in the long term, without intensive monitoring, the feasibility of the PPT diet and the ability to follow the diet recommendations should be investigated. Notably, Ben-Yacov et al,26 reported that pre-diabetes individuals following PPT diet were able to maintain the results during 12-month follow-up as compared with those who followed the MED diet. In addition, as a novel tool, the algorithm is not available for general use which makes it difficult to replicate the intervention. Nevertheless, we do publish the full list of features we use to generate the menus, based on personal and microbiome data (online supplemental table 1).
Lastly, microbiome composition and pathways were recently associated with weight changes and metabolic health parameters, as well as with risk for breast cancer diagnosis and recurrence.35 This may allow us to further explore whether gut microbiome composition and pathways have a predictive role in weight management, metabolic health parameters, glycaemic control and even disease recurrence on the next 5 years after the intervention within patients with breast cancer, although for disease recurrence differences the sample size may not be large enough.
Taken together, our rich dataset including deep phenotyping of each patient may allow us to deeply investigate associations between clinical and Omic data to disease-free survival in early stage HR+ patients with breast cancer and may pave the way to larger studies.
Supplementary Material
Footnotes
Twitter: @ReinMichal
Contributors: MSR, MD and AW conceived the study and designed the intervention. MSR and MD wrote the manuscript. AG is responsible for directing the computational aspects of the study. DK is responsible for the feedback reports and summary reports being sent to participants. MB-G, DM-S and YV coordinate participants’ recruitment and management throughout the intervention and follow-up. AW developed the protocols and directed and performed the microbiome sample sequencing with the help of ML-P. ES and ENG-Y conceived the study, designed the intervention and wrote the manuscript. All authors read and approved the final manuscript.
Funding: This work is supported by Seerave foundation (grant number 713067).
Disclaimer: This funding source had no role in the design of this study and will not have any role during its execution, analyses, interpretation of the data, or decision to submit results.
Competing interests: ENG-Y reports Honoraria and Consulting fees from Pfizer, Novartis, Roche Eli-lilly and AstraZeneca. ES reports scientific consultant fees from Day Two Inc. No pharmaceutical manufacturers or other companies from the industry contributed to the planning, design, or conduct of the trial. No other potential competing interest are relevant to this article were reported.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Vance V, Mourtzakis M, McCargar L, et al. Weight gain in breast cancer survivors: prevalence, pattern and health consequences. Obes Rev 2011;12:282–94. 10.1111/j.1467-789X.2010.00805.x [DOI] [PubMed] [Google Scholar]
- 2.Goodwin PJ, Ennis M, Pritchard KI, et al. Adjuvant treatment and onset of menopause predict weight gain after breast cancer diagnosis. J Clin Oncol 1999;17:120–9. 10.1200/JCO.1999.17.1.120 [DOI] [PubMed] [Google Scholar]
- 3.Ginzac A, Thivat Émilie, Mouret-Reynier M-A, et al. Weight evolution during endocrine therapy for breast cancer in postmenopausal patients: effect of initial fat mass percentage and previous adjuvant treatments. Clin Breast Cancer 2018;18:e1093–102. 10.1016/j.clbc.2018.06.010 [DOI] [PubMed] [Google Scholar]
- 4.Raghavendra A, Sinha AK, Valle-Goffin J, et al. Determinants of weight gain during adjuvant endocrine therapy and association of such weight gain with recurrence in long-term breast cancer survivors. Clin Breast Cancer 2018;18:e7–13. 10.1016/j.clbc.2017.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White AJ, Nichols HB, Bradshaw PT, et al. Overall and central adiposity and breast cancer risk in the sister study. Cancer 2015;121:3700–8. 10.1002/cncr.29552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nyrop KA, Williams GR, Muss HB, et al. Weight gain during adjuvant endocrine treatment for early-stage breast cancer: what is the evidence? Breast Cancer Res Treat 2016;158:203–17. 10.1007/s10549-016-3874-0 [DOI] [PubMed] [Google Scholar]
- 7.Zhu Q-L, Xu W-H, Tao M-H. Biomarkers of the metabolic syndrome and breast cancer prognosis. Cancers 2010;2:721–39. 10.3390/cancers2020721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franzoi MA, Agostinetto E, Perachino M, et al. Evidence-Based approaches for the management of side-effects of adjuvant endocrine therapy in patients with breast cancer. Lancet Oncol 2021;22:e303–13. 10.1016/S1470-2045(20)30666-5 [DOI] [PubMed] [Google Scholar]
- 9.Caan BJ, Emond JA, Natarajan L, et al. Post-Diagnosis weight gain and breast cancer recurrence in women with early stage breast cancer. Breast Cancer Res Treat 2006;99:47–57. 10.1007/s10549-006-9179-y [DOI] [PubMed] [Google Scholar]
- 10.Playdon MC, Bracken MB, Sanft TB, et al. Weight gain after breast cancer diagnosis and all-cause mortality: systematic review and meta-analysis. J Natl Cancer Inst 2015;107:djv275. 10.1093/jnci/djv275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaikh H, Bradhurst P, Ma LX, et al. Body weight management in overweight and obese breast cancer survivors. Cochrane Database Syst Rev 2020;12:CD012110. 10.1002/14651858.CD012110.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abu-Saad K, Endevelt R, Goldsmith R, et al. Adaptation and predictive utility of a Mediterranean diet screener score. Clin Nutr 2019;38:2928–35. 10.1016/j.clnu.2018.12.034 [DOI] [PubMed] [Google Scholar]
- 13.Negrati M, Razza C, Biasini C, et al. Mediterranean diet affects blood circulating lipid-soluble micronutrients and inflammatory biomarkers in a cohort of breast cancer survivors: results from the SETA study. Nutrients 2021;13. 10.3390/nu13103482. [Epub ahead of print: 30 Sep 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Maso M, Maso LD, Augustin LSA, et al. Adherence to the Mediterranean diet and mortality after breast cancer. Nutrients 2020;12:3649. 10.3390/nu12123649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braakhuis A, Campion P, Bishop K. The effects of dietary nutrition education on weight and health biomarkers in breast cancer survivors. Med Sci 2017;5. 10.3390/medsci5020012. [Epub ahead of print: 02 Jun 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plottel CS, Blaser MJ. Microbiome and malignancy. Cell Host Microbe 2011;10:324–35. 10.1016/j.chom.2011.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erdman SE, Poutahidis T. Gut bacteria and cancer. Biochim Biophys Acta 2015;1856:86–90. 10.1016/j.bbcan.2015.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vrieze A, Holleman F, Zoetendal EG, et al. The environment within: how gut microbiota may influence metabolism and body composition. Diabetologia 2010;53:606–13. 10.1007/s00125-010-1662-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shulzhenko N, Morgun A, Hsiao W, et al. Crosstalk between B lymphocytes, microbiota and the intestinal epithelium governs immunity versus metabolism in the gut. Nat Med 2011;17:1585–93. 10.1038/nm.2505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shapira I, Sultan K, Lee A, et al. Evolving concepts: how diet and the intestinal microbiome act as modulators of breast malignancy. ISRN Oncol 2013;2013:1–10. 10.1155/2013/693920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goedert JJ, Jones G, Hua X, et al. Investigation of the association between the fecal microbiota and breast cancer in postmenopausal women: a population-based case-control pilot study. J Natl Cancer Inst 2015;107. 10.1093/jnci/djv147. [Epub ahead of print: 01 06 2015]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menni C, Jackson MA, Pallister T, et al. Gut microbiome diversity and high-fibre intake are related to lower long-term weight gain. Int J Obes 2017;41:1099–105. 10.1038/ijo.2017.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thaiss CA, Itav S, Rothschild D, et al. Persistent microbiome alterations modulate the rate of post-dieting weight regain. Nature 2016;540:544–51. 10.1038/nature20796 [DOI] [PubMed] [Google Scholar]
- 24.Key TJ, Appleby PN, Reeves GK, et al. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst 2003;95:1218–26. 10.1093/jnci/djg022 [DOI] [PubMed] [Google Scholar]
- 25.Zeevi D, Korem T, Zmora N, et al. Personalized nutrition by prediction of glycemic responses. Cell 2015;163:1079–94. 10.1016/j.cell.2015.11.001 [DOI] [PubMed] [Google Scholar]
- 26.Ben-Yacov O, Godneva A, Rein M, et al. Personalized postprandial glucose Response-Targeting diet versus Mediterranean diet for glycemic control in prediabetes. Diabetes Care 2021;44:1980–91. 10.2337/dc21-0162 [DOI] [PubMed] [Google Scholar]
- 27.Harris PA, Taylor R, Minor BL, et al. The REDCap Consortium: building an international community of software platform partners. J Biomed Inform 2019;95:103208. 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mifflin MD, St Jeor ST, Hill LA, et al. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr 1990;51:241–7. 10.1093/ajcn/51.2.241 [DOI] [PubMed] [Google Scholar]
- 29.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American heart association Task force on practice guidelines and the obesity Society. J Am Coll Cardiol 2014;63:2985–3023. 10.1016/j.jacc.2013.11.004 [DOI] [PubMed] [Google Scholar]
- 30.Limon-Miro AT, Lopez-Teros V, Astiazaran-Garcia H. Dietary guidelines for breast cancer patients: a critical review. Adv Nutr 2017;8:613–23. 10.3945/an.116.014423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rein M, Ben-Yacov O, Godneva A, et al. Effects of personalized diets by prediction of glycemic responses on glycemic control and metabolic health in newly diagnosed T2DM: a randomized dietary intervention pilot trial. BMC Med 2022;20:56. 10.1186/s12916-022-02254-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shai I, Schwarzfuchs D, Henkin Y, et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med 2008;359:229–41. 10.1056/NEJMoa0708681 [DOI] [PubMed] [Google Scholar]
- 33.Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, et al. Obesity and adverse breast cancer risk and outcome: mechanistic insights and strategies for intervention. CA Cancer J Clin 2017;67:378–97. 10.3322/caac.21405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anbari AB, Deroche CB, Armer JM. Body mass index trends and quality of life from breast cancer diagnosis through seven years' survivorship. World J Clin Oncol 2019;10:382–90. 10.5306/wjco.v10.i12.382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uzan-Yulzari A, Morr M, Tareef-Nabwani H, et al. The intestinal microbiome, weight, and metabolic changes in women treated by adjuvant chemotherapy for breast and gynecological malignancies. BMC Med 2020;18:281. 10.1186/s12916-020-01751-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-062498supp001.pdf (69.4KB, pdf)
bmjopen-2022-062498supp002.pdf (1MB, pdf)
bmjopen-2022-062498supp003.pdf (169.9KB, pdf)